Oxidative stress plays an important role in the photoreceptor cell death after retinal detachment (RD). Edaravone prevented photoreceptor death after RD by reducing reactive oxygen species.
Abstract
Purpose.
To investigate whether edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a free radical scavenger, would be neuroprotective against photoreceptor cell death in a rat model of retinal detachment (RD).
Methods.
RD was induced in adult Brown Norway rats by subretinal injection of sodium hyaluronate. Edaravone (3, 5, or 10 mg/kg) or physiologic saline was administered intraperitoneally once a day until death on day 3 or 5. Oxidative stress in the retina was assessed by 4-hydroxynonenal staining or ELISA for protein carbonyl content. Photoreceptor death was assessed by TUNEL and measurement of the outer nuclear layer thickness. Western blot analysis and caspase activity assays were performed. Inflammatory cytokine secretion and inflammatory cell infiltration were evaluated by ELISA and immunostaining, respectively.
Results.
RD resulted in increased generation of ROS. Treatment with 5 mg/kg edaravone significantly reduced the ROS level, along with a decrease in TUNEL-positive cells in the photoreceptor layer. A caspase assay also confirmed decreased activation of caspase-3, -8, and -9 in RD treated with edaravone. The level of the antiapoptotic Bcl-2 was increased in detached retinas after edaravone treatment, whereas the levels of the stress-activated p-ERK1/2 were decreased. In addition, edaravone treatment resulted in a significant decrease in the levels of TNF-α, MCP-1, and macrophage infiltration.
Conclusions.
Oxidative stress plays an important role in photoreceptor cell death after RD. Edaravone treatment may aid in preventing photoreceptor cell death after RD by suppressing ROS-induced photoreceptor damage.
There are approximately 30,000 new nontraumatic retinal detachments (RDs) per year in the United States, and it is one of the most common causes of photoreceptor death and vision loss. Treatment options for RD are mainly surgical, such as reattachment with buckle or pneumatic reattachment with gas, and no optimal medical treatment has been found so far. Although the anatomic success rate of reattachment surgery is over 90%, the visual acuity is not always restored after successful reattachment surgery, suggesting functional impairment of the photoreceptors during RD.
The separation of the neurosensory retina from the underlying retinal pigment epithelium (RPE) reduces the photoreceptor outer segments' O2 and nutrient supply, thus causing a relative hypoxic state in the photoreceptor layer.1 These kinds of stress stimuli can lead to the generation of reactive oxygen species (ROS).2 Excessive generation of ROS and the consequent induction of oxidative stress is one of the factors that trigger cellular response to RD3 and is also a major cytotoxic factor for photoreceptor apoptosis.4,5 ROS are usually scavenged by endogenous enzymes such as ascorbate, tocopherol, glutathione, and pyridine nucleotides. However, excessive free radicals, such as those generated during ischemia, can damage the endothelial cells and neurocytes during reperfusion.6,7 Moreover, several studies in animal models of retinal degeneration have highlighted the importance of antioxidants in the inhibition of degenerative disease.8,9 Antioxidants such as diphenylene iodonium sulfate, allopurinol, and superoxide dismutase, which are known to scavenge free radicals, have been studied for the prevention of apoptosis.10–13 However, these chemicals may not be appropriate for use in the clinic because of their toxicity and instability.14,15
Edaravone (3-methyl-1 phenyl-2-pyrazolin-5-one) is a potent hydroxyl radical scavenger that can eliminate hydrogen oxide radicals that induce lipid peroxidation. Several studies have shown that it has antioxidative and antiapoptotic effects.16–18 Moreover, it has been approved in Japan for the treatment of acute brain infarction since 2001, reducing the mortality rate when administered during the acute stage of stroke.19
The purpose of this study was to investigate the role of oxidative stress caused by ROS in a rat model of RD and whether edaravone can prevent death of photoreceptor cells by reducing the level of ROS.
Methods
Animals
All animal experiments followed the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care Committee of the Massachusetts Eye and Ear Infirmary. Male Brown Norway rats (8–10 weeks) were purchased from Charles River Laboratories (Wilmington, MA). A total of 89 rats between 200 and 300 g in weight were used.
Creation of Retinal Detachment
RD was created (Provisc; Alcon, Fort Worth, TX), as previously described.20 Briefly, after anterior chamber puncture was performed via the corneal limbus, to lower intraocular pressure, approximately one-half of the superonasal–inferotemporal neurosensory retina was detached by the subretinal injection of 1% sodium hyaluronate into the subretinal space.
Treatment with Edaravone
Rats were separated into four groups. After induction of RD, three groups (n = 10 each) of rats were given once-daily intraperitoneal injection of 3, 5, and 10 mg/kg of edaravone until the day of death. A fourth group (n = 8) received once-daily intraperitoneal injection of physiologic saline. These doses were selected based on previous studies that had demonstrated the effectiveness of the drug.21,22 Edaravone (Mitsubishi Pharma Corporation, Tokyo, Japan) was dissolved in dimethyl sulfoxide (DMSO) and was diluted with physiologic saline to a concentration of 3 mg/mL.
Immunohistochemical Detection of 4-Hydroxynoneal in the Retina
On days 3 and 5 after RD creation, the eyes were enucleated and embedded in OCT compound (Tissue Tek; Sakura Finetec, Torrance, CA). Serial sections of the eyes in the sagittal plane through the optic nerve head were cut at 10 μm thickness on a cryostat (CM1850; Leica, Heidelberg, Nussloch, Germany) at −20°C and prepared for staining. After fixation in acetone, endogenous peroxidase activity was blocked by 0.3% hydrogen peroxide (Sigma-Aldrich, St. Louis, MO) for 15 minutes and subsequently with 5% skim milk for 1 hour, to block nonspecific binding. Subsequently, sections were incubated with anti-4-hydroxynoneal (HNE) Ab (Alpha Diagnostics, San Antonio, TX) at 4°C overnight. Thereafter, sections were incubated for 30 minutes at room temperature with HRP-conjugated secondary antibody against rabbit IgG (Dako, Carpinteria, CA). For signal detection, the sections were incubated with AEC substrate-chromogen solution for 5 minutes according to the manufacturer's protocol (Envision System-HRP; Dako) and then washed with distilled water. Finally, the sections were counterstained with Mayer's hematoxylin (Sigma-Aldrich). Light microscopy was used to obtain images of the retina at a final magnification of ×200.
TUNEL and Evaluation of ONL Thickness Ratio
A terminal dUTP nick-end labeling (TUNEL) assay was performed according to the manufacturer's protocol (ApoTag Fluorescein In Situ Apoptosis Detection Kit; Chemicon, Temecula, CA) as previously reported.23 The number of TUNEL-positive cells was counted in the photoreceptor layer in nine sections per eye. The center of the detached retina was determined and photographed. To be considered TUNEL-positive, each green fluorescent signal had to correspond precisely to the location of a DAPI-stained cell nucleus and had to be significantly brighter than the faint green background of most cell nuclei. The sum of TUNEL-positive cells in the nine sections of each eye was adjusted for the size of the sections.
The ratio of the ONL thickness to the thickness of neuroretina in the central area of the detached retina was determined by Image J software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) and compared to that in the attached retina.
Immunohistochemistry
For immunostaining of macrophages, the sections were incubated with mouse anti-rat monocytes/macrophage (ED-1) monoclonal antibody (1:100; Millipore, Billerica, MA) overnight at 4°C. Alexa Flour 488-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA) was used as the secondary antibody and incubated at room temperature for 1 hour. Images of the retina were taken with an upright fluorescence microscope (DM RXA; Leica, Solms, Germany), and the number of ED-1-positive cells was counted.
Western Blot Analysis
Retinas from experimental eyes with RD and control eyes without RD were dissected from the RPE-choroid at day 1 after creation of RD. Samples were run on 4% to 12% Bis-Tris gel (NuPAGE; Invitrogen) electrophoresis and transferred onto PVDF membranes (0.45 μm pores; Millipore, Billerica, MA). After they were blocked with 3% nonfat dried milk, the membranes were incubated overnight with primary antibody (Bcl-2 antibody, Bax antibody, phospho-ERK1/2, ERK1/2; 1:1000; Cell Signaling, Danvers, MA). The blotted membranes were then incubated for 30 minutes at room temperature with HRP-labeled anti-rabbit secondary antibody (1:10,000; Jackson ImmunoResearch, West Grove, PA). Immunoreactive bands were visualized by ECL and exposure to film (RX; Fuji-film, Tokyo, Japan).
ELISA for Protein Carbonyl Content, MCP-1, and TNF-α
The amount of carbonyl formation in protein was determined with a protein carbonyl ELISA kit (Oxiselect; Cell Biolabs, San Diego, CA) according to the manufacturer's instructions for retinal lysates. The levels of TNF-α and MCP-1 were determined with rat TNF-α (R&D Systems, Inc., Minneapolis, MN) and MCP-1 (R&D Systems, Inc.) ELISA kits, according to the manufacturer's protocol.
Caspase Activity Assay
The activity of caspases-3, -8, and -9 was assessed by using colorimetric assay kits (Casp-3-C and Casp-8-C for caspase-3 and -8; Sigma-Aldrich; APT173 for caspase-9, Chemicon-Millipore), per the manufacturers' instructions. Fifty micrograms of retinal lysates were incubated with the substrate corresponding to the investigated caspases for 1 hour at 37°C. Optical densities were read at 405 nm and normalized to readings from a blank sample (a well filled with buffer).
Statistical Analysis
The results are expressed as the mean ± SE. Statistical analysis was performed nonparametrically with Mann-Whitney U test (SPSS ver. 17.0; SPSS Inc., Chicago, IL). P < 0.05 was considered statistically significant.
Results
Expression of 4-HNE and Quantification of PCC after RD
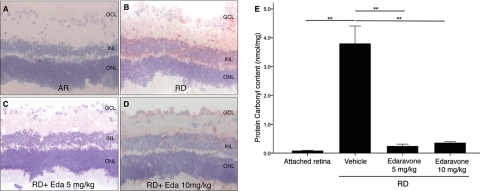
ROS generation leads to DNA, lipid, and protein peroxidation. A major representative of lipid peroxidation is 4-HNE generated from omega 6-poly unsaturated fatty acid. To assess the oxidative retinal damage after RD, we thus performed immunostaining with anti-4-HNE antibody. Three days after RD, 4-HNE staining was detected at the inner plexiform layer (IPL), inner nuclear layer (INL), and the outer plexiform layer (OPL), whereas there was minimal staining in the attached retina (Figs. 1A, 1B). 4-HNE expression in the IPL, INL, and OPL decreased with edaravone treatment (Figs. 1C, 1D).
Figure 1.
Quantification of oxidative retinal damage in retina with HNE immunostaining and ELISA for PCC. Although there was minimal 4-HNE staining in AR (A), increased 4-HNE staining at the IPL and the ONL was noted 3 days after creation of RD (B). Decreased 4-HNE staining was noted after treatment with both (C) 5 (D) and 10 mg/kg edaravone. (E) There was a significant decrease in PCC 3 days after RD in the edaravone treatment group compared with the saline-treated group (P < 0.01). Data are expressed as the mean ± SE; n = 5–7. **P < 0.01. GCL, ganglion cell layer; Eda, Edaravone. Original magnification: ×200.
We next performed ELISA for PCC, which provides a quantitative assessment of oxidative protein in tissue. The carbonyl content per milligram protein in the detached retina was significantly higher than in the attached retina (P = 0.004). In contrast, PCC was significantly decreased in the edaravone-treated group compared with that in those treated with saline after RD (P < 0.01; Fig. 1E)
Inhibition of RD-Induced Photoreceptor Death with Edaravone
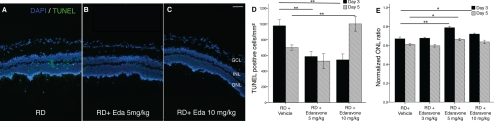
Dose–response experiments with edaravone (3, 5, or 10 mg/kg) to assess protection of photoreceptor cell loss revealed that the optimum protection was obtained at 5 mg/kg. Edaravone at that dose significantly prevented the reduction of the ONL thickness ratio at 3 and 5 days after RD (P < 0.001 and P = 0.041, respectively). Whereas 3 mg/kg did not show any protective effect, 10 mg/kg edaravone showed a protective effect only on day 3 (P = 0.041; Fig. 2E).
Figure 2.
Effect of edaravone on ONL thickness ratio and photoreceptor cell death with TUNEL staining compared with saline treatment 3 and 5 days after RD. Quantification of TUNEL (+) cells showed significantly decreased TUNEL (+) cells/mm2 with treatment of (B) 5 and (C) 10 mg/kg edaravone, compared with the saline-treated group (A) at 3 days after RD creation (both, P < 0.01). (D) Five days after RD creation, 10 mg/kg edaravone treatment showed a significant increase in photoreceptor cell death (P = 0.004). (E) Treatment with 5 mg/kg edaravone significantly prevented the reduction of the ONL thickness ratio on days 3 and 5 after RD (P < 0.001 and P = 0.041, respectively). Whereas treatment with 10 mg/kg showed a protective effect on day 3 (P = 0.041), prolonged treatment until day 5 showed no added protective effect (P = 0.25). Data are expressed as the mean ± SE n = 5–7. *P < 0.05. **P < 0.01. GCL, ganglion cell layer; Eda, Edaravone. Original magnification: (A–C) ×10; scale bar, 100 μm.
Next, we assessed photoreceptor death after RD by TUNEL staining. Doses of 5 and 10 mg/kg edaravone substantially suppressed TUNEL-positive cells in the ONL (585.9 ± 305.7 cells/mm2; P < 0.01; and 543.3.0 ± 300.1 cells/mm2; P = 0.002, respectively) 3 days after RD. On the other hand, 10 mg/kg edaravone showed a significant increase in TUNEL-positive cells 5 days after RD (1000.8 ± 244.5 cells/mm2; P = 0.004), suggesting possible toxicity of high doses of edaravone (Fig. 2D).
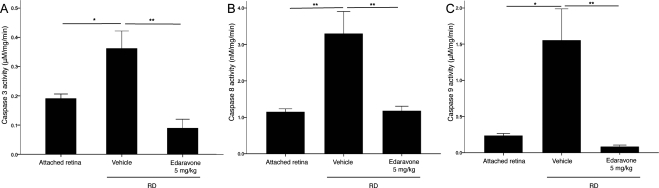
The caspase family is a central regulator of apoptosis. We previously described that caspases are activated in the retina after RD.24 In accordance with that study, RD induced activation of caspase-8, -9, and -3 at 24 hours after RD (P < 0.05). Treatment with 5 mg/kg edaravone significantly suppressed caspase activation (P < 0.01; Fig. 3).
Figure 3.
Decreases in caspase activation after treatment with edaravone 24 hours after RD creation. Activity of caspase-3 (A), -8 (B), and -9 (C) in AR, RD, and RD treated with 5 mg/kg edaravone. RD induced activation of caspase-8, -9, and -3, 1 day after RD, and treatment with 5 mg/kg edaravone significantly suppressed activation of these caspases. Data are expressed as the mean ± SE, n = 6. *P < 0.05, **P < 0.01.
Reduced Inflammatory Cytokine Expression and Macrophage Infiltration after RD with Edaravone
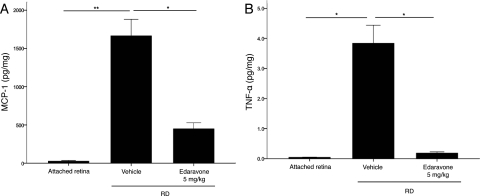
ROS is a critical mediator of the inflammatory cascade.25,26 We previously demonstrated that inflammatory cytokines are markedly elevated in experimental RD23 and that tissue-infiltrated macrophages mediate photoreceptor death.27 Increased levels of MCP-1 and TNF-α were noted with RD, and treatment with 5 mg/kg edaravone significantly reduced their expression 3 days after RD (P < 0.01; Fig. 4). Concurrent with the suppression of inflammatory cytokines, edaravone suppressed the subretinal infiltration of macrophages (ED-1-positive cells) 3 days after RD (P < 0.01; Fig. 5).
Figure 4.
ELISA analysis of TNF-α (A) and MCP-1 (B) activity three days after RD creation. While the level of MCP-1 and TNF-α was increased after RD, treatment with edaravone significantly reduced MCP-1 and TNF-α expression. Data are expressed as the mean ± SE, n = 4–6. *P < 0.05, **P < 0.01.
Figure 5.
Evaluation of macrophage infiltration with ED-1 staining in frozen sections. Three days after RD creation, a significantly decreased number of macrophages (arrowhead) was observed in the edaravone-treated group (B) compared with the saline-treated group (A). Quantification of ED-1 positive cells (C). **P < 0.01. Data are expressed as the mean ± SE n = 5–7. GCL, ganglion cell layer; Eda, Edaravone. (A, B) Original magnification: ×10; scale bar, 100 μm.
Edarvone Downregulates p-ERK1/2 and Upregulates Antiapoptotic Bcl-2 in Detached Retinas
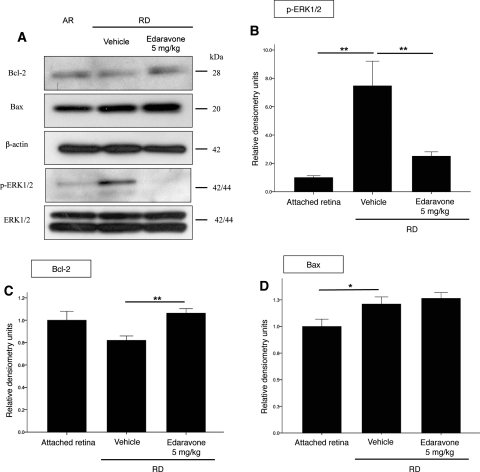
Mitogen-activated protein (MAP) kinases are known to be the transducers of stress stimuli.28,29 Among the three subgroups of MAP kinases, ERK1/2 activation is associated with RD.30 As in a previous study, Western blot analysis showed that expression of phosphorylated ERK1/2 was upregulated with RD, whereas edaravone treatment downregulated phosphorylation of p-ERK1/2 (Figs. 6A, 6B)
Figure 6.
Effect of edaravone on levels of Bcl-2, Bax, p-ERK1/2, and total ERK1/2 in AR, RD, and RD treated with 5 mg/kg edaravone (A). Retinas were harvested at 24 hours after RD. Whereas there was no change in the level of Bcl-2 after RD compared to AR, an increased level of Bcl-2 was noted after treatment with edaravone (C). An increased level of Bax was noted after RD, and it did not change with edaravone treatment (D). There was a marked increase in the level of phosph-ERK1/2 after RD. However, a decrease was noted after edaravone treatment (B). There was no change in the total ERK1/2 levels. *P < 0.05, **P < 0.01. Data are expressed as the mean ± SE n = 4–6.
The Bcl-2 family member proteins Bcl-2 and Bax are thought to be central regulators of ischemia and trauma-associated neuronal apoptosis.31,32 To further confirm mitochondria-mediated apoptosis, we examined the protein levels of the antiapoptotic Bcl-2 and the proapoptotic member of the same family, Bax.
While there was an increase in the expression of Bax 24 hours after RD creation, there was no significant change in the level of Bcl-2. However, edaravone treatment upregulated Bcl-2 expression, whereas it did not affect the expression of Bax (Figs. 6A, 6C, 6D).
Discussion
ROS overproduction has been implicated as a key mediator of photoreceptor death in many ocular diseases, including RD,27 age-related macular degeneration,33 retinitis pigmentosa,34,35 and macular dystrophy.36 We thus wanted to investigate whether ROS scavengers can have an effect on RD-induced photoreceptor cell loss.
In our rodent model of experimental RD, we found evidence of a substantial increase in oxidative stress. Elevated levels of oxidized lipids and proteins in the form of 4-HNE and PCC, respectively, were found in the retina after RD. Treatment with the free radical scavenger edaravone, significantly reduced the expression of both markers (Fig. 1), along with a decrease in TUNEL-positive cells in the photoreceptor layer (Fig. 2). This finding indicates that ROS may be an important therapeutic target in preventing photoreceptor cell death after RD. We should note that the dose of edaravone used in our study was significantly higher than the doses used in patients that suffer stroke, but is similar to that used in other animal studies. The discordance in the doses needed between animals and humans presumably can be attributed to the different pharmacokinetics and route of administration (intravenous in humans versus intraperitoneal in rats).
Along with ROS expression, RD induces proinflammatory cytokine and chemokine secretion. Vitreous samples from patients with RD exhibited significantly higher levels of TNF-α37,38 and MCP-1,39,40 compared with samples from patients with a macular hole or idiopathic premacular fibrosis. In vivo experimental RD studies also showed that RD is strongly associated with the production of MCP-1 and TNF-α.23,27 In the present study, edaravone treatment significantly reduced the expression of the inflammatory cytokines TNF-α and MCP-1 and the number of infiltrating macrophages after RD (Figs. 4, 5).
In experimental RD, the apoptosis of photoreceptor cells has been related to the activation of both intrinsic and extrinsic apoptotic pathways.24,41,42 The present study also confirmed that RD increased the activation of caspase-8, -3, and -9. However, their activity was significantly decreased after edaravone treatment (Fig. 3). This result is in line with the ischemia–reperfusion injury model study which showed that edaravone functions as a neuroprotective agent by blocking cytosolic release of cytochrome c and caspase-3 activation43,44 and by suppressing the Fas-signaling pathway.45 Moreover, these data indicate that reduction of ROS also has an effect in modulating the intrinsic apoptotic pathway. In fact, ROS are known triggers of the intrinsic apoptotic cascade via interactions with proteins of the mitochondrial permeability transition complex.46
Previous studies demonstrated that upregulation in expression of Bcl-2, the antiapoptotic protein located in the outer membrane of the mitochondria, inhibits the opening of the permeability transition complex, thus decreasing apoptotic cell death.47 Furthermore, Bcl-2 overexpression has been found to inhibit photoreceptor degeneration,48 and retinal neurons overexpressing Bcl-2 are protected against axotomy-induced cell death.49 With induction of RD, a trend in decrease of Bcl-2 along with a statistically significant upregulation of Bax (the proapoptotic member of the Bcl-2 family) was noted (Fig. 6). Edaravone treatment lead to increased levels of Bcl-2 protein, whereas the Bax levels were unaffected, thus tilting the balance in favor of the antiapoptotic member of the Bcl-2 family of proteins.
MAP kinase (ERK, JNK, and p38) is a family of stress-related kinases that have been implicated in various neural injuries and diseases.50 Among the members of the family, ERK1/2 has been implicated by some studies as a death-promoting kinase and could be activated by oxidative stress and ROS.51,52 Zacks30 reported that RD is associated with ERK1/2 activation, but not with that of JNK or p38. In the present study, ERK1/2 was activated after RD, and its activation was significantly reduced with edaravone treatment (Fig. 6). This finding suggests that ERK activation may be related to the participation of ROS in neuronal cell death, in concordance with an in vitro study showing that oxidative stress can activate ERK and that complete inhibition of the first phase of ERK1/2 activation can protect cells from oxidative stress–induced cell death.53
In summary, in our rodent model of RD, cell death, as determined by TUNEL positivity, was localized in the photoreceptor layer. After systemic treatment with edaravone, concomitant reduction of ROS, macrophage infiltration, inflammatory cytokines, caspase activation, and photoreceptor cell death were noted in the rat retina. This result leads to the conclusion that edaravone may decrease cell death by inhibiting macrophage function and infiltration. We previously reported that MCP-1 is a key mediator of early infiltration of macrophage/microglia after RD.27 In accordance with this result, MCP-1 upregulation after RD was substantially decreased with edaravone treatment. However, upregulation in the antiapoptotic protein Bcl-2 and downregulation of ERK1/2 with edaravone treatment suggests that edaravone may have a direct effect in preventing photoreceptor cell death.
Edaravone has been studied, not only in in vivo experimental settings, but also in clinical practice. Most of the studies included patients with acute ischemic stroke (AIS). A clinical trial has shown that the administration of edaravone alone within 72 hours of the onset of AIS significantly reduced the infarct volume and produced sustained benefits during a 3-month follow-up period.54 Recently, a study showed that improved visual acuity was observed when edaravone was administered in conjunction with vitrectomy in patients with branch retinal vein occlusion.55 Taken all together, these findings suggest that edaravone could be used in retinal diseases related to oxidative damage characterized by separation of the neurosensory retina from the RPE, including rhegmatogenous RD, AMD, retinal vein occlusion, and diabetic retinopathy. However, there is a report suggesting possible toxicity resulting in renal failure in stroke patients treated with edaravone.56 We observed photoreceptor toxicity with edaravone treatment at higher doses, detecting increased cell death in the photoreceptor layer (Fig. 3). Nevertheless, we did not see any toxic effect with a lower dose when given for 5 days. Therefore, although there is a need for additional studies to determine the most appropriate dosing, these results suggest that reducing oxidative stress may offer a new therapeutic target in treating RD.
In conclusion, this study provides substantial evidence of the role of ROS in photoreceptor cell death after RD. After RD, the ROS level is upregulated, along with increased production of proinflammatory cytokines and subsequent caspase activation resulting in the cell death of photoreceptor cells. We were able to reduce photoreceptor cell loss by reducing ROS with edaravone. It is likely that edaravone absorbed free radicals generated by RD and/or inhibited their functions, thereby preventing free radical–mediated photoreceptor death after RD.
Footnotes
Supported by the Neovascular Research Fund (JM, MIR), the Bacardi Fund (DGV), Research to Prevent Blindness, Inc. (JM, DGV), the Lions Eye Research Fund (DGV), the Onassis Foundation (DGV), a Fight for Sight Grant in Aid (DGV), the Harvard Ophthalmology Department (DGV), and National Eye Institute (NEI) Grant EY014104.
Disclosure: M.I. Roh, None; Y. Murakami, None; A. Thanos, None; D.G. Vavvas, None; J.W. Miller, None
References
- 1. Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000;41:3117–3123 [PubMed] [Google Scholar]
- 2. Bhatt L, Groeger G, McDermott K, Cotter TG. Rod and cone photoreceptor cells produce ROS in response to stress in a live retinal explant system. Mol Vis. 2010;16:283–293 [PMC free article] [PubMed] [Google Scholar]
- 3. Zacks DN, Han Y, Zeng Y, Swaroop A. Activation of signaling pathways and stress-response genes in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2006;47:1691–1695 [DOI] [PubMed] [Google Scholar]
- 4. Carmody RJ, McGowan AJ, Cotter TG. Reactive oxygen species as mediators of photoreceptor apoptosis in vitro. Exp Cell Res. 1999;248:520–530 [DOI] [PubMed] [Google Scholar]
- 5. Rotstein NP, Politi LE, German OL, Girotti R. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Invest Ophthalmol Vis Sci. 2003;44:2252–2259 [DOI] [PubMed] [Google Scholar]
- 6. Peters O, Back T, Lindauer U, et al. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1998;18:196–205 [DOI] [PubMed] [Google Scholar]
- 7. Demopoulos HB, Flamm ES, Pietronigro DD, Seligman ML. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl. 1980;492:91–119 [PubMed] [Google Scholar]
- 8. Feeney L, Berman ER. Oxygen toxicity: membrane damage by free radicals. Invest Ophthalmol. 1976;15(10):789–792 [PubMed] [Google Scholar]
- 9. Li J, Edward DP, Lam TT, Tso MO. Amelioration of retinal photic injury by a combination of flunarizine and dimethylthiourea. Exp Eye Res. 1993;56:71–78 [DOI] [PubMed] [Google Scholar]
- 10. Kono H, Rusyn I, Bradford BU, Connor HD, Mason RP, Thurman RG. Allopurinol prevents early alcohol-induced liver injury in rats. J Pharmacol Exp Ther. 2000;293:296–303 [PubMed] [Google Scholar]
- 11. Kono H, Rusyn I, Uesugi T, et al. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–G1012 [DOI] [PubMed] [Google Scholar]
- 12. Feher J, Lengyel G, Blazovics A. Oxidative stress in the liver and biliary tract diseases. Scand J Gastroenterol Suppl. 1998;228:38–46 [DOI] [PubMed] [Google Scholar]
- 13. Kono H, Fujii H, Matsuda M, Yamamoto M, Matsumoto Y. Gadolinium chloride prevents mortality in hepatectomized rats given endotoxin. J Surg Res. 2001;96:204–210 [DOI] [PubMed] [Google Scholar]
- 14. Nelson SK, Bose SK, McCord JM. The toxicity of high-dose superoxide dismutase suggests that superoxide can both initiate and terminate lipid peroxidation in the reperfused heart. Free Radic Biol Med. 1994;16:195–200 [DOI] [PubMed] [Google Scholar]
- 15. Balcerczyk A, Soszynski M, Rybaczek D, et al. Induction of apoptosis and modulation of production of reactive oxygen species in human endothelial cells by diphenyleneiodonium. Biochem Pharmacol. 2005;69:1263–1273 [DOI] [PubMed] [Google Scholar]
- 16. Kokura S, Yoshida N, Sakamoto N, et al. The radical scavenger edaravone enhances the anti-tumor effects of CPT-11 in murine colon cancer by increasing apoptosis via inhibition of NF-kappaB. Cancer Lett. 2005;229:223–233 [DOI] [PubMed] [Google Scholar]
- 17. Suzuki K, Kazui T, Terada H, et al. Experimental study on the protective effects of edaravone against ischemic spinal cord injury. J Thorac Cardiovasc Surg. 2005;130:1586–1592 [DOI] [PubMed] [Google Scholar]
- 18. Wen J, Watanabe K, Ma M, et al. Edaravone inhibits JNK-c-Jun pathway and restores anti-oxidative defense after ischemia-reperfusion injury in aged rats. Biol Pharm Bull. 2006;29:713–718 [DOI] [PubMed] [Google Scholar]
- 19. Toyoda K, Fujii K, Kamouchi M, et al. Free radical scavenger, edaravone, in stroke with internal carotid artery occlusion. J Neurol Sci. 2004;221:11–17 [DOI] [PubMed] [Google Scholar]
- 20. Hisatomi T, Sakamoto T, Murata T, et al. Relocalization of apoptosis-inducing factor in photoreceptor apoptosis induced by retinal detachment in vivo. Am J Pathol. 2001;158:1271–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimazaki H, Watanabe K, Veeraveedu PT, et al. The antioxidant edaravone attenuates ER-stress-mediated cardiac apoptosis and dysfunction in rats with autoimmune myocarditis. Free Radic Res. 2010;44:1082–1090 [DOI] [PubMed] [Google Scholar]
- 22. Inokuchi Y, Imai S, Nakajima Y, et al. Edaravone, a free radical scavenger, protects against retinal damage in vitro and in vivo. J Pharmacol Exp Ther. 2009;329:687–698 [DOI] [PubMed] [Google Scholar]
- 23. Nakazawa T, Matsubara A, Noda K, et al. Characterization of cytokine responses to retinal detachment in rats. Mol Vis. 2006;12:867–878 [PubMed] [Google Scholar]
- 24. Zacks DN, Hanninen V, Pantcheva M, Ezra E, Grosskreutz C, Miller JW. Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2003;44:1262–1267 [DOI] [PubMed] [Google Scholar]
- 25. Bonne C, Muller A, Villain M. Free radicals in retinal ischemia. Gen Pharmacol. 1998;30:275–280 [DOI] [PubMed] [Google Scholar]
- 26. Winrow VR, Winyard PG, Morris CJ, Blake DR. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull. 1993;49:506–522 [DOI] [PubMed] [Google Scholar]
- 27. Nakazawa T, Hisatomi T, Nakazawa C, et al. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci U S A 2007;104(7):2425–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31:151–174 [DOI] [PubMed] [Google Scholar]
- 29. Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zacks DN. Gene transcription profile of the detached retina (an AOS Thesis). Trans Am Ophthalmol Soc.. 2009;107:343–382 [PMC free article] [PubMed] [Google Scholar]
- 31. Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63–67 [DOI] [PubMed] [Google Scholar]
- 32. Hetts SW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–307 [DOI] [PubMed] [Google Scholar]
- 33. Nicolas MG, Fujiki K, Murayama K, et al. Studies on the mechanism of early onset macular degeneration in cynomolgus (Macaca fascicularis) monkeys. I. Abnormal concentrations of two proteins in the retina. Exp Eye Res. 1996;62:211–219 [DOI] [PubMed] [Google Scholar]
- 34. Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815 [DOI] [PubMed] [Google Scholar]
- 35. Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2006;103:11300–11305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun H, Nathans J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro: implications for retinal disease. J Biol Chem. 2001;276:11766–11774 [DOI] [PubMed] [Google Scholar]
- 37. El-Ghrably IA, Dua HS, Orr GM, Fischer D, Tighe PJ. Detection of cytokine mRNA production in infiltrating cells in proliferative vitreoretinopathy using reverse transcription polymerase chain reaction. Br J Ophthalmol. 1999;83:1296–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Limb GA, Daniels JT, Pleass R, Charteris DG, Luthert PJ, Khaw PT. Differential expression of matrix metalloproteinases 2 and 9 by glial Müller cells: response to soluble and extracellular matrix-bound tumor necrosis factor-alpha. Am J Pathol. 2002;160:1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abu el-Asrar AM, Van Damme J, Put W, et al. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol. 1997;123:599–606 [DOI] [PubMed] [Google Scholar]
- 40. Yoshimura T, Sonoda KH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zacks DN, Zheng QD, Han Y, Bakhru R, Miller JW. FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2004;45(12):4563–4569 [DOI] [PubMed] [Google Scholar]
- 42. Zacks DN, Boehlke C, Richards AL, Zheng QD. Role of the Fas-signaling pathway in photoreceptor neuroprotection. Arch Ophthalmol. 2007;125:1389–1395 [DOI] [PubMed] [Google Scholar]
- 43. Rajesh KG, Sasaguri S, Suzuki R, Maeda H. Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and upregulates Bcl-2 expression. Am J Physiol Heart Circ Physiol. 2003;285:H2171–H2178 [DOI] [PubMed] [Google Scholar]
- 44. Yasuoka N, Nakajima W, Ishida A, Takada G. Neuroprotection of edaravone on hypoxic-ischemic brain injury in neonatal rats. Brain Res Dev Brain Res. 2004;151:129–139 [DOI] [PubMed] [Google Scholar]
- 45. Xiao B, Bi FF, Hu YQ, et al. Edaravone neuroprotection effected by suppressing the gene expression of the Fas signal pathway following transient focal ischemia in rats. Neurotox Res. 2007;12:155–162 [DOI] [PubMed] [Google Scholar]
- 46. Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840 [DOI] [PubMed] [Google Scholar]
- 47. Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci U S A. 1995;92:4507–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen J, Flannery JG, LaVail MM, Steinberg RH, Xu J, Simon MI. bcl-2 overexpression reduces apoptotic photoreceptor cell death in three different retinal degenerations. Proc Natl Acad Sci U S A. 1996;93:7042–7047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bonfanti L, Strettoi E, Chierzi S, et al. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci. 1996;16:4186–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harper SJ, Wilkie N. MAPKs: new targets for neurodegeneration. Expert Opin Ther Targets. 2003;7:187–200 [DOI] [PubMed] [Google Scholar]
- 51. Luo Y, DeFranco DB. Opposing roles for ERK1/2 in neuronal oxidative toxicity: distinct mechanisms of ERK1/2 action at early versus late phases of oxidative stress. J Biol Chem. 2006;281:16436–16442 [DOI] [PubMed] [Google Scholar]
- 52. Stanciu M, Wang Y, Kentor R, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–12206 [DOI] [PubMed] [Google Scholar]
- 53. Glotin AL, Calipel A, Brossas JY, Faussat AM, Treton J, Mascarelli F. Sustained versus transient ERK1/2 signaling underlies the anti- and proapoptotic effects of oxidative stress in human RPE cells. Invest Ophthalmol Vis Sci. 2006;47:4614–4623 [DOI] [PubMed] [Google Scholar]
- 54. The Edaravone Acute Brain Infarction Study Group Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction: randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis.. 2003;15:222–229 [DOI] [PubMed] [Google Scholar]
- 55. Maeno T, Tano R, Takenaka H, Mano T. Edaravone (MCI-186) is effective as a free radical scavenger following arteriovenous sheathotomy for treatment of macular oedema associated with branch retinal vein occlusion. Br J Ophthalmol. 2009;93:1479–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hishida A. Clinical analysis of 207 patients who developed renal disorders during or after treatment with edaravone reported during post-marketing surveillance. Clin Exp Nephrol. 2007;11:292–296 [DOI] [PubMed] [Google Scholar]