The results of this study showed that exposure to desiccating stress stimulates production of cornified envelope precursors and the tissue transglutaminase enzyme that cross-links them via the ocular surface epithelia. These cornifying cells entrap conjunctival goblet cells and block egress of their mucin contents to the ocular surface. This mechanism may contribute to the mucin deficiency that develops in dry eye.
Abstract
Purpose.
To evaluate the effects of desiccating stress on conjunctival goblet cell density and morphology and the expression of cornified envelope precursors by the ocular surface epithelia.
Methods.
Experimental dry eye (EDE) was created in C57BL/6 mice. Real-time PCR evaluated the expression of cornified envelope (CE) precursor proteins (involucrin and small proline-rich [Sprr] -1a, -1b, -2a, -2b, -2f, and -2g proteins), the cross-linking transglutaminase 1 enzyme (Tg-1) and Muc5AC mRNA transcripts by the ocular surface epithelia. Laser scanning confocal microscopy evaluated the expression of the CE precursor proteins Tg-1 and Muc5AC in cryosections. Tg-1 activity was measured by a fluorescein cadaverine assay. Muc5AC concentration was measured by ELISA.
Results.
Levels of involucrin; Sprr-1a, -1b, -2a, -2b, -2f, and -2g; and Tg1–1 mRNA transcripts in ocular surface tissues increased in response to desiccating stress. Expression and activity of Tg in the conjunctiva markedly increased after EDE. Desiccating stress caused progressive loss of mucin-filled goblet cells. The apical portion of the remaining conjunctival goblet cells became entrapped by adjacent stratified apical epithelia expressing increased levels of cornified envelope precursors.
Conclusions.
Exposure to desiccating stress stimulates ocular surface epithelia to produce cornified envelope precursors and the tissue transglutaminase enzyme that cross-links them. This effect is accompanied by loss of mucin-filled goblet cells and entrapment of mucin contents in the remaining ones by cornifying cells that block the egress of mucin contents to the ocular surface. This mechanism may contribute to the conjunctival mucin deficiency that develops in dry eye.
The ocular surface epithelium has an essential function in maintaining comfort and high-quality vision. The cornea and conjunctiva are covered by noncornified stratified epithelia whose apical cells produce mucins that maintain hydration and lubrication of the ocular surface. Furthermore, the conjunctiva contains goblet cells that produce and secrete Muc5AC, a mucin that is a stabilizing factor in the precorneal tear layer. In contrast, the terminally differentiated apical epidermal cells in mammalian skin are lined with a 10-nm-thick layer of proteins called the cornified cell envelope (CE), which forms inside the plasma membrane and becomes insoluble as the result of the cross-linking of constituent proteins by transglutaminase (Tg) enzymes.1 The epidermal CE is composed of several proteins, including scaffold proteins, such as involucrin, and envoplakin and reinforcement proteins, such as loricrin, cystatin A, filaggrin, cysteine-rich protein, and the small proline-rich (Sprr) proteins.2
The stratified ocular surface epithelia bear similarities to the skin epidermis in their pattern of basal-to-superficial polarity,3 but no morphologic evidence of a CE structure has been found. Nevertheless, the CE precursor involucrin has been found to be expressed in the normal corneal epithelium4,5 and the CE precursors involucrin and filaggrin have been found to be elevated in the human conjunctival epithelium in severe ocular surface diseases such as Stevens-Johnson syndrome and after alkali burns.6 It is also known that the corneal epithelium and its basement membrane show Tg cross-linking activity.7
A hallmark of dry eye is poor wettability of the ocular surface and tear film instability. This effect may be due in part to reduced production or secretion of mucins by the ocular surface epithelia, but clinical and immunohistologic evidence suggests that there may be a shift toward a cornified envelope phenotype by the ocular surface epithelia.8 Frank cornification can be observed in severe ocular surface diseases such as Stevens-Johnson6 syndrome, where there is extensive loss of conjunctival goblet cells. We hypothesize that desiccating stress promotes expression of CE precursors and transglutaminase (Tg)-1 by the ocular surface epithelia and alters conjunctival goblet cell mucin secretion.
The purpose of this study was to evaluate the effects of desiccating stress in our experimental murine dry eye model on expression of CE precursors and Tg-1 by ocular surface epithelia, particularly the goblet cell–containing regions of the conjunctiva.
Methods
Experimental Desiccating Ocular Surface Stress
This research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine, and it conformed to the standards in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Experimental dry eye (EDE) was induced in 6- to 8-week-old C57BL/6 mice of both sexes (Jackson Laboratories, Bar Harbor, ME), by a previously reported method.9 Mice were euthanatized after 5 or 10 days of treatment. A group of age- and sex-matched mice that did not receive any treatment to induce dry eye served as untreated (UT) controls.
Histology and Evaluation of Goblet Cell Density
Eyes and ocular adnexa (n = 6 per group) were surgically excised, fixed in 10% formalin, and paraffin-embedded and 8-μm sections were cut. The sections were stained with hematoxylin and eosin for evaluation of morphology and with periodic-Schiff (PAS) reagent for measuring goblet cell density and were examined and photographed with a microscope equipped with a digital camera (Eclipse E400 with a DS-Fi1; Nikon). Goblet cell density in the superior and inferior conjunctiva was measured in three sections from each eye with image-analysis software (NIS Elements Software, version 3.0, BR; Nikon, Tokyo, Japan) and is expressed as the number of goblet cells per 100 μm.
Laser Scanning Confocal Immunofluorescence Microscopy
Immunofluorescent staining was performed with polyclonal antibodies to immunolocalize involucrin (Santa Cruz Biotechnology, Santa Cruz, CA), Sprr-1a (Abcam, Inc., Cambridge, MA), Sprr-2 (Alexis Biochemicals, San Diego, CA), and Tg-1 (Abcam, Inc.) proteins in situ in corneal and conjunctival tissue sections and Muc5AC (a gift from Marcia Jumblatt, University of Louisville, Louisville, KY) in conjunctival sections from normal control and dry eye mice, as previously described.10,11
Briefly, eyes and lids from mice in each group (n = 3 per group) were surgically excised and embedded in OCT compound (VWR, Swannee, GA) and flash frozen in liquid nitrogen. Sagittal 8-μm sections were cut with a cryostat (HM 500; Micron, Waldorf, Germany) and placed on glass slides that were stored at −80°C. They were fixed with either cold acetone (involucrin, Sprr-2, and Tg-1) at −20°C for 5 minutes or with methanol (Sprr-1a, and Muc5AC+Sprr-1a) at 4°C for 5 minutes and then permeabilized with PBS containing 0.1% Triton X-100 for 10 minutes. After blocking with 20% normal goat serum in PBS for 60 minutes (except for involucrin and Muc5AC where 20% horse normal serum was used), primary polyclonal antibodies (dilution 1:100 for all of them, except for involucrin, which was 1:20) were applied, and the sections were incubated for 1 hour at RT. When dual labeling was intended, Alexa-Fluor 488-conjugated goat anti-rabbit IgG and Alexa-Fluor 633-conjugated donkey anti-goat IgG (1:300) secondary antibodies were applied and incubated in a dark chamber for 1 hour. When single labeling was performed, the appropriate Alexa-Fluor 488-conjugated secondary antibody was applied in a similar manner. Nuclei were counterstained with propidium iodide (2 μg/mL in PBS) for 5 minutes. After extensive rinsing, the sections were covered with anti-fade medium (Gel/Mount; Fisher Scientific, Atlanta, GA) and coverslips applied. Digital images (512 × 512 pixels) of cryosections were captured with a laser scanning confocal microscope (LSM 510 with krypton-argon and He-Ne laser; Carl Zeiss Meditec, Inc., Thornwood, NY) with either 488 nm excitation (emission filters, LP505 and LP560) or 488, 543, or 633 nm excitation (BP505-550, BP 560-615, and LP 650 emission filters, respectively). They were acquired with a 40/1.3× oil-immersion objective. Images from treatment and control corneas were captured with identical photomultiplier tube gain settings and processed using the microscope software (LSM-PC; Carl Zeiss Meditec) and image-analysis software (Photoshop 6.0; Adobe Inc., San Jose). The co-localization of Muc5AC (labeled in blue color) and Sprr-1a (labeled green) yielded a turquoise color in merged images.
Transmission Electron Microscopy
C57BL/6 mice were euthanatized and a few drops of 2% glutaraldehyde in 80 mM buffered sodium cacodylate and 330 mOsM/kg fixative12 was immediately applied to the cornea and eyelids (n = 3 per group). The eyes, with lids attached, were carefully removed and immersed in fixative for 4 to 6 hours, to ensure proper cross-linking and preservation of the tissue. After fixation, transverse pieces (1 × 1.5 mm) were cut from both the superior and inferior eyelids. The tissue pieces were washed three times in sodium cacodylate buffer (pH 7.4) at room temperature and left 10 minutes in each wash. Subsequently, the samples were immersed in a freshly prepared 1% solution of osmium tetroxide in 100 mM sodium cacodylate buffer for 1 hour under dim light. Next, the samples were washed several times in sodium cacodylate buffer and left 10 minutes in each wash.
A tissue processor (model EM TP; Leica, Bannockburn, IL) was used for dehydration, transition, infiltration, and embedding. First the tissue samples were dehydrated through a graded alcohol series (30%–100% in six steps) at room temperature. Next, the tissue samples were infiltrated with propylene oxide. Embedding with agitation was achieved through an initial 2:1 mixture of propylene oxide and Araldite resin for 3 hours followed by overnight immersion in a 1:1 mixture of propylene oxide and Araldite resin. Thereafter, the tissue samples were immersed in a propylene oxide and Araldite resin (SPI, West Chester, PA) 1:3 mixture for 4 to 8 hours before being transferred to 100% Araldite resin overnight. The tissue samples were then oriented in embedding molds and left 12 hours for polymerization in an oven at 60°C. An ultramicrotome (MT-7000; Research Manufacturing Co. Inc. Tucson, AZ) was used to cut thick (0.5–1 μm) and ultrathin transverse sections (60–100 nm). The thick sections were stained with 1% toluidine blue for examination with a light microscope (LM) (BX51; Olympus, Center Valley, PA).
For morphologic analysis, ultrathin sections were mounted on parallel-bar copper grids (200MP; Electron Microscopy Sciences, Hatfield, PA). These sections were double stained, first, in 3.5% uranyl acetate for 20 minutes at 60°C, followed by Reynolds lead citrate for 10 minutes at room temperature. The stained grids were examined in a transmission electron microscope (TEM) (Tecnai G2 Bio Twin Spirit; FEI Company, Hillsboro, OR), and micrographs were captured digitally at a magnification of 440×.
Visualization of Endogenous Tg Activity in Ocular Tissues
Tg activity was determined by a cadaverine incorporation assay,7 with modifications. Fluorescein cadaverine (Invitrogen-Molecular Probes, Eugene, OR) is a substrate for Tg, and the intensity of fluorescence is a relative measure of transglutaminase activity. Briefly, cryosections were incubated with a 0.1 M Tris/HCl buffer containing 100 μM of fluorescein cadaverine and 10 mM CaCl2 for 25 hours at room temperature (n = 3 per group). Control sections were incubated with the same fluorescein cadaverine–supplemented buffer containing 10 mM EDTA instead of CaCl2. The reaction was stopped by washing the slides for 5 minutes in PBS containing 10 mM EDTA, and two further washings with plain PBS. Nuclei were counterstained with propidium iodide (2 μg/mL in PBS) for 5 minutes. After extensive rinsing, the sections were covered with antifade medium (Gel/Mount; Fisher, Atlanta, GA) and coverslips applied. Cryosection digital images (512 × 512 pixels) were captured with a laser scanning confocal microscope (LSM 510, with krypton-argon and He-Ne laser; Carl Zeiss Meditec, Thornwood, NY) with 488 nm excitation (emission filters, LP505 and LP560). They were acquired with a 40/1.3× oil-immersion objective. Images from treatment and control corneas were captured with identical photomultiplier tube gain settings and processed (LSM-PC software; Carl Zeiss Meditec, and Photoshop 6.0; Adobe Inc., San Jose, CA).
RNA Isolation and Real-Time PCR
Total RNA was isolated from corneal and conjunctival epithelia that were collected and pooled from 10 eyes at each time point by guanidium thiocyanate-phenol-chloroform extraction (n = 4 per group).13 The RNA concentration was measured by its absorption at 260 nm, and samples were stored at −80°C before use.
First-strand cDNA was synthesized from 1 μg of total RNA with random hexamers, by using M-MuLV reverse transcriptase (Ready-To-Go You-Prime First-Strand Beads; Amersham Pharmacia Biotech, Inc., Piscataway, NJ), as previously described.10,14,15
Real-time PCR was performed using specific MGB probes (Table 1) (Taqman; Applied Biosystems, Inc. [ABI], Foster City, CA), a commercial universal PCR master mix (AmpErase UNG, Taqman; Applied Biosystems), and a thermocycler (Smart Cycler System; Cepheid, Sunnyvale, CA), according to the manufacturer's recommendations. Assays were performed in duplicate for each experiment, and they were repeated on four different sets of mice. A nontemplate control was included in all the experiments to evaluate DNA contamination of the reagent used. The GAPDH gene was be used as an endogenous reference for each reaction, to correct for differences in the amount of total RNA added. The results of quantitative PCR were analyzed by the comparative CT method (Applied Biosystems User Bulletin, No.2; P/N 4303859),10 where the target change ratio was 2−ΔΔCt. The cycle threshold (Ct), the cycle at which the signal crosses a user-defined threshold, was determined by using the primary (fluorescent) signal. The results were normalized by the Ct value of GAPDH, and the relative mRNA level in the C57BL/6 untreated group was used as the calibrator.
Table 1.
Effect of 5 and 10 Days of EDE on the Relative Change of Expression of the CE Components, Cross-Linking Tg-1 and Muc5AC in the Corneal Epithelium from of Six C57BL Mice by Using Real-Time PCR
| Gene Name/Assay ID | EDE-5D | EDE-10D |
|---|---|---|
| Involucrin Mm00515219_s1 | 1.53 ± 0.10 | 1.11 ± 0.23 |
| Sprr-1a Mm01962902_s1 | 2.06 ± 0.14 | 1.96 ± 0.35 |
| Sprr-1b Mm01700275_m1 | 2.93 ± 0.35* | 2.13 ± 0.56† |
| Sprr-2a Mm00845122_s1 | 2.01 ± 0.38 | 3.53 ± 0.70‡ |
| Sprr-2b Mm01978041_s1 | 1.92 ± 0.40 | 2.44 ± 0.70 |
| Sprr-2f Mm00448855_s1 | 2.81 ± 0.32 | 4.09 ± 0.45† |
| Sprr-2g Mm01326062_m1 | 2.81 ± 0.35† | 4.09 ± 0.61* |
| Tg-1 Mm00498375_m1 | 1.72 ± 0.34† | 1.75 ± 0.14‡ |
| Muc5AC Mm01276720_m1 | 1.09 ± 0.55 | 0.28 ± 1.03 |
Assay ID represents the real-time PCR primers and probe for specific genes designed by Applied Biosystems. In the Assay ID, m indicates an assay whose probe spans an exon junction and will not detect genomic DNA; s indicates an assay whose primers and probes are designed within a single exon. Such assays will, by definition, detect genomic DNA. All P values represent EDE vs. untreated control.
P < 0.001.
P < 0.05.
P < 0.01.
Muc5AC Enzyme-Linked Immunosorbent Assay
The Muc5AC concentration in cornea epithelium and whole conjunctiva tissue was assayed with an ELISA kit for mouse mucin 5 subtype AC (Uscn Life Science Inc., Wuhan, Wuhan, China), according to the manufacturer's protocol. Briefly, the corneal epithelium was scraped, and the whole conjunctiva was excised and lysed in RIPA lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 2 mM sodium fluoride, 2 mM EDTA, 0.1% SDS, and an EDTA-free protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN). Each sample consisted of cell extracts pooled from two mice, and three samples were evaluated per group. After sonication, samples were centrifuged at 14,000g for 15 minutes at 4°C, and the supernatants were assayed. The total protein concentrations of the cell extracts were determined by protein assay kit (Micro BCA; Pierce, Rockford, IL) with a microplate reader (Infinite M200; Tecan, Männedorf, Switzerland) equipped with software (Magellan V6.55; Tecan), according to the instruction manual. The microtiter plate provided in this kit has been precoated with an antibody specific to Muc5AC. Standards or samples were added to the designated microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for Muc5AC and incubated overnight at 4°C. Next, avidin conjugated to horseradish peroxidase was added to each microplate well and incubated for 1 hour at 37°C. A TMB substrate solution was added to each well. Only those wells containing Muc5AC bound to biotin-conjugated antibody and enzyme-conjugated avidin exhibited a color change. The enzyme-substrate reaction was terminated by the addition of a sulfuric acid solution, and the color change was measured spectrophotometrically at a wavelength of 450 nm. The concentration of Muc5AC in the samples was determined by comparing the OD of the samples to the standard curve generated with the same microplate reader and software. Muc5AC concentration was calculated per nanogram of protein.
Statistical Analysis
Results are presented as the mean ± SEM of at least four separate experiments. The normal distribution was checked with D'Agostino-Pearson normality tests. The unpaired t-test was used to compare the effect of EDE in C57BL/6 mice. Values of P ≤ 0.05 were considered significant (Prism 3.0 software; GraphPad Software Inc., San Diego, CA).
Results
Effect of Desiccating Stress on Conjunctival GC Density in C57BL/6 Mice
Previous studies using this desiccating stress model have not found any difference in the ocular response between male and female 6 to 8 weeks old C57BL/6 mice. Therefore, a mixture of both male and female mice was used for these experiments.
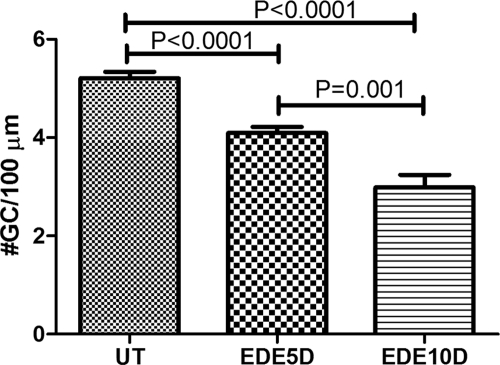
A significant decrease in the number of PAS-positive GCs was noted in the conjunctiva after 5 and 10 days of EDE in C57BL/6 mice (mean ± SD; 4.091 ± 0.1260 and 2.990 ± 0.2538, respectively) compared with UT control samples (5.206 ± 0.1311 GC/100 μm; P < 0.0001 for both). The GC density at 10 days was significantly lower than 5 days (P = 0.001; Fig. 1, n = 6 for each time point).
Figure 1.
Mean ± SD of goblet cell density in UT mice and after 5 and 10 days (D) of EDE.
Effect of EDE on Levels of CE Precursor and Transcripts
The levels of RNA transcripts encoding CE precursor proteins (involucrin and Sprr-1a, -1b, -2a, -2b, -2f, and -2g), CE cross-linking enzyme (Tg-1), as well as the housekeeping gene Gapdh were evaluated by real-time PCR, using pooled total RNA from corneal epithelia or conjunctiva (10 eyes per group) obtained from C57BL/6 mice before and after desiccating stress for 5 or 10 days. Quantitation of gene expression could be performed because the slope of the standard curve for each gene showed a similar efficiency of amplification. Nontemplate control experiments showed an absence of DNA contamination. The comparative Ct method was used to determine the relative change of expression for each gene studied, using the relative mRNA levels in untreated mice as a calibrator. The experiment was performed on four sets of samples, and the results were averaged (Tables 1, 2).
Table 2.
Effect of 5 and 10 Days of EDE on the Relative Expression of the CE Components, Cross-linking Tg-1 and Muc5AC in the Conjunctival Epithelium from C57BL/6 Mice, Determined by Real-Time PCR
| EDE-5D | EDE-10D | |
|---|---|---|
| Involucrin | 1.75 ± 0.27 | 0.79 ± 0.14 |
| Sprr-1a | 1.55 ± 0.14 | 4.82 ± 0.35* |
| Sprr-1b | 1.87 ± 0.35 | 3.21 ± 0.56† |
| Sprr-2a | 2.61 ± 0.65‡ | 2.84 ± 0.61‡ |
| Sprr-2b | 2.77 ± 0.62† | 0.24 ± 0.05 |
| Sprr-2f | 1.17 ± 0.39 | 0.76 ± 0.12 |
| Sprr-2g | 1.58 ± 0.22 | 3.83 ± 0.64† |
| Tg-1 | 1.10 ± 0.11 | 2.84 ± 0.14† |
| Muc5AC m01276720_m1 | 1.15 ± 0.28 | 1.34 ± 0.29 |
Data are the mean ± SEM of results of four experiments. P values are EDE vs. the control.
P < 0.001.
P < 0.01.
P < 0.05.
An increase in the level of expression of RNA transcripts for several CE components was observed in the corneal and conjunctival epithelia after 5 and 10 days of desiccating stress. Significant increases in the corneal epithelium at one of these time points were observed for Sprr-1b, -2a, -2f, and -2g and for Sprr-2b, -2f, and -2g. Significant increases in levels of Sprr-1a, -2a, -2b, and -2g transcripts in the conjunctival epithelium were noted at one of these time points.
The level of transcripts encoding Tg-1 was significantly increased compared with baseline levels after 5 (corneal epithelium) and 10 days (both corneal and conjunctival epithelia) of desiccating stress.
Expression of CE Precursors and Tg-1 Enzyme
The expression of the CE precursor proteins (involucrin, Sprr-1a, and Sprr-2) and Tg-1 was evaluated in cornea and conjunctiva tissue sections by laser scanning confocal immunomicroscopy. Sections incubated with secondary antibody alone exhibited no discernible immunoreactivity in the cornea or conjunctiva (data not shown).
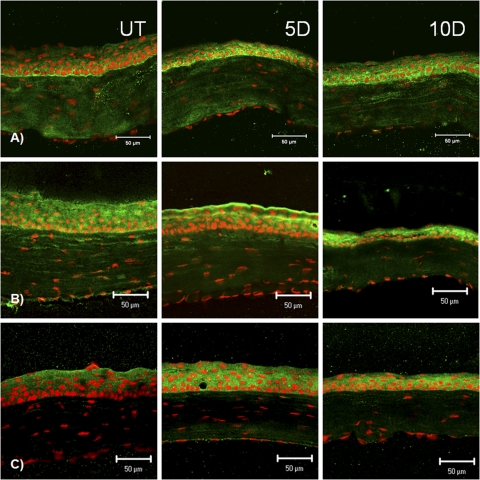
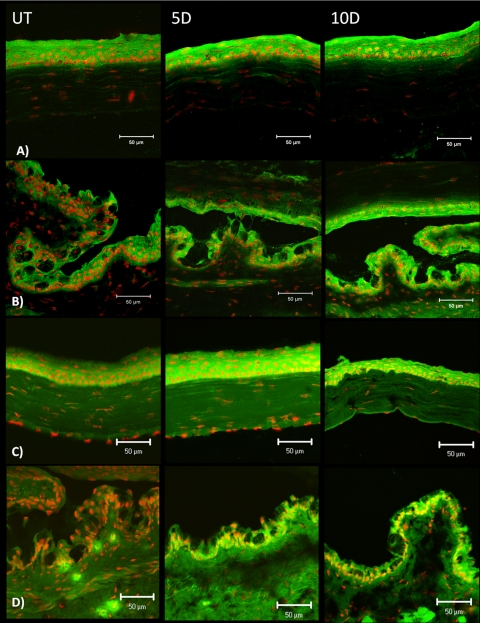
Minimal cytoplasmic staining for involucrin (Fig. 2A) was found in the apical corneal epithelium of unstressed control mice. A marked increase in cytoplasmic staining was observed after 5 days of desiccating stress, and the staining intensity decreased slightly at 10 days (Fig. 2A). A progressive increase in cytoplasmic and membrane involucrin staining was noted in the conjunctiva over the 10-day exposure to a desiccating environment (Fig. 2A).
Figure 2.
Laser scanning confocal immunofluorescence microscopy of cornea cryosections stained for involucrin (A), Sprr-1a (B) and Sprr-2 (C) (all markers in green) with propidium iodide (red) nuclear counterstaining in UT control mice and after 5 and 10 days (D) of EDE. Scale bar, 50 μm.
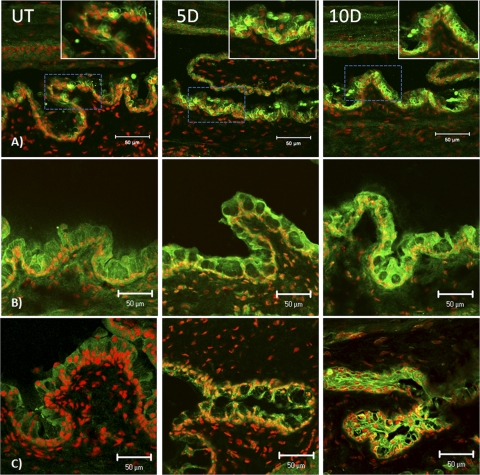
Sprr-1a staining increased in the apical and subapical layers of the corneal epithelium after 5 and 10 days of EDE (Fig. 2B). In the conjunctival epithelium, Sprr-1a expression was noted to surround the goblet cells in unstressed C57BL/6 mice, and the staining intensity dramatically increased surrounding the conjunctival goblet cells, particularly on the apical side in response to desiccation (Fig. 3B).
Figure 3.
Laser scanning confocal immunofluorescence microscopy of conjunctiva cryosections stained for involucrin (A), small proline-rich protein [Sprr]-1a (B) and Sprr-2 (C) (all markers in green) with propidium iodide (red) nuclear counterstaining in UT control mice and after 5 and 10 days (D) of EDE. Insets: higher magnification of areas boxed in blue. Scale bar, 50 μm.
Sprr-2 cytoplasmic immunoreactivity increased in all layers of the cornea after 5 and 10 days of desiccating stress (Fig. 2C). Sprr-2 staining also progressively increased in all layers of the conjunctival epithelium over 10 days of dryness, with the strongest staining intensity noted at 10 days (Fig. 3C).
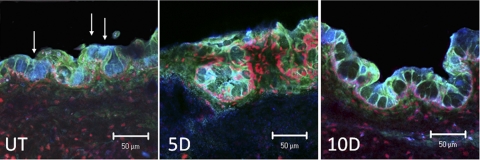
To better evaluate Sprr-1a localization in relation to goblet cells, conjunctival sections were dual labeled for the goblet cell mucin Muc5AC (blue) and Sprr-1a (green) in a color combination that yielded a turquoise color in the merged images (Fig. 4). Sprr-1a was clearly observed to surround the base and sides of many Muc5AC-positive goblet cells in the unstressed (UT) conjunctiva. This pattern was maintained after desiccating stress; however, Sprr-1a was now noted to form a prominent loop around many goblet cells and to trap Muc5AC mucin within certain cells.
Figure 4.
Laser scanning confocal immunofluorescence microscopy of conjunctiva cryosections dual-stained for Muc5AC (blue) and Sprr-1a (green) with propidium iodide (red) nuclear counterstaining in UT control and after 5 and 10 days (D) of EDE. Solid arrows: SPRR-1a staining around the goblet cells. Scale bar, 50 μm.
Transglutaminase -1 expression was observed to increase in corneal (5 and 10 days) and conjunctival epithelia (10 days; Figs. 5A, 5B, respectively). A corresponding increase in Tg activity, measured by the in situ fluorescein cadaverine assay, was observed in corneal and conjunctival epithelia (Figs. 5C, 5D, respectively).
Figure 5.
Laser scanning confocal immunofluorescence microscopy of tissue sections stained for Tg-1 protein in cornea (A) and conjunctiva (B) and with a Tg activity assay in cornea (C) and conjunctival tissue (D) with propidium iodide (red) nuclear counterstaining in UT control mice and after 5 and 10 days (D) of EDE. Scale bar, 50 μm.
Entrapment of Conjunctival Goblet Cells
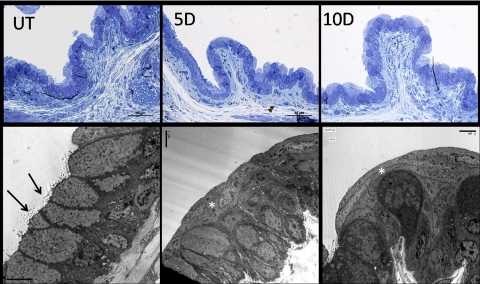
TEM was performed to confirm the immunofluorescence microscopy findings that desiccating stress promotes entrapment of conjunctival goblet cells by surrounding squamous epithelia. In both toluidine blue–stained thick sections and transmission electron micrographs taken of the same area, goblet cells were noted to open to the conjunctival surface in normal nonstressed eyes. In contrast, after 5 and 10 days of exposure to a desiccating environment, the apical opening of many goblet cells was covered by one or more layers of squamous epithelia (Fig. 6).
Figure 6.
Transmission electron microscopy of conjunctiva. The unstressed control (UT) conjunctiva consistently showed areas with contiguous goblet cells that opened to the conjunctival surface (arrows). The goblet cells were packed with secretory vesicles of uniform electron density. The epithelium is two to three cells deep in this view. After 5 and 10 days (D) of EDE fewer goblet cells reached the conjunctival surface. Frequently, mature and maturing goblet cells were covered by one or two layers of thin epithelial cells ( ). Secretory granules in these goblet cells were reduced and varied greatly in electron density compared with those in UT control eyes.
). Secretory granules in these goblet cells were reduced and varied greatly in electron density compared with those in UT control eyes.
Affect of Cornification on Muc5AC Production
No change in the number of Muc5AC transcripts in the conjunctiva was noted over 10 days of EDE. In contrast, the concentration of Muc5AC measured by ELISA significantly decreased after 5 days of EDE in the conjunctiva and after 10 days of EDE in the corneal epithelium. (Fig. 7). The reduced concentration of Muc5AC in the conjunctiva is consistent with the loss of PAS-stained goblet cells and entrapment of some partially filled goblet cells. The decreased Muc5AC in the corneal epithelium may reflect a decrease coating of the corneal surface with conjunctiva-secreted Muc5AC.
Figure 7.
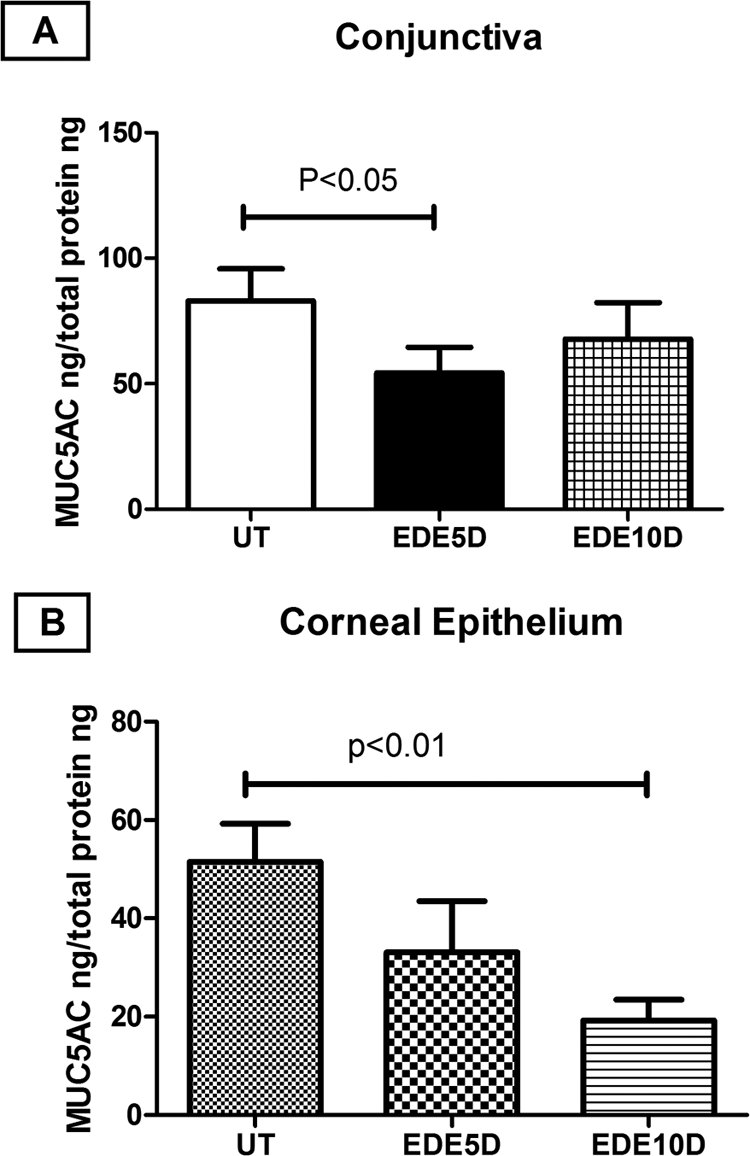
Mean ± SD of MUC5AC concentration measured by ELISA in UT mice and after 5 and 10 days (D) of EDE in conjunctiva (A) and corneal epithelial (B) samples. Concentrations are reported per nanogram of total protein.
Discussion
Certain stratified squamous epithelial cells assemble a specialized protective barrier structure on their periphery termed the cornified cell envelope.16 Skin keratinocytes express several cornified envelope precursors that are subclassified as scaffold proteins (involucrin, envoplakin, and periplakin),17 reinforcement proteins (loricrin,18 small proline-rich [Sprr] proteins,19 and late envelope proteins [LEPs],20 and filaggrin).17As part of the development of the skin epidermis, the cornified envelope precursors become covalently cross-linked to form a 5- to 10-nm mature envelope adjacent to the cell membrane.17 This transamidation process is mediated by transglutaminase (Tg) enzymes, and it occurs sequentially, allowing the classification of precursors into early (involucrin and Sprr) or late proteins (LEP and filaggrin).
SPRR proteins are encoded by a family of at least 17 members located in a closely linked locus on chromosomes 3 and 1 in mice and humans, respectively.19 Each SPRR gene contains two exons, with the entire open reading frame contained in the second exon. Although there are three SPRR subfamilies, most studies of gene expression and function have been performed with a member of the first family, SPRR1a. This gene, along with SPRR2a and SPRR2b, is expressed predominantly in squamous epithelium, where it has been thought to contribute to the formation of the insoluble cornified cross-linked envelope that provides structural integrity and limits permeability. Differential expression of SPRR proteins in various organs correlates with modulation of biomechanical properties (e.g., elasticity and flexibility) of the epithelia, and may change in response to stressful stimuli, such as cigarette smoke or ultraviolet light.21 For instance, SPRR1-positive squamous epithelium is found in tissues that require significant flexibility (e.g., lips, tongue, and esophagus). In addition to their function as structural proteins, nuclear expression of SPRR-encoded proteins, particularly at the time of cell cycle entry into G0 and interactions between SPRR-encoded proteins and nuclear proteins have suggested a role for SPRR family members in gene regulation, possibly in limiting proliferation and promoting differentiation.22
This study evaluated the effects of experimental desiccating stress on expression of early CE precursor proteins and Tg-1 enzyme in the ocular surface epithelia of mice. Both sexes of mice were used because the ocular surface response to this desiccating stress model has been similar in both sexes. Consistent with a previous report by our group, we found a low level of expression of the CE precursor in unstressed ocular surface epithelia. We found that desiccating stress increased the levels of four or more Sprr CE precursor proteins and Tg-1 in both the cornea and conjunctiva.23 The pattern of expression was slightly different between the cornea and the conjunctiva. Perhaps the most interesting finding was the markedly increased expression of cornifying proteins within conjunctival goblet cells and their surrounding squamous epithelia. These changes appeared to prevent egress of goblet cell contents to the ocular surface, and, together with loss of mucin-filled goblet cells, they may be responsible for the observed reduction of the goblet cell mucin Muc5AC in the corneal epithelium after 10 days of dry eye, as well as the decreased concentration of this mucin that has been found in dry eye tears.24,25
In contrast to the epidermis of the skin that has high levels of cornified envelope precursor proteins and Tg-1 in the superficial epithelial cells, the ocular surface epithelia in unstressed mice had low cornified envelope precursors in the superficial epithelium. Furthermore, the Muc5AC-producing goblet cells in the conjunctiva opened directly onto the ocular surface of those eyes. Over 10 days of EDE, increased Sprr-1a staining of the apical epithelium was noted, with apparent localization to the cell membrane, particularly in the goblet cell areas where epithelial cells with strong Sprr-1a immunoreactivity were found to surround goblet cells and block their apical secretory portion.
Increased CE precursor expression was accompanied by increased Tg expression and activity. Tg-1, an enzyme that is activated by high Ca+2 levels, is the primary Tg enzyme responsible for cross-linking CE precursors in the epidermis.26
The cause of desiccation-induced cornification could be the activation of stress signaling pathways such as the c-jun n-terminal kinase (JNK) pathway, which we have found to be activated in response to desiccating and osmotic stress.15,27 Alternatively, cytokines such as IFN-γ released from the CD4+ T cells that infiltrate the conjunctiva in dry eye could be responsible.28 IFN-γ has been noted to regulate the expression of certain CE precursor proteins and Tg-1.29,30 This regulatory effect may explain why conjunctival cornification is worse in ocular surface diseases, such as Stevens-Johnson syndrome,6 mucous membrane pemphigoid and graft-versus-host disease, where the epithelium is heavily infiltrated by activated CD4+T cells.31–33
Footnotes
Supported by National Institutes of Health (NIH) Grants EY11915 (SCP) and EY016928-01(CSDP), an unrestricted grant from Research to Prevent Blindness, The Oshman Foundation, and The William Stamps Farish Fund.
Disclosure: R.M. Corrales, None; C. Sade de Paiva, None; D.-Q. Li, None; W.J. Farley, None; J.T. Henriksson, None; J.P.G. Bergmanson, None; S.C. Pflugfelder, None
References
- 1. Steinert PM, Marekov LN. Initiation of assembly of the cell envelope barrier structure of stratified squamous epithelia. Mol Biol Cell. 1999;10:4247–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steven AC, Steinert PM. Protein composition of cornified cell envelopes of epidermal keratinocytes. J Cell Sci. 1994;107:693–700 [PubMed] [Google Scholar]
- 3. Castro-Munozledo F. Development of a spontaneous permanent cell line of rabbit corneal epithelial cells that undergoes sequential stages of differentiation in cell culture. J Cell Sci. 1994;107:2343–2351 [DOI] [PubMed] [Google Scholar]
- 4. Adhikary G, Crish J, Lass J, Eckert RL. Regulation of involucrin expression in normal human corneal epithelial cells: a role for activator protein one. Invest Ophthalmol Vis Sci. 2004;45:1080–1087 [DOI] [PubMed] [Google Scholar]
- 5. Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakamura T, Nishida K, Dota A, Matsuki M, Yamanishi K, Kinoshita S. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest Ophthalmol Vis Sci. 2001;42:549–556 [PubMed] [Google Scholar]
- 7. Raghunath M, Cankay R, Kubitscheck U, et al. Transglutaminase activity in the eye: cross-linking in epithelia and connective tissue structures. Invest Ophthalmol Vis Sci. 1999;40:2780–2787 [PubMed] [Google Scholar]
- 8. de Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–2856 [DOI] [PubMed] [Google Scholar]
- 9. Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corrales RM, Stern ME, de Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci 2006;47:3293–3302 [DOI] [PubMed] [Google Scholar]
- 11. de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doughty MJ, Bergmanson JPG, Blocker Y. Shrinkage and distortion of the rabbit corneal endothelial cell mosaic caused by a high osmolarity glutaraldehyde-formaldehyde fixative compared to glutaraldehyde. Tissue Cell. 1997;29(5):533–547 [DOI] [PubMed] [Google Scholar]
- 13. Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79 [DOI] [PubMed] [Google Scholar]
- 14. Corrales RM, Calonge M, Herreras JM, Saez V, Mayo A, Chaves FJ. Levels of mucin gene expression in normal human conjunctival epithelium in vivo. Curr Eye Res. 2003;27:323–328 [DOI] [PubMed] [Google Scholar]
- 15. Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301 [DOI] [PubMed] [Google Scholar]
- 16. Segre J. Complex redundancy to build a simple epidermal permeability barrier. Curr Opin Cell Biol. 2003;15:776–782 [DOI] [PubMed] [Google Scholar]
- 17. Bernerd F, Asselineau D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB damages in skin reconstructed in vitro. Dev Biol. 1997;183:123–138 [DOI] [PubMed] [Google Scholar]
- 18. Hohl D, Mehrel T, Lichti U, Turner ML, Roop DR, Steinert PM. Characterization of human loricrin: structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991;266:6626–6636 [PubMed] [Google Scholar]
- 19. Tesfaigzi J, Carlson DM. Expression, regulation, and function of the SPR family of proteins: a review. Cell Biochem Biophys. 1999;30:243–265 [DOI] [PubMed] [Google Scholar]
- 20. Marshall D, Hardman MJ, Nield KM, Byrne C. Differentially expressed late constituents of the epidermal cornified envelope. Proc Natl Acad Sci USA. 2001;98:13031–13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tesfaigzi J, Th'ng J, Hotchkiss JA, Harkema JR, Wright PS. A small proline-rich protein, Sprr1, is upregulated early during tobacco smoke-induced squamous metaplasia in rat nasal epithelia. Am J Respir Cell Mol Biol. 1996;14:478–486 [DOI] [PubMed] [Google Scholar]
- 22. Tesfaigzi J, Carlson DM. Cell cycle-specific expression of G(0)SPR1 in Chinese hamster ovary cells. Exp Cell Res. 1996;228:277–282 [DOI] [PubMed] [Google Scholar]
- 23. Tong L, Corrales RM, Chen Z, et al. Expression and regulation of cornified envelope proteins in human corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47(5):1938–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao H, Jumblatt JE, Wood TO, Jumblatt MM. Quantification of Muc5AC protein in human tears. Cornea. 2001;20:873–877 [DOI] [PubMed] [Google Scholar]
- 25. Argüeso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin Muc5AC in tears of patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011 [PubMed] [Google Scholar]
- 26. Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340 [DOI] [PubMed] [Google Scholar]
- 27. Chen Z, Tong L, Li Z, et al. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008;49(2):539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Paiva CS, Villarreal A, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48(6):2553–2560 [DOI] [PubMed] [Google Scholar]
- 29. Cabral A, Voskamp P, Cleton-Jansen AM, South A, Nizetic D, Backendorf C. Structural organization and regulation of the small proline-rich family of cornified envelope precursors suggest a role in adaptive barrier function. J Biol Chem. 2001;276:19231–19237 [DOI] [PubMed] [Google Scholar]
- 30. Takahashi H, Asano K, Nakamura S, Ishida-Yamamoto A, Iizuka H. Interferon-gamma-dependent stimulation of human involucrin gene expression: STAT1 (signal transduction and activators of transcription 1) protein activates involucrin promoter activity. Biochem J. 1999;344:797–802 [PMC free article] [PubMed] [Google Scholar]
- 31. Caproni M, Torchia D, Schincaglia E, et al. The CD40/CD40 ligand system is expressed in the cutaneous lesions of erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis spectrum. Br J Dermatol. 2006;154:319–324 [DOI] [PubMed] [Google Scholar]
- 32. Rice BA, Foster CS. Immunopathology of cicatricial pemphigoid affecting the conjunctiva. Ophthalmology. 1990;97:1476–1483 [DOI] [PubMed] [Google Scholar]
- 33. Rojas B, Cuhna R, Zafirakis P, et al. Cell populations and adhesion molecules expression in conjunctiva before and after bone marrow transplantation. Exp Eye Res. 2005;81:313–325 [DOI] [PubMed] [Google Scholar]