The present study suggests that PI3K signaling pathway is essential for cone photoreceptor survival in mouse retina.
Abstract
Purpose.
Downregulation of the retinal insulin/mTOR pathway in mouse models of retinitis pigmentosa is linked to cone cell death, which can be delayed by systemic administration of insulin. A classic survival kinase linking extracellular trophic/growth factors with intracellular antiapoptotic pathways is phosphoinositide 3-kinase (PI3K), which the authors have shown to protect rod photoreceptors from stress-induced cell death. The role of PI3K in cones was studied by conditional deletion of its p85α regulatory subunit.
Methods.
Mice expressing Cre recombinase in cones were bred to mice with a floxed pi3k gene encoding the p85α regulatory subunit of the PI3K and were back-crossed to ultimately generate offspring with cone-specific p85α knockout (cKO). Cre expression and cone-specific localization were confirmed by Western blot analysis and immunohistochemistry (IHC), respectively. Cone structural integrity was determined by IHC using peanut agglutinin and an M-opsin–specific antibody. Electroretinography (ERG) was used to assess rod and cone photoreceptor function. Retinal structure was examined by light and electron microscopy.
Results.
An age-related cone degeneration was found in cKO mice, evidenced by a reduction in photopic ERG amplitudes and loss of cone cells. By 12 months of age, approximately 78% of cones had died, and progressive disorganization of synaptic ultrastructure was noted in surviving cone terminals in cKO retinas. Rod viability was unaffected in p85α cKO mice.
Conclusions.
The present study suggests that PI3K signaling pathway is essential for cone survival in the mouse retina.
Class IA phosphoinositide 3-kinase (PI3K) is the principal kinase that, when activated, phosphorylates phosphatidylinositol at the D3 position of the inositol ring.1 This reaction generates the D3 phosphoinositides PI-3-P, PI-3,4-P2, PI-3,5-P2, and PI-3,4,5-P3. These lipid products serve as second messengers that recruit specific phospholipid-binding proteins to the membrane, initiating downstream transduction pathways.2–4 Our laboratory has shown that intact bovine photoreceptor outer segments contain class IA PI3K as an obligatory heterodimeric complex composed of regulatory p85 and catalytic p110 subunits.5 The formation of D3 phosphoinositides generated by PI3K has been demonstrated in intact retinal photoreceptor outer segments from mouse and cattle.6,7 To date, studies have implicated D3 phosphoinositides in a variety of cell activities such as vesicular trafficking, cytoskeletal reorganization, cell growth, adhesion, and survival1,8 and photoreceptor-specific functions such as modulation of phototransduction,9 disc biogenesis,10 protein translocation,11 synaptic ribbon formation,12 and glutamate release.12 Our laboratory has shown that physiological light activates the PI3K/Akt survival pathway through the insulin receptor (IR) in rod photoreceptors.13 Deletion of IR14 and several downstream effector molecules of the IR signaling pathway in the retina, such as IRS-2,15 Akt2,16 and Bcl-xl,17 resulted in photoreceptor degeneration.
Although cone photoreceptors constitute a small percentage (3%–5%) of retinal photoreceptors in humans and rodents,18,19 they are essential in humans for optimal visual acuity, color vision, and visual perception under moderate to high light intensities. In humans, age-related macular degeneration (AMD) and diabetic retinopathy (DR) are the most common disorders affecting cones.20–24 Cones are affected indirectly in diseases such as retinitis pigmentosa (RP) and directly in cone and cone-rod dystrophies.25–27 Specific mechanisms of cone cell death are very different, depending on genetic predispositions and environmental factors.21,28–32 Akt, a canonical prosurvival molecule downstream of PI3K, has been shown to be constitutively active in cone photoreceptors.33 The significance of having constitutively active Akt in cones is unknown. This same PI3K/Akt pathway in rod photoreceptors is only transiently activated during exposure to physiological light or stress conditions such as oxidative, hyperosmotic, or bright light.14,16,33,34 Selective loss of cones has been reported in diabetic retinopathy,23,24 and retinal IR/PI3K/Akt signaling has been shown to be downregulated in diabetes.35,36 However, these studies have not addressed the significance of PI3K in the diabetic retinopathy phenotype.
Recent findings using an animal model of RP showed that as rods die, the remaining cone photoreceptors are starved primarily because of downregulation of the insulin/mTOR signaling pathway.37 Even though this previous study proposes a potential mechanism involved in cone photoreceptor cell death, it does not address the potential importance of the activation and regulation of PI3K to regulation of the insulin/mTOR signaling pathway. The classical link between extracellular signals (e.g., insulin/IR) and intracellular survival pathways (e.g., Akt/mTOR) is PI3K.
Several studies have demonstrated that PI3K functions in maintaining cell viability under oxidative stress conditions in the 661W cone-like cell line and in other neuronal cell lines.38–42 A disadvantage of these studies is that they used chemical inhibitors of PI3K such as LY294002 and wortmannin to inhibit its activity. The class and isoform specificity of these inhibitors is still debatable. Because of the broad specificity of these inhibitors and the wide cellular expression of PI3K, it is difficult to interpret PI3K-specific phenotypic outcomes with respect to a particular cell type. In this study, we set out to identify the importance of PI3K in the maintenance of cone photoreceptor structure and function in vivo using the Cre-lox system of gene inactivation. Here, we demonstrate that normal mature cone photoreceptors express class IA PI3K and that deletion of PI3K in cone photoreceptors leads to the onset of cone functional loss without affecting rod structure or function.
Materials and Methods
Materials
Rabbit polyclonal anti–pan-p85α antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Rabbit polyclonal anti–Akt antibody was purchased from Cell Signaling (Beverly, MA). Mouse monoclonal anti–β-actin antibody was purchased from Affinity BioReagents (Golden, CO). Rabbit polyclonal anti–red/green cone opsin (M-opsin) was purchased from Chemicon (Temecula, CA). Rabbit polyclonal anti–Cre antibody suitable for Western blot analysis was purchased from Novagene (Darmstadt, Germany), and mouse monoclonal anti–Cre antibody suitable for immunohistochemistry was purchased from Abcam (Cambridge, MA). Rabbit polyclonal anti–cone arrestin 4 (Arr4) antibody was generously provided by Cheryl Craft (University of Southern California, Los Angeles, CA). Biotinylated peanut agglutinin (PNA) and secondary antibodies were purchased from Vector Laboratories (Burlingame, CA). DAPI stain used for nuclear staining was purchased from Invitrogen-Molecular Probes (Carlsbad, CA). Anti–rhodopsin (RD14) was a kind gift from Robert Molday (University of British Columbia, Vancouver, Canada). An immortalized mouse cone cell line (661W)43 was a generous gift of Muayyad Al-Ubaidi (University of Oklahoma Health Sciences Center, Oklahoma City, OK). All other reagents used for buffer preparations were of analytical grade and were purchased from Sigma (St. Louis, MO).
Animals
The p85α floxed mice44 were kindly provided by Lewis Cantley (Harvard Medical School, Boston, MA). The Nrl−/− mice were kindly provided by Anand Swaroop (National Eye Institute, National Institutes of Health, Bethesda, MD). The generation of human red/green pigment gene promoter mice was reported previously.45 All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the NIH Guide for the Care and Use of Laboratory Animals. Protocols used were approved by the Institutional Animal Care and Use Committee at the University of Oklahoma Health Sciences Center and the Dean A. McGee Eye Institute. Animals were born and raised in our vivarium and kept under dim cyclic light (40–60 lux, 12-hour light/12-hour dark cycle). For experiments that required enucleating the eye or removing the retina, mice were killed by asphyxiation with CO2 followed by cervical dislocation.
Mice designated wild-type (WT) are controls in which both p85α alleles are floxed. Mice designated conditional knockout (cKO) do not express p85α in cone photoreceptors. In some experiments, additional controls were mice that express only Cre recombinase.
Generation of Cone Photoreceptor-Specific PI3K KO Mice
To produce mice with cone-specific KO of p85α, mice expressing Cre recombinase specifically in cones under the control of the human red/green pigment gene promoter45 were bred with p85α floxed mice in which a 2.6-kb fragment of the mouse pi3k gene containing exon 7 was flanked with loxP sites, which enabled the deletion of all three p85α isoforms (p50, p55, and p85), as previously described44 The desired transgenic mice were generated by back-crossing and were identified by genotyping tail DNA for Cre and floxed p85α using PCR screening. For Cre genotype screening, a forward primer TTG GTT CCC AGC AAA TCC CTC TGA designed within promoter DNA sequence and a reverse primer GCC GCA TAA CCA GTG AAACAG CAT designed within the Cre sequence were used to amplify the PCR product of 411 bp. To distinguish the p85α floxed allele from the WT p85α allele, the primer pair CAC CGA GCA CTG GAG CAC TG and CCA GTT ACT TTC AAA TCA GCA CAG was used to amplify a 252-bp fragment from the WT p85α allele and a 301-bp fragment from the floxed p85α allele.
Immunostaining of Retinal Whole-Mounts
Eyes were enucleated and placed in cold Hanks' balanced salt solution buffered with 25 mM HEPES (pH 7.2). After enucleation, the cornea and lens were removed, and retinas were carefully isolated. Relaxing cuts were made in the retinal margins, and the whole retina was flattened onto a black filter membrane. Whole-mounted retinas were fixed in 4% paraformaldehyde in PBS at 4°C for 2 hours and rinsed in PBS, and nonspecific labeling was blocked using 10% horse serum in PBS. Whole-mounts were incubated in a combination of biotinylated PNA (1:500) and anti–cone arrestin (1:500) overnight at 4°C. Streptavidin conjugated to Texas red (1:250) was used to visualize PNA labeling. Cone arrestin immunoreactivity was visualized using an FITC-conjugated secondary antibody (1:200). Labeling in retinal whole mounts was imaged using either an epifluorescence microscope (either Eclipse E800 [Nikon, Tokyo, Japan] or IX70 [Olympus USA, Center Valley, PA]).
Evaluation of M-Cone Distribution by Quantitative Immunohistochemistry
WT and p85α cKO mice at 6 and 12 months of age were euthanatized by asphyxiation with CO2. Their eyes were enucleated, fixed, and paraffin embedded. Ten-micrometer–thick retinal sections were cut along the vertical meridian and immunostained for M-opsin to label M-cones in the superior and inferior hemispheres. In each hemisphere, all M-opsin–positive cones in the section were counted, starting at the optic nerve head and extending along the vertical meridian toward the superior and inferior ora serrata.
Other Methods
Photoreceptor outer segments were prepared using a discontinuous sucrose gradient, as previously described.46 The structure and morphology of p85α cKO and control retinas were examined after tissue fixation and sectioning, as previously described.16 Retinal function was examined using a Ganzfeld-type ERG recording system (UTAS-E3000; LKC Technologies, Gaithersburg, MD) according to the method described earlier).16 To assess cone function, mice were light adapted for 5 minutes, and the responses to five flashes of 3.57 log scot cd · s/m2 intensity were averaged to generate the photopic ERG response (cone and cone-driven inner retinal responses). The amplitude of the a-wave was measured from baseline to the a-wave trough, whereas the amplitude of the b-wave was measured from the trough of the a-wave to the peak of the b-wave. Lectin cytochemical and immunohistochemical analysis using PNA and anti–cone arrestin-4 (Arr4) was performed47 on whole retinal flat mounts. The Arr4 antibody is specific for cone arrestin and does not cross-react with rod arrestin.47 661W cells43 were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2.
Statistical Analysis
One-way ANOVA and post hoc statistical analysis using Bonferroni's pairwise comparisons were used to determine statistical significance (P < 0.05).
Results
Expression of p85α Regulatory Subunit of PI3K in Mature Mouse Cone Photoreceptors
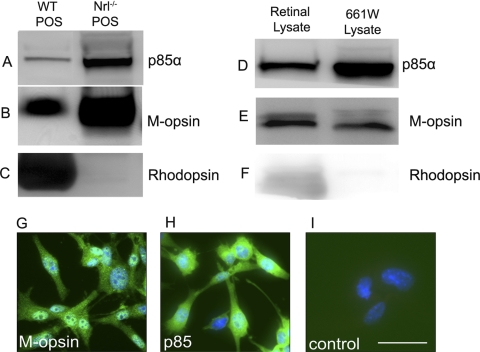
To determine whether mature cone photoreceptors express class IA PI3K in vivo, we took advantage of the Nrl−/− mouse model in which the photoreceptor population consists exclusively of cones by virtue of the absence of the rod differentiation transcription factor Nrl.48 Photoreceptor outer segments (POS) were isolated from WT and Nrl−/− retinas and analyzed for p85α expression by Western blotting. Rod and cone photoreceptor-specific proteins rhodopsin and M-opsin were used as markers. The results indicate that mature cone POS express abundant amounts of p85α (Fig. 1A) compared with POS from WT retinas. High levels of M-opsin were present in Nrl−/− POS (Fig. 1B) compared with WT POS. The rod-specific protein marker opsin was absent from Nrl−/− POS lysates (Fig. 1C), as previously established.48 To further establish the existence of PI3K/Akt pathways in cone photoreceptors, we analyzed lysates of 661W cells (a cone-like transformed cell line43) for p85α expression by Western blot analysis and immunofluorescence microscopy. This cell line has previously been used to study the multiple death pathways in cone photoreceptors.49 Total retinal lysate from WT mice was used as a positive control. The results indicate that 661W cells express high levels of p85α protein (Fig. 1D). Cone-like characteristics of 661W were confirmed by labeling 661W cell lysates (Fig. 1E) or fixed 661W cells with anti–M-cone opsin antibody (Fig. 1G). Further, the 661W cells do not express the rod photoreceptor marker rhodopsin (Fig. 1F). The expression of p85α in 661W cells is shown in Figure 1H. We found that the ratio of p85α expression to M-opsin was higher in 661W cells (Figs. 1D, 1E) than in the POS Nrl−/− mouse (Figs. 1A, 1B). This difference was due to the whole cell lysate of 661W cell compared with POS in the Nrl−/− mouse. The p85α in POS represents only the membrane-bound fraction, whereas in 661W cells it has both soluble and membrane-bound forms of p85α. These experiments provide evidence that cone photoreceptors express the p85α regulatory subunit of PI3K in vivo and in vitro.
Figure 1.
p85α protein levels in cone photoreceptor outer segments and the 661W cone cell line. Western blot analysis of total mouse retinal lysates, POS-enriched extracts from WT and Nrl−/− mouse retinas, and cell extracts from the 661W cone cell line were used to assess expression levels of p85α (A, D) protein. M-cone opsin (B, E) and rhodopsin (C, F) were used as cone and rod photoreceptor markers, respectively. Immunocytochemical analysis of M-cone opsin (G) and p85α (H) expression were determined in the 661W cone cell line. For control, primary antibodies were omitted (I). Nuclei are counterstained with DAPI (blue). Scale bar, 50 μm for all panels.
Generation of Cone Photoreceptor-Specific p85α KO Mice
Systemic deletion of p85α or p110α subunits of PI3K results in embryonic or neonatal lethality.50,51 To circumvent these difficulties, we used Cre/lox technology to generate a cone photoreceptor-specific deletion of pik3r1, which resulted in deletion of the p85α, p55α, and p50α isoforms. Mice expressing floxed p85α alleles were bred with mice expressing Cre in cones (Fig. 2A), driven by the promoter of the human red/green pigment (L/M opsin) gene.45
Figure 2.
Generation of the cone-specific p85 KO mouse model. (A) Cone photoreceptor-specific deletion of Pik3r1, a pan-p85α regulatory subunit of PI3K, was made by cross-breeding floxed p85α mice to cone-specific Cre mice. (B) Expression levels of p85α in POS and Band II (retinal cells enriched with inner segments) from WT, WT-Cre+, p85α-flox, p85α-het Cre+, and p85α KO Cre+ mouse retinas were examined with anti–p85α antibody. (C) Immunohistochemical analysis of Cre recombinase immunolabeling in p85α cKO and WT control retinas harvested from littermates. Red: Cre-positive cone cells (arrowheads). Labeling of blood vessels (bv) is nonspecific. (D) Western blot analysis of duplicate p85α cKO and WT retinal extracts was used to determine the level of Cre expression; β-actin levels were used as a loading control. (E) Assessment of cone outer segment integrity using immunolabeling for M-cone opsin at 1 month of age. ROS, rod outer segment; ONL, outer nuclear layer; INL, inner nuclear layer; POS, photoreceptor outer segment. Scale bar, 100 μm for all panels.
We have shown previously that p85α is expressed in rods,5,13 and our data on the Nrl-KO mice suggest that p85α is also expressed in cones. However, we failed to observe the reduction of p85α protein in POS of cKO mice compared with WT (Fig. 2B). We found a slightly increased p85α in cKO POS (Fig. 2B). The slight increase was likely a compensatory increase of p85α in rods. However, when we bred cKO mice with Nrl−/− mice, we found the deletion of more than 80% of p85α in cKO/Nrl−/− double-knockout mice (data not shown). We did not observe a reduction in p85α in cKO mice compared with WT mice, probably because of the contribution of p85α from rods, which outnumber cones. To ensure that Cre expression in cones was working properly, we assessed Cre protein expression and cellular localization in the retinas of WT and p85α cKO littermates by Western blot analysis and immunofluorescence microscopy using an anti–Cre antibody. Cre expression was localized to cone photoreceptor nuclei in p85α cKO retinas but was absent in WT controls (Fig. 2C). The secondary anti–mouse antibody nonspecifically labeled endogenous IgGs in the blood vessels of both WT and p85α cKO mouse retinas (Fig. 2C). Further, the results indicated Cre expression observed in p85α cKO retinal lysates was absent in WT controls (Fig. 2D). We have shown previously by immunohistochemistry that almost 100% of cone cells in the parent Cre-expressing mouse line express Cre.45 Given that successful Cre-mediated recombination only requires four Cre molecules,52 a protein level that is much lower than the threshold of detection by IHC, and that the locus for PI3K-p85α is not known for unusually low frequency of Cre-mediated recombination,44 deletion of the PI3K-p85α subunit most likely occurred in all cone cells. The parent Cre mouse line we used was previously shown to have normal cone distribution, morphology, and function for up to 10 months of age.45
Effect of p85α Deletion on M-Opsin Localization
As mentioned, cone photoreceptors constitute a small percentage of the total photoreceptor cell population in the mouse retina. Therefore, retinal structural analysis and ONL thickness measurements can give insight into retinal and rod photoreceptor structure and viability but are not informative about the viability of cone photoreceptors. Regarding the role of PI3K in rod photoreceptors, studies have shown that PI3K-generated D3 phosphoinositides function in the proper trafficking of rhodopsin and its assembly into outer segment disc membranes.10 Therefore, we analyzed cone M-opsin localization in cone POS in these animals. As shown in Figure 2E, cone M-opsin in 1-month-old cKO mice showed proper localization to cone outer segments that were similar in morphology and distribution to the M-opsin–positive cone outer segments of WT mice (Fig. 2E).
Effect of p85α Deletion on Retinal Morphology
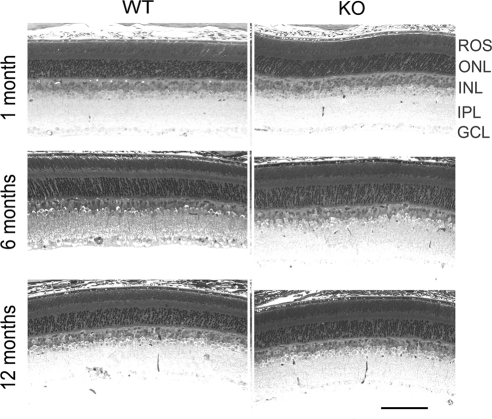
Cone-specific p85α KO mice were generated by crossing cone-specific Cre mice with floxed p85α mice and were characterized at different ages for the cytoarchitectural and functional integrity of their mature retinas. For most studies, retinas from WT and p85α cKO littermates were examined and compared. The overall morphology and structural integrity of the retina were indistinguishable between WT and p85α cKO mice at 1, 6, and 12 months of age (Fig. 3). Therefore, the conditional deletion of PI3K in cone photoreceptors does not affect the gross histologic organization and morphologic structure of the retina.
Figure 3.
Morphology of cone-specific p85 KO retina and assessment of cone outer segment integrity. Morphologic examination of retinas from WT and p85α cKO mice at 1, 6, and 12 month of age.
Effect of p85α Deletion on Retinal Function
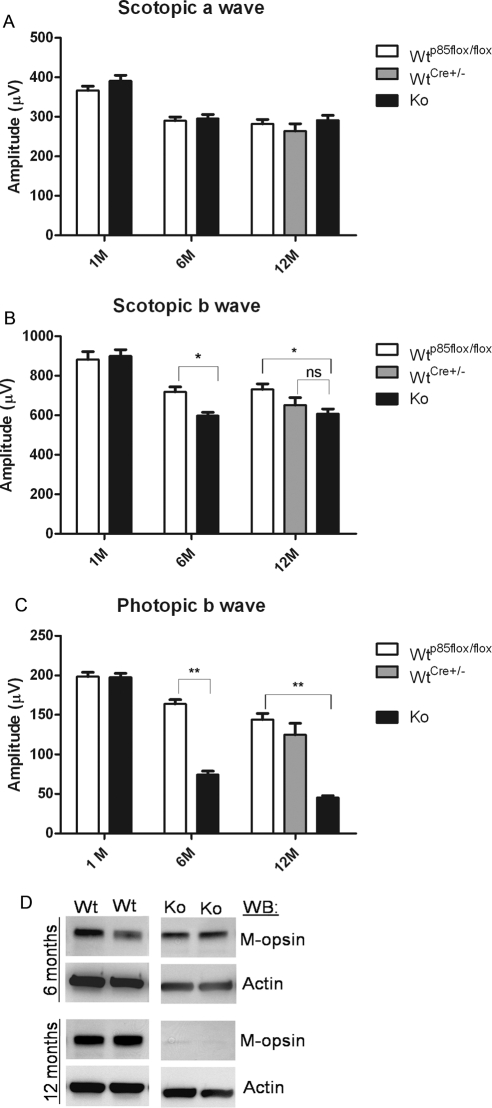
Retinal function of p85α cKO mice was assessed by electroretinography (ERG). Scotopic ERG recordings of rod photoreceptors (scotopic a-wave) were functionally normal and statistically comparable in WT and p85α cKO retinas at 1, 6, and 12 months of age (Fig. 4A). Scotopic b-wave amplitudes of p85α cKO mice were lower 6 and 12 months of age compared with WT (floxed) mice but were not different from WT (Cre±) at 12 months of age (Fig. 4B). Photopic (cone-driven) ERG b-wave recordings indicated the presence of functionally normal signaling of cones to the inner retina cones at 1 month of age (Fig. 4C). However, cone-driven signaling to the inner retina was significantly decreased in p85α cKO mice compared with WT by 6 months of age (Fig. 4C) and decreased progressively in p85α cKO mice to 12 months of age (Fig. 4C). Photopic b-wave amplitudes from 12-month-old mice expressing Cre recombinase alone were comparable to photopic b-wave amplitudes in WT mice expressing two floxed alleles, indicating that Cre expression was not the cause of the loss of function (Fig. 4C). Consistent with the progressive decline of cone photoreceptor function in older animals, Western blot analysis revealed reduced M-opsin expression in p85α cKO retinas at 6 and 12 months of age (Fig. 4D).
Figure 4.
Function of the cone-specific p85α KO retina. Scotopic a- and b-wave (A, B) and photopic b-wave (C) electroretinographic analysis of WT and cKO mice at 1 month (WT p85αflox/flox, n = 5; cKO, n = 5), 6 months (WT p85αflox/flox, n = 14; cKO, n = 14), and 12 months (WT p85αflox/flox, n = 10; WT Cre±, n = 4; cKO, n = 10) of age. Values are mean ± SEM. *P < 0.05 for scotopic b-wave (B) for WT p85αflox/flox vs. cKO at 6 and 12 months of age. P < 0.001 for photopic b-wave (C) for WT p85αflox/flox compared with cKO at 6 and 12 months of age. (D) Western blot analysis of WT and p85α cKO littermate retinal extracts was used to assess M-cone opsin levels at 6 and 12 months of age; β-actin was used as a loading control.
Effect of p85α Deletion on Cone Cell Viability
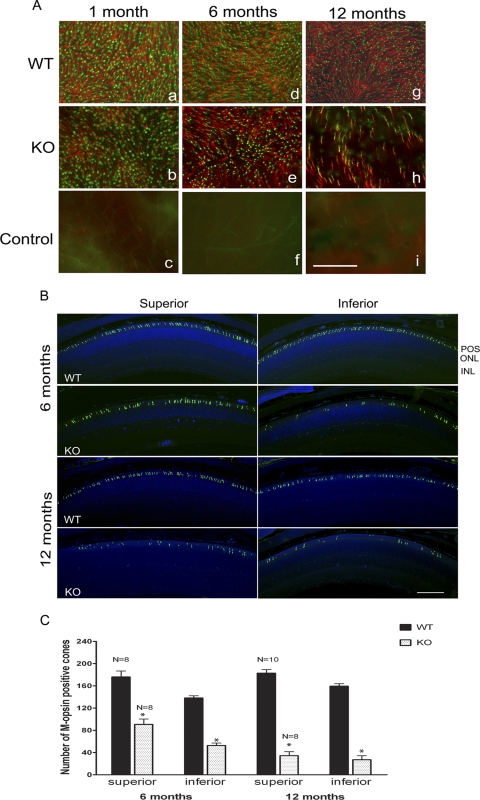
To test whether functional loss is caused by cone structural degeneration and cone cell death, we performed lectin cytochemical and immunohistochemical analysis of whole retinal flat mounts using PNA and anti–cone Arr4 to label cone outer and inner segments,47 respectively. Fluorescence microscopic analysis of WT and p85α cKO retinal flat mounts indicated that the distribution and density of cone photoreceptors were not affected at 1 month of age (Fig. 5A). However, by 6 months, patchy regions devoid of cones appeared in p85α cKO retinas compared with WT mice. Cone photoreceptor loss was further exacerbated in p85α cKO retinas by 12 months of age. Arrestin expression decreases with age in WT mice; however, the functional significance of the decrease is unknown.
Figure 5.
Loss of cone photoreceptors in p85α cKO retinas at 1, 6, and 12 months of age. (A) PNA (red) and anti–cone arrestin (cArr, green) immunofluorescence staining of retinal whole mounts from WT (a, d, g), and p85α cKO (b, e, h) mice (n = 4 each). For a control, primary antibodies were omitted (c, f, i). Scale bar, 100 μm for all panels. (B) Retinal sections of inferior and superior regions from WT and p85α cKO mice at 6 and 12 months of age were stained for M-cone opsin. (C) Quantitative analysis of M-cone opsin–positive cells at 6- and 12-month-old mice from inferior and superior regions of retina. Data are mean ± SEM; sample size is indicated on top of each bar. *P < 0.001, significance between WT and cKO. POS, photoreceptor outer segment; ONL, outer nuclear layer; INL, inner nuclear layer.
To determine whether there were regional differences in cone cell loss, we analyzed cone distribution along the superior/inferior meridian of retinas from 6- and 12-month-old WT and p85α cKO mice by fluorescence microscopy using an anti–M-cone opsin antibody to positively identify cones (Fig. 5B). In WT retinas at 6 and 12 months of age, cone distribution appeared uniform along the entire superior/inferior meridian. However, cone loss was evident in retinas of p85α cKO mice at 6 and 12 months of age, particularly in the inferior region where cone loss was worse (Fig. 5B). Quantitative analysis showed that p85α cKO mice at 6 months of age (Fig. 5C) had significantly fewer M-opsin–labeled cones than WT littermates in both superior and inferior regions, although the inferior region showed greater cone loss. By 12 months of age, cone loss in the p85α cKO retina progressed in both inferior and superior regions, with cone loss in the superior retina similar to loss in the inferior region by this age. Our studies clearly demonstrate that conditional deletion of p85α in cones leads to significant progressive loss of cones, indicating that PI3K signaling is critical to cone survival and cone function.
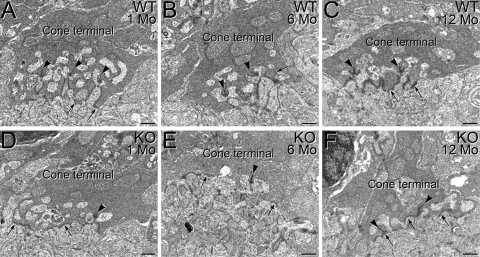
Effect of p85α Deletion on the Organization of Synaptic Ultrastructure in Surviving Cone Terminals
The synaptic terminals of cones in WT mouse retina show normal organization at 1 month (Figs. 6A, 6B) and 12 months (Fig. 6C) of age, with multiple ribbon synaptic complexes and flat contacts on the base of the terminal. However, progressive disorganization of synaptic ultrastructure was observed in the terminals of surviving cones in the p85α cKO retinas. Although cone terminals in the 1-month-old p85α cKO retina appeared ultrastructurally similar to those in WT retinas (Fig. 6D), this was not true at 6 months (Fig. 6E) and 12 months (Fig. 6F). At these ages, cone terminals in the p85α cKO retina ribbon synaptic complexes were reduced, flat contacts were sometimes located adjacent to synaptic ribbons, and interactions with postsynaptic processes often appeared disorganized, indicating aberrant ultrastructural organization of cone synapses in the absence of the p85α subunit of PI3K.
Figure 6.
Effect of conditional deletion of p85α on the organization of synaptic ultrastructure in surviving cone terminals. The synaptic terminals of cones in WT mouse retina show normal organization at 1 month (A), 6 months (B), and 12 months (C) of age, with multiple triadic ribbon synaptic complexes (arrows) and flat contacts along the base of the terminal (arrowheads). A progressive decline in organization of synaptic ultrastructure was evident in the terminals of surviving cones in the p85α cKO retina. Although cone terminals in the 1-month p85α cKO retina appeared ultrastructurally similar to that of the WT retina (D), in the p85α cKO retina at 6 months (E) and 12 months (F), fewer ribbon synaptic complexes were evident. Although flat contacts were present, they were sometimes inappropriately located in apposition to synaptic ribbons (F), indicating diminished ultrastructural organization. Scale bars, 0.5 μm for all panels.
Discussion
Characterization of cone-specific p85α KO mice at 1 month of age did not show any detectable biochemical, morphologic, or functional phenotypes, suggesting that the expression of PI3K is not required for the maturation of adult retina. However, mice at 6 and 12 months of age showed significantly reduced cone photoreceptor function and cone loss because of the cone-specific deletion of p85α. The loss of cone function was not the result of Cre expression (Fig. 4), as we have also previously reported.31 It has been shown previously that phosphoinositides modulate phototransduction.9 However, because photopic ERGs were normal in 1-month-old p85α cKO mice, the functional loss we observed was not attributed to the absence of PI3K-generated phosphoinositides but rather to the progressive loss of cone photoreceptors. These findings suggest that when deprived of active PI3K, cone photoreceptors degenerate faster in them than in WT retinas in an age-dependent manner. Our results also suggest that cone cell death induced by the absence of p85α could not be rescued by surrounding healthy rods and their putative trophic survival signals. Conversely, it is also noteworthy that dying cone photoreceptors did not affect the viability or function of the surrounding rod population (i.e., there was no “bystander” effect).38 Our study suggests that rods may communicate survival signals to cones through the cone PI3K pathway. Further studies are required to establish that cone-PI3K mediates this function.
Previous studies using animal models of RP showed that cones begin to degenerate soon after the death of rod photoreceptors, even when the causal mutation is expressed only in rods.53,54 It is well known that mutations of rod-specific genes that lead to rod malfunction and death also compromise cone function and survival, eventually resulting in complete blindness.21,28–31 Even though the underlying genetic mutations of RP are known, the particular cone survival pathways affected, ultimately leading to cone photoreceptor death, are not well known. Several theories have been advanced to explain the loss of cones in a rod mutation. First, dying rods release toxins that compromise the survival of surrounding cones.54,55 Second, because of the death of rods, cones are deprived of rod-released trophic factors necessary for maintaining cone survival and function.56–60 Third, as rods die, there is an increase of oxygen in the photoreceptor region, which leads to oxidative stress and death in cones.22,61 Fourth, as rods die, contact between RPE and cones is disrupted,37 which ultimately compromises nutrient delivery to and waste elimination from cones.
A recent study using four mouse models of RP showed that cone photoreceptor death was caused by the downregulation of the insulin/mTOR metabolic pathway in cones, as assessed by RNA microarray.37 This study showed that toxins released by dying rods are not directly responsible for cone loss because cone death did not coincide with rod death. That study also suggested that genetically influenced rod death compromises physical interaction between RPE and cones, resulting in cone cells being deprived of RPE-delivered nutrients. Starved cones responded by downregulating energy-dependent metabolic processes such as protein and phospholipid synthesis; with prolonged nutrient deprivation, cones eventually died. The same study showed that supplementation with insulin delayed cone death. This finding supports a key role for the insulin/mTOR pathway in maintaining cone survival and function and suggests that the loss of physical interaction between RPE and cones alone is not responsible for cone loss because supplied insulin was successfully passed on to cones by the RPE and was able to delay cone loss. However, the study did not address the regulation and activation of PI3K as a main link in the insulin/mTOR pathway. Furthermore, nutrient deprivation led to the downregulation of glucose-dependent phospholipid and membrane synthesis in cone outer segments.37 This study explained that shortening of cone outer segments was a result of the reduced rate of membrane synthesis compared with the rate of cone outer segment shedding and phagocytosis by RPE. This statement argues, again, against the loss of RPE and cone interaction. Why, then, do cone photoreceptors die in retinas that express mutations in only rods?
In the present study, we demonstrated that the deletion of p85α in cone photoreceptors led to progressive age-related cone degeneration. We also demonstrated that rod photoreceptor function (a-wave) was normal at all ages tested. The studies cited here showed that exogenous administration of glucose and insulin delayed cone loss but was insufficient to prevent cone death.37 When rods are lost, as occurs in RP, or when PI3K pathways are downregulated, as occurs in diabetic retinopathy, the delivery of trophic/growth factors through the RPE only delayed cone death. Therefore, we propose that if rods release trophic/growth factors that protect cones, they signal specifically through the cone PI3K pathway, which we have shown to be neuroprotective in rods.14
Previous studies have shown that phosphoinositide metabolism is important for proper synaptic function by cone terminals.12 Our studies indicate that cones are able to form ultrastructurally and functionally normal synaptic connections, even in the absence of the p85α subunit. However, the ablation of p85α in cones leads to progressive disorganization of synaptic ultrastructure in surviving cone terminals, indicating that PI3K signaling is critical to the maintenance of synaptic terminals and connections by cones. The disruption of cone terminal organization by the deletion of p85α is also likely to contribute to impaired signaling from cones to the inner retina, indicated by the decreased amplitude of the photopic b-wave in p85α cKO animals. The small reduction in scotopic b-wave amplitude in p85α cKO mice was surprising because there was no difference in the a-wave values. This cannot be attributed to Cre expression because Cre was not expressed in rods. It may be that the synaptic changes noted in the cones have some small effect on rod b-wave function.
In summary, our findings show that PI3K is important for the maintenance of cone viability and function. Our study suggests that rods may communicate survival signals to cones by way of cone PI3K. Activation of the IR (or other receptor)/mTOR/PI3K/Akt pathways may have clinical relevance. Age-related macular degeneration, diabetic retinopathy, and retinitis pigmentosa are retinal diseases that result in the loss of cone function and ultimately in cone death, leading to blindness. These findings may have significance in other chronic neurologic diseases such as Parkinson's, Huntington's, and Alzheimer's disease. In addition, the role of PI3K in synapse organization should have broad applicability to other neuronal cells.
Acknowledgments
The authors thank Michael Elliott for critical review of the manuscript and Dustin Allen for technical assistance.
Footnotes
Supported by grants from the National Institutes of Health (EY016507, EY00871, EY007361, RR17703, and EY12190), by unrestricted departmental grants from Research to Prevent Blindness, Inc., and by the SUNY Eye Institute.
Disclosure: I. Ivanovic, None; R.E. Anderson, None; Y.Z. Le, None; S.J. Fliesler, None; D.M. Sherry, None; R.V.S. Rajala, None
References
- 1. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657 [DOI] [PubMed] [Google Scholar]
- 2. Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665 [DOI] [PubMed] [Google Scholar]
- 3. Fukami K, Endo T, Imamura M, Takenawa T. alpha-Actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase. J Biol Chem. 1994;269:1518–1522 [PubMed] [Google Scholar]
- 4. Fukami K, Sawada N, Endo T, Takenawa T. Identification of a phosphatidylinositol 4,5-bisphosphate-binding site in chicken skeletal muscle alpha-actinin. J Biol Chem. 1996;271:2646–2650 [DOI] [PubMed] [Google Scholar]
- 5. Guo X, Ghalayini AJ, Chen H, Anderson RE. Phosphatidylinositol 3-kinase in bovine photoreceptor rod outer segments. Invest Ophthalmol Vis Sci. 1997;38:1873–1882 [PubMed] [Google Scholar]
- 6. Baranova LA, Grits AN, Volotovskii ID. [Phosphorylation of proteins and phosphoinositides in the axoneme of the bovine rod outer segments: the effect of structural factor]. Biofizika. 1996;41:1258–1263 [PubMed] [Google Scholar]
- 7. Guo XX, Huang Z, Bell MW, Chen H, Anderson RE. Tyrosine phosphorylation is involved in phosphatidylinositol 3-kinase activation in bovine rod outer segments. Mol Vis. 2000;6:216–221 [PubMed] [Google Scholar]
- 8. Rajala RV. Phosphoinositide 3-kinase signaling in the vertebrate retina. J Lipid Res. 2010;51:4–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He F, Mao M, Wensel TG. Enhancement of phototransduction G protein-effector interactions by phosphoinositides. J Biol Chem. 2004;279:8986–8990 [DOI] [PubMed] [Google Scholar]
- 10. Chuang JZ, Zhao Y, Sung CH. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell. 2007;130:535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SJ, Xu H, Kang LW, Amzel LM, Montell C. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 2003;39:121–132 [DOI] [PubMed] [Google Scholar]
- 12. Schmitz F, Drenckhahn D. Li(+)-induced structural changes of synaptic ribbons are related to the phosphoinositide metabolism in photoreceptor synapses. Brain Res. 1993;604:142–148 [DOI] [PubMed] [Google Scholar]
- 13. Rajala RV, McClellan ME, Ash JD, Anderson RE. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J Biol Chem. 2002;277:43319–43326 [DOI] [PubMed] [Google Scholar]
- 14. Rajala A, Tanito M, Le YZ, Kahn CR, Rajala RV. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J Biol Chem. 2008;283:19781–19792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yi X, Schubert M, Peachey NS, et al. Insulin receptor substrate 2 is essential for maturation and survival of photoreceptor cells. J Neurosci. 2005;25:1240–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li G, Anderson RE, Tomita H, et al. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J Neurosci. 2007;27:203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng L, Anderson RE, Agbaga MP, Rucker EB, III, Le YZ. Loss of BCL-XL in rod photoreceptors: increased susceptibility to bright light stress. Invest Ophthalmol Vis Sci. 2006;47:5583–5589 [DOI] [PubMed] [Google Scholar]
- 18. Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina, II: autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979;188:263–272 [DOI] [PubMed] [Google Scholar]
- 19. Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina, I: structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262 [DOI] [PubMed] [Google Scholar]
- 20. Adler R, Curcio C, Hicks D, Price D, Wong F. Cell death in age-related macular degeneration. Mol Vis. 1999;5:31. [PubMed] [Google Scholar]
- 21. Stone EM, Braun TA, Russell SR, et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351:346–353 [DOI] [PubMed] [Google Scholar]
- 22. Shen J, Yang X, Dong A, et al. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol. 2005;203:457–464 [DOI] [PubMed] [Google Scholar]
- 23. Cho NC, Poulsen GL, Ver Hoeve JN, Nork TM. Selective loss of S-cones in diabetic retinopathy. Arch Ophthalmol. 2000;118:1393–1400 [DOI] [PubMed] [Google Scholar]
- 24. Nork TM. Acquired color vision loss and a possible mechanism of ganglion cell death in glaucoma. Trans Am Ophthalmol Soc. 2000;98:331–363 [PMC free article] [PubMed] [Google Scholar]
- 25. Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kellner U, Foerster MH. Pattern of dysfunction in progressive cone dystrophies—an extended classification. Ger J Ophthalmol. 1993;2:170–177 [PubMed] [Google Scholar]
- 27. Kohl S. [Genetic causes of hereditary cone and cone-rod dystrophies]. Ophthalmologe. 2009;106:109–115 [DOI] [PubMed] [Google Scholar]
- 28. Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680 [DOI] [PubMed] [Google Scholar]
- 29. Lem J, Krasnoperova NV, Calvert PD, et al. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci U S A. 1999;96:736–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naash MI, Hollyfield JG, Al Ubaidi MR, Baehr W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc Natl Acad Sci U S A. 1993;90:5499–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsang SH, Gouras P, Yamashita CK, et al. Retinal degeneration in mice lacking the gamma subunit of the rod cGMP phosphodiesterase. Science. 1996;272:1026–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan E, Wang Q, Quiambao AB, et al. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42:589–600 [PubMed] [Google Scholar]
- 33. Johnson LE, van Veen T, Ekstrom PA. Differential Akt activation in the photoreceptors of normal and rd1 mice. Cell Tissue Res. 2005;320:213–222 [DOI] [PubMed] [Google Scholar]
- 34. Rajala RV, Ivanovic I, Dilly AK. Retinal insulin receptor signaling in hyperosmotic stress. Vitam Horm. 2009;80:583–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park SH, Park JW, Park SJ, et al. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46:1260–1268 [DOI] [PubMed] [Google Scholar]
- 36. Reiter CE, Wu X, Sandirasegarane L, et al. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55:1148–1156 [DOI] [PubMed] [Google Scholar]
- 37. Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gomez-Vicente V, Doonan F, Donovan M, Cotter TG. Induction of BIM(EL) following growth factor withdrawal is a key event in caspase-dependent apoptosis of 661W photoreceptor cells. Eur J Neurosci. 2006;24:981–990 [DOI] [PubMed] [Google Scholar]
- 39. Mackey AM, Sanvicens N, Groeger G, Doonan F, Wallace D, Cotter TG. Redox survival signalling in retina-derived 661W cells. Cell Death Differ. 2008;15:1291–1303 [DOI] [PubMed] [Google Scholar]
- 40. Barber AJ, Nakamura M, Wolpert EB, et al. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001;276:32814–32821 [DOI] [PubMed] [Google Scholar]
- 41. Edstrom A, Ekstrom PA. Role of phosphatidylinositol 3-kinase in neuronal survival and axonal outgrowth of adult mouse dorsal root ganglia explants. J Neurosci Res. 2003;74:726–735 [DOI] [PubMed] [Google Scholar]
- 42. Kermer P, Klocker N, Labes M, Bahr M. Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J Neurosci. 2000;20:2–8 [PubMed] [Google Scholar]
- 43. Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:764–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luo J, McMullen JR, Sobkiw CL, et al. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25:9491–9502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Le YZ, Ash JD, Al Ubaidi MR, Chen Y, Ma JX, Anderson RE. Targeted expression of Cre recombinase to cone photoreceptors in transgenic mice. Mol Vis. 2004;10:1011–1018 [PubMed] [Google Scholar]
- 46. Rajala A, Anderson RE, Ma JX, Lem J, Al Ubaidi MR, Rajala RV. G-protein-coupled receptor rhodopsin regulates the phosphorylation of retinal insulin receptor. J Biol Chem. 2007;282:9865–9873 [DOI] [PubMed] [Google Scholar]
- 47. Nikonov SS, Brown BM, Davis JA, et al. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron. 2008;59:462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452 [DOI] [PubMed] [Google Scholar]
- 49. Gomez-Vicente V, Donovan M, Cotter TG. Multiple death pathways in retina-derived 661W cells following growth factor deprivation: crosstalk between caspases and calpains. Cell Death Differ. 2005;12:796–804 [DOI] [PubMed] [Google Scholar]
- 50. Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–10968 [DOI] [PubMed] [Google Scholar]
- 51. Fruman DA, Mauvais-Jarvis F, Pollard DA, et al. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat Genet. 2000;26:379–382 [DOI] [PubMed] [Google Scholar]
- 52. Guo F, Gopaul DN, van Duyne GD. Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse. Nature. 1997;389:40–46 [DOI] [PubMed] [Google Scholar]
- 53. Banin E, Cideciyan AV, Aleman TS, et al. Retinal rod photoreceptor-specific gene mutation perturbs cone pathway development. Neuron. 1999;23:549–557 [DOI] [PubMed] [Google Scholar]
- 54. Ripps H. Cell death in retinitis pigmentosa: gap junctions and the ‘bystander’ effect. Exp Eye Res. 2002;74:327–336 [DOI] [PubMed] [Google Scholar]
- 55. Cronin T, Leveillard T, Sahel JA. Retinal degenerations: from cell signaling to cell therapy; pre-clinical and clinical issues. Curr Gene Ther. 2007;7:121–129 [DOI] [PubMed] [Google Scholar]
- 56. Leveillard T, Mohand-Said S, Lorentz O, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36:755–759 [DOI] [PubMed] [Google Scholar]
- 57. Mohand-Said S, Deudon-Combe A, Hicks D, et al. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci U S A. 1998;95:8357–8362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mohand-Said S, Hicks D, Dreyfus H, Sahel JA. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch Ophthalmol. 2000;118:807–811 [DOI] [PubMed] [Google Scholar]
- 59. Mohand-Said S, Hicks D, Simonutti M, et al. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 1997;29:290–297 [DOI] [PubMed] [Google Scholar]
- 60. Chalmel F, Leveillard T, Jaillard C, et al. Rod-derived cone viability factor-2 is a novel bifunctional-thioredoxin-like protein with therapeutic potential. BMC Mol Biol. 2007;8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2006;103:11300–11305 [DOI] [PMC free article] [PubMed] [Google Scholar]