Abstract
Aged mdx mice represent an important model for studying Duchenne cardiomyopathy. Herein we compared the cardiac phenotypes of 22-month-old male and female mdx mice. Surprisingly, only females displayed the characteristic cardiac dilation on pressure–volume loop analysis. Female mdx mice also exhibited lower contractility, larger Q waves, and higher ratios of heart weight to body weight. Our results reveal significant gender disparity in mdx cardiac function. Gender should be considered when using the mdx model for the study of Duchenne cardiomyopathy.
Keywords: animal models, cardiomyopathy, Duchenne muscular dystrophy, ECG, gender, hemodynamics, mdx
Duchenne muscular dystrophy (DMD) is a fatal X-linked muscle-wasting disease caused by null mutation of the dystrophin gene. The absence of dystrophin causes muscle damage, inflammation and fibrosis. Heart disease is a significant cause of morbidity and mortality in DMD.1,2 Current treatment for Duchenne cardiomyopathy is limited.
A critical step toward improved therapy is the development of appropriate animal models. Dystrophin-deficient mdx mice have been widely used as a DMD model.3–5 Several groups have examined mdx cardiac function. Although young mdx mice exhibit a mild cardiac phenotype, older mdx mice seem to display significant cardiac dysfunction.6–9 In particular, we have found that 20–22-month-old mdx mice show severe dilated cardiomyopathy (DCM) reminiscent of that seen in human patients.8
Animal gender has been recognized as a modulating factor for the dystrophic phenotype in skeletal muscle.10–12 Herein we examined whether gender affects the cardiac phenotype in aged mdx mice. We compared the anatomic features, cardiac fibrosis and electrocardiographic (ECG) profiles, and hemodynamic function of 22-month-old male and female mdx mice. The ratio of heart weight (HW) to body weight (BW) was significantly higher in aged female mdx mice. ECG analysis revealed larger Q waves in female mdx mice. Furthermore, left ventricular hemodynamic function was more severely reduced in female mdx mice. However, the level of cardiac fibrosis was not significantly different between genders. Our results suggest that aged mdx female mice display more severe cardiomyopathy.
Methods
Experimental Mice
All animal experiments were approved by local animal care and use committee and were in accordance with NIH guidelines. Age-matched C57Bl/10SnJ (BL10) and C57Bl/10ScSn-Dmdmdx/J (mdx) mice were originally purchased from the Jackson Laboratory (Bar Harbor, Maine).
Hemodynamic Assay
Left ventricular hemodynamic assay was performed in a closed-chest preparation using a 1.4F Millar microtip pressure–volume catheter (SPR-839; Millar Instruments, Houston, Texas).8,13 Baseline hemodynamic parameters were collected using the Millar Aria-1 PV conductance system and analyzed with PVAN software (Millar Instruments).
ECG Analysis
Non-invasive 12-lead ECG was performed under isoflurane anesthesia as previously described.8,13,14 Signal-averaged ECG tracings were constructed from 1-min recordings using a Model MLA0112S PowerLab system running Chart ECG analysis software (version 5.5.5; AD Instruments, Colorado Springs, Colorado). The lead II tracing was used for all parameters except Q-wave amplitude, which was taken from the lead I tracing. The cardiomyopathy index is calculated as the QT interval divided by the PQ segment (QT/PQ).
Hydroxyproline Content Assay
The heart hydroxyproline content was determined in lyophilized whole mouse heart.13 Briefly, hydrolyzed heart lysate was oxidized with chloramine-T and then reacted with p-dimethylamino-benzaldehyde/perchloric acid. The absorbance at 558 nm was measured, and hydroxyproline content was determined using a standard curve.
Statistical Analysis
Data are presented as mean ± standard error of mean. Data were analyzed by one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc analysis using statistical software (SPSS, Chicago, Illinois). P < 0.05 was considered statistically significant.
Results
Cardiac function in aged mdx mice was significantly worse than in normal BL10 mice.8,13 However, different patterns were observed between male and female mdx mice. Although there was no obvious pressure–volume (PV) loop shift in males (Fig. 1A), the characteristic rightward shift of the PV loops was observed in females (Fig. 1B).
FIGURE 1.
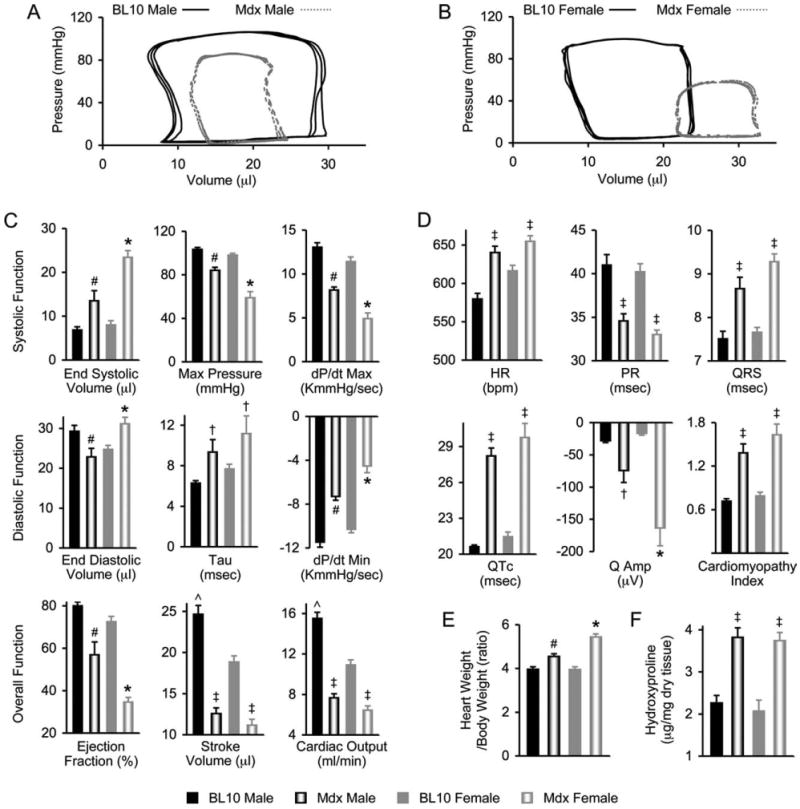
Heart disease in 22-month-old mdx mice is more severe in female mice than in male mice. (A) Representative PV loops from male BL10 and mdx mice. (B) Representative PV loops from female BL10 and mdx mice. (C) Baseline hemodynamic profiles (N = 15 for BL10 males, 16 for mdx males, 16 for BL10 females, 13 for mdx females). (D) ECG profiles (N = 13 for BL10 males, 11 for mdx males, 11 for BL10 females, 12 for mdx females). (E) Ratios of heart weight to body weight (N = 24 for BL10 males, 35 for mdx males, 24 for BL10 females, 44 for mdx females). (F) Hydroxyproline content (N = 7 for BL10 males, 18 for mdx males, 6 for BL10 females, 16 for mdx females). *mdx female significantly different from all other groups; #mdx male significantly different from all other groups; †significantly different from gender-matched group by t-test analysis; ˆBL10 male significantly greater than BL10 female; ‡mdx groups are significantly different from BL10 groups by ANOVA analysis, but not from each other.
Detailed analysis of systolic and diastolic function showed worse hemodynamic function in female mdx mice. Specifically, females had significantly higher end-systolic and end-diastolic volumes. The maximum pressure, maximal rate of left ventricular pressure development (dP/dtmax), and the minimal rate of left ventricular pressure fall (dP/dtmin) were significantly lower in females (Fig. 1C). Interestingly, there was no difference in the time constant of left ventricular isovolumetric pressure decay (τ) between males and females (Fig. 1C).
Ejection fraction was significantly decreased in both male and female mdx mice. However, females fared worse with a much greater reduction. Nevertheless, there was no significant difference between males and females in stroke volume and cardiac output (Fig. 1C, bottom panel).
We also compared the ECG tracings of 22-month-old male and female mdx mice. Both males and females had significantly increased heart rate (HR) compared with normal BL10 mice but showed no difference between genders (Fig. 1D). Analysis of the PR interval, QRS duration, and corrected QT interval (QTc) revealed the same pattern. There were significant changes in mdx mice compared with normal, but there was no gender disparity (Fig. 1D).
Only the lead I Q amplitude (Qamp) revealed a significant gender difference. Both male and female mdx mice Q waves were greater than normal. However, females had significantly larger Q waves than males (Fig. 1D). There was no gender difference in Q-wave amplitudes of normal mice. Analysis of the cardiomyopathy index showed significant increases for both male and female mdx mice, but no gender difference (Fig. 1D).
We also compared the HW/BW ratio and the cardiac hydroxyproline content. Although both genders of mdx mice had significantly increased HW/BW ratios, females had a much greater increase (Fig. 1E). The cardiac hydroxyproline content was significantly elevated in mdx mice, but there was no difference between males and females (Fig. 1F).
Discussion
Mdx mice represent a well-established model for the study of DMD skeletal muscle disease.2 However, until recently the mdx mouse has not been considered a useful model to investigate Duchenne cardiomyopathy. Early studies of young mdx mice found minimal cardiac dysfunction.6,7,15 Recent investigations on aged mdx mice have renewed interest in this model for the study of Duchenne cardiomyopathy.8,13,16 To further characterize the aged mdx mouse model for DMD heart disease, we compared cardiac function between aged male and female mdx mice.
Our findings suggest that gender may significantly affect the mdx cardiac phenotype. Specifically, the classic rightward shift of DCM was found only in females. Females also showed more severe reductions in systolic and diastolic parameters, larger Q waves in their ECG tracings, and a significantly greater increase in HW/BW ratio (Fig. 1).
Gender differences have been increasingly recognized as a critical factor in the incidence and severity of heart disease.17,18 Typically, heart disease is more severe in males than in females. The prevailing hypothesis suggests that estrogen may play a protective role.19 However, there are also instances in which females show more severe heart disease than males.20–22 In patients with diabetes, women have more severe heart disease and an increased risk of DCM and heart failure.21 Studies in calmodulin-induced diabetic mice have revealed more severe cardiac dysfunction in females.22
The mechanisms behind these findings are not completely understood. One intriguing hypothesis may relate to renin–angiotensin system (RAS) alterations. In the case of diabetic cardiomyopathy, it has been shown that females are more sensitive to RAS perturbations.23,24 Interestingly, angiotensin receptor mRNA is elevated in the mdx heart.25 Angiotensin-converting enzyme (ACE) inhibitors also improve mdx hemodynamic profiles.26 In addition, ACE inhibition has been shown to be effective in treating Duchenne cardiomyopathy.27 These findings suggest the RAS may play a role in dystrophic cardiomyopathy. Future studies are needed to clarify the underlying mechanisms.
The aged mdx mouse represents a promising model for developing novel therapies tailored to the heart. Our results suggest that aged female mdx mice may exhibit a more severe cardiac phenotype. Gender should be carefully considered when using this model for studying Duchenne cardiomyopathy.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR-49419 to D.D.) and the Muscular Dystrophy Association (to D.D.).
Abbreviations
- ACE
angiotensin-converting enzyme
- ANOVA
analysis of variance
- BW
body weight
- bpm
beats per minute
- mdx
C57Bl/10SnJ, BL10; C57Bl/10scSn-Dmdmdx/J
- DCM
dilated cardiomyopathy
- DMD
Duchenne muscular dystrophy
- ECG
electrocardiography
- dP/dtmax
maximal rate of left ventricular pressure development
- dP/dtmin
minimal rate of left ventricular pressure development
References
- 1.McNally EM. New approaches in the therapy of cardiomyopathy in muscular dystrophy. Ann Rev Med. 2007;58:75–88. doi: 10.1146/annurev.med.58.011706.144703. [DOI] [PubMed] [Google Scholar]
- 2.Duan D. Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum Mol Genet. 2006;15:R253–R261. doi: 10.1093/hmg/ddl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 4.Bulfield G, Siller WG, Wight PAL, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells DJ, Wells KE. What do animal models have to tell us regarding Duchenne muscular dystrophy? Acta Myol. 2005;24:172–180. [PubMed] [Google Scholar]
- 6.Yue Y, Skimming JW, Liu M, Strawn T, Duan D. Full-length dystrophin expression in half of the heart cells ameliorates β-isoproterenol-induced cardiomyopathy in mdx mice. Hum Mol Genet. 2004;13:1669–1675. doi: 10.1093/hmg/ddh174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danialou G, Comtois AS, Dudley R, Karpati G, Vincent G, Des Rosiers C, et al. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J. 2001;15:1655–1657. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- 8.Bostick B, Yue Y, Long C, Duan D. Prevention of dystrophin-deficient cardiomyopathy in twenty-one-month-old carrier mice by mosaic dystrophin expression or complementary dystrophin/utrophin expression. Circ Res. 2008;102:121–130. doi: 10.1161/CIRCRESAHA.107.162982. [DOI] [PubMed] [Google Scholar]
- 9.Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS, Levin LS. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord. 2004;14:491–496. doi: 10.1016/j.nmd.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Salimena MC, Lagrota-Candido J, Quírico-Santos T. Gender dimorphism influences extracellular matrix expression and regeneration of muscular tissue in mdx dystrophic mice. Histochem Cell Biol. 2000;122:435–444. doi: 10.1007/s00418-004-0707-8. [DOI] [PubMed] [Google Scholar]
- 11.Valentine BA, Cooper BJ, de Lahunta A, O'Quinn R, Blue JT. Canine X-linked muscular dystrophy: an animal model of Duchenne muscular dystrophy: clinical studies. J Neurol Sci. 1988;88:69–81. doi: 10.1016/0022-510x(88)90206-7. [DOI] [PubMed] [Google Scholar]
- 12.Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis. 2008;31:1–19. doi: 10.1016/j.nbd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bostick B, Yue Y, Long C, Marschalk N, Fine DM, Chen J, et al. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged mdx mice. Mol Ther. 2009;17:253–261. doi: 10.1038/mt.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bostick B, Yue Y, Lai Y, Long C, Li D, Duan D. Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum Gene Ther. 2008;19:851–857. doi: 10.1089/hum.2008.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamogawa Y, Biro S, Maeda M, Setoguchi M, Hirakawa T, Yoshida H, et al. Dystrophin-deficient myocardium is vulnerable to pressure overload in vivo. Cardiovasc Res. 2001;50:509–515. doi: 10.1016/s0008-6363(01)00205-x. [DOI] [PubMed] [Google Scholar]
- 16.Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- 17.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 18.Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ. 2007;31:17–22. doi: 10.1152/advan.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konhilas JP, Leinwand LA. The effects of biological sex and diet on the development of heart failure. Circulation. 2007;116:2747–2759. doi: 10.1161/CIRCULATIONAHA.106.672006. [DOI] [PubMed] [Google Scholar]
- 20.Ponten A, Li X, Thoren P, Aase K, Sjoblom T, Ostman A, et al. Transgenic overexpression of platelet-derived growth factor-c in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am J Pathol. 2003;163:673–682. doi: 10.1016/S0002-9440(10)63694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren J, Ceylan-Isik A. Diabetic cardiomyopathy. Endocrine. 2004;25:73–83. doi: 10.1385/ENDO:25:2:073. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Ye G, Duan J, Chen AF, Ren J. Influence of gender on intrinsic contractile properties of isolated ventricular myocytes from calmodulin-induced diabetic transgenic mice. Endocrine Res. 2003;29:227–236. doi: 10.1081/erc-120022318. [DOI] [PubMed] [Google Scholar]
- 23.Duprez DA. Is the female heart more sensitive to aldosterone for early remodeling? Hypertension. 2004;43:936–937. doi: 10.1161/01.HYP.0000124253.98863.86. [DOI] [PubMed] [Google Scholar]
- 24.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, et al. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension. 2004;43:957–962. doi: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura A, Harrod GV, Davies KE. Activation of calcineurin and stress activated protein kinase/p38-mitogen activated protein kinase in hearts of utrophin–dystrophin knockout mice. Neuromuscul Disord. 2001;11:251–259. doi: 10.1016/s0960-8966(00)00201-7. [DOI] [PubMed] [Google Scholar]
- 26.Bauer R, Straub V, Blain A, Bushby K, MacGowan GA. Contrasting effects of steroids and angiotensin-converting-enzyme inhibitors in a mouse model of dystrophin-deficient cardiomyopathy. Eur J Heart Fail. 2009;11:463–471. doi: 10.1093/eurjhf/hfp028. [DOI] [PubMed] [Google Scholar]
- 27.Ramaciotti C, Heistein LC, Coursey M, Lemler MS, Eapen RS, Iannaccone ST, et al. Left ventricular function and response to enalapril in patients with Duchenne muscular dystrophy during the second decade of life. Am J Cardiol. 2006;98:825–827. doi: 10.1016/j.amjcard.2006.04.020. [DOI] [PubMed] [Google Scholar]


