Abstract
Clinical malaria is associated with the proliferation of Plasmodium parasites in human erythrocytes. The coordinated processes of parasite egress from and invasion into erythrocytes are rapid and tightly regulated. Here we found that the plant-like calcium-dependent protein kinase PfCDPK5, which is expressed in invasive merozoite forms of Plasmodium falciparum, was critical for egress. Parasites deficient in PfCDPK5 arrested as mature schizonts with intact membranes, despite normal maturation of egress proteases and invasion ligands. Merozoites physically released from stalled schizonts were capable of invading new erythrocytes, separating the pathways of egress and invasion. The arrest was downstream of cGMP-dependent protein kinase (PfPKG) function and independent of protease processing. Thus PfCDPK5 plays an essential role of PfCDPK5 during the blood-stage of malaria replication.
Plasmodium falciparum malaria remains a leading cause of morbidity and mortality worldwide, and resistance to existing antimalarials is a growing problem. Signal transduction plays a major role in key transitions of the parasite life-cycle. Calcium is thought to be a regulator of parasite egress from and invasion into host cells. Multiple calcium-dependent protein kinases (CDPKs), a sub-family absent from the human genome, have been identified in apicomplexan organisms (1–8).
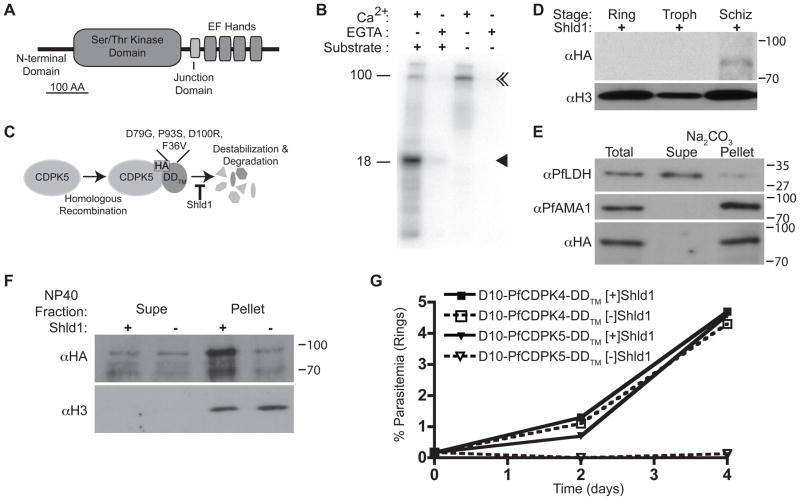
The PfCDPK5 gene (PF13_0211) is transcribed in mature blood-stage schizonts and invasive merozoites (9) suggesting a role in egress and/or erythrocyte invasion (Fig. S1). PfCDPK5 exhibits a canonical multi-domain structure with a serine/threonine kinase domain followed by a C-terminal calmodulin-like domain comprising four Ca2+-binding EF hands (Fig. 1A) (10). This structure is predicted to facilitate rapid kinase activation following locally increased Ca2+ concentration. Recombinant PfCDPK5 phosphorylated an artificial target protein in the presence of Ca2+, and this was inhibited by the calcium chelator EGTA, demonstrating that PfCDPK5 is a bona fide CDPK (Fig. 1B) (11). In the absence of substrate, recombinant PfCDPK5 auto-phosphorylated itself.
Figure 1. PfCDPK5 is an essential calcium-dependent protein kinase.
A) Schematic of PfCDPK5. Scale bar, 100 amino acids. B) Recombinant GST-PfCDPK5–6His (94 kDa) was incubated with or without substrate (myelin basic protein, 18 kDa), with 1.1 mM Ca2+ or 1 mM EGTA, and 32P-g-ATP. In the presence of Ca2+, PfCDPK5 phosphorylates itself (double arrowhead) and substrate (arrowhead). C) PfCDPK5 fused to DDTM is targeted for degradation, but is stabilized by Shld1. D) Protein lysates from D10-PfCDPK5-HA-DDTM ring (0–20h), trophozoite (20–36h), and schizont-stage (36–48h) parasites cultured with Shld1 and probed with anti-HA (PfCDPK5) or anti-Histone H3 (loading control). E) Na2CO3-extracted schizonts were probed with anti-PfLDH (cytoplasmic fraction), anti-PfAMA1 (membrane fraction), or anti-HA. F) D10- PfCDPK5-HA-DDTM parasites were grown [+] or [−] Shld1 until 44 h, incubated with E- 64 for 10 h, harvested, fractionated with 0.6% NP-40, and probed with anti-HA and anti- Histone H3. G) Representative replication curves. D10-PfCDPK5-DDTM and D10- PfCDPK4-DDTM parasites were cultured [+] and [−] Shld1.
We used the destabilizing domain (DD) system to regulate the level of PfCDPK5 expression in P. falciparum (12–15). DD-fusion proteins are expected to be rapidly degraded in the absence of the ligand Shield-1 (Shld1) and stabilized in its presence (Fig. 1C). We genetically fused DDTM, a strongly destabilizing DD derivative (16) to the C-terminus of PfCDPK5, generating the D10-PfCDPK5-DDTM and D10-PfCDPK5-HA-DDTM parasite lines (Fig. S2), which expressed PfCDPK5 in schizonts (Fig. 1D). PfCDPK5 has potential palmitoylation sites but no myristoylation site like its paralog PfCDPK1 (17). Carbonate extraction, which separates soluble from membrane-associated proteins, showed that PfCDPK5 was associated with parasite membranes (Fig. 1E). This may be important for maintaining the kinase in close proximity to its substrate(s).
Newly invaded ring-stage D10-PfCDPK5-DDTM and D10-PfCDPK5-HA-DDTM parasites were maintained in the presence or absence of Shld1 until the mature schizont stage. PfCDPK5 levels were decreased ~60–80% in the absence of Shld1 (Fig. 1F) in the detergent-insoluble fraction. To evaluate the requirement for PfCDPK5 for P. falciparum growth, we examined replication rates of D10-PfCDPK5-DDTM parasites in the presence and absence of Shld1 (Fig. 1G). As a control, we created parasites with DDTM fused to PfCDPK4 (D10-PfCDPK4-DDTM), a paralog not essential for asexual replication (1, 6). Without Shld1, the D10-PfCDPK5-DDTM parasites failed to proliferate, demonstrating that PfCDPK5 is essential. In contrast, D10-PfCDPK4-DDTM parasites grew normally in the absence of Shld1, despite a >90% drop in PfCDPK4 levels (Fig. S3). D10-PfCDPK5-DDTM parasitemia levels remained <0.2% for >10 days after Shld1 removal. However, after 10–12 days, a Shld1-independent revertant population emerged (D10-PfCDPK5-DDTM-Rev, Fig. S4A) with a concomitant genomic alteration at the PfCDPK5 locus (Fig. S2B). We generated PfCDPK5-deficient parasites in the 3D7 strain, demonstrating a similar arrest in proliferation in a different strain of P. falciparum (Fig. S4B).
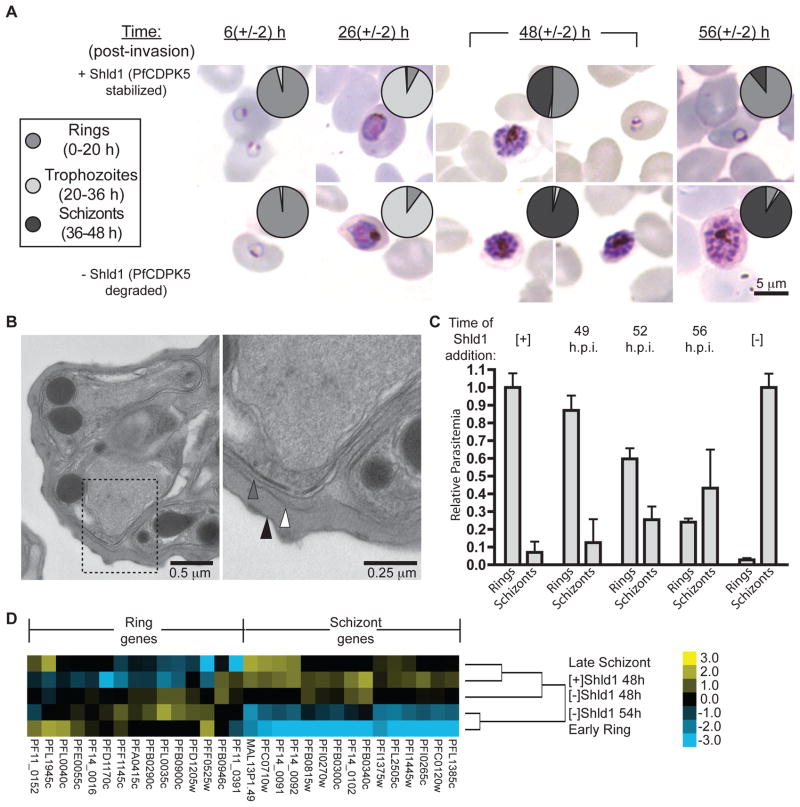
Morphological analysis over the 48 h blood-stage asexual cycle (Fig. 2A) revealed healthy rings 6±2 hours post-invasion (h.p.i.) and trophozoites 26±2 h.p.i. irrespective of the presence of Shld1. At 48±2 h.p.i., schizonts were observed both [+] and [−] Shld1. New rings were observed in the presence of Shld1 but were >95% reduced in the [−]Shld1 cultures. 8 h later, the majority of [+]Shld1 parasites had reinvaded to form rings, but the [−]Shld1 parasites, deficient for PfCDPK5, remained stalled as late schizonts, with a >90% reduction in new ring formation. Thus PfCDPK5 plays an essential role in parasite proliferation and egress from the erythrocyte. Functional knockout of PfCDPK5 did not affect the number of merozoites per schizont (Fig. S5). Arrested schizonts were ultrastructurally normal and the erythrocyte plasma membrane (PM), parasitophorous vacuole membrane (PVM), and parasite PM were intact (Fig. 2B). The block thus occurred after schizont differentiation but prior to PVM rupture.
Figure 2. PfCDPK5-deficient parasite arrest prior to egress.
A) Light microscopy of synchronized D10-PfCDPK5-DDTM parasites grown [+] or [−] Shld1 at indicated hours post invasion (h.p.i.). Pie charts show relative proportions of rings, trophozoites, and schizonts (N=100). B) D10-PfCDPK5-DDTM parasites were grown [−]Shld1 until 56 h.p.i. and visualized by electron microscopy. The erythrocyte PM (black triangle), PVMe), and the parasite PM (grey triangle) were intact. C) Ring-stage D10-PfCDPK5-DDTM parasites were grown [+] or [−] Shld1 until 48 h.p.i.. In parallel [-]Shld1cultures, Shld1 was added back at the indicated times (49, 52, 56 h.p.i). Rings and schizonts were counted 4 h later (mean +/− S.D., N=4). D) RNA expression patterns from 3D7-PfCDPK5-DDTM parasites at 48 h.p.i. [+] and [−] Shld1 and 54 h.p.i. [−]Shld1. Log2- transformed expression pattern shown for 15 late schizont-stage and 15 early ring-stage genes.
To test whether PfCDPK5-deficient schizonts had undergone aberrant differentiation or were irreversibly prevented from egress and/or invasion, we performed Shld1 rescue experiments (Fig. 2C). Upon observation of reinvaded rings in the [+]Shld1 parasites (48 h.p.i.), we allowed parallel [−]Shld1 parasites to incubate for an additional 1, 4, or 8 h prior to adding back Shld1; new ring formation and levels of non-ruptured schizonts were measured 4 h after the addition of Shld1. Newly invaded rings were observed, albeit at lower levels, even in parasites that had been PfCDPK5-deficient for 52 h.p.i and 56 h.p.i.. Thus, PfCDPK5-deficient arrested schizonts had differentiated normally and retained the ability to egress and reinvade. Gene expression profiles in PfCDPK5-deficient parasites at 46±2 h.p.i. correlated most closely with the expression pattern for late schizonts (r = 0.83) (9). Focusing on a subset of 30 differentially expressed genes between late schizonts and early rings, the expression pattern of the arrested parasites progressed towards the early ring phenotype (Fig. 2D, file S1).
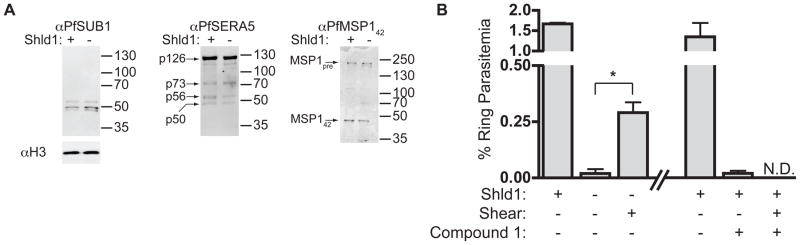
Recent studies have identified a parasite protease cascade required for egress from erythrocytes (18, 19) and maturation of merozoite surface proteins. PfSUB1, released from specialized secretory organelles called exonemes, cleaves several proteins including the protease PfSERA5 and the invasion ligand merozoite surface protein 1 (PfMSP1) (19, 20). In our egress-blocked parasites, processing of PfSUB1 (mature form: ~47 kDa), PfSERA5 (mature form: ~50 kDa), and PfMSP1 (mature form: ~42 kDa) were all unaffected (Fig. 3A), and in the PfCDPK5-deficient arrested parasites, PfSUB1 discharge and enzyme activity was unaffected. While PfSERA5 processing is necessary for parasite egress, it is not sufficient. PfCDPK5 mediates a separate and downstream signal to the protease cascade required for parasite egress.
Figure 3. PfCDPK5-deficient parasites are fully mature and invasion-competent.
A) D10-PfCDPK5-HA-DDTM parasites were grown [+] and [−] Shld1, harvested by Percoll-purification at 48 h.p.i., and analyzed by western blot with anti-PfSUB1, anti-PfSERA5, and anti-PfMSP142. B) D10-PfCDPK5-DDTM parasites were grown until 48 h.p.i. ([+]/[−]0.5 μM Shld1, [+]/[−] 4 μM compound 1). Subsets of parasites were sheared through a 28.5G needle. Ring parasitemia was determined 4 h later. (mean, +/− S.D. [or data range], N=2–7, *p < 0.05, unpaired t-test.)
Selective inhibition of the cGMP-dependent kinase PfPKG wit h 4-[2-(fluorphenyl)-5-(1-methylpiperidine-4-yl)-1H pyrrol-3-yl]pyridine (compound 1) blocks egress (21). Treatment of schizonts with compound 1 inhibited processing of PfMSP1 by PfSUB1 (Fig. S6A). However, compound 1 did not inhibit PfSUB1 enzyme activity directly (Fig. S6B), suggesting that compound 1 treatment interferes with access of PfSUB1 to its endogenous substrates, perhaps by preventing exoneme discharge.
To test if PfCDPK5-deficient merozoites are capable of erythrocyte invasion, we mechanically disrupted arrested schizonts by needle-shearing. Newly invaded rings were observed at a significantly higher rate in the sheared cultures compared with unsheared controls (Fig. 3B). Thus the egress trigger may be distinct from the erythrocyte invasion trigger. Isolation of viable PfCDPK5-deficient merozoites provides a valuable source for studies of erythrocyte invasion. In contrast to the invasion-competent PfCDPK5-deficient merozoites, mechanically disrupted compound 1-treated parasites did not generate newly invaded rings (Fig. 3B), consistent with these parasites being blocked at an earlier step.
We propose a modified model of P. falciparum egress from erythrocytes (Fig. S7). The protease cascade is necessary but not sufficient for parasite egress. Following an egress trigger, a transient increase in Ca2+ concentration activates PfCDPK5 to amplify and transmit the signal for parasite egress. The final stages of PVM and erythrocyte PM rupture probably involve molecules downstream of PfCDPK5 activation, such as perforin-like proteins, membrane channels, and/or proteases (22, 23).
Supplementary Material
This file contains spreadsheet of the entire data set from gene expression microarrays of 3D7-PfCDPK5-DDTM parasites [+] and [-] Shld1.
Size: 0.8 MB
Materials and Methods
SOM Text
Fig. S1 Blood-stage asexual replication of P. falciparum
Fig. S2 Generation of transgenic parasite lines
Fig. S3 Characterization of D10-PfCDPK4-DDTM parasites
Fig. S4 Long-term replication curves
Fig. S5 Merozoite nuclei in arrested PfCDPK5-deficient schizonts
Fig. S6 PfPKG and Compound 1 are upstream of PCDPK5 arrest
Fig. S7 Model of P. falciparum egress
Supplemental References
Size: 6.9 MB
Acknowledgments
We thank K. Kettleborough, S. Osborne, and colleagues at MRC Technology for provision of compound 1, S. Badrinath, E. Duraisingh, C. Merrick, and B. Coleman for critical reading of the manuscript, and D. Wirth for essential guidance and support. This work was supported by Pediatric Scientist Development Program Fellowship awards K12-HD000850 (JDD and SDP), a National Institutes of Health R01 grant R01AI057919 (MTD), a Burroughs Wellcome Fund New Investigator in the Pathogenesis of Infectious Diseases Fellowship (MTD), the Medical Research Council UK (MJB), a Wellcome Trust Project Grant 086550 (CRC), the NIH GM073046 (TJW), and an EU FP7 Grant MALSIG 223044 (DAB).
Footnotes
One sentence summary:
The Plasmodium falciparum calcium-dependent protein kinase PfCDPK5 is essential for blood-stage proliferation of the human malaria parasite.
REFERENCES AND NOTES
- 1.Billker O, et al. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 2.Coppi A, et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe. 2007;2:316. doi: 10.1016/j.chom.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Färber PM, Graeser R, Franklin RM, Kappes B. Molecular cloning and characterization of a second calcium-dependent protein kinase of Plasmodium falciparum. Mol Biochem Parasitol. 1997;87:211. doi: 10.1016/s0166-6851(97)00052-2. [DOI] [PubMed] [Google Scholar]
- 4.Green JL, et al. The motor complex of Plasmodium falciparum: phosphorylation by a calcium-dependent protein kinase. J Biol Chem. 2008;283:30980. doi: 10.1074/jbc.M803129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol. 2006;59:1175. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- 6.Kato N, et al. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat Chem Biol. 2008;4:347. doi: 10.1038/nchembio.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kieschnick H, Wakefield T, Narducci CA, Beckers C. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem. 2001;276:12369. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Kappes B, Franklin RM. Gene structure and expression of an unusual protein kinase from Plasmodium falciparum homologous at its carboxyl terminus with the EF hand calcium-binding proteins. J Biol Chem. 1993;268:4347. [PubMed] [Google Scholar]
- 9.Le Roch KG, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 10.Harper JF, Harmon A. Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol. 2005;6:555. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- 11.Information on materials and methods is available on Science Online.
- 12.Armstrong CM, Goldberg DE. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat Methods. 2007;4:1007. doi: 10.1038/nmeth1132. [DOI] [PubMed] [Google Scholar]
- 13.Banaszynski LA, Chen L-C, Maynard-Smith LA, Ooi AGL, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herm-Götz, et al. Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nat Methods. 2007;4:1003. doi: 10.1038/nmeth1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo I, Oksman A, Vaupel B, Goldberg D. A calpain unique to alveolates is essential in Plasmodium falciparum and its knockdown reveals an involvement in pre-S-phase development. Proc Natl Acad Sci USA. 2009;106:1554. doi: 10.1073/pnas.0806926106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu BW, Banaszynski LA, Chen LC, Wandless TJ. Recent progress with FKBP-derived destabilizing domains. Bioorganic & Medicinal Chemistry Letters. 2008;18:5941. doi: 10.1016/j.bmcl.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Möskes C, et al. Export of Plasmodium falciparum calcium-dependent protein kinase 1 to the parasitophorous vacuole is dependent on three N-terminal membrane anchor motifs. Mol Microbiol. 2004;54:676. doi: 10.1111/j.1365-2958.2004.04313.x. [DOI] [PubMed] [Google Scholar]
- 18.Arastu-Kapur S, et al. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat Chem Biol. 2008;4:203. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- 19.Yeoh S, et al. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell. 2007;131:1072. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 20.Koussis K, et al. A multifunctional serine protease primes the malaria parasite for red blood cell invasion. EMBO J. 2009;28:725. doi: 10.1038/emboj.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor H, et al. The malaria parasite cGMP-dependent protein kinase plays a central role in blood stage schizogony. Eukaryotic Cell. 2009;9:37. doi: 10.1128/EC.00186-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandramohanadas R, et al. Apicomplexan Parasites Co-Opt Host Calpains to Facilitate Their Escape from Infected Cells. Science. 2009;324:794. doi: 10.1126/science.1171085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kafsack B, et al. Rapid Membrane Disruption by a Perforin-Like Protein Facilitates Parasite Exit from Host Cells. Science. 2008;323:530. doi: 10.1126/science.1165740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains spreadsheet of the entire data set from gene expression microarrays of 3D7-PfCDPK5-DDTM parasites [+] and [-] Shld1.
Size: 0.8 MB
Materials and Methods
SOM Text
Fig. S1 Blood-stage asexual replication of P. falciparum
Fig. S2 Generation of transgenic parasite lines
Fig. S3 Characterization of D10-PfCDPK4-DDTM parasites
Fig. S4 Long-term replication curves
Fig. S5 Merozoite nuclei in arrested PfCDPK5-deficient schizonts
Fig. S6 PfPKG and Compound 1 are upstream of PCDPK5 arrest
Fig. S7 Model of P. falciparum egress
Supplemental References
Size: 6.9 MB