The protective effect of time outdoors in lowering the risk of myopia onset suggests that vitamin D metabolism may be altered in myopia. This study provides evidence that genetic variants within the vitamin D receptor gene VDR may be associated with myopia in white subjects with milder levels of myopia. Replication is needed, as well as establishing whether there is any biological relevance of these variations to myopia.
Abstract
Purpose.
Epidemiologic evidence indicates that time outdoors reduces the risk of myopia, suggesting a possible role for vitamin D. This case–control study was conducted to determine whether single-nucleotide polymorphisms (SNPs) within VDR at 12q13.11 and GC at 4q12-13 are associated with myopia.
Methods.
The primary analysis was conducted on 81 white adult control subjects between 18 and 50 years of age with a spherical equivalent refractive error between +0.50 and +2.00 D in both eyes and less than 1.50 D of astigmatism. Affected myopic subjects were 289 unrelated white adults at least 18 years of age with at least −0.75 D myopia in both principal meridians of both eyes.
Results.
One SNP within VDR was significantly associated with myopia in the multivariate analysis of the primary sample (rs2853559: odds ratio = 1.99, P = 0.003). In a subsample of less severely myopic white subjects between −0.75 and −4.00 D, three SNPs within VDR were significantly associated in a multivariate model after adjustment for multiple comparisons (rs2239182: odds ratio = 2.17, P = 0.007; rs3819545: odds ratio = 2.34, P = 0.003; rs2853559: odds ratio = 2.14, P = 0.0035), accounting for 12% of model variance over age alone.
Conclusions.
Polymorphisms within VDR appear to be associated with low to moderate amounts of myopia in white subjects. Future studies should determine whether this finding can be replicated and should explore the biological significance of these variations with respect to myopia.
Myopia is a common trait characterized by an inability to see clearly at a distance without a refractive correction such as glasses, contact lenses, or refractive surgery. The prevalence of myopia in the United States has recently been estimated at 33.1% of adults.1 The annual costs associated with distance visual impairment from refractive errors such as myopia are high—on the order of $3.8 billion.2 Myopia most often has its onset during the school years with a peak incidence beginning at about age 9 years that then falls off by the late teens.3,4 There are numerous theories regarding the etiology of myopia, with the major putative risk factors most easily divided into hereditary and environmental.5
The influence of heredity can be seen in the significant odds ratio for myopia in children whose parents are myopic. Estimates vary, but the odds that children will become myopic are on the order of five times higher if both parents are myopic than if no parents are myopic.6,7 Heritabilities calculated from twin studies are also high at 0.8 to nearly 1.0.8–11 The hereditary component of myopia is also supported by positive findings from numerous molecular genetics studies. Currently, there are 18 named myopia loci (http://www.genenames.org/index.html). Environmental factors may modify this genetic risk. Greater amounts of reading and other forms of close work have often been associated with existing myopic refractive errors, but increased near work has not been a significant risk factor in longitudinal studies for the onset of new cases of myopia.7,12 Interest in environmental influences on refractive error has shifted recently away from near work toward the effect of time spent outdoors. Several groups studying myopia in various parts of the world have found that myopic children spend less time outdoors than do nonmyopic children.13–15 Unlike the negative results for near work, longitudinal data are consistent with these cross-sectional findings and show that more time spent in outdoor or sports activity is protective against the onset of myopia.7 This environmental effect interacts with the underlying genetic risk, reducing the probability of myopia onset in children with two myopic parents from 0.60 if time outdoors is under 5 hours per week to 0.20 if time outdoors is over 14 hours per week.7
Recently, we reported that SNP rs1635529 on chromosome 12, region q13.11, showed highly significant overtransmission to myopic individuals in families participating in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study.16 This chromosomal locus is in the vicinity of the genes COL2A1 (collagen, type II, alpha 1) and VDR (vitamin D [1,25-dihydroxyvitamin D3] receptor). That there is little type II collagen in the sclera of the postnatal eye reduces the likelihood that COL2A1 has strong physiologic significance,17 but there are several findings that make VDR an intriguing candidate gene for myopia. One is the aforementioned CLEERE Study result that outdoor and sports activities are protective against myopia onset in children.7 The precise outdoor activity that conveys the protective effect is unknown, but it is noteworthy that recent results from Singapore and Australia suggest that merely being outdoors is more significant than any specific physical activity such as exercise.13,15 The progression of myopia is also faster in the autumn and winter when there are fewer hours of daylight and slower in the sunnier spring and summer months.18 Increased levels of cutaneously derived vitamin D from more time spent outdoors and longer hours of daylight might be the source of these protective effects. A recent study has found that myopes have 20% lower circulating blood levels of vitamin D than do nonmyopes.19 Speculating further, changes in diet affecting circulating levels of vitamin D may also affect the onset or progression of myopia. The prevalence of myopia appears to be on the rise in Asian populations20 at the same time that traditional sources of vitamin D from fish may be being replaced by other sources of protein and calories in the Asian diet.21 In Taiwan, for example, men aged 45 to 64 years have a dietary intake of 3.39 μg/d of vitamin D from fish, but 6- to 12-year-old boys obtain only 1.74 μg/d from this major food source of vitamin D.22
Taken together, epidemiologic and genetic data suggest a role for vitamin D in myopia. Considering that the most vitamin D does not circulate freely in the plasma but is bound to vitamin D-binding protein that may alter functional properties23 or plasma levels,24 the group-specific component (vitamin D–binding protein) gene GC is also of interest. Data on environmental exposures and plasma vitamin D were not collected in the present study, but adult participants in the CLEERE Study genetics phase were carefully phenotyped for refractive error. The purpose of this study was to conduct a case–control analysis of associations between myopia and variations in SNPs within VDR and GC in affected myopic adults compared to nonrelated, adult, emmetropic controls.
Methods
Recruitment of subjects took place at five clinical centers: The Ohio State University College of Optometry, The University of Alabama at Birmingham School of Optometry, The University of Houston College of Optometry, the Southern California College of Optometry, and the University of Arizona Department of Ophthalmology and Vision Science. Each site's institutional review board reviewed and approved the protocol. The research was performed in accordance with the tenets of the Declaration of Helsinki. Subjects gave written informed consent after all study procedures were explained.
Refractive error criteria were chosen to present clearly defined phenotypes of affected myopic and unaffected nonmyopic refractive errors. Affected subjects were at least 18 years of age with a minimum refractive error of at least −0.75 D of myopia in both principal meridians in both eyes, as measured by cycloplegic autorefraction (model WR-5100K; Grand Seiko, Hiroshima, Japan). This degree of myopia creates symptoms of distance blur, would most likely be corrected by most clinicians, and is beyond the measurement error of the autorefractor used in the study.25,26 Because results may vary by the level of refractive error,27 myopia was further divided into milder myopia between −0.75 and −4.00 D (inclusive) and more severe myopia worse than −4.00 D. This cut point was chosen on the basis of our hypothesis that vitamin D's potential relevance to refractive error may be limited to ametropias generated by essentially normal but disproportionate ocular components (i.e., correlation ametropia), and −4.00 D is the classic dividing line between correlation and component ametropia.28 Because case–control analyses may be biased by ethnic variation, our primary analysis was of white subjects, first for all levels of myopia, with a secondary analysis in a subgroup with myopia between −0.75 and −4.00 D. Ethnic group was designated by self-report from one of the following six ethnic designations: American Indian or Alaskan Native; Asian or Pacific Islander; black, not of Hispanic origin; Hispanic; white, not of Hispanic origin; and other, or unknown.
Unaffected control subjects were between 18 and 50 years of age with a spherical equivalent refractive error of at least +0.50 D but less than +2.00 D of hyperopia in each eye and less than 1.50 D of astigmatism. The lower limits for age and hyperopia were chosen to minimize the likelihood of future conversion to myopia. The upper limit for hyperopia was chosen to avoid clinically significant hyperopia, to make the comparison between myopia and minimal nonmyopic refractive error. The upper age restriction for unaffected subjects was chosen to avoid misclassification due to age-related hyperopic shifts in refractive error that tend to begin after age 50 years.29,30 Cycloplegia was achieved using 1 drop of proparacaine 0.5% followed by 2 drops of tropicamide 1% five minutes apart. Ten autorefractor readings were taken 30 minutes after instillation of the first drop of tropicamide. Subjects had no history of ocular surgery and no ongoing orthokeratologic treatment for myopia (if treated previously, treatment must have ceased 6 months before participation in the study). Current contact lens wear was acceptable. Subject characteristics are listed in Table 1.
Table 1.
Characteristics of Study Participants
| Cases | Controls | P | |
|---|---|---|---|
| Whites* | |||
| n | 289 | 81 | |
| Sex | 182 female | 41 female | 0.05 |
| 107 male | 40 male | ||
| Age, y | 32.3 ± 11.3 | 24.2 ± 7.5 | 3.2 × 10−13 |
| Whites† | |||
| n | 146 | 81 | |
| Sex | 87 female | 41 female | 0.19 |
| 59 male | 40 male | ||
| Age, y | 31.4 ± 11.5 | 24.2 ± 7.5 | 2.3 × 10−8 |
| Entire Sample* | |||
| N | 472 | 98 | |
| Sex | 312 female | 48 female | 0.002 |
| 160 male | 50 male | ||
| Age, y | 30.8 ± 10.2 | 24.6 ± 7.8 | 1.2 × 10−10 |
| Ethnicity | 289 white; | 81 white; | 5.7 × 10−5 |
| 182 nonwhite; | 17 nonwhite | ||
| 1 unknown |
Cases of more myopia than −0.75 D.
Cases of myopia between −0.75 D and −4.00 D.
Up to 10 mL of venous blood was collected from each participant. Whole blood DNA was processed with amplification kits (QiaAmp; Qiagen, Inc., Valencia, CA). Genotyping was performed (TaqMan SNP Genotyping Assays, Prism 7900HT; Applied Biosystems, Inc. [ABI], Foster City, CA) with a protocol slightly modified from the manufacturer's instructions. The SNP selection was performed using HapMap and Haploview (www.hapmap.org). The SNPs were first chosen if they had a possible functional role (rs10735810)31,32 or if they had high heterozygosity or were tagging SNPs from the two major haplotype blocks encompassing the exonic structure of the VDR gene and the region of low linkage disequilibrium lying between these two blocks. Two of the three SNPs within GC were selected because of a previous report that their variation correlated with differences in plasma levels of vitamin D (rs4588 and rs7041).24 The third GC SNP was selected to provide more complete genetic coverage of the haplotype block structure, taking it as a tagging SNP from HapMap. Genotyping was performed using 384-well plates containing dried DNA samples, and the scoring of the alleles was performed with a sequence detection system (SDS ver. 2.3; ABI). The data were uploaded into a database (Progeny Software, LLC, South Bend, IN) and analyzed (SAS Institute, Cary, NC).
Traditional case–control analysis was performed for allelic association and for genotypic association. Using these preliminary results, we identified the associated allele by noting when the observed frequency in cases was greater than in controls. The SNPs were coded with the genotype of homozygous for the unassociated allele as the reference genotype, with a dose–response indicated as the genotype progressed to heterozygous and homozygous for the associated allele. The effects of age, sex, and ethnicity on myopia were assessed by logistic regression. SNPs were placed into a multivariate logistic model with these demographic variables and evaluated for main effects and two-way interactions by using likelihood ratio tests of significance of the change in the model due to the inclusion or removal of individual SNPs. Models were evaluated with consideration of the number of terms in the model using Akaike's Information Criterion, Wald and Score statistics, and the change in model R2. Further analyses were performed on the subset of subjects with mild myopia (defined as a spherical equivalent at least −0.75 D and equal to but not more myopic than −4.00 D), who were classified as white by self-report. The models were first constructed with an α level of 0.05. Adjustment for multiple comparisons was performed using the approach of Benjamini and Hochberg.33 With 12 SNPs analyzed, the lowest raw P-value tested would need to be less than 0.00417 to be considered significant. Each successively higher P-value tested would need to be less than successive integer multiples of 0.00417 (e.g., 0.00833, 0.0125, and so forth).
Results
The cases differed from the controls in several demographic respects. The cases were older, more often female, and less often white than were the controls (Table 1). Subsequent analyses were conducted using multivariate adjustment for age, sex, and ethnicity, when appropriate. The adjusted results that follow were essentially no different from those when subject ages were limited to less than 50 years as an alternate method for minimizing effects from age-related shifts in refractive error (results not shown).29,30 Hardy-Weinberg P-values ranged from 0.10 to 1.00, with the exception of rs10877013 at 2 × 10−5, rs2298849 at 0.01, and rs4516053 at 0.04. None of these SNPs were found to be significantly associated with myopia in the subsequent analyses. Minor allele frequencies ranged from 0.22 to 0.50, and genotyping success ranged from 94.3% to 99.7% (Table 2).
Table 2.
SNPs Evaluated
| Chromosome | SNP number | Position* | Hardy-Weinberg P | % Genotyped | Minor Allele Freq. | Alleles (Common: Minor) |
|---|---|---|---|---|---|---|
| 12 | rs7975232 | 48238837 | 0.78 | 96.7 | 0.50 | C:A |
| 12 | rs2239182 | 48255411 | 0.57 | 94.7 | 0.49 | A:G |
| 12 | rs2189480 | 48263828 | 0.66 | 96.2 | 0.40 | C:A |
| 12 | rs3819545 | 48265006 | 0.10 | 99.1 | 0.41 | T:C |
| 12 | rs3782905 | 48266167 | 0.83 | 99.7 | 0.30 | G:C |
| 12 | rs10735810† | 48272895 | 1.00 | 99.3 | 0.39 | G:A |
| 12 | rs2853559 | 48282805 | 0.35 | 95.2 | 0.36 | C:T |
| 12 | rs4516035 | 48299826 | 0.04 | 94.3 | 0.34 | T:C |
| 12 | rs10877013 | 58165085 | 2 × 10−5 | 97.9 | 0.39 | C:T |
| 4 | rs4588 (DBP-2) | 72618323 | 0.72 | 98.5 | 0.28 | C:A |
| 4 | rs7041 (DBP-1) | 72618334 | 0.29 | 98.6 | 0.48 | T:G |
| 4 | rs2298849 | 72648851 | 0.01 | 98.8 | 0.22 | T:C |
UCSC Genome Browser http://genome.ucsc.edu/build 37.1 (GRCh37), dbSNP build 131 (University of California at Santa Cruz).
rs10735810 has been merged into rs2228570 at the same position with alleles C and T (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene; National Institutes of Health, Bethesda, MD).
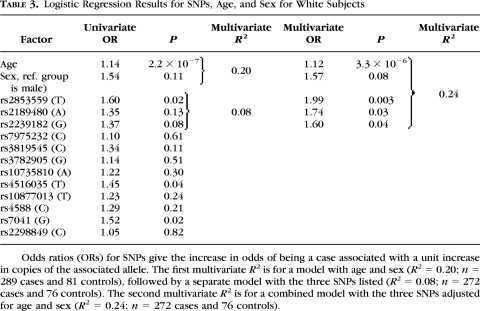
Univariate logistic regression for white control subjects (n = 81) and all levels of myopia (n = 289) indicated that three SNPs were of interest for significance at P = 0.05: VDR SNPs rs2853559 (P = 0.02) and rs4516035 (P = 0.04), and GC SNP rs7041 (P = 0.02; Table 3). All SNPs were placed into a multivariate logistic model with age, sex, and ethnicity. All two-way interactions with the SNPs and the demographic factors were tested. No interaction terms added substantially to the model R2, and they were not analyzed further. In a multivariate model with age and sex, VDR SNP rs2853559 maintained significance after adjustment for multiple comparisons (odds ratio = 1.99, P = 0.003). Two additional VDR SNPs in the multivariate model were not significant after adjustment for multiple comparisons (rs2189480 and rs2239182, P = 0.03 and 0.04, respectively). The model R2 increased by a small 4 percentage points over the model with the addition of the three SNPs with the demographic factors alone, from 0.20 to 0.24 (Table 3).
Table 3.
Logistic Regression Results for SNPs, Age, and Sex for White Subjects
Odds ratios (ORs) for SNPs give the increase in odds of being a case associated with a unit increase in copies of the associated allele. The first multivariate R2 is for a model with age and sex (R2 = 0.20; n = 289 cases and 81 controls), followed by a separate model with the three SNPs listed (R2 = 0.08; n = 272 cases and 76 controls). The second multivariate R2 is for a combined model with the three SNPs adjusted for age and sex (R2 = 0.24; n = 272 cases and 76 controls).
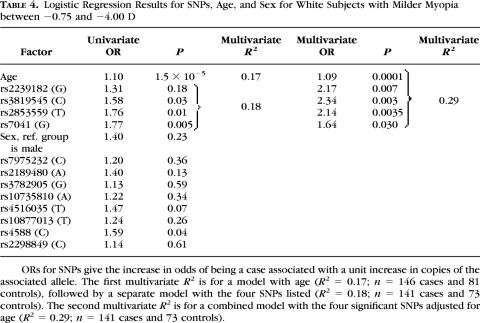
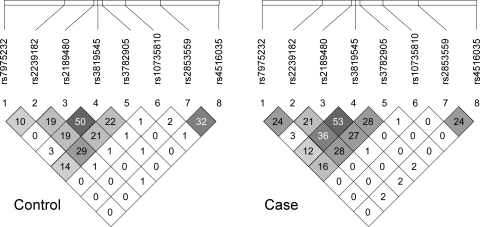
Univariate logistic regression results from white control subjects and with myopic spherical equivalent between −0.75 and −4.00 D are displayed in Table 4. Eight SNPs showed no significant association, but four were of interest for significance at the 0.05 level: VDR SNPs rs3819545 and rs2853559 and GC SNPs rs7041 and rs4588 (P = 0.03, 0.01, 0.005, and 0.04, respectively). All SNPs were placed into a multivariate logistic model with age and sex (Table 4). No two-way interaction terms added substantially to the model R2 and were not analyzed further. The multivariate model for the sample as a whole resulted in a significant effect for age, no effect for sex, and significant effects for three SNPs within VDR (rs2239182, rs3819545, and rs2853559) and one within GC (rs7041). Three of these four SNPs are mentioned above for the white sample as a whole. SNP rs3819545 showed the greatest linkage disequilibrium, albeit limited, with rs2189480 (Fig. 1) and replaced it here compared with results for white subjects at all levels of myopia. The P-values for the three significant SNPs rs2239182, rs3819545, and rs2853559, within VDR in the multivariate analysis (0.007, 0.003, and 0.0035; Table 4), were all significant after adjustment for multiple comparisons; however, SNP rs7041 within GC was not significant after adjustment for multiple comparisons. The model R2 increased by a substantial 12 percentage points with the addition of the four SNPs over the model with the demographic factors alone, from 0.17 to 0.29. Multivariate odds ratios for these SNPs are presented in Table 5. There was some evidence of dose–response behavior in the VDR SNPs by virtue of the numerically increasing odds ratios, but the 95% confidence intervals for one and two copies of the risk alleles were overlapping.
Table 4.
Logistic Regression Results for SNPs, Age, and Sex for White Subjects with Milder Myopia between −0.75 and −4.00 D
ORs for SNPs give the increase in odds of being a case associated with a unit increase in copies of the associated allele. The first multivariate R2 is for a model with age (R2 = 0.17; n = 146 cases and 81 controls), followed by a separate model with the four SNPs listed (R2 = 0.18; n = 141 cases and 73 controls). The second multivariate R2 is for a combined model with the four significant SNPs adjusted for age (R2 = 0.29; n = 141 cases and 73 controls).
Figure 1.
Linkage disequilibrium measures expressed as R2 for controls and cases in the sample as a whole.
Table 5.
Multivariate Odds Ratios
| Multivariate OR (95% CI) | |
|---|---|
| rs2239182 | |
| GG | 4.79 (1.52–15.08) |
| AG | 1.67 (0.71–3.93) |
| AA | Reference |
| rs3819545 | |
| CC | 4.64 (1.46–14.80) |
| CT | 2.88 (1.27–6.55) |
| TT | Reference |
| rs2853559 | |
| TT | 4.01 (1.29–12.51) |
| TC | 2.67 (1.33–5.36) |
| CC | Reference |
| rs7041 | |
| GG | 2.74 (1.10–6.86) |
| GT | 2.54 (1.10–5.87) |
| TT | Reference |
ORs are associated with one or two copies of the associated allele in the sample of white subjects with milder myopia between −0.75 and −4.00 D, adjusted for age. 95% CI, 95% confidence interval.
Variations within VDR were weakly associated with myopia more severe than −4.00 D in 143 white cases compared to 81 white controls. No single SNP was significant in a univariate analysis. Three SNPs within VDR were only marginally significant in a multivariate analysis adjusted for age (rs2239182, rs2189480, and rs2853559, P = 0.05, 0.04, and 0.03, respectively). VDR SNP rs3819545 and GC SNP rs7041 were not significant in this subsample. At only 0.09, the model R2 for these three SNPs alone in white subjects with myopia worse than −4.00D was half that for the four SNPs associated with myopia between −0.75 and −4.00 D. Their addition to a model with age and sex improved the R2 by only 2 percentage points, from 0.27 to 0.29. None of the SNPs in the multivariate analysis reached the 0.00417 level required for adjustment for multiple comparisons.
To examine the potential relevance of these variations to ethnicities other than white, univariate logistic regression was also preformed on the sample as a whole. Ten SNPs showed no significant association with myopia, but two within VDR were of interest for significance at the 0.05 level (rs3819545, P = 0.02; rs2189480, P = 0.04). The multivariate model for the sample as a whole resulted in significant effects for age, sex, and ethnicity (R2 = 0.19), in addition to three SNPs within VDR (rs2239182, rs3819545, and rs2853559; R2 for the three SNPs by themselves = 0.04). No SNPs within GC were significantly associated with myopia in the multivariate analysis. The model R2 increased only 2 percentage points with the addition of the three SNPs over the model with the demographic factors alone, from 0.19 to 0.21. The final three-SNP model was of questionable significance; however, with adjustment for multiple comparisons, none of the SNPs in the multivariate analysis reached the 0.00417 level.
Discussion
We have identified three SNPs within VDR (rs2239182, rs3819545, and rs2853559) that differed significantly between white adult cases with myopia between −0.75 and −4.00D and white nonmyopic controls. Associations between myopia and variations within VDR and perhaps an additional SNP within GC (rs7041) seemed most applicable to this group. Only one SNP, rs2853559, was significant for white subjects when the range of myopia was unrestricted. None were significant when either the sample as a whole including all ethnicities or when white subjects with myopia worse than −4.00 D were considered. Odds ratios ranged from 1.64 to 2.34 with a substantial increase in the model R2, from 0.17 with age alone to 0.29 with the four SNPs adjusted for age. The size of these effects is somewhat larger than recent genome-wide association studies of myopia with a much larger sample size. Nakanishi et al.34 found odds ratios of 1.24 to 1.50 for SNPS within 11q24.1 in a genome-wide study of 830 cases with pathologic myopia and 1911 controls. More recently Hysi et al.35 and Solouki et al.36 reported associations between SNPs and myopia assessed as part of very large-scale, genome-wide scans: rs8027411 at 15q2535 (odds ratio for homozygous for risk allele = 1.16) and rs634990 at 15q14 (odds ratio for homozygous for risk allele = 1.83).36
To our knowledge, no genome-wide scans for genes related to myopia have identified VDR or GC as significantly related to myopia. That they have not is somewhat surprising, given the very large sample size, replication, and SNP density in recent reports.35–36 One possibility is that the present study results are false positives, a concern that can only be addressed through replication. We estimated the false-positive rate for SNPs with the allele frequencies observed in the study to be 3.8% through performing over 1000 simulations, making the probability of finding three significant SNPs by chance alone equal to 5.4 × 10−5. An important difference is that the present study is more a candidate gene approach using a multivariate analysis of several, mostly independent, SNPs showing weak to no linkage disequilibrium. The effects shown in Table 5 are from multivariate odds ratios. In agreement with recent reports,35,36 none of the SNPs from the present study showed genome-wide significance as singletons. The multiple SNP analysis we used might be better suited to this candidate gene approach, given the prohibitively high number of multiple SNP combinations in a genome-wide study.
Interestingly, GC is in the vicinity of the MYP9 locus at 4q12 but has not been named as a candidate gene.37 G was the risk allele for rs7041 in the present study. This result was unexpected, given that being homozygous for the G allele for rs7041 has been associated with higher plasma levels of 25(OH) vitamin D,24 assumed to be beneficial given the protective effects of time spent outdoors.7 The regions recently identified as associated with myopia that are closest to VDR have been at 12q21.2-24.12 (MYP3 locus)38 and SNPs within COL2A1 at 12q13.1116,27; however, linkage between COL2A1 and VDR seems unlikely and none of the SNPs within VDR or GC used in the present study was part of a panel used previously (Linkage Panel IVb; Illumina, San Diego, CA).38 The recent, detailed investigation of SNPs within and near COL2A1 did not include SNPs within VDR.27 One group has reported that the BsmI polymorphism within VDR (rs1544410, not evaluated in the present study) is associated with myopia.39 In addition, SNPs within LRP5 (low-density lipoprotein receptor-related protein 5) located on chromosome 11, region q13.4, have recently been associated with refractive error (Simpson CL, et al. IOVS. 2009;50:ARVO E-Abstract 2817). This finding is relevant, because the LRP5 gene has been shown to interact with the vitamin D receptor and vitamin D, modifying osteoblast function in the mouse.40 Further study is needed to determine whether an interaction between VDR and LRP5 is important in human refractive error.
Replication studies are needed to confirm the importance of these polymorphisms to myopia; pending replication, these results should be interpreted with caution. The sample size was relatively small, and a case–control design may be subject to false-positive findings if differences in ancestry between cases and controls create differences in allele frequencies that are more related to ancestry than to myopia. In addition, self-reported ethnicity and the limited number of ethnic categories used may have resulted in misclassification. With this caution in mind, our results were consistent across attempts to control for ancestry by adjusting for ethnicity in the multivariate analysis of the entire sample and by conducting the analysis in the white subsample. Obviously, such adjustments are not perfect, nor do they constitute a complete set of potential confounding variables. Unmeasured factors related to myopia such as time spent outdoors,7 personality,41,42 and IQ43 may interact with or be more directly associated with these polymorphisms. A more complete study of a role for VDR and vitamin D in myopia would compare myopes to nonmyopes, not only for genetic polymorphisms but also for differences in dietary intake of vitamin D, circulating blood levels of vitamin D, IQ, and visual activity profiles at various times of year to untangle the specific contributions of each factor and the interactions between them.
With one exception, the SNPs within VDR are located in intron 7 of the gene in a region without strong linkage disequilibrium and that lies between the two major haplotype blocks that encompass the coding sequence of VDR. While there are multiple adjacent regions to the associated SNPs of strong sequence conservation within 1000 bp or fewer that are suggestive of regulatory elements, none of the three SNPs is itself resident in a conserved element. One SNP, rs10735810 (rs228570), is outside of intron 7 and has a C-to-T nucleotide change that creates a 424-amino-acid VDR protein compared with a longer 427-amino-acid protein made in the presence of the C allele. Studies have shown the 424-amino-acid protein results in more transactivation of the vitamin D response element than for the longer protein,31,32 yet this SNP showed no association with myopia in any analyses, leaving the mechanism of action unclear.
Additional studies are needed to determine whether these polymorphisms have any biological significance in vitamin D receptor function or vitamin D metabolism relevant to the eye. Vitamin D is known to be a powerful regulator of cellular differentiation with strong anticancer and antiproliferative effects.44 As a member of the nuclear receptor family, the vitamin D receptor regulates gene transcription, forming heterodimeric partnerships with retinoid X receptors (RXR).45 The presence of 1,25-dihydroxyvitamin D3 initiates the formation of the vitamin D receptor/RXR heterodimer. Thus, an abnormality of the vitamin D receptor or a lack of vitamin D in the diet might affect transcription, and perhaps ocular growth, through these abnormalities. Retinoic acid receptors also form heterodimers with RXR, with retinoic acid and 1,25-dihydroxyvitamin D3 engaging in some crosstalk in signaling and cell-cycle regulation through overlapping binding specificities.45 Retinoic acid is noteworthy because it has been shown to be a bidirectional regulator of eye growth.46,47 In addition, feeding experimental animals large amounts of retinoic acid leads to increased eye growth.48,49 Levels of retinoic acid receptor-α mRNA have also been reported to increase in form deprivation myopia and recovery from myopia in the chick.50 Perhaps this crosstalk between vitamin D and retinoic acid exists in signaling pathways affecting eye growth. While speculative at this stage, these potential mechanisms suggest several experimental avenues to pursue.
In summary, we found that polymorphisms within the vitamin D receptor gene VDR were associated with myopia, particularly in white subjects with low myopia, accounting for a large proportion of model variance (12%) over age alone. The repeatability of this finding and the biological significance of these variations with respect to myopia are yet to be determined.
Appendix
The members of the CLEERE Study Group responsible for data collection were Monica Chitkara, The Ohio State University College of Optometry, Columbus, OH; Susan A. Cotter, Southern California College of Optometry, Fullerton, CA; Robert N. Kleinstein, School of Optometry, University of Alabama at Birmingham, Birmingham, AL; Ruth E. Manny, University of Houston College of Optometry, Houston, TX; and J. Daniel Twelker, University of Arizona Department of Ophthalmology and Vision Science, Tucson, AZ.
Footnotes
Supported by National Institutes of Health/National Eye Institute Grants U10-EY08893 and R24-EY014792, the Ohio Lions Eye Research Foundation, and the E. F. Wildermuth Foundation.
Disclosure: D.O. Mutti, None; M.E. Cooper, None; E. Dragan, None; L.A. Jones-Jordan, None; M.D. Bailey, None; M.L. Marazita, None; J.C. Murray, None; K. Zadnik, None
References
- 1. Vitale S, Ellwein L, Cotch MF, Ferris FL, Sperduto R. Prevalence of refractive error in the United States 1999–2004. Arch Ophthalmol. 2008;126:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vitale S, Cotch MF, Sperduto R, Ellwein L. Costs of refractive correction of distance vision impairment in the United States 1999–2002. Ophthalmology. 2006;113:2163–2170 [DOI] [PubMed] [Google Scholar]
- 3. Blum HL, Peters HB, Bettman JW. Vision Screening for Elementary Schools: The Orinda Study.. Berkeley: University of California Press; 1959 [Google Scholar]
- 4. Kleinstein RN, Jones LA, Hullett S, et al. Refractive error and ethnicity in children. Arch Ophthalmol. 2003;121:1141–1147 [DOI] [PubMed] [Google Scholar]
- 5. Mutti DO, Zadnik K, Adams AJ. Myopia: the nature versus nurture debate goes on. Invest Ophthalmol Vis Sci. 1996;37:952–957 [PubMed] [Google Scholar]
- 6. Pacella R, McLellan J, Grice K, et al. Role of genetic factors in the etiology of juvenile-onset myopia based on a longitudinal study of refractive error. Optom Vis Sci. 1999;76:381–386 [DOI] [PubMed] [Google Scholar]
- 7. Jones LA, Sinnott LT, Mutti DO, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teikari JM, Kaprio J, Koskenvuo MK, Vannas A. Heritability estimate for refractive errors: a population-based sample of adult twins. Gen Epidemiol. 1988;5:171–181 [DOI] [PubMed] [Google Scholar]
- 9. Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci. 2001;42:1232–1236 [PubMed] [Google Scholar]
- 10. Lyhne N, Sjolie AK, Kyvik KO, Green A. The importance of genes and environment for ocular refraction and its determiners: a population based study among 20–45 year old twins. Br J Ophthalmol. 2001;85:1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dirani M, Chamberlain M, Shekar SN, et al. Heritability of refractive error and ocular biometrics: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci. 2006;47:4756–4761 [DOI] [PubMed] [Google Scholar]
- 12. Saw SM, Shankar A, Tan SB, et al. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47:1839–1844 [DOI] [PubMed] [Google Scholar]
- 13. Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285 [DOI] [PubMed] [Google Scholar]
- 14. McBrien NA, Young TL, Pang CP, et al. Myopia: recent advances in molecular studies; prevalence, progression and risk factors; emmetropization; therapies; optical links; peripheral refraction; sclera and ocular growth; signalling cascades; and animal models. Optom Vis Sci. 2009;86:45–66 [Google Scholar]
- 15. Dirani M, Tong L, Gazzard G, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009;93:997–1000 [DOI] [PubMed] [Google Scholar]
- 16. Mutti DO, Cooper ME, O'Brien S, et al. Candidate gene and locus analysis of myopia. Mol Vis. 2007;13:1012–1019 [PMC free article] [PubMed] [Google Scholar]
- 17. Keeley FW, Morin JD, Vesely S. Characterization of collagen from normal human sclera. Exp Eye Res. 1984;39:533–542 [DOI] [PubMed] [Google Scholar]
- 18. Fulk GW, Cyert LA, Parker DA. Seasonal variation in myopia progression and ocular elongation. Optom Vis Sci. 2002;79:46–51 [DOI] [PubMed] [Google Scholar]
- 19. Mutti DO, Marks AR. Blood levels of vitamin D in teens and young adults with myopia. Optom Vis Sci.. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin LL, Shih YF, Hsiao CK, et al. Epidemiologic study of the prevalence and severity of myopia among schoolchildren in Taiwan in 2000. J Formos Med Assoc.. 2001;100:684–691 [PubMed] [Google Scholar]
- 21. Zhai F, Wang H, Du S, et al. Lifespan nutrition and changing socio-economic conditions in China. Asia Pac J Clin Nutr. 2007;16(suppl 1):374–382 [PubMed] [Google Scholar]
- 22. Lee MS, Li HL, Hung TH, et al. Vitamin D intake and its food sources in Taiwanese. Asia Pac J Clin Nutr. 2008;17:397–407 [PubMed] [Google Scholar]
- 23. Chun RF, Adams JS, Hewison M. Back to the future: a new look at ‘old’ vitamin D. J Endocrinol. 2008;198:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sinotte M, Diorio C, Berube S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr. 2009;89:634–640 [DOI] [PubMed] [Google Scholar]
- 25. Davies LN, Mallen EA, Wolffsohn JS, Gilmartin B. Clinical evaluation of the Shin-Nippon NVision-K 5001/Grand Seiko WR-5100K autorefractor. Optom Vis Sci. 2003;80:320–324 [DOI] [PubMed] [Google Scholar]
- 26. Bailey MD, Twa MD, Mitchell GL, et al. Repeatability of autorefraction and axial length measurements after laser in situ keratomileusis. J Cataract Refract Surg. 2005;31:1025–1034 [DOI] [PubMed] [Google Scholar]
- 27. Metlapally R, Li YJ, Tran-Viet KN, et al. COL1A1 and COL2A1 genes and myopia susceptibility: evidence of association and suggestive linkage to the COL2A1 locus. Invest Ophthalmol Vis Sci. 2009;50:4080–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sorsby A, Benjamin B, Davey JB, Sheridan M, Tanner JM. Emmetropia and its aberrations. Medical Research Council, Special Report Series.. London: Her Majesty's Stationery Office; 1957 [PubMed] [Google Scholar]
- 29. Mutti DO, Zadnik K. Age-related decreases in the prevalence of myopia: longitudinal change or cohort effect? Invest Ophthalmol Vis Sci. 2000;41:2103–2107 [PubMed] [Google Scholar]
- 30. Lee KE, Klein BE, Klein R, Wong TY. Changes in refraction over 10 years in an adult population: the Beaver Dam Eye study. Invest Ophthalmol Vis Sci. 2002;43:2566–2571 [PubMed] [Google Scholar]
- 31. Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156 [DOI] [PubMed] [Google Scholar]
- 32. Arai H, Miyamoto K, Taketani Y, et al. A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res. 1997;12:915–921 [DOI] [PubMed] [Google Scholar]
- 33. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B. 1995;57:289–300 [Google Scholar]
- 34. Nakanishi H, Yamada R, Gotoh N, et al. A genome-wide association analysis identified a novel susceptible locus for pathological myopia at 11q24.1. PLoS Genet. 2009;5:e1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hysi PG, Young TL, Mackey DA, et al. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet. 2010;42:902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Solouki AM, Verhoeven VJ, van Duijn CM, et al. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet. 2010;42:897–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hammond CJ, Andrew T, Mak YT, Spector TD. A susceptibility locus for myopia in the normal population is linked to the PAX6 gene region on chromosome 11: a genomewide scan of dizygotic twins. Am J Hum Genet. 2004;75:294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li YJ, Guggenheim JA, Bulusu A, et al. An international collaborative family-based whole-genome linkage scan for high-grade myopia. Invest Ophthalmol Vis Sci. 2009;50:3116–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sandhya A. Human Genome Meeting 2008, Hyderabad, India, September 27–30, 2008 Poster 441 [Google Scholar]
- 40. Fretz JA, Zella LA, Kim S, Shevde NK, Pike JW. 1,25-Dihydroxyvitamin D3 induces expression of the Wnt signaling co-regulator LRP5 via regulatory elements located significantly downstream of the gene's transcriptional start site. J Steroid Biochem Mol Biol. 2007;103:440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beedle SL, Young FA. Values, personality, physical characteristics, and refractive error. Am J Optom Physiol Opt. 1976;53:735–739 [PubMed] [Google Scholar]
- 42. van de Berg R, Dirani M, Chen CY, Haslam N, Baird PN. Myopia and personality: the genes in myopia (GEM) personality study. Invest Ophthalmol Vis Sci. 2008;49:882–886 [DOI] [PubMed] [Google Scholar]
- 43. Saw SM, Tan SB, Fung D, et al. IQ and the association with myopia in children. Invest Ophthalmol Vis Sci. 2004;45:2943–2948 [DOI] [PubMed] [Google Scholar]
- 44. Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004;26:21–28 [DOI] [PubMed] [Google Scholar]
- 45. Tavera-Mendoza L, Wang TT, Lallemant B, et al. Convergence of vitamin D and retinoic acid signalling at a common hormone response element. EMBO Reports. 2006;7:180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70:519–527 [DOI] [PubMed] [Google Scholar]
- 47. Troilo D, Nickla DL, Mertz JR, Summers Rada JA. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci. 2006;47:1768–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McFadden SA, Howlett MHC, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44:643–653 [DOI] [PubMed] [Google Scholar]
- 49. McFadden SA, Howlett MH, Mertz JR, Wallman J. Acute effects of dietary retinoic acid on ocular components in the growing chick. Exp Eye Res. 2006;83:949–961 [DOI] [PubMed] [Google Scholar]
- 50. Morgan I, Kucharski R, Krongkaew N, et al. Screening for differential gene expression during the development of form-deprivation myopia in the chicken. Optom Vis Sci. 2004;81:148–155 [DOI] [PubMed] [Google Scholar]