Abstract
Although tailored health interventions can be more effective in eliciting positive behavior change then generic interventions, the underlying neural mechanisms are not yet understood. Ninety-one smokers participated in a functional magnetic resonance imaging (fMRI) session and a tailored smoking-cessation program. We found that increases in activations in self-related processing regions, particularly dorsomedial prefrontal cortex, to tailored messages predicted quitting during a 4-month follow-up.
Over the past decade, a powerful convergence of health communication techniques and information technology has fueled the rapid development of tailored health intervention programs, including web-based programs. Such widely available and affordable health intervention programs can help reduce the staggering health and societal costs of preventable illnesses. Emerging evidence suggests that tailored interventions can be more effective than one-size-fits-all interventions in eliciting positive health-behavior changes, including smoking cessation and weight management1-2. Thus, understanding the underlying mechanisms is an important next step.
We hypothesized that self-related processing is a central psychological mechanism underlying the enhanced efficacy of tailored message interventions. Tailored messages typically make references to an individual's life, needs, and interests, as well as to specific obstacles to achieving a desired change. Tailoring itself involves increasing the proportion of recognizable features of the individual in the message, and providing feedback about the individual3. Thus, an individual receiving tailored messages is expected to engage in self-referential processing and to perceive the messages as self-relevant4. In turn, heightened perception of self-relevance could enhance learning and memory, plausibly by greater elaboration during encoding and by fostering a more systematic organization in storage5. Thus, self-relevant messages should be more persuasive and effective in producing desired behavioral changes.
If the enhanced efficacy of tailored messages involves self-related processing, then it should also engage brain regions involved in self-related processing. Meta-analyses of functional neuroimaging studies suggest that self-related processing is mediated by a cortical network, including medial prefrontal cortex [mPFC] and precuneus/posterior cingulate regions6,7. Although we previously demonstrated that highly tailored smoking-cessation messages indeed activate mPFC and precuneus/posterior cingulate regions8, it was unclear whether these activations were associated with self-related processing. In the present study, we examined whether the neural response to tailored smoking-cessation messages overlaps with self-related processing regions, determined via an independent Self task and, more critically, whether the activation in these regions predicts subsequent real-life quitting.
We recruited ninety-one smokers interested in quitting (44 females and 47 males; mean age 37.5 years; mean number of cigarettes per day 16.7) for the study, which included 3 sessions and a follow-up phone interview (Supplementary Methods). In Session 1, participants gave written informed consent, and completed a standard assessment about their health, demographic, and psychosocial characteristics relevant to smoking cessation. We used the responses to create tailored smoking-cessation messages for a subsequent intervention. In Session 2, participants completed two fMRI tasks: a Messages Task, to examine brain regions associated with processing of smoking-cessation messages, and a Self-Appraisal Task, to identify brain regions involved in self-related processing. In Session 3, participants completed a web-based tailored smoking-cessation program9 and were instructed to quit smoking. Four months after the intervention, we interviewed the participants over the phone to determine their smoking cessation status using the standard 7-day point prevalence abstinence (cigarette free for the past 7 days)10.
During the Messages Task, participants passively received audio-visual blocks of three types of messages: Tailored smoking-cessation messages, Untailored smoking-cessation messages, and Neutral messages (Supplementary Table 1 for examples). Neutral messages were not related to smoking cessation. Untailored messages were smoking-cessation statements applicable to smokers in general, whereas Tailored messages were generated based on the individual's information and thus varied across participants. Untailored and Neutral messages were identical for all participants. In the Self-Appraisal Task11, participants had to decide using key press whether an adjective presented described them or not (self-referential evaluation condition, “Self”) or whether the adjective was of positive valence (valence judgment condition, “Valence”).
The smoking-cessation outcome was available from 87 out of the 91 participants. We categorized participants who remained abstinent during the 4-month follow-up as “Quitters” (n = 45) and the participants who did not succeed in quitting as “Non-Quitters” (n = 42).
Performance on a surprise memory test demonstrated overall better memory for Tailored than Untailored or Neutral messages, but the memory performance was not associated with quitting (Supplementary Results). We examined brain activation regions associated with self-related processing, tailored message processing, and untailored message processing (Supplementary Figs. 1-2 and Tables 2-4). To identify overlapping brain foci between regions preferentially engaged by tailored message processing (Supplementary Fig. 3 and Table 5) and self-related processing, we used a conjunctive mask of Tailored > Untailored and Self-Appraisal (Self > Valence) contrasts to identify the common regions in the two processes (Supplementary Fig. 4). Three regions – dorsomedial prefrontal cortex (dmPFC), precuneus, and angular gyrus – were preferentially engaged by Tailored messages and by self-related processing. We then examined whether the neural response to Tailored messages in these functionally defined regions predicted smoking-cessation outcome at the 4-month follow-up. The average beta parameter estimate for all voxels in the three identified regions for the Tailored > Neutral contrast was used as a predictor in a logistic regression. Greater neural response to Tailored messages in the entire common region significantly predicted the odds of quitting smoking at 4 months, (ß = .31, S.E. = 14, Wald χ2 = 5.21, p = .022; O.R. = 1.36). We then examined the three regions individually to see if activation in each region predicted quitting. The average beta across the voxels in each of the three regions was used as a predictor in logistic regression models. We found that greater activation in dmPFC during Tailored messages (BA 9,10; peak xyz coordinates: 0, 54, 30; k = 128; ß = .27, S.E. = .11, Wald χ2 = 5.71, p = .017; O.R. = 1.31) significantly predicted the odds of quitting smoking. Similarly, greater activation in the precuneus (BA 31, 7; peak xyz coordinates = −6, −51, 36; k = 30; ß = .22, S.E. = .12, Wald χ2 = 3.11, p = .078; O.R. = 1.24) marginally correlated with smoking abstinence at 4-months (Fig.1). The activation in angular gyrus did not predict quitting (BA 39; peak xyz coordinates: −51, −63, 33; k = 30; ß = .06, S.E. = .12, Wald χ2 = 0.34, ns; O.R. = 1.07). In the above logistic regression models, we controlled for the effects of number of cigarettes per day, which was independently negatively correlated with quitting, r = −.23, p = .033.
Fig. 1.
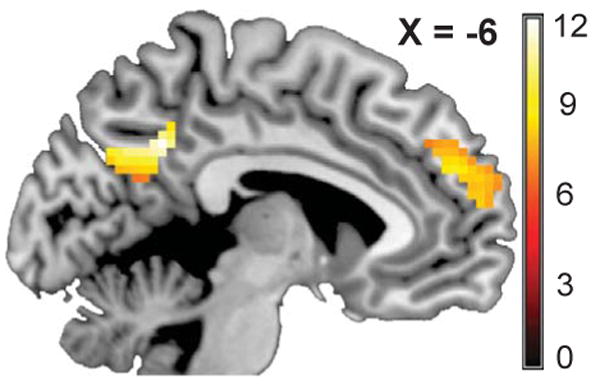
Brain region activations during Tailored messages associated with quitting. Greater dmPFC activation predicted quitting and greater precuneus activation marginally predicting quitting. Regions are defined by areas preferentially engaged during Tailored messages in contrast to Untailored messages and also self-related processing in the Self-Appraisal task. The color map depicts the t-score and image coordinates are in MNI space.
To our knowledge, this is the first study to demonstrate a direct link between the neural response to tailored messages and real-life smoking cessation. Greater activation in regions preferentially engaged by tailored messages and self-related processing was associated with smoking abstinence at 4 months, following a tailored-message smoking-cessation intervention.
Our findings suggest that the advantage of tailored health messages in promoting a desirable health-behavior change stems at least in part from enhanced engagement of self-related processes evoked by tailoring. Specifically, the dmPFC region has been associated with the evaluative and decision making aspects of self-related processing12, which could underlie the efficacy of tailored message interventions. The engagement of self-related processing could allow for deeper processing and more efficient integration of the newly formulated health-change goals into one's learning, self-schema and action plans, culminating in behavioral change. Indeed, follow-up reports indicate that Quitters were more likely to change their behavioral response to stress and to avoid situations that trigger smoking following the tailored smoking-cessation program (see Supplement).
This study provides a first and important step in identifying neural mechanisms associated with eliciting a real-life health-behavior change through tailored health communications. It is our belief that the addition of neuroimaging methods to the already powerful convergence of health communication techniques and information technology will promote rapid progress in facilitating psychoneurobiological understanding of behavior change leading to improved tailored health intervention programs.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Drug Abuse Grant R21-DA024429-01 to Drs. Hannah Faye Chua and Victor J. Strecher.
Footnotes
Author Contributions: H.F.C performed all aspects of this study, including experimental design, data collection, analyses, interpretation, and manuscript preparation. S.S.H. and A.J.J. assisted in the data analyses and interpretation and manuscript preparation. T.A.P., R.C.W., and I.L contributed to the experiment design, results interpretation, and manuscript preparation. V.J.S. contributed to the experiment design.
References
- 1.Lancaster T, Stead LF. Cochrane Database Syst Rev. 2005:CD001118. doi: 10.1002/14651858.CD001118.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Brug J, Campbell M, van Assema P. Patient Educ Couns. 1999;36:145–156. doi: 10.1016/s0738-3991(98)00131-1. [DOI] [PubMed] [Google Scholar]
- 3.Dijkstra A. Health Educ Res. 2005;20:527–539. doi: 10.1093/her/cyh014. [DOI] [PubMed] [Google Scholar]
- 4.Strecher V. Annu Rev Clin Psychol. 2007;3:53–76. doi: 10.1146/annurev.clinpsy.3.022806.091428. [DOI] [PubMed] [Google Scholar]
- 5.Symons CS, Johnson BT. Psychol Bull. 1997;121:371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- 6.Northoff G, Bermpohl F. Trends Cogn Sci. 2004;8:1364–6613. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Northoff G, et al. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Chua HF, Liberzon I, Welsh RC, Strecher VJ. Biol Psychiatry. 2009;65:165–168. doi: 10.1016/j.biopsych.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strecher VJ, et al. Am J Prev Med. 2008;34:373–381. doi: 10.1016/j.amepre.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velicer WF, Prochaska JO. Addict Behav. 2004;29:51–60. doi: 10.1016/s0306-4603(03)00084-4. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz TW, Johnson SC. Neuroimage. 2006;30:1050–1058. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Meer L, Costafreda S, Aleman A, David AS. Neurosci Biobehav Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


