Sonoporation, a technique by which ultrasound is combined with diagnostic microbubbles to enhance the intracellular uptake of drugs, is shown to enhance the delivery, and thus the efficacy, of chemotherapy against retinoblastoma cells in vitro.
Abstract
Purpose.
To study the ability of ultrasound (US) and microbubbles (MB) to enhance chemotherapeutic efficacy against retinoblastoma Y79 cells in vitro.
Methods.
The experiment was performed in three stages. The authors first compared cell viability of Y79 cells exposed to doxorubicin versus cells exposed to doxorubicin combined with low-intensity, low-frequency US + MB. They then evaluated enhanced cell permeability by studying the intensity of intracellular fluorescence in cells exposed to doxorubicin versus those exposed to doxorubicin with US + MB. Lastly they evaluated the morphologic characteristics of the cells by scanning electron microscopy (SEM) to identify the presence of pores.
Results.
The Y79 cells exposed to doxorubicin with US + MB showed a significant decrease in cell viability at 72 hours compared with those exposed to doxorubicin alone (P = 0.02). Cells also showed immediate increased permeability to doxorubicin with the addition of US + MB compared with doxorubicin alone, which continued to increase over 60 minutes. SEM did not demonstrate physical pores at the lowest US + MB intensity shown to enhance intracellular doxorubicin fluorescence.
Conclusions.
US + MB facilitates the uptake of chemotherapy in retinoblastoma Y79 cells in vitro. This occurs in the absence of visible pores, suggesting a possible secondary mechanism for increased drug delivery. This experiment is the first step toward enhancing chemotherapy with sonoporation in the treatment of intraocular tumors. This technique may lead to more effective chemotherapy treatments with less collateral damage to ocular tissues and may allow reduced systemic dosage and systemic side effects.
Retinoblastoma is considered a curable cancer in the developed world, yet it can cause significant morbidity and, rarely, mortality.1 In bilateral cases, treatment most often consists of primary enucleation of the more involved eye, with systemic chemotherapy plus local therapy or with local treatment alone for the fellow eye, depending on group classification. Local treatment modalities include laser photocoagulation, transpupillary thermotherapy, cryotherapy, and brachytherapy.2–4 Advanced tumors, especially in the presence of vitreous seeding, require systemic combination chemotherapy for tumor reduction with consolidative focal therapy once the tumor burden decreases.4,5 Recurrent intraocular tumors remain a challenge, ultimately leading to enucleation in 25% to 30% of eyes in which attempted salvage fails.5 Tumor regrowth after systemic chemotherapy most likely reflects transient and inadequate levels of the agents achieved in the vitreous after systemic administration of chemotherapy. Recent clinical efforts to improve eye-salvaging therapies and minimize systemic side effects include intra-ophthalmic artery chemotherapy6–9 and periocular injections of carboplatinum.10 Sequestered delivery of agents from an episcleral reservoir11 promises sustained delivery of higher therapeutic drug levels while eliminating the dynamic barrier12 that has prevented viable transscleral drug delivery in the past. Pre-clinical trials for this drug delivery method are underway and clinical enrollment is expected to begin in Fall 2011 (NIH-funded Grant 1RC3CA150730-01).
Recently, it has been demonstrated that ultrasound (US), when combined with diagnostic microbubbles (MB) can enhance intracellular uptake, with wide implications for gene therapy and drug delivery.13–15 MB are approved by the United States Food and Drug Administration (FDA) for cardiac imaging. They are composed of a lipid shell with a gas core that oscillates in the presence of ultrasound, allowing enhanced detection of blood flow in vessels and better delineation of ischemic tissue.16 The ability of MB to enhance drug delivery is thought to be due to cavitation: the alternate growing and shrinking of MB under the influence of an ultrasonic field.14,16–18 When the intensity of US reaches a certain threshold, the MB implode and cause microjets that are thought to transiently perforate the membranes of nearby cells.15 This, in turn, can have therapeutic implications by enhancing the intracellular uptake of drugs through these pores.18 Previous studies have shown that MB-enhanced US promotes chemotherapy uptake in rat models19 and is effective against malignant melanoma of the eyelid in a mouse model.20 Although this technique has been shown to increase vascular permeability in the eye,21 it has never been attempted to enhance chemotherapy to tumors located inside the eye.
Vincristine, etoposide, and carboplatin are the agents commonly used systemically in the treatment of retinoblastoma. The side effects of these agents, including abdominal pain, nausea, vomiting, and myelosuppression, cause significant morbidity to pediatric patients and significantly limit dosing.22 We undertook this study to determine whether US + MB could increase chemotherapeutic efficacy in retinoblastoma cell lines in vitro. The successful translation of this approach into clinical practice could allow significant reduction in the dosage and systemic side effects of current therapy, with simultaneous enhancement of local delivery of chemotherapy to the intraocular tumor.
Materials and Methods
Cell Culture
Retinoblastoma Y79 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; VWR International, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; VWR International) at 37°C in a humidified environment with 5% carbon dioxide.
Fetal retinal pigment epithelial (RPE) cells were cultured from fetal eyes obtained from Advanced Bioscience (Alameda, CA).23 Typically, gestation lasts between 18 and 22 weeks. Eyes were soaked in phosphate-buffered saline (PBS; VWR International) containing 5% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO). The RPE layer was gently peeled off under sterile conditions, and only the large pieces were passed through a 70-μm filter (VWR International), then through a 40-μm filter, isolating only the larger pieces. These pieces of RPE were spun down for 5 minutes at 1200 rpm and were resuspended in DMEM (VWR International) supplemented with 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Omega Scientific, Ventura, CA), obtaining at least 25% FBS (Omega Scientific). Cells were plated on laminin-coated plates (Sigma-Aldrich). Cells were confirmed to be RPE cells by immunocytochemical staining using antibodies against cytokeratin and endothelial cell antigen Factor VIII (DAKO, Carpinteria, CA).
US + MB Procedure
Cells were sonoporated in 2 mL medium in six-well plates, at a concentration of 4 × 105 cells/well. Cells were gently pipetted immediately before ultrasound to ensure they were in suspension. The US probe (Sonitron; Artison Corp., Inola, OK) was placed into each well, and the tip was submerged into the medium. The US frequency used was 1 MHz, the pulse repetition frequency was 10 kHz, and the duty cycle was set at 20% throughout all experiments. We used Artison microbubbles (Artison Corp.), which are 2.4 μm in diameter. They are composed of a lipid shell and a perfluorocarbon gas core. Microbubbles (0.1 mL) at a stock concentration of 13 × 108 microbubbles/mL were added to the medium. Therefore approximately 13 × 107 microbubbles were added to 2 mL medium and cells for a final concentration of 6.2 × 107 microbubbles/mL. The total fluid volume in each well after the addition of microbubbles was 2.1 mL.
The duration and intensity of US exposure varied by experiment. For the cell viability experiments, the US exposure was 10 seconds. The intensities used were 1, 5, and 10 W/cm2 for US alone. With the addition of MB, lower US intensities—0.3, 0.6, and 1 W/cm2—were used. The chemotherapy experiments were performed with 10 seconds of US exposure and 0.3 W/cm2 of US intensity. The SEM experiment was divided into two groups; one group of Y79 cells was exposed to 10 seconds of US with variable intensities. The second group was exposed to 60 seconds of US with the intensity of 0.3 W/cm2 (0.94 mPa).
Cell Counts and Analysis of Cell Viability and Chemotherapeutic Efficacy
To determine cell viability, Y79 cells were retrieved and counted using the trypan blue exclusion assay. Cells were counted using a hemocytometer and a light microscope. Every cell that stained blue after the addition of trypan blue was marked as dead. Each experiment was repeated six times, and cell viability was calculated from the number of live cells divided by the number of total cells. This was done for cells exposed to US only (before and after US exposure) and those exposed to US + MB.
The experiment was repeated with doxorubicin (Sigma-Aldrich), which was chosen as a chemotherapeutic agent because of its inherent property of autofluorescence. The doxorubicin was dissolved in DMSO to 10 mM and diluted with medium to 1 μM; it was then stored at 4°C. Four microliters of doxorubicin was added to each plate, and Y79 cells were added by gentle pipetting. The remainder of the experiment proceeded as described in US + MB procedure. Cells were counted at 0, 24, 48, and 72 hours. Controls included cells with no exposures, cells exposed to doxorubicin alone, and cells exposed to US + MB without doxorubicin.
Analysis of RPE Cell Permeability Using Doxorubicin with or without US + MB
Doxorubicin was diluted to 1 μM in media (DMEM + 10% FBS + 100 U penicillin/mL + 100 μg streptomycin/mL + 2 mM l-glutamine [Omega Scientific] at 37°C [95% air, 5% CO2]). This was then pipetted into a glass-bottom culture dish (MatTek Corporation, Ashland, MA) containing the RPE cells in 2 mL media. Fetal RPE cells23 were treated with 6.04 μL of 1 μM doxorubicin, 100 μL of MB, and US at 0.3 W/cm2 for 10 seconds. Control cells were treated with 5.75 μL of 1 μM doxorubicin.
The US probe was placed in the middle of a glass-bottom cell culture dish followed by the addition of MB and doxorubicin. Fluorescence and bright-field images of treated cells were acquired every 30 minutes for a period of 1 hour, using a spinning disc confocal (UltraVIEW ERS; PerkinElmer, Waltham, MA) with Zeiss microscope (Carl Zeiss, Inc., Thornwood, NY) and a 40× water immersion objective lens (C-Apochromat; Zeiss) with a numerical aperture of 1.2. The fluorescent doxorubicin was stimulated with a 568-nm laser, using the same setting and exposure times for each image. An emission discrimination filter of 600 to 615 nm was used. Exposure time was 2000 ms for fluorescence images and 250 ms for bright-field images. The same exposure times and gain values were used for all images.
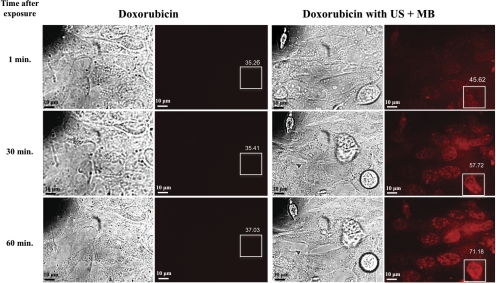
The intensity of the fluorescence was measured with the “analyze” function of 3D image analysis software24 (Volocity, version 5.4; PerkinElmer). A region of interest (ROI) of equal dimensions was outlined in each image, and the mean intensity was then recorded for each ROI (see Fig. 4).
Figure 4.
Bright-field and fluorescent microscopic images of cells exposed to doxorubicin alone versus doxorubicin with ultrasound and microbubbles (US + MB). As early as 1 minute after US + MB exposures, the cells showed increased intracellular fluorescence that increased over 60 minutes. Cells exposed only to doxorubicin showed trace intracellular fluorescence at 60 minutes. Boxes represent ROIs for measuring levels of fluorescence, and values indicate mean intensity of fluorescence within the ROI.
Scanning Electron Microscopy
The Y79 cells were examined by SEM for morphologic evaluation. MB were added just before the application of US. As stated in the US + MB procedure, the cells were divided into two groups—cells exposed to 10 seconds and cells exposed to 60 seconds of US. The first group of cells was fixed at time (t) = 10 seconds and t = 13 seconds. The second group was fixed at t = 60 seconds. Control cells were fixed at t = 0 seconds. To fix the cells, 100 μL Karnovsky solution was aliquotted to each of the various treatment wells. Cells were incubated in this solution for 1 hour. Cells were spun down using high-speed centrifugation for 3 minutes, and then these samples were serially washed with PBS three times and left in PBS overnight at 4°C. Cells were washed in distilled water and refixed in osmium tetroxide and cacodylate buffer for 30 minutes. The cells were then dehydrated through successive serial washes with ethanol at 4°C (15 minutes at 25%, 50%, 75%, 95%, and then twice in 100% ethanol). Cells were placed on porous filter paper within a Petri dish of 100% ethanol and loaded into a critical point dryer for further processing. After dehydration, cells were gently sprinkled onto a carefully cut section of double-sided tape mounted on a specimen stub. Caution was taken to ascertain that all the available cells were transferred. The edges of the double-sided tape were then covered with fast-drying colloidal silver paint to maintain electrostatic integrity. Finally, each of the specimen stubs was sputter-coated with gold before examination with the variable pressure scanning electron microscope (JSM-6390LV/LGS; JEOL, Tokyo, Japan).
Statistical Analysis
For the entire data set, we used the mean ± SE. P was ascertained using the Student's t-test when comparing data groups. P < 0.05 was considered statistically significant.
Results
Effects of US Alone
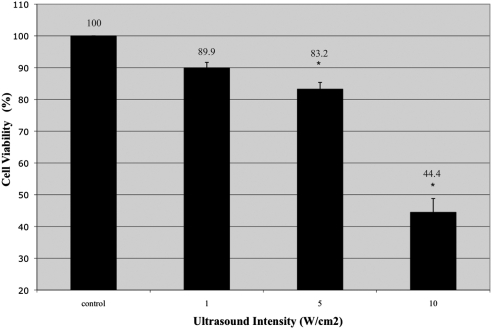
To ascertain a safe dose of US that would not be directly toxic to cells, we studied 1-MHz frequency, 20% duty cycle, 10-kHz pulse repetition frequency, and a range of intensities from 1 W/cm2 to 10 W/cm2. We evaluated cell counts before and after ultrasound (Table 1). At 10 W/cm2, there was a precipitous (60%) decrease in cell viability seen on trypan blue exclusion compared with controls (Fig. 1). At that intensity, there was also a 70% decrease in total cell population after ultrasound exposure. This result represented an order of magnitude decrease compared with the other intensities (Table 1), possibly indicating that at such high intensities, the cell membranes lose all integrity and become emulsified. At 5 W/cm2, there was a 15% decrease in cell viability (Fig. 1) associated with minimal decrease in total cell counts (Table 1). At 1 W/cm2, there was minimal effect on cell viability (Fig. 1).
Table 1.
Cell Counts and Trypan Blue Exclusion Assay of Y79 Cells Exposed to Ultrasound Alone
| Before Ultrasound |
After Ultrasound |
|||||
|---|---|---|---|---|---|---|
| No. Cells Alive | No. Cells Dead | Total Cells | No. Cells Alive | No. Cells Dead | Total Cells | |
| Control | 3.4 × 105 | 0 | 3.4 × 105 | 3.2 × 105 | 0 | 3.2 × 105 |
| 1 W/cm2 | 3.8 × 105 | 3000 | 3.83 × 105 | 3.17 × 105 | 3.3 × 104 | 3.5 × 105 |
| 5 W/cm2 | 3.43 × 105 | 1000 | 3.44 × 105 | 2.7 × 105 | 5.0 × 104 | 3.2 × 105 |
| 10 W/cm2 | 3.1 × 105 | 0 | 3.1 × 105 | 4.0 × 104 | 5.0 × 104 | 9.0 × 104 |
Ultrasound intensity >1 W/cm2 was directly toxic to Y79 cells; a 60% decrease in cell viability was seen from exposure to ultrasound alone at an intensity of 10 W/cm2.
Figure 1.
Cell viability after exposure to a range of ultrasound intensities (n = 6). Compared with controls, cell viability decreased with exposure to ultrasound in a linear fashion. Higher intensity ultrasound caused a proportionally larger decrease in cell viability. *P < 0.01. Bars represent SE.
Safe Intensities of US + MB for Sonoporation
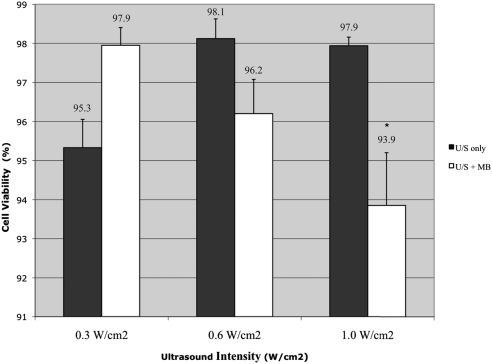
The addition of MB has been shown to lower the threshold for cell damage; because the previous experiment identified 1 W/cm2 as minimally damaging to cells, we explored cell viability after the addition of MB for intensities ≤1 W/cm2. At 1 W/cm2, the addition of MB induced a statistically significant decrease in cell viability compared with ultrasound alone (P = 0.016; Fig. 2). At intensities of 0.3 W/cm2 and 0.6 W/cm2 in the presence of MB, there was no statistically significant decrease in cell viability (Fig. 2). Based on these results, we limited the subsequent experiments to 0.3 W/cm2 because there was minimal loss of cell viability, thus limiting collateral damage to the cells from the US + MB treatment.
Figure 2.
The effect of low-intensity ultrasound with MB on cell viability (n = 6). At 1 W/cm2 there is a statistically significant decrease in cell viability with the addition of MB. *P < 0.05. Bars represent SE.
Increasing the Efficacy of Chemotherapy
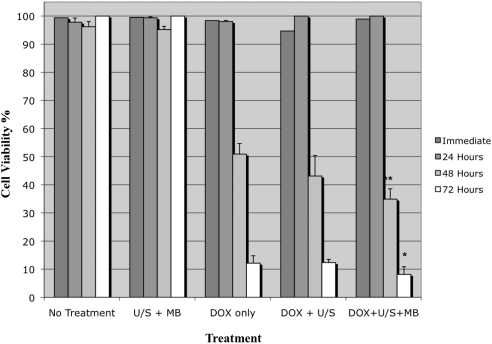
One of the main hypotheses of this study was that US + MB is able to enhance the efficacy of chemotherapy. To examine this hypothesis, we compared the viability of Y79 cells exposed to doxorubicin alone versus cells exposed to doxorubicin + US + MB versus untreated cells. There was no significant effect on cell viability in any of the experimental groups within the first 24 hours. At 48 to 72 hours, however, cell viability decreased significantly in all groups that were exposed to doxorubicin. At 48 hours, cells exposed to doxorubicin + US + MB had significantly decreased cell viability (34.9%) compared with those exposed to doxorubicin alone (50.9%) (P = 0.05). This trend continued at 72 hours with 12.1% cell viability in the doxorubicin group compared with 8.1% cell viability for cells exposed to doxorubicin + US + MB. (P = 0.02; Fig. 3). Hence, although doxorubicin leads to cell death by 48 hours, the addition of US + MB can lead to statistically significant enhancement of chemotherapeutic effects.
Figure 3.
Cell viability for cells exposed to doxorubicin (DOX), ultrasound (US), and microbubbles (MB) in various combinations. The cells exposed to doxorubicin + US + MB had a statistically significant decrease in cell viability compared with cells exposed to doxorubicin alone or with ultrasound. Each experiment was repeated three times. *P < 0.05 when compared to DOX only treatment group. Bars represent SE.
Analysis of Sonoporation Efficacy
To further support our hypothesis that the addition of US + MB increased the uptake of chemotherapy, we measured the intracellular fluorescence of doxorubicin alone compared with that for doxorubicin + US + MB. As early as 1 minute after sonoporation, cells treated with doxorubicin + US + MB showed increased intracellular fluorescence compared with cells exposed to doxorubicin alone. The mean intensity of fluorescence in the control at 1 minute was 35.26 vs. 45.62 in cells exposed to US + MB. This effect increased further at 30 minutes, where the mean intensity of fluorescence was 35.41 in control cells versus 57.72. At 60 minutes, the mean intensity was 37.03 in controls versus 71.18 in cells treated with US + MB. Figure 4 shows that compared with the early fluorescence seen in cells exposed to US + MB, cells treated with doxorubicin alone showed only trace intracellular fluorescence at 60 minutes.
Visualization of Pores
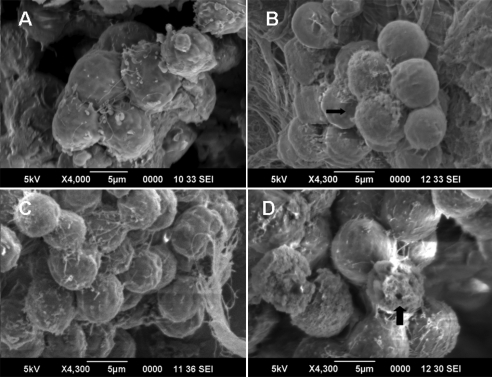
To investigate the duration and mechanism of increased permeability, we used SEM to image cells exposed to US + MB. The experiments were carried out in two groups: cells exposed to 10 seconds versus cells exposed to 60 seconds of US + MB. We were able to visualize pores (∼1 μm in size) in cells exposed to US + MB for 60 seconds (Fig. 5d). However, these pores were not visible in cells exposed to the same intensity of US + MB for 10 seconds only (Figs. 5b, 5c).
Figure 5.
SEM of cells. (A) t = 0, control cells not exposed to ultrasound. (B) t = 10, cells exposed to 10 seconds of 0.3 W/cm2 ultrasound and microbubbles (US + MB). No pores were visible. (C) t = 13, photographs taken 3 seconds after ultrasound was stopped. No pores were identified. (D) t = 60, cells exposed to 60 seconds of 0.3 W/cm2 US + MB. Pores (arrow) were identified in cells after 60 seconds of US + MB exposure.
Discussion
This study shows that the chemotherapeutic efficacy of doxorubicin against retinoblastoma cells in vitro is enhanced with the addition of MB and low-intensity (0.3 W/cm2), low-frequency (1 MHz) US for 10 seconds. We showed a statistically significant decrease in cell viability at 48 and 72 hours in cells treated with this therapy compared with cells treated with chemotherapy alone. Although targeted drug delivery of chemotherapy with sonoporation has been studied in primary cutaneous melanoma,18,20 lymphoma,25 prostate cancer,26 and oral cancer,27,28 this is the first study to show the effectiveness of this treatment against retinoblastoma cells in vitro.
Another aim of this study was to identify whether physical poration was linked to enhanced drug delivery. We performed SEM on cells that were fixed at different time points during exposure to US + MB. We identified pores in cells exposed to 60 seconds of US + MB but not during or after 10 seconds of US + MB. Interestingly, in vitro fluorescence showed that doxorubicin uptake significantly increased immediately after exposure to 10 seconds of US + MB. It is possible that the pores were smaller than we were able to identify with SEM or that they were in the process of resealing and were not seen, but, overall, these findings suggest that the presence of physical pores may not be a prerequisite to enhanced drug entry into the cells. Sonoporation has been thought to induce a temporary and reversible opening of mechanical channels or pores during US + MB.15,29 Other cellular changes have also been shown to occur, such as transient changes in the electrical resting potential of cells, which then returns to baseline when the treatment ceases.30,31 Meijering et al.32 demonstrated US + MB induced endocytosis, which may be another mechanism by which this therapy increases drug uptake. Our experiment showed increased uptake of doxorubicin with increased intracellular fluorescence in cells exposed to 10 seconds of US + MB in the absence of physical pores on SEM. It is possible that transient electrical changes, endocytosis, or other yet unidentified mechanisms contribute to the increase in cell permeability. Thus, further research is needed to investigate the mechanism of enhanced drug delivery associated with this treatment.
Based on previous research and our present results, we believe that enhanced drug delivery with US + MB may be limited by the size of the molecule that can be taken up by cells during sonoporation. Recently, Zhou et al.33 showed that with exposure to 0.2 second of ultrasound with a frequency of 1.075 MHz and 0.3 MPa amplitude, the mean pore size was 110 nm with an SD of 40 nm. Previous studies have estimated pore size in a broad range from 50 to 2500 nm measured with SEM or by the uptake of various sizes of FITC-dextran.30,34–36 We used non–liposomal-based doxorubicin, which is approximately 60 nm. This formulation of doxorubicin should easily fit through the smallest of these pores. The small size of free doxorubicin may explain the increased uptake after only 10 seconds of US + MB. Although this short treatment duration may be effective for small molecules, such as doxorubicin, these parameters may not be adequate for larger molecules, such as liposomal-based doxorubicin.
Low-intensity (0.692–2.87 W/cm2) US at 1-Mhz frequency with MB has been suggested to be most effective at sonoporation.37 Other studies have used even lower frequencies (20–70 kHz) at intensities between 1 and 2 W/cm2 and showed a significant therapeutic effect in murine tumor models compared with controls.19 However, US frequencies in kilohertz cannot be safely applied to ocular tissues because of the significant cavitation effects; notably, these are the frequencies used for phacoemulsification.38 In cutaneous melanoma, 1 MHz US at 1 W/cm2 has been shown to be effective in vitro and in vivo.20,27 Similarly, effective sonoporation in lymphoma cells has been achieved with pulsed 1-MHz US for 60 seconds, with intensities between 0.2 and 0.5 W/cm2.25 Oral squamous cell carcinoma was effectively treated in mouse models with bleomycin and 1 MHz US + MB for 20 seconds at intensities between 1 and 2 W/cm2.27,28 We have found that 1 MHz US (without MB) can decrease cell viability at intensities above 1 W/cm2. At 5 W/cm2 (mechanical index = 0.4), the viability of Y79 cells decreased to 85%; and at 10 W/cm2, the viability decreased further to 45%. Low-intensity US alone (1 W/cm2) caused minimal cell death; however, when MB were added, there was a statistically significant increase in cell death at 1 W/cm2. This is interesting because the mechanical index at 1 W/cm2 is 0.1, well below FDA allowance for ultrasound exposures in the eye, which is 0.23. This invites caution when using US + MB in ocular settings. For this very reason, we designed our chemotherapy experiment using 0.3 W/cm2, allowing for a safety margin, because we did not observe any detrimental effect on cell viability at intensities ≤0.6 W/cm2. This was lower than the intensities used in other studies. It is our impression that the final US parameters will be tumor and chemotherapeutic dependent and that the parameters studied herein may be useful as a starting point when treating tumors isolated to the eye in vivo. At this intensity, we can hope to avoid collateral damage to the surrounding healthy retinal and neural tissue while still effectively increasing drug delivery to tumor cells. It will be crucial to perform further studies exploring the optimal US + MB parameters that are effective at enhancing various chemotherapeutic drugs in vivo yet safe for normal ocular tissues before this technique can be transferred to clinical practice.
Sonoporation therapy is a promising tool for locally enhancing chemotherapeutic drug delivery, with the potential to augment cancer treatments for many primary malignancies.18,20,25–28 The treatment of retinoblastoma has been increasingly focused on localizing therapy to the eye. This approach has the potential advantage of allowing the eye to be salvaged by preserving some level of functional vision while minimizing systemic side effects. Thus, sonoporation may prove to be a valuable adjuvant to chemotherapy in retinoblastoma, specifically in the setting of vitreous seeding, where treatment is often limited by drug delivery. This study is the first to attempt this approach in retinoblastoma. Our results are consistent with other studies that have shown the efficacy of low-frequency, low-intensity US + MB in facilitating chemotherapeutic entry into cells. Our study shows that drug delivery is enhanced even at intensities and durations of US + MB that are not associated with the presence of visible pores, suggesting there may be alternate mechanisms for drug entry into cells at this dosage. We further show that 1 MHz US at intensities >1 W/cm2 in the presence of MB may cause decreased cell viability not mediated by chemotherapy. Although further research is still needed to identify the optimal US parameters and chemotherapeutic agents before this approach can be transferred to the clinic, our study provides a promising first step toward US-based targeted and localized therapies for retinoblastoma.
Acknowledgments
The authors thank Susan Clarke for careful review of the manuscript and Chris Spee and Jennifer Aparicio for their excellent help with cell culture experiments.
Footnotes
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2010, and at the annual meeting of the American Academy of Ophthalmology, Chicago, Illinois, October 2010.
Supported by National Institutes of Health Grant EY03040; a University of Southern California James H. Zumberge Individual Faculty Research Award (AAF); and an unrestricted grant from Research to Prevent Blindness, Inc.
Disclosure: N.G. Lee, None; J.L. Berry, None; T.C. Lee, None; A.T. Wang, None; S. Honowitz, None; A.L. Murphree, None; N. Varshney, None; D.R. Hinton, None; A.A. Fawzi, None
References
- 1. Boubacar T, Fatou S, Fousseyni T, et al. A 30-month prospective study on the treatment of retinoblastoma in the Gabriel Toure Teaching Hospital, Bamako, Mali. Br J Ophthalmol. 2010;94:467–469 [DOI] [PubMed] [Google Scholar]
- 2. Shields CL, Shields JA. Retinoblastoma management: advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy. Curr Opin Ophthalmol. 2010;21:203–212 [DOI] [PubMed] [Google Scholar]
- 3. MacPherson D, Dyer MA. Retinoblastoma: from the two-hit hypothesis to targeted chemotherapy. Cancer Res. 2007;67:7547–7550 [DOI] [PubMed] [Google Scholar]
- 4. Lin P, O'Brien JM. Frontiers in the management of retinoblastoma. Am J Ophthalmol. 2009;148:192–198 [DOI] [PubMed] [Google Scholar]
- 5. Chan HS, Gallie BL, Munier FL, Beck Popovic M. Chemotherapy for retinoblastoma. Ophthalmol Clin North Am. 2005;18:55–63, viii [DOI] [PubMed] [Google Scholar]
- 6. Abramson DH. Super selective ophthalmic artery delivery of chemotherapy for intraocular retinoblastoma: ‘chemosurgery’ the first Stallard lecture. Br J Ophthalmol. 2010;94:396–399 [DOI] [PubMed] [Google Scholar]
- 7. Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115:1398–1404 [DOI] [PubMed] [Google Scholar]
- 8. Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery). Ophthalmology. 2010;117:1623–1629 [DOI] [PubMed] [Google Scholar]
- 9. Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Bilateral superselective ophthalmic artery chemotherapy for bilateral retinoblastoma: tandem therapy. Arch Ophthalmol. 2010;128:370–372 [DOI] [PubMed] [Google Scholar]
- 10. Abramson DH. Periocular chemotherapy for retinoblastoma: success with problems? Arch Ophthalmol. 2005;123:128–129, author reply 129 [DOI] [PubMed] [Google Scholar]
- 11. Pontes de Carvalho RA, Krausse ML, Murphree AL, Schmitt EE, Campochiaro PA, Maumenee IH. Delivery from episcleral exoplants. Invest Ophthalmol Vis Sci. 2006;47:4532–4539 [DOI] [PubMed] [Google Scholar]
- 12. Kim SH, Lutz RJ, Wang NS, Robinson MR. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007;39:244–254 [DOI] [PubMed] [Google Scholar]
- 13. Casey G, Cashman JP, Morrissey D, et al. Sonoporation-mediated immunogene therapy of solid tumors. Ultrasound Med Biol. 2010;36:430–440 [DOI] [PubMed] [Google Scholar]
- 14. Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol. 2009;54:R27–R57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Wamel A, Kooiman K, Harteveld M, et al. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release. 2006;112:149–155 [DOI] [PubMed] [Google Scholar]
- 16. Dayton PA, Rychak JJ. Molecular ultrasound imaging using microbubble contrast agents. Front Biosci. 2007;12:5124–5142 [DOI] [PubMed] [Google Scholar]
- 17. Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol. 1996;22:1131–1154 [DOI] [PubMed] [Google Scholar]
- 18. Lentacker I, Geers B, Demeester J, De Smedt SC, Sanders NN. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol Ther. 18:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG. Ultrasonically activated chemotherapeutic drug delivery in a rat model. Cancer Res. 2002;62:7280–7283 [PubMed] [Google Scholar]
- 20. Sonoda S, Tachibana K, Uchino E, et al. Inhibition of melanoma by ultrasound-microbubble-aided drug delivery suggests membrane permeabilization. Cancer Biol Ther. 2007;6:1276–1283 [DOI] [PubMed] [Google Scholar]
- 21. Hirokawa T, Karshafian R, Pavlin CJ, Burns PN. Insonation of the eye in the presence of microbubbles: preliminary study of the duration and degree of vascular bioeffects—work in progress. J Ultrasound Med. 2007;26:731–738 [DOI] [PubMed] [Google Scholar]
- 22. Rizzuti AE, Dunkel IJ, Abramson DH. The adverse events of chemotherapy for retinoblastoma: what are they? Do we know? Arch Ophthalmol. 2008;126:862–865 [DOI] [PubMed] [Google Scholar]
- 23. Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamilton N. Quantification and its applications in fluorescent microscopy imaging. Traffic. 2009;10:951–961 [DOI] [PubMed] [Google Scholar]
- 25. Yoshida T, Kondo T, Ogawa R, et al. Combination of doxorubicin and low-intensity ultrasound causes a synergistic enhancement in cell killing and an additive enhancement in apoptosis induction in human lymphoma U937 cells. Cancer Chemother Pharmacol. 2008;61:559–567 [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Liu Z, Li T, Ye G. Ultrasonic sonoporation can enhance the prostate permeability. Med Hypotheses. 2010;74:449–451 [DOI] [PubMed] [Google Scholar]
- 27. Iwanaga K, Tominaga K, Yamamoto K, et al. Local delivery system of cytotoxic agents to tumors by focused sonoporation. Cancer Gene Ther. 2007;14:354–363 [DOI] [PubMed] [Google Scholar]
- 28. Maeda H, Tominaga K, Iwanaga K, et al. Targeted drug delivery system for oral cancer therapy using sonoporation. J Oral Pathol Med. 2009;38:572–579 [DOI] [PubMed] [Google Scholar]
- 29. Liang HD, Tang J, Halliwell M. Sonoporation, drug delivery, and gene therapy. Proc Inst Mech Eng H. 2010;224:343–361 [DOI] [PubMed] [Google Scholar]
- 30. Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound Med Biol. 2004;30:519–526 [DOI] [PubMed] [Google Scholar]
- 31. Zhou Y, Shi J, Cui J, Deng CX. Effects of extracellular calcium on cell membrane resealing in sonoporation. J Control Release. 2008;126:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meijering BD, Juffermans LJ, van Wamel A, et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ Res. 2009;104:679–687 [DOI] [PubMed] [Google Scholar]
- 33. Zhou Y, Kumon RE, Cui J, Deng CX. The size of sonoporation pores on the cell membrane. Ultrasound Med Biol. 2009;35:1756–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, Prausnitz MR. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med Biol. 2006;32:915–924 [DOI] [PubMed] [Google Scholar]
- 35. Zhao YZ, Luo YK, Lu CT, et al. Phospholipids-based microbubbles sonoporation pore size and reseal of cell membrane cultured in vitro. J Drug Target. 2008;16:18–25 [DOI] [PubMed] [Google Scholar]
- 36. Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Plasma membrane poration induced by ultrasound exposure: implication for drug delivery. J Control Release. 2005;104:213–222 [DOI] [PubMed] [Google Scholar]
- 37. Feril LB, Jr, Kondo T. Biological effects of low intensity ultrasound: the mechanism involved, and its implications on therapy and on biosafety of ultrasound. J Radiat Res (Tokyo). 2004;45:479–489 [DOI] [PubMed] [Google Scholar]
- 38. Fine IH, Packer M, Hoffman RS. New phacoemulsification technologies. J Cataract Refract Surg. 2002;28:1054–1060 [DOI] [PubMed] [Google Scholar]