In diabetes, increased accumulation of MMP2 in the retinal mitochondria degrades their membranes by modulating Hsp60 and damaging the gap junction protein connexin 43. Cytochrome c leaks out of the mitochondria and activates the apoptotic machinery, resulting in accelerated loss of retinal capillary cells.
Abstract
Purpose.
In the pathogenesis of diabetic retinopathy, retinal mitochondria become dysfunctional, their DNA is damaged, and capillary cells undergo accelerated apoptosis. Matrix metalloproteinase-2 (MMP2) becomes activated and proapoptotic, and the therapies that inhibit the development of diabetic retinopathy alleviate MMP2 activation. The authors sought to elucidate the possible mechanism by which activated MMP2 contributes to mitochondrial dysfunction.
Methods.
The effect of the regulation of MMP2 on mitochondrial dysfunction and the subcellular localization of the molecular chaperone important for mitochondrial integrity (Hsp60) and gap junction protein connexin 43 were investigated in retinal endothelial cells. The results were confirmed in retinal mitochondria isolated from diabetic mouse overexpressing MnSOD and in the retinas of normal rats that received intravitreal administration of MMP2.
Results.
High glucose increased MMP2 and decreased connexin 43 in the mitochondria of retinal endothelial cells. Although the Hsp60 gene transcript was increased, its abundance in the mitochondria was decreased, and its interaction with MMP2 was increased. In mice, the overexpression of MnSOD protected retinal mitochondria from diabetes-induced increases in MMP2 and decreases in Hsp60 and connexin 43. MMP2 administration in normal rats damaged the retinal mitochondria, decreased Hsp60 and connexin 43, and accelerated the apoptosis of retinal capillary cells.
Conclusions.
Elevated MMP2 in the mitochondria degrades its membranes by modulating Hsp60 and damaging connexin 43, and this activates the apoptotic machinery. Better understanding of MMP2-mediated mitochondrial damage could help identify new strategies for the treatment of this blinding disease.
Retinopathy, one of the most common microvascular complications of diabetes, is the leading cause of acquired blindness in young adults. Many biochemical and molecular mechanisms have been proposed to explain its development, but the exact molecular mechanism remains elusive. In the pathogenesis of diabetic retinopathy, retinal microvascular cells (pericytes and endothelial cells) and other cells, including glial cells and neuronal cells, are lost selectively through apoptosis.1–3 Retinal capillary cell apoptosis precedes the appearance of the microvascular histopathology characteristic of diabetic retinopathy,2,4,5 suggesting that accelerated apoptosis can account for the pericyte “dropout” and the formation of “ghosts.” Increased oxidative stress and inflammatory mediators are linked to this accelerated loss of capillary cells,6–8 but its mechanism is not clearly understood.
Retinal mitochondria become dysfunctional, superoxide levels are elevated, and their pore transition is significantly increased in diabetes.4,9–11 In mice, these mitochondrial abnormalities and the development of diabetic retinopathy can be prevented by the overexpression of manganese superoxide dismutase (MnSOD), suggesting a major role of mitochondria in the development of diabetic retinopathy.11,12 In addition, superoxide generated by mitochondria are considered to act as unifying molecules in regulating major pathways implicated in the development of diabetic retinopathy.13 However, how diabetes damages the mitochondria remains to be explored.
Matrix metalloproteinase (MMP), a member of proteinase family, regulates major biological functions, including tissue repair and cell signaling. Among the MMPs, MMP2 is the most ubiquitous.14 Our recent studies have shown that the activation of MMP2 in diabetes has a proapoptotic role in the loss of retinal capillary cells.11,15 High glucose–induced MMP2 activation damages mitochondria, which can be attenuated by the inhibition of MMP2 activation.11 However, there is little information regarding the crosstalk between MMP2 activation and mitochondrial dysfunction.
Heat shock proteins (Hsps) are considered to maintain cell homeostasis against oxidative stress,16 and, in the early stages of diabetes in rodents, the expression of these proteins in the retina is altered.17 Hsp60, a member of the Hsp family, is localized primarily in the mitochondria and helps to maintain mitochondrial function and integrity by acting as a chaperone for the transport of proteins into the mitochondria.18,19 Its regulation in the tumor cell is shown to result in the loss of mitochondria membrane potential and in the activation of caspase-dependent apoptotic machinery.20 How alterations in mitochondrial Hsp60 contribute to the development of diabetic retinopathy is not clearly understood.
The present study was designed to elucidate the plausible mechanism by which MMP2 contributes to the mitochondrial dysfunction of retinal capillary cells, leading to their accelerated loss. Using isolated retinal endothelial cells in culture, we investigated the effect of the regulation of the MMP2 gene on mitochondrial dysfunction and subcellular localization of Hsp60 and the role of connexin 43, a gap junction protein, in the mitochondrial damage. The in vitro results are confirmed in retinal mitochondria from the diabetic mouse, in which mitochondrial dysfunction and the development of retinopathy are protected by the overexpression of MnSOD. To further confirm the relationship among MMP2, Hsp60, and connexin 43, the effects of the administration of MMP2 in the vitreous of normal rats on retinal mitochondrial Hsp60, connexin 43, and retinal capillary cell apoptosis are investigated.
Materials and Methods
Retinal Endothelial Cells
Bovine retina endothelial cells (BRECs) were prepared and were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 15% fetal calf serum (heat inactivated), 5% replacement serum (Nu-serum; BD Biosciences, San Jose, CA), heparin (50 μg/mL), endothelial growth supplement (25 μg/mL), and antibiotic/antimycotic in an environment of 95% O2 and 5% CO2 in Petri dishes coated with 0.1% gelatin.4
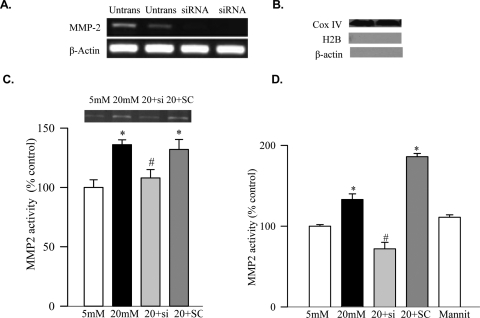
Endothelial cells from passages 3 to 5 were transfected with MMP2 siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) using the methods routinely used in our laboratory.11 Briefly, siRNA duplex (0.25–1 μg) was diluted into 100 μL siRNA transfection medium; the cells were washed and incubated with the transfection complex (consisting of the siRNA and transfection reagent) for 8 hours at 37°C. Parallel incubations were carried out using nontargeting scrambled siRNA. At the end of the incubation, the medium containing transfection complex was replaced with fresh incubation medium that was supplemented with 20 mM glucose or 5 mM glucose. Cells were incubated for 4 additional days, and the incubation medium was replaced with fresh medium every 48 hours. Transfection efficiency was evaluated by quantifying the gene expression of MMP-2, and, as shown in Figure 1A, the gene expression of MMP-2 was silenced in the cells transfected with siRNA. Cells incubated in 20 mM mannitol, instead of glucose, served as osmolar control.
Figure 1.
Effect of high glucose on mitochondrial MMP2 and its regulation in retinal endothelial cells. BRECs were transfected with MMP2-siRNA (si) or scrambled siRNA (SC) and were incubated in 5 mM or 20 mM glucose for 4 days. (A) To determine transfection efficiency, gene expression of MMP2 was quantified by semiquantitative PCR. Shown is an agarose gel image from two sets of normal (untrans) and MMP2-si–transfected (siRNA) cells. Mitochondria were prepared from the MMP2-siRNA transfected and untransfected cells incubated in normal or high glucose. (B) To evaluate the purity of mitochondria, protein expressions of Cox IV, H2B, and β-actin were quantified, and the Western blot is from two sets of normal untransfected cells. The activity of MMP2 was quantified in the mitochondrial fraction by (C) in situ zymography and (D) ELISA. Each measurement was made in duplicate in three to four different cell preparations, and the values obtained from the untransfected cells incubated in 5 mM glucose are considered as 100%. 5 mM, 5 mM glucose, 20 mM, 20 mM glucose; 20+si, cells transfected with MMP2-siRNA followed by 20 mM glucose treatment; 20+SC, cells transfected with scrambled RNA and treated with 20 mM glucose; mannit, 20 mM mannitol. *P < 0.05 compared with the values obtained from the cells incubated in 5 mM glucose or 20 mM mannitol. #P < 0.05 compared with untransfected or scrambled RNA-transfected cells treated with 20 mM glucose.
Mice
Hemizygous MnSOD transgenic mice with a C57BL/6 background, developed using human β-actin-MnSOD expression construct 2, were generated by microinjecting fertilized eggs harvested from female B6C3 (C57BL/6 × C3H) F1 hybrid mice mated with male B6C3 F1 mice. The hemizygous MnSOD-Tg mice were bred for 7 to 8 generations with wild-type C57BL/6 mice to generate experimental animals, and the litters were genotyped by Southern blot analysis. A group of MnSOD-overexpressing mice (Tg) and their wild-type (WT) littermates (8–10 weeks old) were made diabetic by streptozotocin injection (55 mg/kg) for 5 consecutive days.12 The mice, with blood glucose levels 250 mg/dL or higher 3 days after the last injection, were considered as diabetic. These mice are routinely used in our laboratory.11,12,15 Approximately 6 months after the induction of diabetes, they were killed by overdose of pentobarbital (120 mg/kg), and the retinas were immediately isolated under a dissecting microscope and stored in liquid nitrogen. Treatment of the animals conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Intravitreal Injection of MMP2
Wistar rats (weight range, 220–225 g) were kept under deep anesthesia, and a sterilized solution of recombinant active MMP2 (5 ng/5 μL; Enzo Life Sciences International, Inc., Plymouth Meeting, PA) was injected into the vitreous of the right eye, as previously described by us.21 For the control, the left eye received 5 μL sterile PBS. The animals were killed 4 days after intravitreal administration, and either the eyes were suspended in 10% formalin to prepare retinal microvasculature (trypsin digestion) or the retina was carefully dissected to prepare the mitochondria as described. The animals were used 4 days after the intravitreal administration of MMP2 because at 4 days after intravitreal injection, erythropoietin has been shown to normalize the upregulation of proapoptotic and proinflammatory genes in the diabetic rat retina.22
Isolation of Mitochondria and Cytosol
Mitochondria were isolated using a mitochondria isolation kit (Pierce, Rockford, IL) according to the manufacturer's instruction.12 Briefly, retina or BRECs were homogenized in reagent A solution using 10 to 12 strokes of the Dounce homogenizer. An equal volume of reagent C was added to the homogenate, mixed by inverting, and was centrifuged at 700g for 10 minutes at 4°C. The supernatant was centrifuged at 12,000g for 15 minutes, and the pellet (mitochondria fraction) was washed with PBS by centrifuging at 12,000g for 5 minutes. The resultant mitochondrial pellet was suspended in mitochondria lysis buffer (2% CHAPS in 25 mM Tris, 0.15 M NaCl, pH 7.2), and the supernatant was centrifuged at 105,000g for 90 minutes to obtain the cytosolic fraction. Protein concentrations were determined by the bicinchoninic acid assay (Sigma-Aldrich, St. Louis, MO). Nuclear contamination in the mitochondria fraction was determined by quantifying the expression histone H2B23 and cytosolic contamination by measuring two proteins that are found largely in the cytosol, β-actin24 and glyceraldehyde dehydrogenase (GAPDH). Figure 1B shows that the mitochondria fraction was devoid of nuclear and cytosolic contamination, as evidenced by the expressions of H2B and β-actin, and the activity of GAPDH23 in the mitochondria was undetectable.
Activity of MMP2
For quantifying the gelatinase activity of MMP2, mitochondrial protein was analyzed by electrophoresis on SDS-polyacrylamide gels containing 1 mg/mL gelatin. Samples were loaded on the gels without denaturation in the presence of reducing agents. The gels were washed with 2.5% Triton X-100 and stained with 2% (vol/vol) Coomassie blue G-250. After destaining, the bands were photographed on a light box.11
The activity of MMP2 was also quantified with an ELISA kit (Matrix Metalloproteinase-2 Biotrak Activity Assay System; Amersham, Buckinghamshire, UK) according to the manufacturer's instructions. Briefly, MMP2 standards (0.5–4 ng) or 25 μg mitochondria were incubated overnight at 4°C in wells precoated with anti-MMP2. Active MMP2 was detected (without p-aminophenylmercuric acetate) using a specific peptide chromogen medium by reading the resulting color at 405 nm.
Protein Expression
The protein expression of Hsp60, connexin 43, Bax, Bcl-xl, and cytochrome c were determined by the Western blot analysis. Mitochondrial or cytosolic protein (25–30 μg) was separated on a 4% to 20% SDS-PAGE. After transferring the proteins onto nitrocellulose membrane, the membranes were blocked in 5% nonfat milk and incubated with the antibody against the target protein (obtained from Santa Cruz Biotechnology). The target protein was enhanced with ECL reagent and determined by autoradiography. Membranes were reprobed with Cox IV or β-actin to evaluate the lane-loading control for mitochondria or cytosol, respectively. Band intensities were quantified by graph digitizing software (Un-Scan-It; Silk Scientific, Orem, UT), as reported previously.11,12
Coimmunoprecipitation
BRECs, incubated in 5 mM or 20 mM glucose for 4 days, were rinsed with cold PBS and homogenized in the lysis buffer containing 30 mM Tris-HCl buffer pH containing 10 mM EGTA, 5 mM EDTA, 1% Triton X-100, 250 mM sucrose, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and 1 mM Na3VO4. Protein (l20 μg) was incubated overnight at 4°C with 2 μg of Hsp6O or MMP2 antibody, followed by incubation of the protein-antibody complex for 1 hour at 4°C with A/G agarose beads (Santa Cruz Biotechnology) that had been prewashed with the lysis buffer.23 The beads were then boiled in SDS sample loading buffer, and MMP2 and connexin 43, respectively, were detected by Western blot analysis.
Gene Expression
Gene expression of Hsp60 was determined using conventional semiquantitative PCR. cDNA template (0.5 μL) was added to 1 U DNA polymerase (GoTaq; Promega, Madison, WI), and 10 pmol of forward and reverse primers (BRECs HSP60: forward, 5′-TGGTCTTCAAGTTGTGGCAG-3′; reverse, 5′- CTTTCAAGAGCATGGCATCA-3′; Mouse HSP60: forward, 5′-TATTGAACAGAGTTGGGGAAAAGTCC-3′; reverse, 5′-GCTCATCATTCAGGGTTTTTCCATC-3′). β-Actin was used as an internal standard. The standard PCR conditions included 2 minutes at 95°C followed by 30 to 35 cycles of denaturation at 95°C for 1 minute, annealing at 50° to 55°C for 2 minutes, and extension at 72°C for 1 minute. After completing the amplification, the PCR products were analyzed on a 1.2% agarose gel, and a 100-bp DNA ladder was used as a marker. The bands were visualized with imaging software (Bio-Doc it Imaging System; UVP LLC, Upland, CA), and band intensities were quantified by graph digitizing software (Un-Scan-It; Silk Scientific).
Apoptosis and Histopathology in Retinal Microvessels
Retinal microvessels were isolated by incubating the formalin-fixed retina in 3% crude trypsin solution (prepared in Tris-HCl buffer, pH 7.8) containing 0.2 M sodium fluoride. Detection of apoptotic vascular cells was performed by terminal deoxyribonucleotide transferase (TdT)-mediated dUTP nick end labeling (TUNEL; In Situ Cell Death kit; Roche Molecular Biochemicals, Indianapolis, IN). After TUNEL staining, the microvessels were stained with periodic acid-Schiff–hematoxylin and examined by light microscopy.7,12
Statistical Analysis
Data are reported as the mean ± SD, and experimental groups were compared using the nonparametric Kruskal-Wallis test followed by the Mann-Whitney test for multiple-group comparison. Similar conclusions were reached by using ANOVA with the Fisher or Tukey test.
Results
Retinal Endothelial Cells
High Glucose Activates Mitochondrial MMP2.
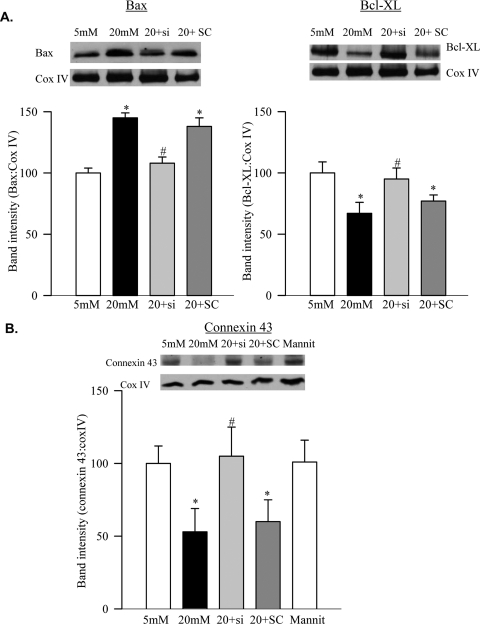
Gelatinase activity of MMP2 was increased by 35% in mitochondria obtained from the cells incubated in high glucose (Fig. 1C), and this was confirmed by ELISA (Fig. 1D). In addition, the cells exposed to high glucose had damaged mitochondrial integrity, as evidenced by a 45% increase in Bax, a 30% decrease in Bcl-XL, and a 50% decrease in connexin 43 in their mitochondria (Figs. 2A, 2B).
Figure 2.
Effect of MMP2-siRNA on mitochondrial function. Mitochondrial content of (A) Bax and Bcl-XL and (B) connexin 43 was quantified by Western blot analysis using Cox IV as a loading protein. Each measurement was performed in at least three different cell preparations. The values, represented as mean ± SD, obtained from the cells incubated in 5 mM glucose are considered as 100%. *P < 0.05 compared with the values obtained from the cells incubated in 5 mM glucose or 20 mM mannitol. #P < 0.05 compared with the cells untransfected or transfected with scrambled RNA and incubated with 20 mM glucose.
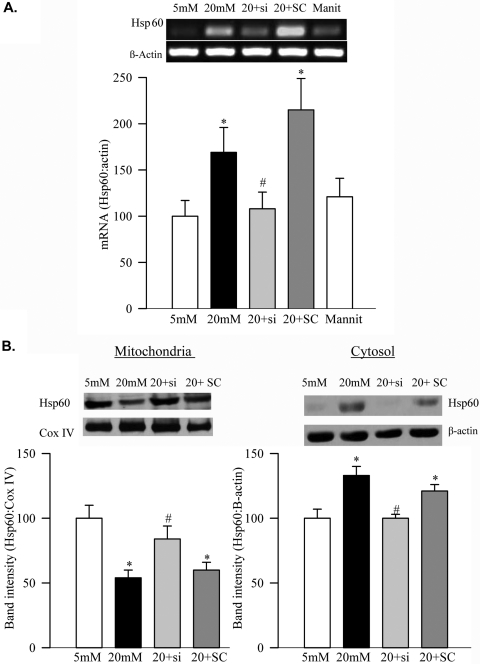
Exposure of retinal endothelial cells to high glucose significantly increased the gene expression of Hsp60 (Fig. 3A), but its protein expression was decreased in the mitochondria and increased in the cytosol fraction (Fig. 3B).
Figure 3.
Effect of high glucose on Hsp60 and its regulation by MMP2. (A) Gene expression of Hsp60 was quantified by semiquantitative PCR and was adjusted to the mRNA levels of β-actin in each sample. (B) Mitochondrial and cytosolic fractions were prepared by differential centrifugation. Hsp60 was quantified by Western blot analysis, and the band intensity of Hsp60 was adjusted to the expression of the Cox IV and β-actin (mitochondrial and cytosolic fractions, respectively). Western blots are representative of three different experiments. *P < 0.05 compared with the values obtained from the cells incubated in 5 mM glucose or 20 mM mannitol. #P < 0.05 compared with values obtained from the cells untransfected or transfected with scrambled RNA and incubated with 20 mM glucose.
Regulation of Mitochondrial Dysfunction by MMP2.
To determine the effect of MMP2 on mitochondrial damage, the MMP2 gene was manipulated by MMP2 siRNA. The transfection of BRECs with MMP2-siRNA prevented the glucose-induced activation of mitochondrial MMP2 (Figs. 1C, 1D) and also protected mitochondrial integrity (Figs. 2A, 2B). This was accompanied by the amelioration of glucose-induced decreased accumulation of connexin 43 and Hsp60 in the mitochondria (Figs. 2B, 3B). Values obtained from the cells incubated in high glucose, but transfected with MMP2 siRNA or with scrambled RNA, were significantly different (P < 0.05) from each other.
Interactions of MMP2 with Hsp60 and with Connexin 43.
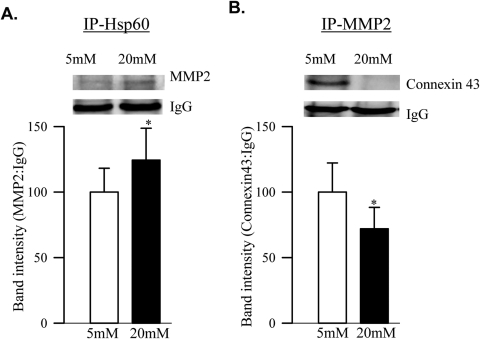
Coimmunoprecipitation studies were performed on BRECs incubated in 5 mM glucose or 20 mM glucose for 4 days. Interaction of MMP2 with Hsp60 was significantly increased in the cells exposed to high glucose (Fig. 4A), but interaction with connexin 43 was decreased (Fig. 4B) compared with the values obtained from cells incubated in normal glucose.
Figure 4.
Immunoprecipitation of Hsp60 and MMP2. BRECs, incubated in 5 mM or 20 mM glucose for 4 days, were immunoprecipitated with anti-Hsp60 or anti-MMP2. Relative abundance of MMP2 in the Hsp60 immunoprecipitates (A) and that of connexin43 in MMP2 immunoprecipitates (B) were determined by Western blot analysis using IgG as the loading control. Results are expressed as mean ± SD of three or more experiments. Values obtained from cells incubated in 5 mM glucose are considered as 100%.
Retinas of Diabetic Mice
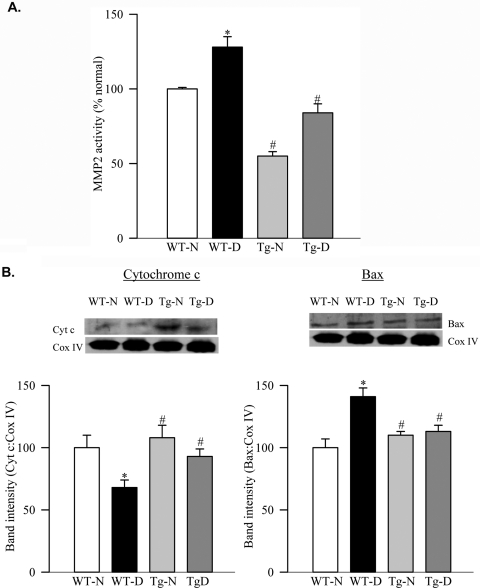
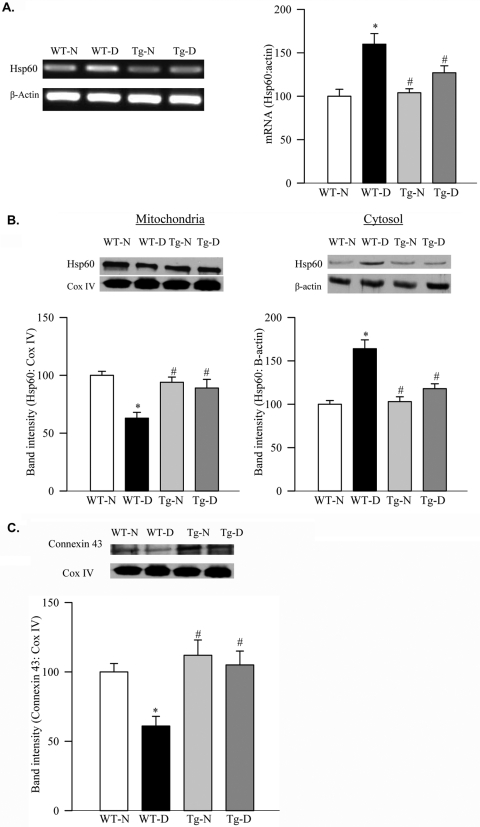
Six months of diabetes in mice increased MMP2 activity in the retinal mitochondria by approximately 25% (Fig. 5A). The integrity of mitochondria was compromised as evidenced by decreased mitochondrial cytochrome c and increased Bax expression (Fig. 5B). In the same retina, although Hsp60 gene expression was increased by 60% (Fig. 6A), its abundance in the mitochondria was decreased with the concomitant increase in its cytosolic levels (Fig. 6B). This was accompanied by the decreased expression of connexin 43 in the retinal mitochondria (Fig. 6C).
Figure 5.
Effect of MnSOD overexpression on mitochondrial MMP2 and dysfunction in the mouse retina. (A) MMP2 was quantified by ELISA using 20 to 25 μg retinal mitochondria obtained from MnSOD-Tg mice kept diabetic for 6 months. (B) Cytochrome c and Bax contents were determined by Western blot analysis in the retinal mitochondria, and the band intensities were adjusted to the expression of the Cox IV in each lane. Measurements were made in duplicate in five to seven mice in each group. Western blots are representative of three different experiments. Results are expressed as mean ± SD. Values obtained from WT nondiabetic mice are considered as 100%. WT-N, wild-type nondiabetic mice; WT-D, wild-type diabetic mice; Tg-N, MnSOD transgenic nondiabetic mice; Tg-D, MnSOD transgenic diabetic mice. *P < 0.05 compared with WT nondiabetic mice. #P < 0.05 compared with WT diabetic mice.
Figure 6.
Effect of MnSOD overexpression on retinal Hsp60 and connexin 43. (A) Gene expression of Hsp60 was quantified in the retina by semiquantitative PCR using β-actin as an internal control. Hsp60 (B) and connexin 43 (C) abundance were determined by Western blot in freshly prepared mitochondrial and cytosolic fractions using Cox IV (for mitochondria) and β-actin (cytosol) as loading controls. The Western blots are representative of three different experiments. Values obtained from WT nondiabetic mice are considered as 100%. *P < 0.05 compared with the values obtained from the samples obtained from WT normal mouse retina. #P < 0.05 compared with those obtained from WT diabetic mice.
The overexpression of MnSOD prevented a diabetes-induced increase in mitochondrial MMP2 in the retina; values obtained from WT-diabetic mice and Tg-diabetic mice were significantly different from each other (P < 0.05; Fig. 5A). In the same animals, overexpression of MnSOD also protected retinal mitochondrial dysfunction; the mitochondrial expressions of cytochrome c, Bax, Hsp60 and connexin 43 were not different from those obtained from WT-normal or Tg-normal mice (Figs. 5B, 6B, 6C).
Intravitreal Administration of MMP2 in Rat
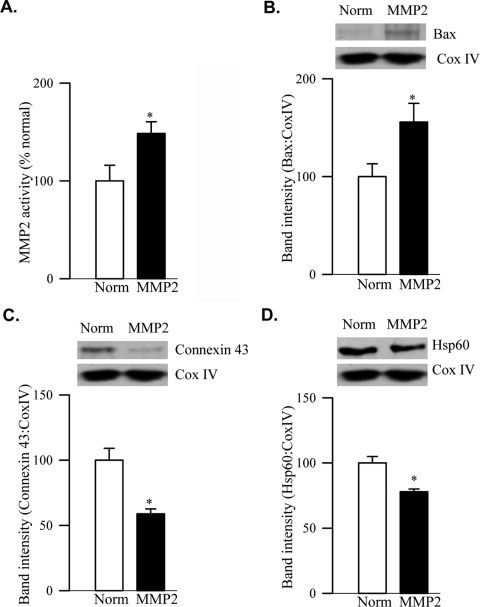
The effect of MMP2 on retinal mitochondrial HSP60, connexin 43, and vascular apoptosis was determined by administering MMP2 in the vitreous of normal rats. As shown in Figure 7A, MMP2 activity was increased in the retinal mitochondria by approximately 50% compared with the values obtained from the collateral eye that received PBS alone. In the same mitochondria fraction, MMP2 injection resulted in a 55% increase in Bax expression, a 40% decrease in connexin 43, and a 30% decrease in Hsp60 expression (Figs. 7B–D).
Figure 7.
Effect of MMP2 administration on mitochondrial dysfunction, Hsp60, and connexin 43. Retinal mitochondria, prepared from normal rats that received intravitreal injections of MMP2 or PBS, was analyzed for (A) MMP2 activity (ELISA) (B) dysfunction by quantifying the Bax content, and (D) Hsp60 and (C) connexin 43 abundance. Western blot analyses are representative of three different experiments. Norm and MMP2 represent retinas of rats that received intravitreal injections of PBS and MMP2 respectively. *P < 0.05 compared with the values obtained from retinal mitochondria of PBS-injected eyes.
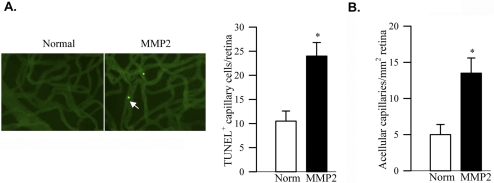
To directly relate MMP2 to the capillary cell apoptosis and histopathology characteristic of diabetic retinopathy, TUNEL staining of retinal trypsin digests showed that the administration of MMP2 in the vitreous of the normal rat increased the number of TUNEL-positive cells by 2.5-fold (Fig. 8A). In the same retinal microvasculature, the number of acellular capillaries was also increased by more than 2.5-fold (Figs. 8A, 8B) compared with the values obtained from the PBS-injected eye.
Figure 8.
Effect of MMP2 on rat retinal vascular apoptosis and histopathology. (A) TUNEL-positive capillary cells were counted in the retinal microvasculature prepared by trypsin-digestion. (B) The same microvasculature was stained with periodic acid-Schiff-hematoxylin to count acellular capillaries. Basement membrane tubes lacked cell nuclei and maintained at least one-fourth the normal capillary caliber over their lengths. Arrow: TUNEL-positive capillary cell.
Discussion
MMP2 has a proapoptotic role in the loss of retinal capillary cells in diabetes, and the inhibition of MMP2 ameliorates mitochondrial damage.11,15 Now we present data to show that retinal mitochondria are the target of the diabetes-induced increase in MMP2 and provide mechanistic details by which activated MMP2 damages mitochondria. Our results demonstrate that hyperglycemia activates MMP2, resulting in decreased Hsp60 in the mitochondria, decreased connexin 43, and damaged mitochondrial transport pores. Bax translocates into the mitochondria, and cytochrome c leaks out, activating the apoptosis machinery. This is confirmed by our coimmunoprecipitation data demonstrating that glucose induces increased interactions between Hsp60 and MMP2 but decreased interactions between MMP2 and connexin 43. The regulation of MMP2 by its genetic manipulation protects mitochondrial integrity and prevents apoptosis. In support of these in vitro data, our in vivo results show that the regulation of mitochondrial integrity by the overexpression of MnSOD protects the diabetes-induced activation of MMP2 in the retinal mitochondria. This is accompanied by amelioration in the reduced levels of Hsp60 and connexin 43, and the leakage of cytochrome c from the mitochondria is prevented. Furthermore, our exciting data show that the administration of MMP2 in the vitreous of normal rats damages the retinal mitochondria and increases the apoptosis of retinal capillary cells and the formation of acellular capillaries, directly supporting the role of MMP2 in mitochondrial damage by Hsp60 and connexin 43.
Mitochondria are the principal endogenous source of superoxide, and increased superoxide accumulation in diabetes initiates a cascade of damaging events through the production of additional superoxide and other damaging radicals.9,12 Mitochondrial dysfunction of the retinal capillary cells is shown to play a major role in the pathogenesis of diabetic retinopathy by activation of the apoptotic pathway.10 Oxidative stress is one of the major activators of mitochondrial MMPs.25 Here, in an extension of our recent study showing a proapoptotic role for MMP2 in the loss of retinal capillary cells in diabetes,11,15 we show for the first time that hyperglycemia activates MMP2 in the mitochondria, damaging their integrity, and that the process is mediated by the regulation of Hsp60 and connexin 43. This is also confirmed by our in vivo experiment; the intravitreal injection of MMP2 to a normal rat increases MMP2 activity and induces the modulation of Hsp60 and connexin43 content in the mitochondria.
Retinal cells, including capillary cells and nonvascular cells, undergo accelerated apoptosis in diabetes, and this precedes the histopathology characteristic of diabetic retinopathy.1,3 Given that retinopathy is a slowly progressing complication of diabetes and that apoptosis is a rapidly consummated phenomenon,26 even a small number of apoptotic capillary cells observed in the diabetic retina2,4 can have a major impact on the formation of acellular capillaries and pericyte ghosts. Mitochondrial dysfunction is considered one of the possible mechanisms responsible for the accelerated apoptosis of capillary cells.4,10 Increased superoxide in the retina and its capillary cells damages mitochondrial membranes,11,12,27 the membranes lose their potential, Bax translocates to the mitochondria, and cytochrome c leaks out.4,12,28 Here, our results from MnSOD-Tg mice confirm that the damage to the mitochondria occurs possibly by way of the superoxide-mediated activation of mitochondrial MMP2, which initiates a cascade of events resulting in apoptosis. In support, others have shown that the activation of MMP2 in cardiomyocytes damages mitochondria and releases cytochrome c into the cytosol.29
Heat-shock proteins, a family of highly conserved proteins, function as intracellular molecular chaperones of newly synthesized polypeptide chains by helping them translocate across subcellular membranes to their appropriate cellular compartments. These proteins also reduce cellular stress by clearing the damaged proteins.30,31 Hsp60 is mainly a mitochondrial protein important in facilitating protein folding and maintaining mitochondrial integrity, and in stress state, it accumulates in the cytosol.20,32 Depending on the cell type and insult, Hsp60 can act as a proapoptotic or a prosurvival protein.33–35 Reduced expression of Hsp60 by an antisense oligonucleotide is shown to release cytochrome c into the mitochondria and to induce apoptosis.36 Because the apoptosis of retinal capillary cells is an early predictor of histopathology characteristic of diabetic retinopathy, the inhibition of Hsp60 translocation from the mitochondria to the cytosol by MMP2-siRNA implies that Hsp60 is one of the mediators by which mitochondrial MMP2 contributes to apoptosis. This is also confirmed by our coimmunoprecipitation results showing increased interactions between Hsp60 and MMP2 in high-glucose conditions. Hsp60 can also interact with another member of the heat shock protein family, Hsp7037; this is implicated in a conformational change in Bax and in the regulation of cytochrome c release from the mitochondria.38 Diabetes increases Bax expression in the retinal mitochondria.4,11 Here we show that the translocation of Bax and cytochrome c across the mitochondria is under the control of MMP2-Hsp60 interactions, further confirming the role of Hsp60 in mitochondrial damage. Our results are consistent with those reported by others showing increased gene expression of Hsp60 in the retina 7 days after the induction of diabetes in rodents.17 However, the data presented here clearly demonstrate that, despite the increased expression of retinal Hsp60 in diabetes, its mitochondrial accumulation is significantly decreased.
Connexin 43, a member of the Gap junction protein that helps in cell-cell communication and transfer of ions, is shown to be downregulated in the retina in diabetes, and this downregulation is considered an early trigger in the apoptosis of retinal capillary cells.39 In cardiac tissue, mitochondrial connexin 43 is shown to regulate mitochondrial physiology and myocyte apoptosis.40 Our data show that high glucose decreases connexin 43 expression in the mitochondria, which can be prevented by the regulation of MMP2. Thus, it is plausible that MMP2 activation induces the loss of mitochondrial potential by modulating the abundance of Hsp60. This opens up mitochondrial transition pores by disrupting connexin 43. The apoptotic machinery is activated, and the cells start to undergo accelerated apoptosis. In support, high-glucose exposure, in addition to increasing interactions between Hsp60 and MMP2, decreases interactions between MMP2 and connexin 43. This is further strengthened by our results from the injection of MMP2 in the vitreous of normal rats; MMP2 administration damages retinal mitochondria and decreases Hsp60 and connexin 43 in the mitochondria, accompanied by increased capillary cell apoptosis and acellular capillaries, a hallmark of diabetic retinopathy.
Diabetes-induced retinal mitochondrial dysfunction and retinopathy development are prevented by the overexpression of superoxide dismutase.12 Overexpression of MnSOD in mice, in addition to protecting the activation of mitochondrial MMP2, also regulates Hsp60 and connexin 43 and strongly suggests that the activation of mitochondrial MMP2 has a major role in the development of diabetic retinopathy by accelerating capillary cell apoptosis.
In summary, we have provided a mechanism by which activated MMP2 in the mitochondria results in the accelerated apoptosis of retinal capillary cells in diabetes. The activation of mitochondrial MMP2 damages the retinal mitochondria by modulating Hsp60 and connexin 43. This allows cytochrome c to leak out and activate the apoptotic machinery. Better understanding of MMP-2–mediated mitochondria damage pathways will help identify novel targets for the treatment of this blinding disease.
Acknowledgments
The authors thank Yakov Shamailov and Doug Putt for technical assistance.
Footnotes
Supported in part by grants from the National Institutes of Health, the Juvenile Diabetes Research Foundation, the Thomas Foundation, and Research to Prevent Blindness.
Disclosure: G. Mohammad, None; R.A. Kowluru, None
References
- 1. Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kern TS, Tang J, Mizutani M, Kowluru R, Nagraj R, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978 [PubMed] [Google Scholar]
- 3. Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J Clin Invest. 1998;102:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Inves Ophthalmol Vis Sci. 2003;44:5327–5334 [DOI] [PubMed] [Google Scholar]
- 5. Kowluru RA, Abbas SN, Odenbach S. Effect of re-institution of good metabolic control on oxidative stress in the kidney of diabetic rats. J Diabetes Complications. 2004;18:282–288 [DOI] [PubMed] [Google Scholar]
- 6. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 7. Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia, VII: effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942 [DOI] [PubMed] [Google Scholar]
- 8. Kowluru RA. Diabetic retinopathy, oxidative stress and antioxidants. Curr Topics Nutrac Res. 2005;3:209–218 [Google Scholar]
- 9. Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Rad Biol Med. 2003;35:1491–1499 [DOI] [PubMed] [Google Scholar]
- 10. Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxidants Redox Signaling. 2005;7:1581–1587 [DOI] [PubMed] [Google Scholar]
- 11. Mohammad G, Kowluru RA. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab Invest. 2010;90:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811 [DOI] [PubMed] [Google Scholar]
- 13. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 14. Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701 [DOI] [PubMed] [Google Scholar]
- 15. Kowluru RA, Kanwar M. Oxidative stress and the development of diabetic retinopathy: contributory role of matrix metalloproteinase-2. Free Rad Biol Med. 2009;46:1677–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677 [DOI] [PubMed] [Google Scholar]
- 17. Joussen AM, Huang S, Poulaki V, et al. In vivo retinal gene expression in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:3047–3057 [PubMed] [Google Scholar]
- 18. Motoyama S, Saito S, Itoh H, et al. Methylprednisolone-induced expression of mitochondrial heat shock protein 60 protects mitochondrial membrane potential in the hypoxic rat liver. Shock. 2004;22:234–239 [DOI] [PubMed] [Google Scholar]
- 19. Richardson A, Landry SJ, Georgopoulos C. The ins and outs of a molecular chaperone machine. Trends Biochem Sci. 1998;23:138–143 [DOI] [PubMed] [Google Scholar]
- 20. Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J Biol Chem. 2008;283:5188–5194 [DOI] [PubMed] [Google Scholar]
- 21. Kowluru RA, Odenbach S. Role of interleukin-1β in the development of diabetic retinopathy in rats: effects of antioxidants. Inves Ophthal Vis Sci. 2004;45:4161–4166 [DOI] [PubMed] [Google Scholar]
- 22. Chu Q, Zhang J, Wu Y, et al. Differential gene expression pattern of diabetic rat retina after intravitreal injection of erythropoietin. Clin Exp Ophthalmol. 2011;39:142–151 [DOI] [PubMed] [Google Scholar]
- 23. Kanwar M, Kowluru R. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes. 2009;58:227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marabese M, Mazzoletti M, Vikhanskaya F, Broggini M. HtrA2 enhances the apoptotic functions of p73 on bax. Cell Death Differ. 2008;15:849–858 [DOI] [PubMed] [Google Scholar]
- 25. Moshal KS, Metreveli N, Frank I, Tyagi SC. Mitochondrial MMP activation, dysfunction and arrhythmogenesis in hyperhomocysteinemia. Curr Vasc Pharmacol. 2008;2:84–92 [DOI] [PubMed] [Google Scholar]
- 26. Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700 [DOI] [PubMed] [Google Scholar]
- 27. Xie L, Zhu X, Hu Y, et al. Mitochondrial DNA oxidative damage triggering mitochondrial dysfunction and apoptosis in high glucose-induced HRECs. Invest Ophthalmol Vis Sci. 2008;49:4203–4209 [DOI] [PubMed] [Google Scholar]
- 28. Oshitari T, Yamamoto S, Hata N, Roy S. Mitochondria and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy. Br J Ophthalmol. 2008;92:552–556 [DOI] [PubMed] [Google Scholar]
- 29. Menon B, Singh M, Ross RS, Johnson JN, Singh K. beta-Adrenergic receptor-stimulated apoptosis in adult cardiac myocytes involves MMP-2-mediated disruption of beta1 integrin signaling and mitochondrial pathway. Am J Physiol Cell Physiol. 2006;290:C254–C261 [DOI] [PubMed] [Google Scholar]
- 30. Udelsman R, Blake MJ, Stagg CA, Li DG, Putney DJ, Holbrook NJ. Vascular heat shock protein expression in response to stress: endocrine and autonomic regulation of this age-dependent response. J Clin Invest. 1993;91:465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rossi MR, Somji S, Garrett SH, Sens MA, Nath J, Sens DA. Expression of hsp 27, hsp 60, hsc 70, and hsp 70 stress response genes in cultured human urothelial cells (UROtsa) exposed to lethal and sublethal concentrations of sodium arsenite. Environ Health Perspect. 2002;110:1225–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lau S, Patnaik N, Sayen MR, Mestril R. Simultaneous overexpression of two stress proteins in rat cardiomyocytes and myogenic cells confers protection against ischemia-induced injury. Circulation. 1997;96:2287–2294 [DOI] [PubMed] [Google Scholar]
- 33. Arya R, Mallik M, Lakhotia SC. Heat shock genes—integrating cell survival and death. J Biosci. 2007;32:595–610 [DOI] [PubMed] [Google Scholar]
- 34. Chandra D, Choy G, Tang DG. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J Biol Chem. 2007;26:31289–31301 [DOI] [PubMed] [Google Scholar]
- 35. Xanthoudakis S, Roy S, Rasper D, et al. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999;18:2049–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He L, Lemasters JJ. Heat shock suppresses the permeability transition in rat liver mitochondria. J Biol Chem. 2003;279:16755–16760 [DOI] [PubMed] [Google Scholar]
- 37. Cappello F, Conway de Macario E, Marasa L, Zummo G, Macario AJL. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther. 2008;7:801–809 [DOI] [PubMed] [Google Scholar]
- 38. Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739 [DOI] [PubMed] [Google Scholar]
- 39. Bobbie MW, Roy S, Trudeau KM, Munger SJ, Simon A, Roy S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest Ophthalmol Vis Sci. 2010;51:3758–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goubaeva F, Mikam M, Giardina S, Ding B, Abe J, Yang J. Cardiac mitochondrial connexin 43 regulates apoptosis. Biochem Biophys Res Commun. 2007;352:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]