Abstract
While adult born neurons in the olfactory bulb (OB) and the dentate gyrus (DG) subregion of the hippocampus have fundamentally different properties, they may have more in common than meets the eye. Here, we propose that new granule cells in the OB and DG may function as modulators of principal neurons to influence pattern separation and that adult neurogenesis constitutes an adaptive mechanism to optimally encode contextual or olfactory information.
Introduction
Making sense of our external world requires us to continuously assess if our day-to-day experiences are different or similar to those previously encountered. In this way, we can differentiate today’s car parking location from that of yesterday and two beach vacations from one another. Conversely, we may vividly remember a beach vacation when we see palm trees or recall a traumatic bicycle accident when we see a bicycle on a street. The balance between keeping similar episodes separate while retrieving previous memories based on environmental cues is thought to require two opposing processes, pattern separation and pattern completion. Pattern separation is defined as the process by which overlapping or similar inputs (representations) are transformed into less similar outputs whereas pattern completion is the reconstruction of complete stored representations from partial inputs that are part of the stored representation (Colgin et al., 2008; Wilson, 2009). Understanding how neural circuits within the hippocampus and the olfactory system subserve these processes has received considerable attention in this last decade.
Experimental evidence for a role of the DG in pattern separation first came from lesion studies in rodents showing that ablation of the DG impaired discrimination of two spatial locations based on distal environmental cues (Gilbert et al., 2001). More recent studies relying on genetic approaches to specifically manipulate DG functions have yielded similar results (McHugh et al., 2007). Collectively, these studies suggest that the DG is required to minimize interference between overlapping spatial or contextual information (Figure 1). Multitetrode recordings of hippocampal ensemble activity have begun to identify the neuronal correlates of pattern separation in the DG. Subtle morphing of a rat’s environment is sufficient to elicit remapping of firing rates of place cells in the DG suggesting that small changes in spatial input can produce highly divergent output (Leutgeb et al., 2007). However, multitetrode recordings do not capture the activity of the entire DG neuronal population and circuit based genetic approaches that permit visualization and manipulation of neuronal activity at a population level along the entire DG will prove invaluable. Neurocognitive testing and fMRI studies in humans have also suggested a role for the DG in pattern separation (Bakker et al., 2008; Lacy et al., 2010).
Figure 1. Neurogenesis and pattern separation in the dentate gyrus and olfactory bulb.
The dentate gyrus and the olfactory bulb (blue boxes) are required for discrimination between similar contexts and similar odors (A and B), respectively. This process is termed pattern separation and is modulated by adult neurogenesis which is the generation of new neurons throughout life (yellow box). When adult neurogenesis is blocked either by irradiation (X-ray) or by genetic ablation, discrimination is impaired leading to generalization and an inability to distinguish A from B. In contrast when neurogenesis is stimulated by genetic manipulations (iBax) or exercise, discrimination is enhanced.
Like the hippocampus, the olfactory system deals with complex spatial and temporal patterns (Figure 1). Both individual molecules and complex molecular mixtures can evoke highly overlapping spatial patterns within the OB and separation of these patterns is required for high acuity odor discrimination. Using analysis of ensemble single-unit activity, Wilson and colleagues (Barnes et al., 2008; Wilson, 2009) have demonstrated an apparent segregation of pattern recognition functions between the olfactory bulb and anterior piriform cortex (PC), remarkably similar to that described for contextual pattern recognition in DG and hippocampal area CA3 (Leutgeb et al., 2007). As in most other sensory systems, olfactory perceptual acuity is experience-dependent. Humans (Rabin, 1988) and other animals (Cleland et al., 2002; Fletcher and Wilson, 2002) can improve discrimination of molecularly similar odorants through training, and this perceptual learning appears to modulate pattern separation within olfactory bulb local circuits.
Neurogenesis modulates pattern separation in the dentate gyrus and olfactory bulb
The continuous modification of circuitry of the DG and the OB by integration of new neurons suggests that adult-born neurons may functionally contribute to these two regions. Since both of these populations of adult-born neurons exhibit critical periods of plasticity during a specific window of their maturation, it is unlikely that adult neurogenesis simply adds new equivalent units but instead expands the capacity for plasticity in the DG and OB. Here, we review growing evidence suggesting that new neurons in both the DG and OB contribute to pattern separation.
Dentate Gyrus
Although the general pattern of afferent and efferent connectivity of new neurons in the DG recapitulates that of developmentally generated dentate granule neurons, new neurons exhibit distinct physiological properties relative to mature neurons during a specific window of their maturation. Specifically, young 4–8 week old adult-born neurons show greater synaptic plasticity, increased excitability and a unique contribution to behavior (Denny et al., 2011); see also Ming and Song, this issue). These features of young adult-born neurons may be critical for their recently discovered role in pattern separation (Figure 1).
Clelland and colleagues used a two-choice touch screen spatial discrimination task and a delayed nonmatching to place radial arm maze task to first demonstrate the impact of blocking hippocampal neurogenesis on spatial pattern separation (Clelland et al., 2009). In both tests, the correct choice that mice were required to make relied on discrimination of small or large spatial separations. Consistent with previous studies using DG lesions, the authors found that blockade of adult hippocampal neurogenesis by hippocampal x-irradiation impaired the animal’s ability to make fine, but not large, spatial discriminations. More recently, Sahay and colleagues used a contextual fear discrimination learning task, previously shown to require pattern separation in DG, to test whether new neurons are required for distinguishing between similar contextual representations (Sahay et al., 2011) . The authors found that hippocampal x-irradiated mice exhibited similar levels of freezing behavior in both the shock associated training context and a similar no-shock “safe” context, unlike controls that learned to discriminate between the two contexts (x-ray, Figure 1). A second study using the same contextual fear discrimination learning task yielded analogous results (Tronel et al., 2010). Together, these studies show that new neurons are required for pattern separation in three different DG dependent behavioral paradigms and raise the possibility that increasing adult hippocampal neurogenesis may enhance pattern separation. To directly address this possibility, Sahay and colleagues developed a genetic strategy to selectively increase adult neurogenesis (Sahay et al., 2011). In the contextual fear discrimination learning task, mice with more functionally integrated adult-born dentate granule neurons (iBax mice, Figure 1) were better at distinguishing between two similar contexts. These results indicate that increasing adult hippocampal neurogenesis is sufficient to improve pattern separation.
Although behavioral assays that explicitly measure pattern separation have been critical in establishing the link between new neurons and pattern separation, behavioral paradigms which test memory interference have also proven invaluable. For example, tests of spatial relational memory requiring an animal to disambiguate overlapping room cues to find a hidden platform necessitate recruitment of the dentate gyrus. Recent studies showing a role for adult-born neurons in such tasks are consistent with the idea that neurogenesis contributes to pattern separation (Dupret et al., 2008; Garthe et al., 2009; Wojtowicz et al., 2008).
The convergence of these loss-of-function and gain-of-function studies causally linking bidirectional changes in levels of adult hippocampal neurogenesis with pattern separation efficiency bolsters the hypothesis that the unique properties of young adult-born neurons are instrumental to their functions. How do a relatively small number of highly plastic young adult-born neurons influence pattern separation? Efforts to address this question are underway, and there are currently two distinct but non-mutually exclusive hypotheses. The first model emphasizes a cell autonomous function for new neurons as individual encoding units (Aimone et al., 2010). The new neurons may be the preferred substrate for place cell remapping in response to subtle changes in the animal’s environment. Through their mossy fibers, new neurons may also influence place cell remapping in CA3. To begin to address this possibility, we need to know how dentate granule place cell properties or mossy fiber-CA3 pyramidal neuron synapse maturation track with the development of adult-born neurons. Ensemble recordings from behaving mice in which adult neurogenesis has been bidirectionally manipulated will provide insight into how adult-born neurons sculpt place cell dynamics in DG and CA3.
The second model proposes a modulatory role for young-adult-born neurons and is predicated upon the lower threshold for firing of young adult-born neurons, their insensitivity to GABAergic inhibition, and their connections with interneurons and mossy cells in the DG hilar microcircuit (Figure 2)(Sahay and Hen, 2007) (Lacefield et al., 2010) see also Ming and Song, this issue). Within this framework, young adult-born neurons preferentially respond to subtle changes in context (weak inputs) because of their lower threshold of activation and influence the firing probability of mature dentate granule neurons through feedback inhibition conveyed either directly by local inhibitory interneurons or indirectly via mossy cells. Since mossy cells also form monosynaptic excitatory contacts onto dentate granule neurons, the summation of feedback inhibition and excitation relative to the strength of perforant path inputs will dictate the degree of sparseness of activity in the DG and consequently, pattern separation efficiency (Treves et al., 2008). Moreover, neurogenesis-dependent feedback inhibition may also enhance the divergence between given overlapping inputs into the DG and corresponding outputs. In addition, through recruitment of interneurons whose dendrites and axons ramify in the molecular layers of the DG, new neurons may directly modulate afferent inputs and dendritic excitability. Preliminary support for a modulatory or non-cell autonomous function for new neurons comes from a study showing that ablation of adult-born hippocampal neurons results in an increase in gamma oscillatory activity suggestive of increased coordinated network activity in the DG (Lacefield et al., 2010). A second study found a reduction in inhibitory inputs to the DG following ablation of adult-born neurons (Singer et al., 2011). Analysis of mature granule cell activity and levels of inhibition in the DG of mice in which adult neurogenesis levels are manipulated is required to demonstrate that new neurons modulate the activity of mature granule cells to influence pattern separation.
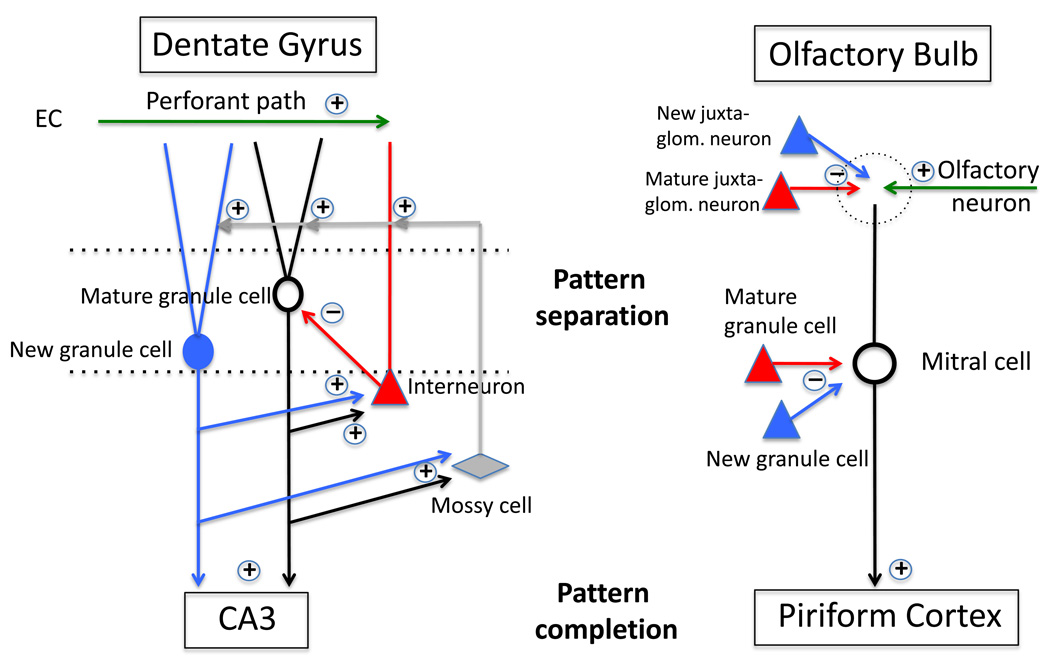
Figure 2. Neural circuits underlying pattern separation in the dentate gyrus and olfactory bulb.
Contextual information arrives in the dentate gyrus from the entorhinal cortex (EC) via the perforant path. In the dentate gyrus, the granule cell layer (space between the dotted lines) is composed of mature granule cells (black neuron) born during development and new granule cells born in adulthood (blue neuron). Both mature and new granule cells activate a variety of interneurons which include inhibitory neurons such as the basket cells (red triangular-shaped neuron) and excitatory neurons such as the mossy cells (grey diamond-shaped neuron). Mossy cells send axons to the molecular layer where they activate both inhibitory interneurons and excitatory granule cells. Inhibitory interneurons, in turn, inhibit mature granule cells but not new granule cells (at least when they are less than 4 weeks old). Activation of new granule cells may therefore result in a preferential inhibition of mature granule cells and consequently, increase sparseness of activity in the dentate gyrus which may improve pattern separation.
Sensory information arrives in the olfactory bulb through axons of olfactory sensory neurons, which synapse onto dendrites of principal cells (mitral or tufted cells) in a structure called a glomerulus (dotted circle). A variety of inhibitory interneurons (red triangles) inhibit the mitral or tufted cell either at the level of the glomeruli (mature juxtaglomerular neuron) or at dendrodendritic synapses (mature granule cell). Adult neurogenesis generates new granule cells and new juxtaglomerular neurons (blue triangles), which also inhibit mitral/tufted cells. In addition, adult-born granule cells contact other granule and juxtaglomerular cells (not shown here). Like in the dentate gyrus, the addition of new neurons in the olfactory bulb may therefore result in increased inhibition and possibly as a result, increased pattern separation.
Pattern completion has been proposed to take place in the projection zone of the dentate gyrus and olfactory bulb, which are the hippocampal CA3 field for contextual information and the piriform cortex for olfactory information.
Symbols in Dentate Gyrus; Black oval-shaped cell: mature granule cell; Blue oval- shaped cell: young adult-born granule cell; both young and mature granule cells excite CA3 pyramidal neurons well as hilar inhibitory interneurons such as basket cells (red triangular-shaped cell), and hilar mossy cells (grey diamond-shaped cell) ; +: excitatory projections; −: inhibitory projections.
Symbols in Olfactory Bulb; Black round-shaped cell: mitral cells and tufted cells which send excitatory projections to the piriform cortex; red triangular-shaped cell: mature granule cells and mature juxtaglomerular cells (periglomerular cells and short axon cells) which are all inhibitory; blue triangular-shaped cell: young adult-born granule cells and young adult-born juxtaglomerular cells (periglomerular cells and short-axon cells) which are all inhibitory.
In addition to these proposed active roles for new neurons in pattern separation, neurogenesis may also influence encoding in other ways. For instance, the competition between new and old neurons for perforant path inputs (Toni et al., 2007) and potential post-synaptic targets may result in a redistribution of synaptic weights. Furthermore, a recent study showed that varying levels of neurogenesis dictated the temporal extent of hippocampal dependence of memories (Kitamura et al., 2009). Thus, neurogenesis may ensure that an appropriate amount of space is available in the DG for encoding information by transferring memories out of the DG to the neocortex.
Olfactory Bulb
Odor acuity is in part dependent on pattern separation in the olfactory bulb, and olfactory bulb pattern separation is modulated by, and dependent on, local inhibitory interneurons, many of which are generated in adulthood. There are two populations of adult generated interneurons in the olfactory bulb, juxtaglomerular neurons (periglomerular and short axon cells) and inhibitory granule cells (Lazarini and Lledo 2011), that contribute to lateral inhibition and the spatio-temporal structure of olfactory bulb output activity. This inhibition helps enhance contrast between similar inputs (Luo and Katz, 2001; Schoppa and Urban, 2003; Yokoi et al., 1995), and thus enhances separation between similar patterns of olfactory sensory neuron input (Figure 2).
Prolonged odor exposure and odor conditioning not only induce a memory for the experienced odor, but also enhance acuity for that odor relative to other similar odors. This memory and enhanced olfactory acuity are associated with modified newborn granule cell survival (Moreno et al., 2009; Rochefort et al., 2002; Rochefort and Lledo, 2005). In fact, given the spatial organization of odor-evoked activity across the olfactory bulb, cell survival is also spatially selective, with cells surviving primarily in the region activated by the exposure odor (Mandairon and Linster, 2009). In contrast, olfactory deprivation, which can impair pattern separation in the olfactory bulb (Wilson and Sullivan, 1995), reduces survival of adult generated granule cells (Mandairon and Linster, 2009)(Yamaguchi and Mori, 2005).
Reduced olfactory bulb neurogenesis disrupts normal synaptic inhibition and stimulus evoked gamma frequency oscillations (Breton-Provencher et al., 2009), which should impair odor-evoked activity patterns. Furthermore, as in the DG, young granule cells show more robust synaptic plasticity than mature granule cells (Nissant et al., 2009), and young granule cells are more responsive to novel odors (Mandairon and Linster, 2009). Interestingly, adult-born cells synapse on to all major cell types in the OB (Bardy et al., 2010; Carleton et al., 2003; Panzanelli et al., 2009). Thus, this pool of neurons may be particularly effective at shaping responses to novel odors in a manner which enhances pattern separation. Therefore, it is surprising that unlike manipulations of developmental bulbar neurogenesis (Bath et al., 2008; Gheusi et al., 2000), most manipulations of adult neurogenesis (Breton-Provencher et al., 2009; Imayoshi et al., 2008; Lazarini et al., 2009; Valley et al., 2009) have not found impairments in olfactory discrimination. However, two studies have shown a role for adult-born granule cells in olfactory discrimination (Moreno et al., 2009; Mouret et al., 2009). These discordant findings could be due to compensatory effects following chronic blockade of adult neurogenesis, underscoring the need to acutely manipulate adult-born neurons. Alternatively, it is possible that a role for adult-born granule cells in pattern separation is uncovered only in the most difficult tasks used to probe olfactory discrimination (Moreno et al., 2009). Indeed, blockade of neurogenesis does not impair discrimination of perceptually or molecularly distinct odors where pattern separation may be less critical (Breton-Provencher et al., 2009).
Neurogenesis as adaptation to the environment
Experience-dependent plasticity is a common feature of most central circuits, yet is most commonly mediated by changes in synaptic strength, membrane excitability or remodeling of synaptic or dendritic structure. These kinds of changes can be rapidly induced (seconds to hours) and rapidly reversed. Using neurogenesis to track and record experience, in contrast, functions on a timescale of weeks, suggesting that neurogenesis-based circuit changes may be most relevant for reflecting long-term, adaptive responses to the changing environment.
Dentate Gyrus
The exquisite regulation of adult hippocampal neurogenesis by environmental factors has been well documented (See Ming and Song for a review, this issue), but how environmentally induced changes in levels of neurogenesis functionally relate to the organism is much less understood. Here, we propose that changing levels of neurogenesis constitute a long-term adaptive response to different environments by shifting the balance between pattern separation and pattern completion (Figure 3). For example, enriched environments are generally thought to promote exploration and learning and may exert greater cognitive demands by requiring encoding of details and generation and maintenance of multiple non-overlapping representations. Increased levels of adult neurogenesis following environmental enrichment may serve two adaptive functions. First, it may facilitate the faithful processing of the complex environment through enhanced pattern separation thereby also ensuring rapid recognition of such environments in the future (Kempermann, 2008). Second, it may prevent the DG from being overburdened with contextual information by rapidly transferring memories out to the cortex (Kitamura et al., 2009). In contrast, decreased neurogenesis due to stressful environments may lead to generalization of individual features and enable the organism to avoid similar situations by restraining exploration. For example, increased generalization in a hostile environment may be adaptive because it promotes avoidance of most potential threats. It should be emphasized that changes in neurogenesis are not required for acute responses to the environment for which other synaptic modifications in the DG may suffice. Instead, environment-dependent changes in neurogenesis may prepare the organism for novel habitats on longer time scales.
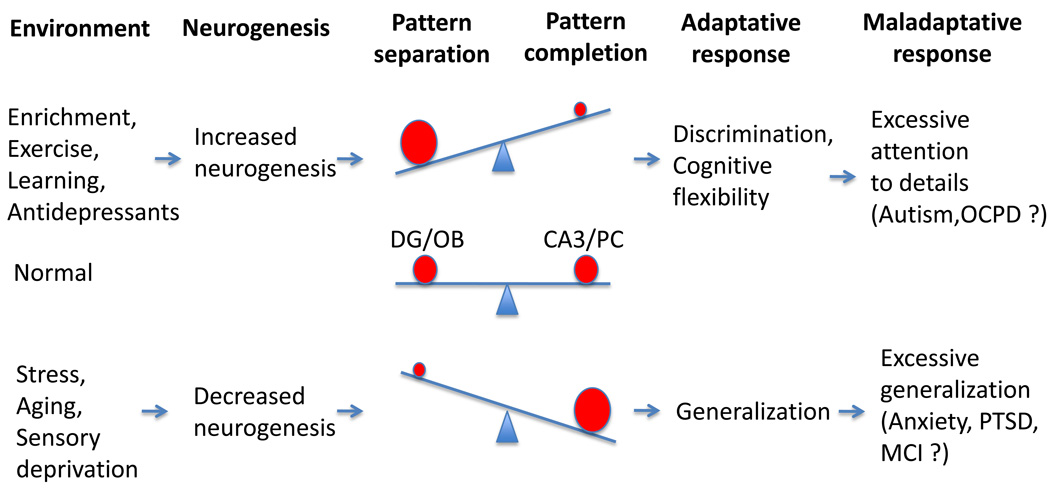
Figure 3. Environmental influences on neurogenesis and pattern separation.
Environments rich in odors or contexts stimulate neurogenesis in the olfactory bulb (OB) and dentate gyrus (DG), respectively. Similarly, learning stimulates neurogenesis in the OB and DG depending on whether the modalities are olfactory or contextual and spatial. Other manipulations such as exercise or antidepressants stimulate neurogenesis primarily in the DG. Stress, aging and sensory deprivation result in a decrease in neurogenesis in both the DG and OB. We propose that an increase in neurogenesis favors pattern separation, which alters the balance that normally exists between pattern separation (taking place in DG or OB) and pattern completion (taking place in CA3 or PC). Conversely, a decrease in neurogenesis impairs pattern separation, which shifts the balance in favor of pattern completion and results in generalization. These shifts may be a part of the normal adaptive response to changing environments. In an enriched environment, discrimination and cognitive flexibility (which result from increased pattern separation) are advantageous because they favor exploration and learning; in contrast, in a dangerous environment, generalization (which results from decreased pattern separation) may be advantageous because it favors avoidance of new and potentially dangerous situations. However, these normal adaptive responses when exaggerated may lead to pathologies: excessive generalization may for example lead to anxiety disorders such as Post Traumatic Stress Disorder (PTSD) or to the impairments that often accompany aging such as Mild Cognitive Impairment (MCI). Similarly, excessive pattern separation may lead to an excessive attention to details such as seen in some psychiatric disorders such as autism and obsessive-compulsive personality disorder (OCPD).
Disruption in these normal adaptive regulations of neurogenesis may occur in pathological contexts resulting in excessive or impaired pattern separation (Figure 3). Excessive pattern separation may impede normal integration of environmental information as the individual will devote too much attention to individual contextual and sensory features at the detriment of the “big picture”. Behaviorally, this may manifest as cognitive inflexibility, increased preoccupation with minutiae and unrestrained fixation on fine details as seen in autism spectrum disorders (Mottron et al., 2006; Soulieres et al., 2009) or obsessive-compulsive personality disorder (DSM-IVTR, 2000). In contrast, impaired pattern separation may lead to excessive generalization, which would cause an organism to lump multiple contexts or items together even if the similarity between them is minimal. Such a maladaptive response may underlie the increased generalization of new “innocuous” experiences with previously encountered aversive events seen in individuals with panic disorder (Lissek et al., 2010) and posttraumatic stress disorder (PTSD)(Peri et al., 2000). For example, in somebody who developed PTSD as a result of 9/11, the simple sight of a plane flying over New York City may be sufficient to trigger a panic attack. In addition, impaired pattern separation has also been reported during aging and in individuals with mild cognitive impairment (Toner et al., 2009; Yassa, 2010; Yassa et al., 2010). Finally, perturbed interpretation of ambiguous cues when combined with a negative response bias may be a predisposing factor in depression (Beck, 2008; Becker and Wojtowicz, 2007; Enkel et al., 2010). Deficits in pattern separation may therefore represent a circuit-based endophenotype for these different disorders.
The finding that increasing adult hippocampal neurogenesis is sufficient to improve pattern separation suggests that neurogenesis may be harnessed to treat disorders with pattern separation deficits (Sahay et al., 2011) Interestingly, naturalistic interventions such as voluntary exercise have been shown to improve pattern separation in rodents (Creer et al., 2010). It is possible that increased adult hippocampal neurogenesis mediates some of the beneficial effects of exercise on pattern separation. Chronic antidepressant treatments, which are known to stimulate adult hippocampal neurogenesis, may also exert some of their behavioral effects through enhancing pattern separation; however this is yet to be demonstrated. Unraveling the molecular mechanisms underlying the plasticity of neural stem cells and adult-born neurons and identification of proneurogenic small molecules (Pieper et al., 2010; Wurdak et al., 2010) will catalyze the development of novel strategies to treat pattern separation deficits.
Olfactory Bulb
Olfaction is at the heart of mammalian life, playing critical and often necessary roles in mother-infant attachment, kin recognition, mate selection and recognition, food selection, predator avoidance and homing. Each of these basic functions can include prolonged changes in internal state and the external chemical environment, and often require remarkably precise separation of highly overlapping odorant stimulus patterns. Enhanced survival of newly generated olfactory bulb interneurons due to a springtime eruption of novel environmental odors could coincide with the need for enhanced pattern separation and the perceptual acuity necessary for navigating this rich olfactory world. In fact, prolonged enrichment of the odor environment enhances the survival (Rochefort et al., 2002) of adult generated olfactory bulb interneurons and odor perceptual learning and memory (Mandairon and Linster, 2009). Together, these findings suggest that adult neurogenesis in the OB, as in the DG, may provide a mechanism for adapting to relatively stable changes in the environment, allowing for shifts in olfactory pattern separation and ultimately olfactory acuity.
Perturbed experience dependent regulation of olfactory bulb neurogenesis may result in unlimited pattern separation, which could come at the expense of pattern completion and perceptual stability. Given the ephemeral nature of odors, excessive pattern separation could lead to an overrepresentation of feature representation in slightly shifting stimuli, with each successive presentation of even the same stimulus being perceived as unique. Abnormal prominence of unique feature sets and stimulus analysis, at the expense of synthetic processing of odor objects could just as easily impair discrimination and memory as impaired pattern separation ((Mottron et al., 2006; Soulieres et al., 2009); Figure 3).
As in the DG, environmental chronic stress impairs neurogenesis and reduces the population of newborn neurons in the olfactory bulb granule cell layer (Hitoshi et al., 2007). These findings suggest that chronic stress may also impair olfactory bulb pattern separation and odor acuity for highly similar odors. Olfactory impairments are associated with a wide range of disorders including Mild Cognitive Impairment, Alzheimers disease, Parkinsons disease and schizophrenia. Normal aging can also both reduce OB neurogenesis and impair fine odor discrimination (Enwere et al., 2004). Although the level of olfactory bulb neurogenesis in humans is still debated, it is unclear why olfactory dysfunction would be comorbid with disorders having such diverse etiologies. Thus, investigation of olfactory pattern separation in these disorders is warranted.
Conclusion
Here, we propose a common role in pattern separation for adult neurogenesis in the olfactory bulb and hippocampus. Specifically, in both regions, new granule cells may modulate inhibition of principal cells either directly (OB) or via interneurons (DG) and this inhibition may contribute to pattern separation. We also propose that different levels of neurogenesis represent an adaptation to environmental changes in cognitive demands such as those that take place with changing seasons, exposure to enriched environment, or in response to stress and adversity. When exaggerated, these adaptive changes may lead to pathologies associated with dysregulated pattern separation. For example, the excessive generalization observed in anxiety disorders may stem from impaired pattern separation while the excessive attention to details seen in individuals with autism spectrum disorders may result from excessive pattern separation.
Major questions remain unanswered. For example, if adult neurogenesis is such an effective strategy for promoting pattern separation, why is it not more widespread in the brain? Is neurogenesis the privilege of neural circuits devoted to encoding but not storage? Are there costs (such as erosion of memories) that preclude its inclusion in other circuits, or is adult neurogenesis in the OB and DG simply an evolutionary hold-over not available to other regions (Kaslin et al., 2008)? Is the potential for neurogenesis latent in other parts of the brain? Addressing these questions will undoubtedly continue to transform our ideas regarding the regenerative potential of the adult mammalian brain
Acknowledgements
We thank Susanne Ahmari and Mazen Kheirbek for comments on the manuscript. The work was supported by NIMH Grant 5K99MH086615-02 (A.S.), NIDCD Grant R01-DC003906 (D.A.W) and NARSAD, the New York Stem Cell Initiative (NYSTEM), NIH R01 MH068542 Grants (R.H.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends in cognitive sciences. 2010;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy C, Alonso M, Bouthour W, Lledo PM. How, when, and where new inhibitory neurons release neurotransmitters in the adult olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:17023–17034. doi: 10.1523/JNEUROSCI.4543-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nat Neurosci. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends in cognitive sciences. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V, Lemasson M, Peralta MR, 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, DM S, Hen R, Drew MR. week old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2011:4–6. doi: 10.1002/hipo.20964. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-IVTR. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition - Text Revision. DSM-IV TR; 2000. [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkel T, Gholizadeh D, von Bohlen Und Halbach O, Sanchis-Segura C, Hurlemann R, Spanagel R, Gass P, Vollmayr B. Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology. 2010;35:1008–1015. doi: 10.1038/npp.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 2002;22:RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, Ikenaka K. Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. Journal of neuroscience research. 2007;85:3574–3585. doi: 10.1002/jnr.21455. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Ganz J, Brand M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008;363:101–122. doi: 10.1098/rstb.2006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2010 doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learning & memory (Cold Spring Harbor, N.Y. 2010;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Mouthon MA, Gheusi G, de Chaumont F, Olivo-Marin JC, Lamarque S, Abrous DN, Boussin FD, Lledo PM. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS ONE. 2009;4:e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Katz LC. Response correlation maps of neurons in the mammalian olfactory bulb. Neuron. 2001;32:1165–1179. doi: 10.1016/s0896-6273(01)00537-2. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Linster C. Odor perception and olfactory bulb plasticity in adult mammals. J Neurophysiol. 2009;101:2204–2209. doi: 10.1152/jn.00076.2009. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci U S A. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mouret A, Lepousez G, Gras J, Gabellec MM, Lledo PM. Turnover of newborn olfactory bulb neurons optimizes olfaction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12302–12314. doi: 10.1523/JNEUROSCI.3383-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nature Neuroscience. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- Panzanelli P, Bardy C, Nissant A, Pallotto M, Sassoe-Pognetto M, Lledo PM, Fritschy JM. Early synapse formation in developing interneurons of the adult olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15039–15052. doi: 10.1523/JNEUROSCI.3034-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, et al. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin MD. Experience facilitates olfactory quality discrimination. Percept Psychophys. 1988;44:532–540. doi: 10.3758/bf03207487. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Lledo PM. Short-term survival of newborn neurons in the adult olfactory bulb after exposure to a complex odor environment. Eur J Neurosci. 2005;22:2863–2870. doi: 10.1111/j.1460-9568.2005.04486.x. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26:501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- Singer BH, Gamelli AE, Fuller CL, Temme SJ, Parent JM, Murphy GG. Compensatory network changes in the dentate gyrus restore long-term potentiation following ablation of neurogenesis in young-adult mice. Proc Natl Acad Sci U S A. 2011;108:5437–5442. doi: 10.1073/pnas.1015425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulieres I, Dawson M, Samson F, Barbeau EB, Sahyoun CP, Strangman GE, Zeffiro TA, Mottron L. Enhanced visual processing contributes to matrix reasoning in autism. Hum Brain Mapp. 2009;30:4082–4107. doi: 10.1002/hbm.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & memory (Cold Spring Harbor, N.Y. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Treves A, Tashiro A, Witter ME, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2010 doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- Valley MT, Mullen TR, Schultz LC, Sagdullaev BT, Firestein S. Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Frontiers in neuroscience. 2009;3:51. doi: 10.3389/neuro.22.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Pattern separation and completion in olfaction. Ann N Y Acad Sci. 2009;1170:306–312. doi: 10.1111/j.1749-6632.2009.04017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J Neurosci. 1995;15:5574–5581. doi: 10.1523/JNEUROSCI.15-08-05574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Wurdak H, Zhu S, Min KH, Aimone L, Lairson LL, Watson J, Chopiuk G, Demas J, Charette B, Halder R, et al. A small molecule accelerates neuronal differentiation in the adult rat. Proc Natl Acad Sci U S A. 2010;107:16542–16547. doi: 10.1073/pnas.1010300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9697–9702. doi: 10.1073/pnas.0406082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010 doi: 10.1002/hipo.20808. 28 JUN 2010, DOI : 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]