Principle component analyses of infrared spectra of human meibum indicate that human meibum collected from normal donors is less ordered than meibum from donors with meibomian gland dysfunction. Model studies indicate that changes in the amount of lipid saturation, rather than the amount of cholesterol esters, could be a factor in the observed conformational differences.
Abstract
Purpose.
Instability of the tear film with rapid tear break-up time is a common feature of aqueous-deficient and evaporative dry eye diseases, suggesting that there may be a shared structural abnormality of the tear film that is responsible for the instability. It may be that a change in the normal meibum lipid composition and conformation causes this abnormality. Principle component analyses of infrared spectra of human meibum indicate that human meibum collected from normal donors (Mn) is less ordered than meibum from donors with meibomian gland dysfunction (Md). In this study the conformation of Md was quantified to test this finding.
Methods.
Changes in lipid conformation with temperature were measured by infrared spectroscopy. There were two phases to our study. In phase 1, the phase transitions of human samples, Mn and Md, were measured. In phase 2, the phase transitions of model lipid standards composed of different waxes and cholesterol esters were measured.
Results.
The phase-transition temperature was significantly higher (4°C) for the Md compared with the Mn of age-matched donors with no history of dry-eye symptoms. Most (82%) of the phase-transition temperatures measured for Md were above the values for Mn. The small change in the transition temperature was amplified in the average lipid order (stiffness) at 33.4°C. The average lipid order at 33.4°C for Md was significantly higher (30%, P = 0.004) than for Mn. The strength of lipid–lipid interactions was 72% higher for Md than for Mn. The ability of one lipid to influence the melting of adjacent lipids is termed cooperativity. There were no significant differences between Mn and Md in phase-transition cooperativity, nor was there a difference between Mn and Md in the minimum order or maximum order that Mn and Md achieved at very low and very high temperatures, respectively. The model wax studies showed that the phase transition of complex mixtures of natural lipids was set by the level of unsaturation. A double bond decreased the phase-transition temperature by approximately 40°C. The addition of a second CH CH moiety decreased the phase-transition temperature by approximately 19°C. Unsaturated waxes were miscible with saturated waxes. When a saturated wax was mixed with an unsaturated one, the saturated wax disproportionately increased the phase transition of the mixture by approximately 30°C compared with the saturated wax alone. Cholesterol ester had little effect on the phase-transition temperature of the waxes. Model studies indicated that changes in the amount of lipid saturation, rather than the amount of cholesterol esters, could be a factor in the observed conformational changes.
CH moiety decreased the phase-transition temperature by approximately 19°C. Unsaturated waxes were miscible with saturated waxes. When a saturated wax was mixed with an unsaturated one, the saturated wax disproportionately increased the phase transition of the mixture by approximately 30°C compared with the saturated wax alone. Cholesterol ester had little effect on the phase-transition temperature of the waxes. Model studies indicated that changes in the amount of lipid saturation, rather than the amount of cholesterol esters, could be a factor in the observed conformational changes.
Conclusions.
Meibum lipid compositional changes with meibomian gland dysfunction reflect changes in hydrocarbon chain conformation and lipid–lipid interaction strength. Spectroscopic techniques are useful in studying the lipid–lipid interactions and conformation of lipid from individual patients. (ClinicalTrials.gov number, NCT00803452.)
Meibomian gland and aqueous-deficient dry eye diseases affect 6 to 7 million people in the United States alone and can cause chronic and severe symptoms, as well as secondary changes that can lead to visual disturbance.1,2 Among many factors, maintenance of the integrity of tear lipids is essential to the prevention of dry-eye symptoms (see reviews by Foulks3 and others4–15). It is generally believed that the predominant cause of evaporative dry eye is meibomian gland dysfunction (MGD), although blink abnormalities also contribute to the disorder.16
The correlation between dry eye and an abnormal lipid layer of the tear film has been made by the use of interference microscopy17–22 and other techniques.23–25 Moreover, when the meibomian gland, which produces most of the tear film lipid, is dysfunctional, delivery of oil to the lid margin is reduced26–28 and dry-eye conditions occur.8,11 Changes in meibum composition that may contribute to and be diagnostic of dry eye symptoms have been reported.1,29–32
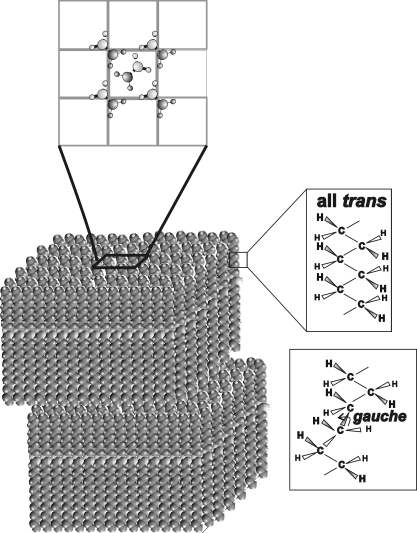
One of the earliest therapies for MGD has been to warm the eyelid to 45°C (113°F).33–35 and conformational changes observed in the hydrocarbon chains of Mn with temperature suggested that the observed increased delivery of meibum lipid with eyelid therapeutic heating could be related to the increased disorder in the packing of the hydrocarbon tails.36 Conformation is defined as the arrangement of the atoms in a molecule in space. The conformation of the lipid hydrocarbon chain changes with increasing temperature. When lipids are in an ordered state (at low temperatures) the hydrocarbon chains contain more trans rotomers (Fig. 1). Rotomers are conformations that can change with the rotation of a molecule. Order refers to a phase where lipids are in a more solid (but not completely) gel like phase. When lipids become disordered, they are in a fluid, liquid–crystalline phase and contain gauche rotomers. When lipids go from an ordered state to a disordered one, they are said to undergo a phase transition. The lipid phase transition is different from the melting of a solid, because the lipids are not completely solid and not completely liquid, but rather are more like a gel or liquid crystal. Phase transitions may be described by four parameters: the order of the most ordered and disordered state; the transition temperature at which half of the lipid molecules undergo a phase change; and the relative cooperativity. Cooperativity describes how a lipid molecule influences the order of neighboring lipids. The broader the phase transition, the smaller the absolute value for cooperativity. The phase-transition temperature is the temperature at which half the lipids undergo the phase transition. When the conformation of the hydrocarbon chains is all trans, the lipids are able to pack more closely together because the hydrocarbon chain is extended (Fig. 1) and Van der Waal's interactions between the lipids are at their maximum. The strength of the lipid–lipid interactions is measured in kilocalories per mole.
Figure 1.
Multilamellar monolayers of ordered (solid, or unmelted) wax molecules that are arranged in an orthorhombic crystal structure in which the hydrocarbon chain conformation is all trans. The hydrocarbon chains of fluid waxes contain gauche rotomers.
The hydrocarbon chains of Mn become more disordered at higher temperatures (Fig. 1).36 It is reasonable that inspissation (more ordered lipid) of the meibum results in stronger lipid–lipid interactions that reduce the flow of meibum from the meibomian gland orifice. We tested this hypothesis in the present study.
Spectroscopic techniques have been used to characterize human meibum.37 Some applications of infrared spectroscopy to biological systems can be found in the textbook by Mantsch and Chapman.38 Temperature-induced phase transitions of meibum lipid were experimentally reproducible and were similar in multiple samples collected from the same person.39 Our studies showed that at ambient lid temperature, the lipid is approximately 37% ordered, between solid (gel phase) and liquid (liquid–crystalline phase).37 As the temperature increased from 25°C to 45°C, lipid delivery to the margins was observed to increase with a concomitant decrease in the refractive index,40 hydrocarbon disorder,36 and meibum lipid hydrocarbon motion.39 These findings suggest that hydrocarbon chain order and motion determine the delivery of meibum lipid from the meibomian glands to the lid margins and subsequently to the tear film.
Mn from the same person was approximately 40% less ordered at 33.5°C than was lipid extracted from tears, indicating that tears do not have the same lipid composition as Mn.36 In Mn and muscle, retina, and lens tissues, lipid saturation and lipid order at physiological temperature were linearly related to the lipid phase-transition temperature.41 Mn order and phase-transition temperatures decrease with age, a trend that may be attributable to lipid compositional changes.40,42 Our infrared spectral studies, along with principal component analysis, enable the quantification of the variance among the lipid spectra, which allowed us to identify and quantify protein associated with human meibum.43 The spectra were used to discriminate between Mn and meibum from donors with MGD (Md) with an accuracy of 93%.43 More important, this high degree of accuracy shows that the infrared spectra contain compositional and structural information about the changes that occur with meibomian gland dysfunction. Md was found to contain more protein and relatively less methyl groups (CH3) and cis double bonds (cis CH) than did Mn.43 The amount of protein was confirmed from relative infrared band intensities.43
CH) than did Mn.43 The amount of protein was confirmed from relative infrared band intensities.43
We tested Md both before and after 1 month of therapy with topical azithromycin44 and determined that this therapy reduces elevated phase-transition temperature (a general biomarker) and lipid order (another biomarker) of abnormal secretions to normal levels. Md conformation was measured with temperature to characterize its thermodynamic properties for comparison with those of Mn.
MGD is defined as “a condition of progressive obstruction due to ductal hyperkeratinization or inspissation of secretion.”3 Until this study, inspissation (thickening) of meibum lipid had been assessed only qualitatively. One would expect that the lipid–lipid interactions would be greater in thicker secretions. In this study, the thickening of meibomian secretion was quantified by infrared spectroscopy. This technology has been applied to assessment of the molecular structure/conformation and packing of meibum lipid hydrocarbon chains.36–40,44
In phase 1 of our study, infrared spectroscopy was used to characterize the biophysical and thermodynamic properties of Md in terms of lipid order, lipid–lipid interaction strength and phase-transition temperature parameters. In phase 2 of our study, model wax and cholesterol ester mixtures were studied to determine the major compositional factors that contribute to changes in the thermodynamic properties of meibum.
Methods
Materials
Silver chloride windows for infrared spectroscopy were obtained from Crystran Ltd. (Poole, UK). Wax esters, oleyloleate (OO) and palmityloleate (PO), palmitylpalmitate (PP), sterylpalmitate (SP), and the cholesterol ester cholesterylpalmitate were purchased from the Sigma-Aldrich (St. Louis, MO).
Diagnosis of Normal and MGD Status
Normal status was assigned when the patient's meibomian gland orifices showed no evidence of keratinization or plugging with turbid or thickened secretions, and no dilated blood vessels were observed on the eyelid margin. Normal donors did not recall having dry eye symptoms. The diagnosis of MGD was made according to the criteria of Foulks and Bron.26 Plugging of the meibomian glands in at least 5 of 10 orifices in the central portion of the upper eyelid was a requirement for diagnosis. The character of meibomian gland–expressed secretion had to be turbid, turbid with clumps, or pastelike. Inflammation of the eyelid margin, as evidenced by swelling of the eyelid margin and 2+ vascular injection of the posterior lid margin, was necessary for diagnosis. The presence of telangiectasia of the posterior eyelid margin was confirmatory of chronic disease but was not required for study enrollment. Inclusion criteria were the presence of symptomatic meibomian gland dysfunction in subjects between 18 and 80 years of age who were not taking systemic or topical antibiotics and were not using topical anti-inflammatory medications. Exclusion criteria were a history of allergy to azithromycin, altered lid anatomy (with the exception of meibomian gland dysfunction), wearing eye makeup, inability to comprehend the informed consent, or inability to complete the prescribed therapy and follow-up. Dietary history was not assessed, but none of the subjects were taking omega-3 essential fatty acid supplements. All subjects underwent a complete examination of the eyelids and anterior segment of the eye, including measurement of intraocular pressure before entry into the study. Symptoms were measured on a four-point categorical scale of none, mild, moderate, or severe, according to the subject's response to questions regarding itching, burning, foreign body sensation, eyelid redness, and eyelid swelling (Table 1). Signs evaluated were slit lamp–observed conjunctival injection, fluorescein tear breakup time, ocular surface staining with fluorescein, appearance of the eyelid margin, and character of meibomian gland orifices (Table 2). All signs were graded on a four-point categorical scale, as summarized in Table 2, with the exception of tear breakup time, which was measured in seconds after a complete blink that followed instillation of 5 μL topical 1% fluorescein solution.
Table 1.
Grading of Clinical Symptom in Donors with MGD
| Mean Grade | Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|---|
| Itching | 1.1 ± 0.2 | None | Awareness | Desire to rub | Frequent rub |
| Fbs | 1.4 ± 0.2 | None | Awareness | Desire to rub | Desire to close lid |
| Dryness | 0.7 ± 0.2 | None | Awareness | Need for drop | Frequent drops |
| Burning | 0.7 ± 0.2 | None | Awareness | Need to rub | Frequent rub |
| Swelling | 0.73 ± 0.08 | None | Noticeable | Obvious | Decrease in aperture width |
Values are average ± SEM. Fbs, foreign body sensation.
Table 2.
Grading of Clinical Signs in Donors with MGD
| Sign | Mean Grade | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|---|
| Plugged, n | 9.0 ± 0.4* | ||||
| Lid margin redness | 2.0 ± 0.2 | None | Pink | Light red | Bright red |
| Swelling | 0.9 ± 0.2 | None | Rounded | >1 mm | >2 mm |
| Plugging | 3.2 ± 0.2 | Touch | Light pressure | Moderate pressure | Occluded |
| Mg secretion | 3.2 ± 0.2 | Clear | Turbid | Turbid with clumps | Solid paste |
| TBUT | 6.0 ± 0.3† |
Values are average ± SEM.
MG plugging, number of plugged orifices within the central 10 glands of upper lid.
TBUT, number of seconds to first breakup following complete blink.
Written informed consent was obtained from all donors and protocols, and procedures were approved by the University of Louisville Institutional Review Board as well as the Louisville Veterans Affairs Hospital Institutional Review Board. All procedures were in accordance with the Declaration of Helsinki.
Collection of Tear Lipids
Meibum lipid was obtained from living subjects, as described by Kilp et al.,22 except that meibomian gland excreta were collected with a platinum spatula with attention toward avoiding scraping of the eyelid margin. Meibum was expressed from both free and plugged meibomian glands. We visually estimated that approximately 1 mg of meibum was collected per individual for direct spectroscopic study.
Fourier Transform Infrared Spectroscopy
For phase 1, the study of human meibum, expressed meibum was placed directly from the platinum spatula onto an AgCl window without any further manipulation or extraction. For phase 2, the study of model waxes, standard wax was dissolved in tetrahydrofuran/methanol (3:1, vol/vol), and approximately 1 mg was applied to an AgCl infrared window. The solvent was evaporated under a stream of nitrogen gas, and the window was placed in a lyophilizer for 4 hours to remove all traces of solvent.
Infrared spectra were measured using a Fourier transform infrared spectrometer (Nicolet 5000 Magna Series; Thermo Fisher Scientific, Inc., Waltham MA). Meibum or wax sample on the AgCl window was placed in a temperature-controlled infrared cell. The cell was jacketed by an insulated water coil connected to a circulating water bath (model R-134A; Neslab Instruments, Newton NH). The sample temperature was measured and controlled by a thermistor touching the sample cell window. The water bath unit was programmed to measure the temperature at the thermistor and to adjust the bath temperature so that the sample temperature could be set to the desired value. The rate of heating or cooling (1°C/15 minutes) at the sample was also adjusted by the water bath unit. Temperatures were maintained within ±0.01°C. Exactly 150 interferograms were recorded and averaged. Spectral resolution was set to 1.0 cm−1. Phase transitions for lipid standards were run at least twice with care taken to obtain data points in the sharp phase-transition temperature region. Replicate runs were combined and then the phase-transition parameters were calculated.
Infrared data analysis was then performed (GRAMS/386 software; Galactic Industries, Salem, NH). The frequency of the CH2 band near the symmetric CH2 stretching band (2850 cm−1) was used to estimate the content of trans and gauche rotomers in the hydrocarbon chains. Although the 2954-cm−1 asymmetric CH2 stretching band is useful for measuring phase-transition parameters, we chose to use the 2850-cm−1 band rather than the near 2954-cm−1, because measurement of the asymmetric band frequency is complicated by the adjacent CH3 symmetric stretching band near 2955 cm−1 and the CH2 symmetric stretching band near 2852 cm−1. The symmetric stretch (ν̃sym) was calculated by first baseline leveling the OH-CH stretching region between 3500 and 2700 cm−1. The center of mass of the CH2 symmetric stretching band was calculated by integrating the top 10% of the intensity of the band. The baseline for integrating the top 10% of the intensity of the band was parallel to the OH-CH region baseline.
Lipid CH2 groups in the hydrocarbon chains are present as gauche rotomers, prevalent in disordered hydrocarbon chains, or trans rotomers, more abundant in ordered hydrocarbon chains (Fig. 1). Thus, lipid hydrocarbon chain order may be evaluated in terms of the relative amount of CH2 trans rotomers. The frequency of the CH2 symmetric stretch, ν̃sym, is dependent on the amount of trans or gauche rotomers45 and has been used to characterize lipid phase transitions36–40,42–53 and to measure the trans rotomer content of lipid hydrocarbon chains with changes in temperature.36–40,42–44,47–50,53 Since rotomers are either in trans or gauche conformations, phase transitions can be described by a two-state sigmoidal equation, as described by Borchman et al.36 Lipid order at 33.4°C was calculated by extrapolating the ν̃sym at 33.4°C from the fit of the phase transition and then converting ν̃sym to the percentage of trans rotomers, a measure of lipid conformational order (see Borchman et al.36,40). The data for percentage of trans rotomer were used to calculate the phase-transition enthalpy and entropy from the slopes of Arrhenius plots, as described in Borchman et al.36,40 Arrhenius plots from tear–lipid phase transitions were linear, with correlation coefficients greater than 0.998.
Because we calculated the percentage of trans rotomers at 33.4°C and calculated the enthalpy and entropy to convert a mole of gauche rotomers to trans rotomers, we could calculate the strength of lipid–lipid interactions at 33.4°C by multiplying lipid order (%) by the enthalpy of the transition and dividing by 100.
Statistics
Data are presented as the average ± the SEM. Significance was determined with the Student's t-test or the correlation coefficient from the linear regression best fit. Differences reaching P < 0.01 were considered to be statistically significant.
Results
Phase 1: Human Meibum Study
Human Material.
Human donors of meibum were recruited from the members and associates of DB's laboratory and patients of GNF's at the Kentucky Lions Eye Center and the Veterans Affairs Hospital, Louisville Kentucky. Donor data are presented in Table 3. The number of donors is different in Tables 3 and 4, because Table 3 lists all donors and Table 4 lists only age-matched normal donors.
Table 3.
Donor Data
| Normal | Meibomian Gland Dysfunction | |
|---|---|---|
| Male | 81/26 | 62/31 |
| Caucasian | 78/25 | 78/39 |
| Black | 3/1 | 12/6 |
| Hispanic | 0/0 | 2/1 |
| Asian | 16/5 | 0/0 |
| Unknown | 3/1 | 8/4 |
| Total number of donors* | 32 | 50 |
Data are the percentage/number of the total group. A few donors donated as many as five times.
Data from repeat donors was averaged and considered as n = 1.
Table 4.
Lipid Phase-Transition Parameters
| Phase-Transition Parameter | Normal (Age-Matched) | MGD | Significance (P) |
|---|---|---|---|
| Minimum frequency, cm−1 | 2849.90 ± 0.09 | 2849.68 ± 0.06 | 0.09 |
| Maximum frequency, cm−1 | 2853.6 ± 0.1 | 2853.48 ± 0.07 | 0.41 |
| Cooperativity | −9.2 ± 0.5 | −8.5 ± 0.4 | 0.40 |
| Phase transition | 28.9 ± 0.4 | 32.2 ± 0.5 | 0.002 |
| Temperature, °C | |||
| Enthalpy, kcal/mol | 180 ± 15 | 157 ± 7 | 0.15 |
| Entropy, kcal/mol/deg | 0.56 ± 0.05 | 0.51 ± 0.02 | 0.29 |
| Order % at 33.4°C | 35 ± 2 | 47 ± 2* | 0.004 |
| Donors, n | 13 | 49 |
Order values are predicated on meibum from 50 donors.
Lipid Phase Transitions and the Infrared CH Stretching Region.
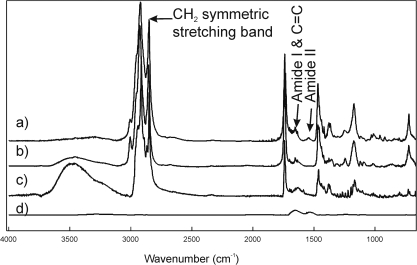
The CH2 stretching bands were predominant in the infrared spectra of lipids due to the large number of CH2 groups in their hydrocarbon chains (Fig. 2). The CH stretching region is composed of six major bands.39 In this study, we used ν̃sym near 2850 cm−1 to estimate the trans to gauche rotomer content of the hydrocarbon chains (spectrum a). The spectrum of lysozyme (d) was scaled to the amide II band (a), to show that even if all the intensity of the band marked amide II in (a) were from protein, there would be no significant overlap with the lipid CH stretching bands. The ν̃sym increased with the number of gauche rotomers, concurrent with a decrease in intensity.
Figure 2.
(a) A typical infrared spectrum in a 20-year-old man without dry eye symptoms. The infrared spectrum of meibum closely resembles the infrared spectrum of the wax oleyloleate (b) and the spectrum of cholesterylpalmitate (c). (d) The spectrum of lysozyme is scaled to the amide II band in (a) to show that even if all the intensity of the band marked amide II in (a) were from protein, there would be no significant overlap with the lipid CH stretching bands.
Meibum lipid phase transitions were quantified by fitting them to a four-parameter, two-state, sigmoidal equation, as described by Borchman et al.39 The four parameters fitted were the minimum and maximum ν̃sym in the phase transition that correspond to the most ordered and disordered states, respectively; the transition temperature at which half of the lipid molecules underwent a phase change; and the relative cooperativity. The broader the phase transition, the smaller the absolute value of the cooperativity. Cooperativity describes how the order of a lipid influences that of neighboring lipids.
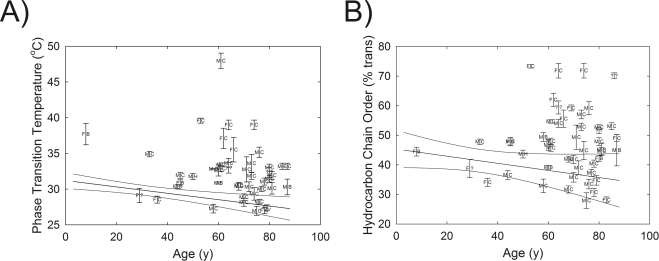
The four phase-transition parameters necessary for defining the phase transition and other parameters calculated from the defining parameters are listed in Table 4. The phase-transition temperature was significantly (4°C) higher (P < 0.01) for Md than for Mn of age-matched donors with no history of dry eye symptoms (Table 4). The individual phase-transition temperatures versus age are shown in Figure 3A. Only 18% of the phase-transition temperatures measured for Md were within the 95% confidence limits for Mn (Fig. 3A). Note that the lowest values for the phase-transition temperature decreased with age at the same rate as the values for Mn reported previously (Fig. 3A).38 None of the nine phase-transition temperatures measured for Mn was above the average phase-transition temperature for Md. There were no significant differences in the other three phase-transition parameters between Mn and Md (Table 4).
Figure 3.
(A) The relationship between donor age and phase-transition temperature for meibum lipid from donors with MGD. (B) The relationship between donor age and hydrocarbon chain order at lid temperature, 33.4°C, from meibum lipid from donors with MGD. Error bars are ± SE. Lines: linear fit and 95% confidence limits of data from meibum lipid donors without a history of dry eye symptoms, as reported in Reference 40. F, female; M, male; B, black; C, Caucasian; H, Hispanic.
Lipid order was measured close to but not exactly at the temperature of the eye lid, 33.4°C, so lipid order at exactly 33.4°C was calculated by extrapolating the ν̃sym at 33.4°C from the fit of the phase transition and then converting ν̃sym to the percentage of trans rotomers (Table 4).38 The average lipid order at 33.4°C for Md was significantly (30%; P < 0.0001) higher than for Mn (Fig. 3B, Table 4). The individual lipid orders at 33.4°C versus age are shown in Figure 3B. Note that the lowest Md values for the lipid order at 33.4°C decreased with age at the same rate as the values reported for Mn reported previously (Fig. 3B).40 No sex- or race-related differences between the phase-transition parameters of Md were evident.
The large interdonor distribution of parameters (Fig. 3) was not due to large variations in the precision of testing protocols. Phase-transition temperatures spanned 12°C from sample to sample, which is much larger than the ±2°C precision of the measurements (Fig. 3). The variability from sample to sample must have been due to differences in lipid composition differences.
The enthalpy, 157 ± 7 kcal/mole, and entropy, 0.51 ± 0.02 kcal/mole/deg, of the phase transitions of Md were not significantly different from those of Mn (Table 4) and did not change with age. The strength of lipid–lipid interactions was 72% higher for Md (43 ± 6 kcal/mole) than for Mn (74 ± 4 kcal/mole).
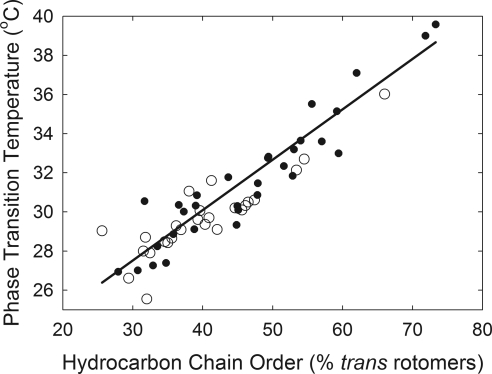
The order versus phase-transition temperature data (Fig. 4) were fit by linear regression analysis, and the slope of the fit was 0.26, with an intercept of 19.8 (r = 0.76, P < 0.005). This result indicates that the order of human meibum at 36°C is directly related to the phase-transition temperature within the range of phase-transition temperatures measured. When the lipid reaches its maximum or minimum order, the phase-transition temperature is at a critical point, above or below which the lipid order will not change.
Figure 4.
Linear relationship between lipid phase-transition temperature and order of meibomian lipid from donors with meibomian-gland dysfunction (●) and donors without a history of dry-eye symptoms (○).
Phase 2: Model Lipid/Wax Study
Elongated waxes are tube shaped, forming multilamellar monolayers that are closely packed (2.5–3.2 Å; Fig. 1).54,55 When waxes are ordered (stiff, or unmelted), the hydrocarbon chains align in a trans conformation and pack in a perpendicular orthorhombic crystal structure (Fig. 1, top).54 Structural analysis has not been done on disordered (fluid, or melted) waxes that take on gauche conformations. In this study, we examined the influence of double bonds and cholesterol ester on wax mixtures, since wax is the major component of meibum.56
The Phase-Transition Temperature and the Infrared CH Stretching Region (between 3100 and 2700 cm−1).
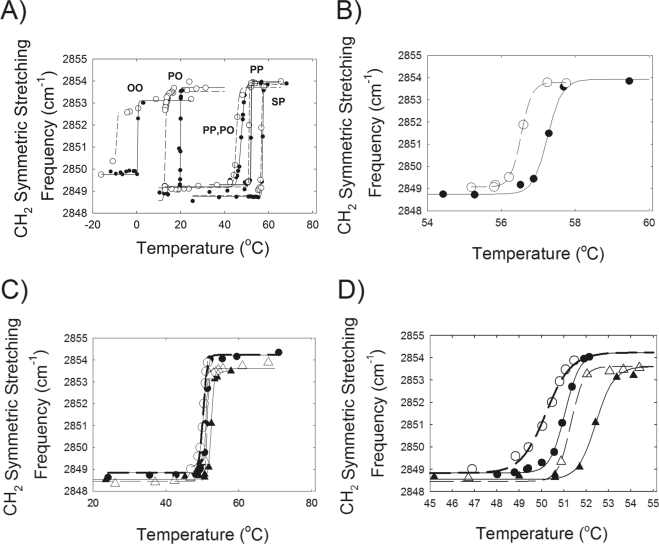
Hydrocarbon order-to-disorder phase transitions were measured for four model standard wax esters over a temperature range of −20°C to 70°C (Fig. 5A). Hysteresis was noted in all samples. Compared to the heating curves, the phase-transition temperatures for the cooling curves were approximately 7°C lower for the unsaturated wax esters oleyloleate (OO) and palmityloleate (PO) (Table 5). Hysteresis in the phase transitions of the saturated waxes palmitylpalmitate (PP) and sterylpalmitate (SP) was less (∼0.8°C) than that for the unsaturated waxes, but measurable (Fig. 5B, Table 5). Compared to PP, an additional CH2—CH2 moiety in the palmityl hydrocarbon chain of SP raised the melting temperature (Tm) approximately 5°C (Table 5). A double bond in PO decreased the phase-transition temperature of SP by approximately 40°C. In comparison, the addition of a second CH CH moiety in OO decreased the phase-transition temperature of PO by approximately 19°C.
CH moiety in OO decreased the phase-transition temperature of PO by approximately 19°C.
Figure 5.
(A) Wax phase transition curves for OO oleyloleate, PO palmityloleate, PP palmitylpalmitate, SP sterylpalmitate. (●) Heating curve; (○) cooling curve. Lines are the fit of the data to equation 1 in Borchman et al.36 The lower the CH2 symmetric stretching frequency, the more ordered the wax hydrocarbon chains. (B) Melting curve for sterylpalmitate. The scale is enlarged from (A) so that hysteresis between the cooling (○) and heating (●) curves can be observed. (C) Equal molar mixture of sterylpalmitate and oleyloleate (●) heating and (○) cooling curve. Sterylpalmitate:oleyloleate:cholesterylpalmitate 2:2:1 (m:m:m) where m is moles. (▴) heating curve, (▵) cooling curve. (D) Enlargement of the scale in (C).
Table 5.
Phase-Transition Parameters*
| Sample | Transition Temp. (°C) | Minimum Frequency (cm−1) | Maximum Frequency (cm−1) | Cooperativity (No Units) |
|---|---|---|---|---|
| Oleyloleate | ||||
| Heating | 0.5 ± 0.3 | 2849.84 ± 0.03 | 2853.11 ± 0.04 | 12 ± 14 |
| Cooling | −10 to −4 | |||
| Palmityloleate | ||||
| Heating | 19.96 ± 0.02 | 2849.1 ± 0.1 | 2853.7 ± 0.2 | 283 ± 58 |
| Cooling | 13.1 ± 0.1 | 2848.6 ± 0.6 | 2853.5 ± 0.4 | 43 ± 16 |
| Palmitylpalmitate | ||||
| Heating | 51.98 ± 0.02 | 2849.31 ± 0.05 | 2854.0 ± 0.1 | 480 ± 178 |
| Cooling | 51.06 ± 0.01 | 2849.21 ± 0.05 | 2853.91 ± 0.04 | 435 ± 73 |
| Stearylpalmitate | ||||
| Heating | 57.23 ± 0.02 | 2848.75 ± 0.03 | 2853.92 ± 0.08 | 292 ± 25 |
| Cooling | 56.54 ± 0.01 | 2849.07 ± 0.03 | 2853.78 ± 0.03 | 386 ± 21 |
| Palmitylpalmitate/palmityloleate, 1:1, m:m | ||||
| Heating | 47.61 ± 0.07 | 2849.20 ± 0.04 | 2853.98 ± 0.09 | 56 ± 3 |
| Cooling | 45.28 ± 0.09 | 2849.16 ± 0.06 | 2853.7 ± 0.1 | 81. ± 10 |
| Stearylpalmitate/oleyloleate, 1:1, m:m | ||||
| Heating | 51.04 ± 0.03 | 2848.83 ± 0.05 | 2853.21 ± 0.08 | 152 ± 12 |
| Cooling | 50.20 ± 0.08 | 2848.84 ± 0.09 | 2853.23 ± 0.10 | 89.4 ± 58 |
| Stearylpalmitate/oleyloleate/cholesterylpalmitate 2:2:1, m:m:m | ||||
| Heating | 52.44 ± 0.06 | 2848.54 ± 0.06 | 2853.60 ± 0.09 | 130 ± 17 |
| Cooling | 51.31 ± 0.07 | 2848.46 ± 0.07 | 2853.61 ± 0.06 | 184 ± 24 |
Phase transitions for lipid standards were run at least twice with care to obtain data points in the sharp phase-transition temperature region. Replicate runs were combined, and then the phase-transition parameters were calculated.
The unsaturated waxes OO and PO were miscible with the saturated waxes PP and SP, as is evident by there being only one phase transition (Figs. 5A, 5C). When a saturated wax, PP, was mixed with an unsaturated one, PO, the saturated wax, disproportionately increased the phase transition of the mixture by approximately 30°C compared with PO alone (Table 5, Figs. 5A). In comparison, PO lowered the phase-transition temperature of the mixture by only approximately 5°C compared with PP alone. Similarly, when the saturated wax SP was mixed with an unsaturated one, OO, the saturated wax disproportionately increased the phase-transition temperature of the mixture by approximately 50°C, compared with OO alone (Table 5, Fig. 5C). In comparison, OO lowered the phase-transition temperature of the mixture by only approximately 6°C compared with SP alone. The cooperativity of the PO/PP or OO/SP mixtures was lower than that of PP or SP alone (Table 5).
Cholesterylpalmitate (20 mole percent) increased the Tm of the equimolar OO/SP wax mixture by slightly more than 1°C in the heating and cooling curves (Fig. 5D, Table 5). At 24°C, the CH2 symmetric stretching frequency was 2849.6 cm−1, indicating that the hydrocarbons of cholesterol alone were ordered.
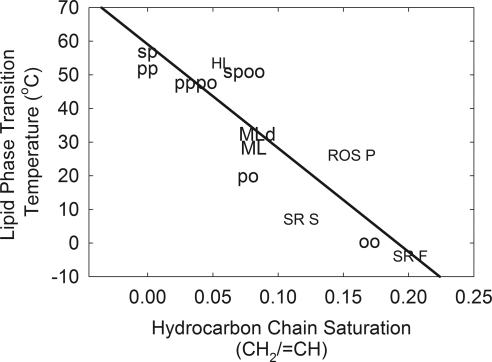
The phase-transition temperature of the standard waxes measured in this study were linearly related to the degree of saturation, and similar to the linear relationship between saturation and phase-transition temperature measured for human meibum, which is predominantly wax and other natural membranes (Fig. 6). The data were fit to the line with a slope of −308 and a y intercept of 59.1 (r = 0.87).
Figure 6.
Relationship between phase-transition temperature and hydrocarbon chain saturation. Samples measured in this study: OO oleyloleate, PO palmityloleate, PP palmitylpalmitate, SP sterylpalmitate, PPPO equimolar mixture of PP and PO, SPOO equimolar mixture of SP and OO. Data are reported in Ref. 39. Solid line: least-squares linear regression fit to all the data. Human meibum donor data are presented in Table 6. HL human lens lipid, ROS P bovine rod outer segment plasma membrane, SR F fast twitch rabbit muscle sarcoplasmic reticulum membrane, SR S slow twitch rabbit muscle sarcoplasmic reticulum membrane.
The Phase-Transition Temperature and the Infrared Carbonyl, CH2 Stretching, and C—C Rocking Bands.
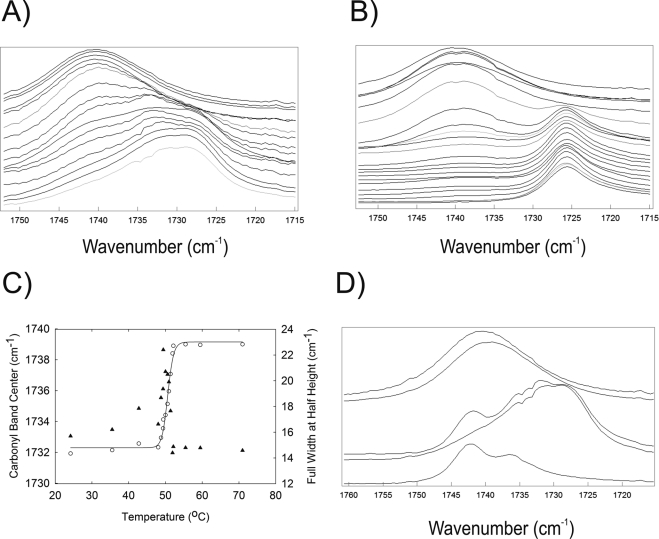
The frequency of the carbonyl band increased when the hydrocarbon chains transitioned from an ordered to a disordered phase (Fig. 7C). The bandwidth of the carbonyl band peaked dramatically at the phase-transition temperature (Fig. 7C), because at the phase-transition temperature, two carbonyl bands were evident in the mixture of SP and OO (Fig. 7A), especially for PO alone (Fig. 7B). The carbonyl bands for PO (Fig. 7B) were better resolved than for the mixture of SP and OO (Fig. 7A). The difference in the phase-transition temperature for the carbonyl band and CH symmetric stretching band in an equimolar mixture of PS and OO was only 0.4°C (Tables 5, 7). Cholesterylpalmitate exhibited a carbonyl band near 1743 cm−1, with a shoulder at 1737 cm−1 (Fig. 7D, bottom). Below the Tm, the carbonyl band of cholesterol ester appeared as a shoulder at 1741 cm−1 in the spectra of the mixture cholesterylpalmitate/SP/OO (Fig. 7D). Above the phase-transition temperature the cholesterol ester-carbonyl band was not visible in the SP/OO mixture. (Fig. 7D, top).
Figure 7.
Infrared spectra of the carbonyl region for (A) sterylpalmitate/oleyloleate, 1:1, m:m. Temperatures (°C) from top to bottom: 70.96, 59.47, 55.45, 52.14, 51.91, 51.37, 50.93, 50.57, 50.01, 49.41, 49.35, 48.78, 48.02, 42.74, 35.5, and 24.17. (B) Palmityloleate temperatures (°C) from top to bottom: 24.00, 20.25, 20.17, 20.14, 20.09, 20.02, 19.92, 19.85, 19.78 19.69, 19.63, 19.55 19.36, 19.16, 18.72, 18.47, 18.38, 18.35, 17.64, 16.93, 16.80, 13.59, and 12.83. (C) The carbonyl bandwidth at half height (▴) increased then decreased at the transition temperature measured by the carbonyl band center (○). All data are from heating curves. (D) Infrared spectra of the C O region: (top) sterylpalmitate/oleyloleate, 1:1, m:m, 70.96°C; sterylpalmitate/oleyloleate/cholesterylpalmitate, 2:2:1, m:m:m, 70.96°C; sterylpalmitate/oleyloleate/cholesterylpalmitate, 2:2:1, m:m:, 26.07°C; sterylpalmitate/oleyloleate, 1:1, m:m, 24.17°C; (bottom) cholesterylpalmitate 24°C.
O region: (top) sterylpalmitate/oleyloleate, 1:1, m:m, 70.96°C; sterylpalmitate/oleyloleate/cholesterylpalmitate, 2:2:1, m:m:m, 70.96°C; sterylpalmitate/oleyloleate/cholesterylpalmitate, 2:2:1, m:m:, 26.07°C; sterylpalmitate/oleyloleate, 1:1, m:m, 24.17°C; (bottom) cholesterylpalmitate 24°C.
Table 7.
Phase-Transition Parameters for the Infrared Carbonyl Band
| Sample | Transition Temp. (°C) | Minimum Frequency (cm−1) | Maximum Frequency (cm−1) | Cooperativity (No Units) | |
|---|---|---|---|---|---|
| Stearylpalmitate/Oleyloleate (1:1, m:m) | Heating | 50.6 ± 0.1 | 1732.3 ± 0.2 | 1739.2 ± 0.2 | 64 ± 8 |
Table 6.
Human Meibum Donor Data Used in Figure 6
| Group | Age/Sex/Ethnicity* |
|---|---|
| Group 1, meibum below 14 y | 3MC, 4FC, 6MC, 8FC, 10 MC, 12FC, 13FC |
| Group 2, meibum 14 to 30 y | 17MC, 19MC, 19FC, 20FA, 20MA, 21MC, 21MC, 23MC, 23 MC, 24MC, 26 MA, 32MC |
| Group 3, meibum above 30 y | 52FC, 54MC, 62MC, 65MC, 67MC, 80FB, 83MC, 88MC |
M, male; F, female; A, Asian; B, black; C, Caucasian.
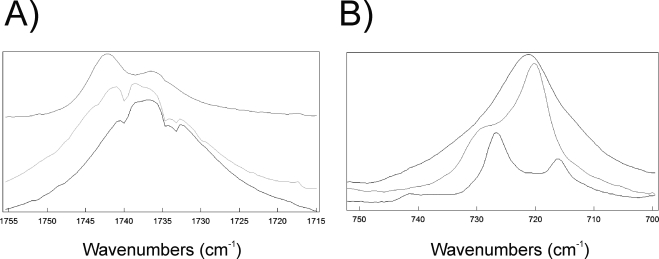
The center of the infrared carbonyl band of human meibum was 1736.0 cm−1 below the Tm, at 15°C. It was only 2.2 cm−1 higher than the phase-transition temperature at 55°C. The difference in frequency above and below the phase-transition temperature was small, compared with the 7-cm−1 shift in the infrared carbonyl band frequency for standard waxes (Fig. 7C). The magnitude of the change in the full bandwidth at half height of the carbonyl band on melting was also small for human meibum, relative to that of the standard waxes. The full bandwidth at half height changed from 16.0 cm−1 below the phase-transition temperature to 17.0 cm−1 above the phase-transition temperature. Two shoulders on both sides of the central band were observed in the human meibum at 1741 and 1732 cm−1 (Fig. 8A).
Figure 8.
Average infrared spectra of human meibum showing (A) the carbonyl band region. Top: cholesteryl palmitate; middle: above the Tm at 55°C; bottom: below the Tm at 15°C. (B) The C—C rocking band region. Top: above the Tm at 55°C; middle: below the Tm at 15°C; bottom: cholesteryl palmitate.
In all the wax samples, two CH2 bending bands were evident when the hydrocarbon chains were ordered below the phase-transition temperature (Fig. 9Ad). This is characteristic of crystal field splitting and orthorhombic packing (Fig. 1, top). The two CH2 bending bands converged into one band slightly above the phase-transition temperature (Fig. 9Aa). Two CH2 bending bands were present in the spectra of cholesterylpalmitate alone at 24°C (Fig. 9Ae) and below the phase-transition temperature when cholesterylpalmitate was present in the mixture SP/OO (Figs. 1A, 9Ac). The characteristics of the CH2 bending bands for the SP/OO mixture with cholesterol were indistinguishable from those of the mixture of SP/OO and PO without cholesterolpalmitate (Fig. 9A).
Figure 9.
(A) Infrared spectra of the CH2 bending region for: (a) sterylpalmitate/oleyloleate, 1:1, m:m, 70.96°C; (b) sterylpalmitate/oleyloleate/cholesterylpalmitate, 2:2:1, m:m:m, 70.96°C; (c) sterylpalmitate/oleyloleate/cholesterylpalmitate, 2:2:1, m:m:, 26.07°C; and (d) sterylpalmitate/oleyloleate, 1:1, m:m, 24.17°C; (e) cholesterylpalmitate 24°C. Infrared spectra of the C—C rocking region for: (B) sterylpalmitate/oleyloleate, 1:1, m:m. Temperatures (°C) from top to bottom: 70.96, 59.47, 55.45, 52.14, 51.91, 51.37, 50.93, 50.57, 50.01, 49.41, 49.35, 48.78, 48.02, 42.74, 35.5, and 24.17. (C) Infrared spectra of the C—C rocking region: (a) sterylpalmitate/oleyloleate, 1:1, m:m, 70.96°C; (b) sterylpalmitate/oleyloleate/cholesterylpalmitate, 2:2:1, m:m:m, 70.96°C; (c) sterylpalmitate/oleyloleate/cholesterylpalmitate, 2:2:1, m:m:, 26.07°C; (d) sterylpalmitate/oleyloleate, 1:1, m:m, 24.17°C; and (e) cholesterylpalmitate 24°C. (D) C—C rocking bands of sterylpalmitate/oleyloleate, 1:1, m:m converge at the transition temperature.
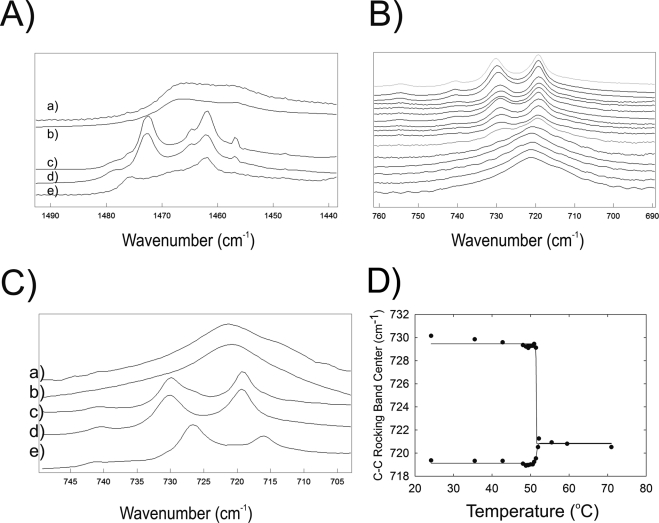
Like the CH2 bending bands discussed above, the two C—C rocking bands confirmed orthorhombic packing below the phase-transition temperature (Fig. 9B). At and above the phase-transition temperature, the two C—C rocking bands converged into one band (Figs. 9B, 9D). Two C—C rocking bands were present in the spectra of cholesterylpalmitate alone at 24°C (Fig. 9C, bottom) and below the phase-transition temperature when cholesterylpalmitate was present in a mixture of SP/OO (Fig. 9C(e)). The characteristics of the C—C rocking bands in the mixture of SP/OO with cholesterol were indistinguishable from the mixture of SP/OO and PO without cholesterolpalmitate (Fig. 15B).
The difference between the center of the infrared band C—C rocking bands of human meibum, above and below the phase-transition temperature, was only 1 cm−1—720 cm−1 below the phase-transition temperature at 15°C and 721 cm−1 above the phase transition at 55°C. A shoulder at 729 cm−1 was present below the phase-transition temperature (Fig. 8A) that was not as well resolved as the two bands at 730 and 719 cm−1 in the spectra of standard waxes below the phase-transition temperature (Figs. 9C, 9D).
Discussion
Phase 1: Human Meibum Study
MGD was defined as “a condition of progressive obstruction due to ductal hyperkeratinization or inspissation of secretion.”3 Until this study, inspissation (thickening) of meibum has primarily been assessed only qualitatively in a clinical setting. Our results indicate that thickening of meibomian secretion can now be quantified by infrared spectroscopy. A major finding of this study is that the average order of Md at the temperature of the eyelid was 30% higher than that of Mn from age-matched donors. Lipid conformational order of Md can be defined as the percentage of trans rotomers (Fig. 1). Because the phase-transition temperature of human meibum is near physiological temperature, a small 4°C change in phase-transition temperature is enough to cause a 30% increase in lipid order and a 72% increase in the strength of lipid–lipid interactions for Md compared to Mn (this study and Ref. 40). Carbons attached to a C—C bond are in a trans conformation when they lie in the same plane and are on opposite sides of the C—C bond (Fig. 1). Any other conformation is considered a gauche conformation (Fig. 1). Lipid conformational order of Md was 47% trans at 33.4°C. Hydrocarbon chains in a trans conformation can pack more tightly. For a laymen's comparison, the hydrocarbon chain stiffness of Md is about halfway between that of olive oil, which is fluid, and butter, which is stiff. Meibum lipid with ordered hydrocarbon chains are less mobile than those with disordered chains.39
As noted in the introduction, when the meibomian glands, which produce most of the tear film lipid, are dysfunctional, delivery of oil to the lid margin is reduced26–28 and dry eye conditions ensue.8,11 It is therefore reasonable to expect that stiffer, more ordered lipids would flow out of the meibomian ducts more slowly, if at all, compared with fluid lipids. When the meibomian glands in patients with dry eye are expressed, the lipid layer thickness increases, as does tear stability.5,9,27,57,58 One of the earliest therapies for MGD was to warm the eyelid.33–35 As shown in this study, on warming, the Md hydrocarbon chains become more disordered with increasing temperature up to approximately 50°C, above which changes in lipid disorder are minimal. We noted the linear relationship between the amount of lipid on the lid margin and meibum lipid hydrocarbon chain disorder.36 The present study confirms the hypothesis that Md is more ordered than Mn and becomes more disordered with increasing temperatures. The present study thus provides a biophysical basis for the observations that heating the eyelid increases the flow and amount of meibum lipid on the lid margin.59
Compositional changes that could cause Md to become more ordered than Mn include increased hydrocarbon chain lengths60–62 and saturation41 or changes in cholesterol ester content.63 Using model waxes we found that the change from one double bond to none raised the phase-transition temperature of standard waxes by 30°C (see detailed discussion below). Cholesterol esters caused no change in wax conformation, so a decrease in double bonds rather than a change in the composition of cholesterol esters is more likely to account for the higher lipid order observed for Md than for Mn.
Proteins may also influence meibum conformation. Infrared spectroscopy detected significant levels of protein in human meibum before its interaction with tear proteins. In Md, the amount of protein indirectly correlated with lipid order.43 Studies to directly correlate meibum conformation with the amount of meibum protein are currently being pursued.
Eighteen percent of the patients diagnosed with MGD exhibited meibum lipid phase-transition temperatures that were similar to those for normal donors. Md ordering in these patients could have been transient, coincidental, or associated with other factors, such as ductal hyperkeratinization or inflammation, which contributed to the dry-eye symptoms. Md order at 33.4°C was strongly related to the lipid phase-transition temperature. Factors that contribute to changes in the phase-transition temperature are likely to change Mn and Md order at 33.4°C. The close correlation between phase-transition temperatures and order provides further evidence that the large interdonor variations (Fig. 3) in these parameters are not due to large variations in the precision of testing protocols. Phase-transition temperatures span 12°C from sample to sample which is much larger than the ±2°C precision of the measurements (Fig. 3A). The variability from sample to sample must be due to lipid compositional differences.
The lipid phase-transition temperature is similar but not identical with melting. Mn and Md in the disordered state at 65°C are not completely disordered as a liquid (melted solid) and are considered liquid crystals. Similarly, Mn and Md in an ordered state at 5°C are not completely ordered as are solids and so we use the term phase-transition temperature rather than melting temperature.
The average phase-transition temperature for Md was approximately 4°C higher than that for age-matched Mn. Terada et al.64 reported the only study that evaluated Md “melting” at 34.0 ± 1.3°C, but this melting temperature was assessed visually. “Melting” of Mn from age-matched controls ranged from 32°C to 37°C in Terada et al.64 and the SE was too large to be significantly different from the melting of Md. The phase-transition temperature of Mn, 28.5°C (Table 4), was below the temperature of the eyelid, 33.1°C to 33.4°C,65,66 ensuring that the Mn is predominantly disordered at lid temperature. The phase-transition temperature of Md, 32.2°C, is similar to the eyelid temperature, resulting in 47% of the lipid being ordered at lid temperature.
Insight into the functional relevance of a more ordered Md compared with Mn may be gained from two very early studies. Brown and Dervichian67 found that meibum lipid that are ordered do not spread when placed on the surface of aqueous saline but spread above 35°C, the melting temperature of the meibum. Remarkably, with only a looped piece of thread, they were able to measure the surface tension of meibum lipid layered on saline solution to be 15 dynes/cm, similar to the value measured by modern instruments. Based on these observations, it is reasonable to expect that ordered Md would not spread or perhaps would spread more slowly on the tear film surface even if it were to be expressed from meibomian glands. Like Brown and Dervichian, Holly68 reported that meibum lipid on saline has “poor spreading characteristics” and “a low film pressure therefore (high surface tension)” The situation in vivo is more complicated than in these in vitro studies. The aqueous tear film contains proteins and surfactants that interact with the meibum lipid. In Holly's seminal paper, he reported that mucin increases the surface pressure of meibum lipid threefold.68 Recent Langmuir trough experiments have shown that above the phase-transition temperature, the area of the meibum film per molecule is significantly larger.69 These results provide insight, but one must be cautious in comparing them with ours, since the trough experiments use chloroform, which alters lipid packing and denatures the proteins present in native meibum. As a consequence of Md's having 72% stronger lipid–lipid interactions than Mn, the Md would be expected to have a lower film pressure than Mn and would not spread on the surface of tears as readily as Mn.68 Brown and Dervichian67 proposed that meibum lipid disordered by temperature spreads more readily on a saline surface because surface active molecules are free to diffuse and “spread on the watery surface and carry on their back the thick nonpolar hydrocarbon portion of the oil.” It is likely that lysozyme, lipocalin, and mucin bind to meibum at the surface, adding stability to the film.68–73 The tear proteins lowered the surface tension and increased surface pressure by causing a compression of the lipids. It has yet to be determined how more ordered Md would interact with the tear proteins.69–73
Phase 2: Model Lipid/Wax Study
The characteristics of the lipid phase transitions of human meibum change with age,40 meibomian gland disease (this study), and therapy.44 The biophysical changes reflect compositional changes that have been elusive to quantify. For instance, six studies have shown that wax esters compose from 13% to 68% of meibum, hydrocarbons 1% to 38%, sterol esters 8% to 39%, and triglycerides 2% to 43%.56 Infrared39 and Raman spectra41 suggest that wax and sterol esters predominate in human meibum, so in this study, the phase characteristics of pure waxes and simple mixtures were examined to gain insight on the compositional factors that are likely to contribute to the changes in the phase-transition parameters of human meibum with age, disease, and therapy.
Carbonyl Region.
The carbonyl band frequency of standard waxes shifted over 7 cm−1 on melting, but the carbonyl band is not a good indicator of lipid phase transitions for human meibum which shifted by only 2 cm−1. The carbonyl full bandwidth at half height and frequency are dependent on the environment of the carbonyl band and not on conformation.74 When the carbonyl is in an environment with a low dielectric constant such as n-hexane (ε = 1.89) its frequency is 1750 cm−1 with a narrow bandwidth of 12.74 When the carbonyl is in an environment with a stronger dielectric constant such a CCl4 (ε = 2.24) or CHCl3 (ε = 4.8) the carbonyl band frequency is lower, 1742 and 1732 cm−1, and the full bandwidth at half height is higher, 13 and 20 cm−1, respectively.74 The carbonyl band therefore may be used as an internal probe of the environment surrounding the carbonyl group.
Below the phase-transition temperature, the carbonyl moieties of standard waxes are in an environment similar to CHCl3. On melting, there is a dramatic change to a more hydrophobic environment similar to that of CCl4. Waxes are tubelike structures that pack together in multilamellar monolayers (Fig. 1). In a system of standard waxes, there are only two environments that the wax carbonyl moiety can experience: the hydrophobic environment of the hydrocarbon chains that have a low dielectric constant and the ester region of adjacent waxes that has a higher dielectric constant. For standard waxes below the phase-transition temperature, the acyl linkages may be in close proximity to the acyl linkages of adjacent waxes. Above the phase-transition temperature, the carbonyl is in proximity to the hydrocarbon region of adjacent waxes.
Below the phase-transition temperature, the carbonyl groups in native human meibum are in an environment with a higher dielectric constant between that of CCl4 and CHCl3. Above the phase-transition temperature the environment changes slightly to a more hydrophobic environment with a lower dielectric.
Cholesterol Esters.
The frequency of the carbonyl of the cholestrylpalmitate ester near 1740 cm−1 is high relative to waxes and meibum, indicating that the ester moiety is in the environment of the hydrocarbon chains of adjacent waxes or the palmitate hydrocarbon region of the cholesterol ester. The small carbonyl shoulder at 1737 cm−1 indicates that a portion of ester moieties are adjacent to the acyl linkages of neighboring wax or cholesterol esters. The CH2 symmetric stretching band frequency of 1749.6 cm−1 for cholestrylpalmitate indicates that the palmitatyl CH2 groups are mostly ordered, in trans conformations.
Cholesterol ester had a minimal affect on the phase transition of a mixture of SP and OO. Cholesterol ester at a concentration of 20% increased the Tm of wax without cholesterol ester by only slightly more than 1°C. It is doubtful that smaller changes in cholesterol ester with age and MGD could account for the 4°C shift in the phase-transition temperature of meibum observed with age40 and MGD (this study).
It would be interesting to study the structure of cholesterol esters in meibum and wax using infrared spectroscopy and deuterated and 13C isoforms of cholesterol esters. Because cholesterol esters caused little change in the phase-transition temperature of waxes, such a study was deferred.
Phase-Transition Temperature.
In the present study the amount of hydrocarbon trans and gauche rotomers was used to quantify the phase-transition temperature. A change in phase from an ordered gel phase with more trans rotomers, to a disordered liquid–crystalline phase with more gauche rotomers was accompanied by the disappearance of orthorhombic packing (two bands to one band, Fig 9A). A change in hydrocarbon chain length could cause a change in the phase-transition temperature. In this study an additional CH2—CH2 moiety raised the phase-transition temperature approximately 5°C. A similar result using a more extensive group of waxes was found with infrared spectroscopy61 and a less precise melting point determination.62 Shorter chain, saturated waxes (myristyl or lauryl stearate) are tilted relative to the lattice plane. Longer chain, saturated waxes (palmitylopalmitate or palmitylstearate) are vertical relative to the lattice plane.55 Relative to saturated waxes, the addition of one CH CH moiety dramatically lowered the phase-transition temperature by approximately 40°C, and the addition of a second CH
CH moiety dramatically lowered the phase-transition temperature by approximately 40°C, and the addition of a second CH CH moiety to a monounsaturated wax decreased the phase-transition temperature by approximately 19°C. The dramatic decrease in phase-transition temperature with unsaturation was expected based on results in previous studies.61
CH moiety to a monounsaturated wax decreased the phase-transition temperature by approximately 19°C. The dramatic decrease in phase-transition temperature with unsaturation was expected based on results in previous studies.61
In the present study a unique and unexpected result was that, in mixtures of saturated and unsaturated waxes, the phase-transition temperature of the mixture was disproportionately 30°C to 50°C higher than that of the pure unsaturated waxes, but only 6°C below the phase-transition temperature of pure saturated waxes. The cooperativity of the mixtures was lower than that of pure saturated waxes. Therefore, even though unsaturation has a profound affect on the phase-transition temperature of pure waxes, the effect is much less pronounced in mixtures. In an x-ray diffraction study, a disproportionate difference in the tilt angle of the hydrocarbon chains in mixtures of waxes was noted.55 The hydrocarbon chains of saturated lipids used in the present study, PP and PS, align vertically (A form) with reference to the lattice planes.55 The hydrocarbon chains of lower melting point waxes such as myristylstearate or laurylstearate have a tilted alignment (B form) with reference to the lattice planes.55 When the higher melting A form is mixed with the lower melting B form, the mixture always accepts the B form.55
We believe that in general, the phase-transition temperature of complex mixtures of natural lipids is determined by the level of unsaturation of lipids (Fig. 5A). It is interesting that human lens, muscle and rod outer segment lipids that do not contain waxes fit so closely to the phase-transition, temperature-saturation curve of standard waxes used in this study (Fig. 5A). Patel et al.61 noted that, regardless of whether the lipid was a wax, hydrocarbon, phospholipid, fatty acid, or triglyceride, every carbon atom added to the hydrocarbon chains raised the phase-transition temperature by 1°C to 2°C. The ester linkage decreases the phase-transition temperature by approximately 15°C relative to hydrocarbons containing the same number of carbon atoms.61,75 Changes in lipid saturation and hydrocarbon chain length could account for the 4°C shift in the phase-transition temperature of meibum observed with age40 and meibomian gland dysfunction (this study). Saturation levels of a limited sample of Mn and Md measured using Raman spectroscopy indicates that meibum saturation does not change dramatically with age and meibomian gland dysfunction.41 In the same study, the level of highly unsaturated carotenoids decreased with age and meibomian gland dysfunction.41 An increase in overall chain length by as little as two carbons could increase the phase-transition temperature by 5°C.
The functional relevance of Md's having a higher phase-transition temperature and order than age-matched Mn, as discussed above, is speculative at this time. Therefore, studies are under way to determine the relationships between meibum lipid composition, structure/conformation, and function. Infrared and Raman spectroscopies are ideal for this purpose, since they both provide conformational and limited compositional information but are nondestructive. It might be informative to spike Md with disordered, unsaturated waxes or Mn with ordered, saturated waxes to determine whether spiked lipids could restore lower lipid order in Md or elevate lipid order in Mn. Nuclear magnetic resonance (NMR) spectroscopy and matrix-assisted mass spectrometry are ideal for quantifying meibum lipid classes and specific composition, respectively. Further studies employing these techniques should elucidate the composition/structure contribution to ordering of the lipids.
Footnotes
Supported by Public Health Service research grant EY017094-01 (Bethesda, MD), the Kentucky Lions Eye Foundation, and an unrestricted grant from Research to Prevent Blindness Inc. Much of the study was supported with the resources and use of the facilities at the Louisville Veterans Affairs Medical Center, Louisville, KY.
Disclosure: D. Borchman, None; G.N. Foulks, None; M.C. Yappert, None; J. Bell, None; E. Wells, None; S. Neravetla, None; V. Greenstone, None
References
- 1. Shine WE, McCulley JP. Meibomianitis: polar lipid abnormalities. Cornea. 2004;23:781–783 [DOI] [PubMed] [Google Scholar]
- 2. Ishida R, Kojima T, Dogru M, et al. The application of a new continuous functional visual acuity measurement system in dry eye syndromes. Am J Ophthalmol. 2005;139:253–258 [DOI] [PubMed] [Google Scholar]
- 3. Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52:369–374 [DOI] [PubMed] [Google Scholar]
- 4. Bron AJ, Tiffany JM. The meibomian glands and tear film lipids; structure, function, and control. Adv Exp Med Biol. 1998;438:281–295 [DOI] [PubMed] [Google Scholar]
- 5. Craig JP, Blades K, Patel S. Tear lipid layer structure and stability following expression of the meibomian glands. Ophthalmic Physiol Opt. 1995;15:569–574 [PubMed] [Google Scholar]
- 6. Korb DR, Baron DF, Herman JP, et al. Tear film lipid layer thickness as a function of blinking. Cornea. 1994;13:354–359 [DOI] [PubMed] [Google Scholar]
- 7. Linton RG, Curnow DH, Riley WJ. The meibomian glands: an investigation into the secretion and some aspects of the physiology. Br J Ophthalmol. 1961;45:718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathers WD. Ocular evaporation in meibomian-gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351 [DOI] [PubMed] [Google Scholar]
- 9. McCulley JP, Sciallis GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977;84:788–793 [DOI] [PubMed] [Google Scholar]
- 10. Milder B. Clinical applications. In: Mossa F, Hart WM. eds. Adler's Physiology of the Eye.. St. Louis: Mosby; 1987:15–35 [Google Scholar]
- 11. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian-gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270 [DOI] [PubMed] [Google Scholar]
- 12. Wolff E. Muco-cutaneous function of the lid margin and the distribution of the tear fluid. Trans Ophthalmol Soc UK. 1946;66:291–308 [Google Scholar]
- 13. Young G, Efron N. Characteristics of the pre-lens tear film during hydrogel contact lens wear. Ophthalmic Physiol Opt. 1991;11:53–58 [PubMed] [Google Scholar]
- 14. Korb DR. Tear film-contact lens interactions. Adv Exp Med Biol. 1994;350:403–410 [DOI] [PubMed] [Google Scholar]
- 15. Norm MS. Tear break-up time: a review. In: Holly FJ. ed. The Preocular Tear Film in Health, Disease and Contact Lens Wear.. Lubbock TX: Dry Eye Institute; 1986:52–55 [Google Scholar]
- 16. Lemp MA. Epidemiology and classification of dry eye. Adv Exp Med Biol. 1998;438:791–803 [DOI] [PubMed] [Google Scholar]
- 17. Yokoi N, Takehisa Y, Kinoshita S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am J Ophthalmol. 1996;122:818–824 [DOI] [PubMed] [Google Scholar]
- 18. Norm MS. Semiquantitative interference study of fatty layer of pre corneal film. Acta Ophthalmol (Copenh).. 1979;57:766–774 [DOI] [PubMed] [Google Scholar]
- 19. Hamano H, Hamano T, Hori M, Kawabe H, Mitsunga S. Observations of the pre corneal tear film of Sjogren's syndrome by a bio differential interference microscope (in Japanese). Folia Ophthalmol Jpn. 1980;31:753–755 [Google Scholar]
- 20. Doane MG. Lacrimal Gland, Tear Film, and Dry Eye Syndrome.. Sullivan DA. ed. New York: Plenum Press; 2004:489–493 [Google Scholar]
- 21. Lee SY, Araki K, Hamano T. Meibomian secretions and tears (in Japanese). Rinsho Ganka. 1994;1941–1944 [Google Scholar]
- 22. Kilp H, Schmid E, Kirchner L, Zipf-Pohl A. Tear film observation by reflecting microscopy and differential interference contrast microscopy. In: Holly FJ. ed. The Preocular Tear Film in Health, Disease and Contact Lens Wear.. Lubbock, TX: Dry Eye Institute; 1986:564–569 [Google Scholar]
- 23. Franck C. Fatty layer of the precorneal film in the ‘office eye syndrome’. Acta Ophthalmol. 1991;69:737–743 [DOI] [PubMed] [Google Scholar]
- 24. Guillon JP. Abnormalities of the structure of the superficial lipid layer on the in vivo dry-eye tear film. In: Holly FJ. ed. Tear Film Structure and Contact Lenses: The Preocular Tear Film in Health, Disease, and Contact Lens Wear.. Lubbock, TX: Dry Eye Institute; 1986;914–939 [Google Scholar]
- 25. Josephson JE. Appearance of the preocular tear film lipid layer. Am J Optom and Physiol Opt. 1983;60:883–887 [Google Scholar]
- 26. Foulks GN, Bron AJ. Meibomian-gland dysfunction: a clinical scheme for description, diagnosis, classification and grading. Ocul Surf. 2003;1:17–36 [DOI] [PubMed] [Google Scholar]
- 27. Bron AJ, Sci FM, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–165 [DOI] [PubMed] [Google Scholar]
- 28. Foulks GN. Blepharitis: lid margin disease and the ocular surface. In: Holland FJ, Mannis MJ. eds. Ocular Surface Disease.. New York: Springer; 2001:39–48 [Google Scholar]
- 29. Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996;15:340–346 [DOI] [PubMed] [Google Scholar]
- 30. Shine WE, McCulley JP. Association of meibum oleic acid with meibomian seborrhea. Cornea. 2000;19:72–74 [DOI] [PubMed] [Google Scholar]
- 31. Joffre C, Souchier M, Leclere L, et al. Branched-chain fatty acids, increased in tears of blepharitis patients, are not toxic for conjunctival cells. Br J Ophthalmol. 2009;93:1391–1395 [DOI] [PubMed] [Google Scholar]
- 32. Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol.. 1998:116;849–852 [DOI] [PubMed] [Google Scholar]
- 33. Harrison DA, Lawlor D. Experiences in treating patients for blepharitis. Arch Ophthalmol. 1998;116:1133–1134 [PubMed] [Google Scholar]
- 34. Mori A, Oguchi Y, Goto E, et al. Efficacy and safety of infrared warming of the eyelids. Cornea. 1999;18:188–193 [DOI] [PubMed] [Google Scholar]
- 35. Thygeson P. The etiology and treatment of blepharitis: a study in military personnel. Mil Surg.. 1946:98;191–203 [PubMed] [Google Scholar]
- 36. Borchman D, Foulks GN, Yappert MC, Tang D, Ho DV. Temperature-induced conformational changes in human tear lipids hydrocarbon chains. Biopolymers Biospectrosc. 2007;87:124–133 [DOI] [PubMed] [Google Scholar]
- 37. Foulks GN, Borchman D. Lipid abnormalities of the Eye. In: Davie BG. ed. Ocular Disease: Mechanisms and Management.. Oxford, UK: Elsevier, Ltd.; 2010 [Google Scholar]
- 38. Mantsch HH, Chapman D. eds. Infrared Spectroscopy of Biomolecules, New York: Wiley-Liss; 1996 [Google Scholar]
- 39. Borchman D, Foulks GN, Yappert MC, Tang D, Ho DV. Spectroscopic evaluation of human tear lipids. Chem Phys Lipids. 2007;147:87–102 [DOI] [PubMed] [Google Scholar]
- 40. Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid hydrocarbon chain conformational and thermodynamic changes with age. Ophthalmic Res. 2010;44:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oshima Y, Sato H, Zaghloul A, Foulks GN, Yappert MC, Borchman D. Characterization of human meibum lipid using Raman spectroscopy. Curr Eye Res. 2009;34:824–835 [DOI] [PubMed] [Google Scholar]
- 42. Borchman D, Foulks GN, Yappert MC. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr Eye Res. 2010;35:778–786 [DOI] [PubMed] [Google Scholar]
- 43. Borchman D, Foulks GN, Yappert MC. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp Eye Res. 2010;91:246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foulks GN, Borchman D, Yappert MC, Sung-Hye K, McKay JW. Topical Azithromycin therapy of meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29:781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moore DJ, Wyrwa M, Reboulleau CP, Mendelsohn R. Quantitative IR studies of acyl chain conformational order in fatty acid homogeneous membranes of live cells of Acholeplasma laidlawii B. Biochemistry. 1993;32:6281–6287 [DOI] [PubMed] [Google Scholar]
- 46. Mantsch HH, McElhaney RN. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem Phys Lipids. 1991;57:213–226 [DOI] [PubMed] [Google Scholar]
- 47. Borchman D, Yappert MC, Afzal M. Lens lipids and maximum lifespan. Exp Eye Res. 2004;79:761–768 [DOI] [PubMed] [Google Scholar]
- 48. Borchman D, Cenedella RI, Lamba OP. Role of cholesterol in the structural order of lens membrane lipids. Exp Eye Res. 1996;62:191–197 [DOI] [PubMed] [Google Scholar]
- 49. Kóta Z, Debreczeny M, Szalontai B. Separable contributions of ordered and disordered lipid fatty acyl chain segments to nuCH2 bands in model and biological membranes: a Fourier transform infrared spectroscopic study. Biospectroscopy. 1999;5:169–178 [DOI] [PubMed] [Google Scholar]
- 50. Borchman D, Yappert MC, Herrell P. Structural characterization of human lens membrane lipid by infrared spectroscopy. Invest Ophthalmol Vis Sci. 1991;32:2404–2416 [PubMed] [Google Scholar]
- 51. Popova AV, Hincha DK. Intermolecular interactions in dry and rehydrated pure and mixed bilayers of phosphatidylcholine and digalactosyldiacylglycerol: a Fourier transform infrared spectroscopy study. Biophys J. 2003;85:1682–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Casal HL, Mantsch HH. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim Biophys Acta. 1984;779:381–401 [DOI] [PubMed] [Google Scholar]
- 53. Borchman D, Tang D, Yappert MC. Lipid composition, membrane structure relationships in lens and muscle sarcoplasmic reticulum membranes. Biospectroscopy. 1999;5:151–167 [DOI] [PubMed] [Google Scholar]
- 54. Simpson TD, Miwa TK. X-ray study of hydrogenated jojoba wax. Chem Soc. 1977;54:54–58 [Google Scholar]
- 55. Kreger DR, Schamhart C. On the long crystal-spacings in wax esters and their value in micro-analysis of plant cuticle waxes. Biochim Biophys Acta. 1956;19:22–44 [DOI] [PubMed] [Google Scholar]
- 56. Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids: a review. Curr Eye Res. 2008;33:405–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tiffany JM, Dart JKG. Normal and abnormal functions of meibomian secretion In: Trevor-Roper PD. ed. The Cornea in Health and Disease.. London: Royal Society of Medicine and Academic Press; 1981:1061–1064 [Google Scholar]
- 58. Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment of meibomian-gland dysfunction. In: Sullivan DA. ed: Lacrimal Gland, Tear Film, and Dry Eye Syndromes.. New York, NY, Plenum Press; 1994:293–298 [DOI] [PubMed] [Google Scholar]
- 59. Nagymihalyi A, Dikstein S, Tiffany JM. The influence of eyelid temperature on the delivery of meibomian oil. Exp Eye Res. 2004;78:367–370 [DOI] [PubMed] [Google Scholar]
- 60. Casal HL, McElhaney RN. Quantitative determination of hydrocarbon chain conformational order in bilayers of saturated phosphatidylcholines of various chain lengths by Fourier transform infrared spectroscopy. Biochemistry.. 1990. 29:5423–5427, 1990 [DOI] [PubMed] [Google Scholar]
- 61. Patel S, Nelson DR, Gibbs AG. Chemical and physical analysis of wax ester properties. J Insect Sci. 2001;1.4:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Iyengar BTR, Schlenk H. Melting points of synthetic wax esters. Lipids. 1969;4:28–30 [DOI] [PubMed] [Google Scholar]
- 63. Lipert JL, Peticolas WL. Laser Raman investigation of the effect of cholesterol on conformational changes in dipalmitoyl lecithin multilayers. Proc Nat Acad Sci USA. 1971;68:1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Terada O, Chiba K, Senoo T, Obara Y. Ocular surface temperature of meibomian-gland dysfunction patients and the melting point of meibomian gland secretions (in Japanese). Nippon Ganka Gakkai Zasshi. 2004;108:690–693 [PubMed] [Google Scholar]
- 65. Morgan PB, Tullo AB, Efron N. Infrared thermography of the tear film in dry eye. Eye. 1995;9:615–618 [DOI] [PubMed] [Google Scholar]
- 66. Shellock FG, Schatz CJ. Increased corneal temperature caused by MR imaging of the eye with a dedicated local coil. Radiology. 1992;185:697–699 [DOI] [PubMed] [Google Scholar]
- 67. Brown SI, Dervichian DG. The oils of the meibomian glands: physical and surface characteristics. Arch Ophthalmol. 1969;82:537–540 [DOI] [PubMed] [Google Scholar]
- 68. Holly FJ. Formation and rupture of the tear film. Exp Eye Res. 1973;15:515–525 [DOI] [PubMed] [Google Scholar]
- 69. Butovich IA, Arciniega JC, Wojtowicz JC. Meibomian lipid films and the impact of temperature. Invest Ophthalmol Vis Sci.. 2010;51:5508–5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2009;50:140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mudgil P, Millar TJ. Adsorption of apo- and holo-tear lipocalin to a bovine Meibomian lipid film. Exp Eye Res.. 2008;86:622–628 [DOI] [PubMed] [Google Scholar]
- 72. Mudgil P, Torres M, Millar TJ. Adsorption of lysozyme to phospholipid and meibomian lipid monolayer films. Colloids Surf B Biointerfaces. 2006;48:128–137 [DOI] [PubMed] [Google Scholar]
- 73. Millar TJ, Tragoulias ST, Anderton PJ, et al. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Cornea. 2006;25:91–100 [DOI] [PubMed] [Google Scholar]
-
74.
Blume A, Hübner W, Messner G. Fourier transform infrared spectroscopy of 13C
 O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988;27:8239–8249 [DOI] [PubMed] [Google Scholar]
O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988;27:8239–8249 [DOI] [PubMed] [Google Scholar] - 75. Gibbs A, Pomonis JG. Physical properties of insect cuticular hydrocarbons: the effects of chain length, methyl-branching and unsaturation. Comp Biochem Physiol B. 1995;112:243–249 [Google Scholar]