Abstract
One of the most frequent symptoms of unilateral stroke is aphasia, the impairment or loss of language functions. Over the past few years, behavioral and neuroimaging studies have shown that rehabilitation interventions can promote neuroplastic changes in aphasic patients that may be associated with the improvement of language functions. Following left-hemisphere strokes, the functional reorganization of language in aphasic patients has been proposed to involve both intrahemispheric interactions between damaged left-hemisphere and perilesional sites and transcallosal interhemispheric interactions between the lesioned left-hemisphere language areas and homotopic regions in the right hemisphere. A growing body of evidence for such reorganization comes from studies using transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), two safe and noninvasive procedures that can be applied clinically to modulate cortical excitability during poststroke language recovery. We discuss a hierarchical model for the plastic changes in language representation that occur in the setting of dominant hemisphere stroke and aphasia. We further argue that TMS and tDCS are potentially promising tools for enhancing functional recovery of language and for further elucidating mechanisms of plasticity in patients with aphasia.
Keywords: aphasia, stroke, neuroplasticity, transcranial magnetic stimulation, transcranial direct current stimulation, neurorehabilitation, interhemispheric interactions
Introduction
Aphasia is a common consequence of stroke that typically results from injury to an extended network of cortical and subcortical structures perfused by the middle cerebral artery in the left hemisphere (Alexander, 1997; McNeil & Pratt, 2001). Most patients who experience aphasia in the setting of acute stroke show some degree of spontaneous recovery, most notably during the first 2–3 months following stroke onset (Laska, Hellblom, Murray, Kahan, & Von Arbin, 2001; Lendrem & Lincoln, 1985; Nicholas, Helm-Estabrooks, Ward-Lonergan, & Morgan, 1993). However, the majority of patients with post-stroke aphasia are left with some degree of chronic deficit for which current rehabilitative treatments are marginally effective (Basso & Marangolo, 2000; Nickels, 2002; Robey, 1994, 1995; Robey, Schultz, Crawford, & Sinner, 1999).
A number of factors have been shown to influence aphasia recovery, including lesion site and size, and the existence of prior strokes (Lazar, Speizer, Festa, Krakauer, & Marshall, 2008). Recent neuroimaging and behavioral data indicate that considerable changes in the cortical representation of language processing can occur in the days, weeks, and months following stroke in the left hemisphere (Horn et al., 2005), and that language recovery after stroke depends significantly on the degree of plastic change observed in the brains of patients after injury (Cherney & Small, 2006; Musso et al., 1999; Thompson, 2000; Thompson et al., 1997). TMS and tDCS are safe noninvasive methods that can be used to induce or enhance neuroplastic changes in bran activity (Antal, Nitsche, & Paulus, 2001): a small but growing body of evidence indicates that noninvasive brain stimulation can have beneficial effects in the treatment of aphasia after stroke. These studies also inform our understanding of potential mechanisms of language recovery following injury to language networks.
Current evidence suggests that three kinds of changes in neural activity after stroke may be most relevant for aphasia recovery: (1) Recruitment of lesioned and perilesional left hemisphere regions for language-related tasks, (2) acquisition, unmasking or refinement of language processing ability in the nondominant right hemisphere, and (3) dysfunctional activation of the nondominant hemisphere that may interfere with language recovery. We will examine the evidence for each of these kinds of plasticity in language recovery after stroke. Importantly, we will emphasize that these mechanisms are not mutually exclusive, but rather may comprise a hierarchical framework of interacting language recovery mechanisms. Finally, we will consider how evidence from studies that employ TMS and tDCS contributes to the understanding of language recovery mechanisms.
Recruitment of the left hemisphere in aphasia recovery
There is considerable evidence that perilesional areas of the left hemisphere acquire or reacquire language ability in the weeks and months following injury. It has long been accepted that the size of left hemisphere infarction in perisylvian language areas correlates with initial aphasia severity and inversely with aphasia recovery (Kertesz, Harlock, & Coates, 1979). A number of functional imaging studies of nonfluent aphasic patients have also demonstrated that better spontaneous language recovery is associated with greater activation of left-hemisphere structures (Karbe, Thiel, & Weber-Luxenburger, 1998a; Karbe et al., 1998b; Miura et al., 1999; Warburton, Price, Swinburn, & Wise, 1999). Left hemisphere activation has been associated with better language improvement among nonfluent aphasic patients who undergo speech therapy (Cornelissen et al., 2003). In patients with fluent aphasia it has been observed that efficient restoration of language is more frequently achieved if left temporal language networks are relatively well-preserved (Gainotti, 1993).
While the mechanisms underlying increased perilesional activation in language recovery have not been fully elucidated, one important contributor may be the release of inhibitory input from the lesioned cortex, leading to increased activity in nearby cortical areas. Evidence indicates that unilateral injury—such as left-hemisphere lesions that give rise to aphasia—can lead to cortical disinhibition in at least two regions: 1) neighboring ipsilesional cortical areas and 2) contralesional homotopic areas connected via the corpus callosum (Bütefisch, Kleiser, & Seitz, 2006; Lang, Nitsche, Paulus, Rothwell, & Lemon, 2004; Shimizu et al., 2002). In the case of the ipsilesional left hemisphere, release from cortical inhibition in the setting of focal injury may facilitate activation of these areas during language tasks. Animal studies of cortical plasticity suggest that persistent recruitment of cortical areas during specific tasks may result in functional modifications that allow perilesional networks to engage more efficiently in the service of those tasks (Nudo & Friel, 1999). Activity-dependant plasticity, facilitated by ipsilesional disinhibition, may thus promote the recruitment and functional reorganization of perilesional regions of the left hemisphere to subserve language processing.
The beneficial role of the right hemisphere in aphasia recovery
While most evidence suggests that ipsilateral perilesional activation in chronic aphasic patients is associated with better language recovery, the role of right hemisphere recruitment during language tasks is more controversial. By some accounts, the right hemisphere plays a beneficial role in language recovery by assuming functions previously represented in the left hemisphere, while other evidence suggests that activation of the right hemisphere during language tasks in patients with chronic aphasia is a reflection of inefficient mechanisms of language processing and may be detrimental to aphasia recovery. Others have argued that functional activation of right hemisphere areas in aphasic patients during language tasks is epiphenomenal, and neither facilitates nor hinders language recovery (Thiel et al., 2001).
The notion that the right hemisphere may play a facilitative role in language recovery after left hemisphere stroke dates as far back as the late 19th century. Barlow (1877) described the case of a 10-year old boy who lost but then recovered the capacity for speech after a left hemisphere stroke, only to lose it again after acquiring a second, right-hemisphere lesion (Finger, Buckner, & Buckingham, 2003). Other reported cases have shown that new right-hemisphere lesions acquired after functional recovery in aphasia can cause deterioration of language (Basso, Gardelli, Grassi, & Mariotti, 1989; Gainotti, 1993; Gowers, 1887). Amobarbital studies have demonstrated that for healthy right-handed adults, language functions are suspended after left-sided carotid injections; however, for aphasic patients with extensive left-hemisphere strokes, residual speech may be suspended by right- and not left-sided carotid injections (Kinsbourne, 1971). Furthermore, some patients who have undergone surgical left hemispherectomy have shown substantial language recovery (Vargha-Khadem et al., 1997) indicating that the right hemisphere possesses the capacity to process language information in the absence of a functioning left hemisphere.
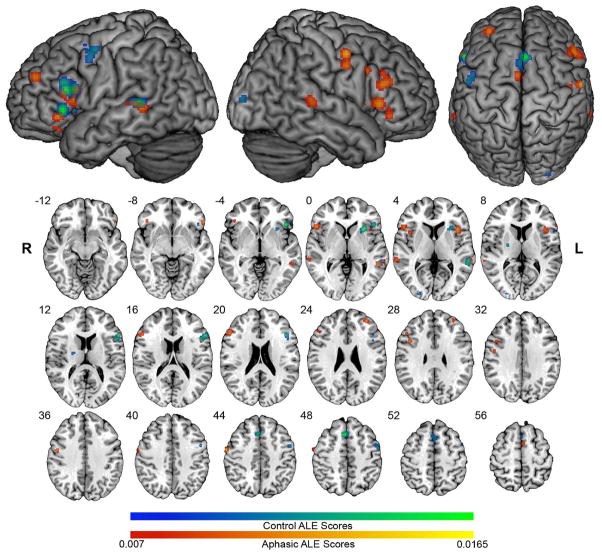
It has been proposed that the capacity for language processing exists in right hemisphere regions that are homotopic to left hemisphere perisylvian structures, but is usually masked by transcallosal interhemispheric inhibition from the dominant left-hemisphere (Karbe et al., 1998b). According to this hypothesis, language recovery after left-hemisphere stroke is associated with a release from inhibition of latent, right-hemisphere language functions. A number of neuroimaging studies involving language tasks have revealed that there is, in addition to activation of left-hemisphere language regions, robust activation in homotopic right hemisphere regions after left-hemisphere stroke (Basso et al., 1989; Buckner, Corbetta, Schatz, Raichle, & Petersen, 1996; Gold & Kertesz, 2000; Ohyama et al., 1996; Rosen et al., 2000; Warburton et al., 1999; Weiller et al., 1995). We recently pursued an investigation of fMRI and PET studies in patients with aphasia using Activation Likelihood Estimation (ALE) meta-analysis in which we analyzed 240 activation foci from 104 aphasics, and 197 foci from 129 controls (See Figure 1). We found that performance on language production tasks in aphasic patients is reliably associated with activation of regions in the right inferior frontal gyrus, whereas comprehension tasks are associated with activation of the right middle temporal gyrus (Turkeltaub, Messing, Norise, & Hamilton, Submitted). In addition to determining the degree to which the location of right hemisphere activation in aphasic patients mirrors that in the left hemisphere, we also examined the degree to which these right hemisphere areas were functionally homologous, that is, the degree to which they activated during the same tasks as left hemisphere areas in normal subjects. We found that among patients with chronic left inferior frontal lesions, patterns of activation in the right inferior frontal gyrus (specifically in the pars opercularis and pars orbitalis) were both homotopic to left inferior frontal gyrus sites in control patients and functionally homologous with respect to the tasks that activated them. Further evidence of functional homology is provided by recent diffusion tensor imaging (DTI) data that indicate that connections between inferior frontal and temporal language regions seen in the left hemisphere are mirrored in homotopic regions of the right hemisphere (Kaplan et al., 2010). These similarities in activation patterns and connectivity support the notion that the right hemisphere possesses and utilizes the functional architecture needed to assume language operations after left hemisphere injury.
Figure 1.
We submitted activation foci from fMRI and PET studies using the same language tasks on both aphasic patients and control subjects to Activation Likelihood Estimation (ALE) analysis. Control ALE clusters are in blue-green scale, and show left hemisphere language and motor activity. Aphasic ALE clusters are in red-yellow scale, and show bilateral activations with right hemisphere areas that are homotopic to control ALE clusters. ALE maps are overlaid on the standard Colin brain in MNI space, using an FDR q = .01 critical threshold, and minimum cluster size of 100 mm3. (Reproduced with permission from Turkeltaub et al., submitted)
The potential for the right hemisphere to acquire or unmask language abilities is the central principle behind at least two behavioral approaches to aphasia treatment. Crosson and colleagues (2009) have described a naming task designed to stimulate reorganization of word production to the right lateral frontal lobe. This task involves subjects making a complex left-hand movement to initiate picture naming attempts, with the rationale that the hand movement activates intention mechanisms in the right medial frontal lobe (Coslett, 1999; Picard & Strick, 1996) that subsequently engage right lateral frontal structures that participate in naming (Crosson et al., 2007). Limited fMRI evidence suggests that improvement in naming in patients who utilize this technique is accompanied by increased right frontal lobe activity (in particular the motor and premotor cortex, and pars opercularis). Melodic intonation therapy (MIT)—a therapeutic approach that relies on the exaggeration of the musical qualities of speech—is another treatment technique that is predicated on recruitment of the right hemisphere for language (Albert, Sparks, & Helm, 1973; Sparks, Helm, & Albert, 1974). Recently, Schlaug and colleagues (2009) have shown using DTI that intense treatment with MIT results in an increase in white matter fibers and volume in the right arcuate fasciculus correlating with subjects’ degree of improvement. This finding further supports the notion that the functional architecture of right hemisphere language areas may mirror that of the left hemisphere perisylvian network (Kaplan et al., 2010), and suggests that these right hemisphere networks may be modified beneficially with training.
A number of additional factors may be important determinants of the degree to which right hemisphere networks are engaged in language recovery. Two of the most important determinants of right hemisphere involvement are lesion size and location. In patients with chronic aphasia, larger lesions involving eloquent cortex of the left hemisphere are associated with greater recruitment of the right hemisphere during language tasks (Heiss & Thiel, 2006; Kertesz et al., 1979). Evidence also suggests that premorbid differences in language lateralization may be a strong predictor of susceptibility to unilateral brain lesions, and may complicate interpretations of left- and right-hemisphere plasticity during poststroke recovery (Andoh & Martinot, 2008; Humphreys & Praamstra, 2002; Knecht et al., 2002). Additionally, it has been argued that hemispheric involvement may be a dynamic process that changes during the course of recovery as a function of time from aphasia onset, patient age, and specific task demands (Finger et al., 2003; Hillis, 2007). In one longitudinal imaging study it was shown that in patients with acute stroke and nonfluent aphasia neither hemisphere is activated during attempted performance of a language task. In the subacute phase, the right hemisphere exhibited stronger involvement in language functions, whereas in the chronic phase, the left hemisphere appeared to regain dominance (Saur et al., 2006). These findings are supported by two studies by Winhuisen and colleagues (2005, 2007), who employed PET and rTMS in the same cohort of aphasic stroke patients within two weeks and again 8 weeks following acute stroke. These authors found that the majority of patients showed bilateral activation of the inferior frontal gyrus during a verbal semantic task, but that over time the proportion of patients in whom inhibitory rTMS of the right inferior frontal lobe disrupted performance decreased. Taken together, these findings indicate both the potential of the right hemisphere to engage in language-related tasks after left-hemisphere stroke as well as its likely evolving role over time (Winhuisen et al., 2005; Winhuisen et al., 2007).
The extent to which the right hemisphere may be able to compensate efficiently after left-hemisphere damage can also depend on the timecourse of injury. For example, Thiel and colleagues (2006) used functional neuroimaging and TMS to elucidate the transferred representation of language functions to the right hemisphere in patients with left-hemisphere tumors. Due to the insidious progression of left-hemisphere injury in these patients, gradual neuroplastic changes may have allowed for adaptive reorganization of language ability in the right hemisphere to an extent that does not occur after acute stroke (Thiel et al., 2006). Cerebral reorganization of language may also depend in part on the age of left hemisphere stroke onset. Elkana and colleagues (2011) reported that pediatric patients who had previously acquired language but then suffered from left hemisphere strokes exhibited right hemisphere activation during language tasks, but that greater proficiency with these language tasks was associated with greater left hemisphere activation. Moreover a shift toward left hemisphere activation during language tasks was observed in a single young patient who they followed over the course of years, suggesting that language reorganization, at least as seen in younger individuals, is a dynamic process that may last for years after stroke onset (Elkana et al., 2011).
The detrimental role of the right hemisphere in aphasia recovery
Increased right hemisphere activity seen after stroke in patients with aphasia may not represent an entirely beneficial change. One alternative account is that right hemisphere involvement after left hemisphere stroke and aphasia reflects inefficient or maladaptive plastic changes in neural activity that have emerged during language reorganization (Belin et al., 1996). According to this model, ineffective changes in language representation may interfere with the reacquisition of more efficient language processing by recovering left-hemisphere cortical networks. Consistent with this argument, it has been shown that increased activation in the right hemisphere in aphasic patients is not always coupled with improved language performance (Naeser, Theoret, & Kobayashi, 2002; Rosen et al., 2000; Saur et al., 2006). In at least one recent fMRI study, increased right hemisphere activity was associated with worse performance on an overt naming task (Postman-Caucheteux et al., 2010).
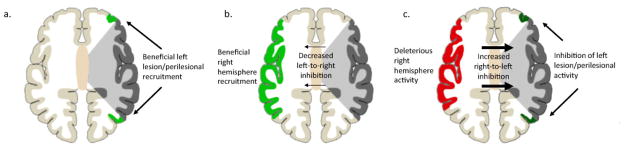
Another hypothesis that further extends the notion of the maladaptive right hemisphere is that increased right hemisphere activation after left hemisphere stroke results in abnormally increased and deleterious transcallosal inhibition of the already damaged left hemisphere. As has been observed with unilateral lesions leading to other deficits such as hemiparesis and neglect, increased contralesional activity after left hemisphere injury may reflect loss of interhemispheric inhibitory influence from damaged language areas in the left hemisphere to right-sided homologues (Martin et al., 2004; Rosen et al., 2000; Shimizu et al., 2002). This release of inhibition and resulting upsurge in right-hemisphere activity may thus result in increased interhemispheric inhibitory influences from the right hemisphere on left hemisphere perisylvian areas, which may exacerbate language symptoms and impede recovery from aphasia (Figure 2).
Figure 2.
Differing accounts of plasticity in language systems in chronic aphasia. 2a) After unilateral left hemisphere stroke (grey), some language functions may be subserved by recovered lesional areas or recruited perilesional areas (light green). 2b) Right perisylvian areas (light green) may be recruited to subserve some language functions, a process facilitated by decreased transcallosal inhibition of the right hemisphere by the damaged left hemisphere. 2c) By contrast, right hemisphere activity may be deleterious. Released from interhemispheric inhibition, right hemisphere structures (red) may exert increased inhibitory influence on left perisylvian areas, impeding functional recovery of lesional and perilesional areas in the left hemisphere (dark green).
Noninvasive brain stimulation techniques: TMS and tDCS
Transcranial magnetic stimulation (TMS) is a technology that can be used to manipulate cortical activity focally, creating either transient or enduring changes in patterns of brain activity (Bailey, Karhu, & Ilmoniemi, 2001; Walsh & Pascual-Leone, 2003). TMS employs the principle of electromagnetic induction and involves the generation of a rapid time-varying magnetic field in a coil of wire. When this coil is held to the head of a subject, the magnetic field penetrates the scalp and skull and induces a small current parallel to the plane of the coil in the brain that is sufficient to depolarize neuronal membranes and generate action potentials. Different TMS paradigms employ various combinations of pulse frequencies, intensities, and stimulation locations. Repetitive TMS (rTMS) involves the application of a series of pulses at a predetermined frequency and can produce effects that outlast the application of the stimulation. Evidence suggests that rTMS delivered at a low frequency (0.5–2 Hz) tends to focally decrease cortical excitability, whereas higher frequencies (faster than 5 Hz) tend to increase excitability (Maeda & Pascual-Leone, 2003). Repetitive TMS has been employed in numerous experiments examining the role of specific cortical areas in the execution of specific linguistic functions (Devlin & Watkins, 2007),
Transcranial direct current stimulation (tDCS) involves the application of small electrical currents (typically 1–2 mA) to the scalp through a pair of surface electrodes. Current flows from the anode, through the cortex, and out through the cathode. Unlike TMS, which induces currents of sufficient magnitude to stimulate action potentials, the weak electrical currents employed in tDCS are thought to modulate the resting membrane potentials of neurons (Nitsche & Paulus, 2000, 2001). The effect of tDCS depends on which electrode is applied to the scalp: cathodal stimulation is associated with decreased cortical excitability due to hyperpolarization of cortical neurons, while anodal stimulation is associated with increased cortical excitability due to subthreshold depolarization. These effects may last for minutes to hours depending on the intensity, polarity, and duration of stimulation (Antal et al., 2001). A growing number of studies have employed of tDCS as an experimental means for manipulating performance in a variety of cognitive domains, and investigators have started to explore the use of tDCS as a possible neurorehabilitation tool for patients with post-stroke deficits (Fregni et al., 2005; Hummel et al., 2005).
Noninvasive brain stimulation informs models of aphasia recovery
A small but growing body of evidence suggests that noninvasive brain stimulation techniques may provide a supplementary treatment approach for certain language deficits in patients with chronic stroke-induced aphasia (See Table 1). Several TMS studies have employed low frequency inhibitory stimulation of the right hemisphere with the goal of focally diminishing neural activity in the intact contralesional hemisphere. Here the work of Naeser and colleagues (Martin et al., 2004; Naeser et al., 2005a; Naeser et al., 2002) has been central. In an initial investigation, 1Hz inhibitory rTMS was applied to four different points on right-hemisphere perisylvian regions of six chronic nonfluent aphasia patients at 90% of motor threshold for 10 minutes. Following stimulation of the anterior portion of the right-hemisphere homologue of Broca’s area (pars triangularis) they observed significant but transient improvement in accuracy and reduction in reaction time with respect to picture naming (Naeser et al., 2002). Conversely, application of 1 Hz rTMS to the posterior portion of the right-hemisphere homologue of Broca’s area (pars opercularis) was associated with a transient decrease in picture naming accuracy and an increase in reaction time. Extending these findings, the same investigators stimulated the right pars triangularis for 20 minutes five days a week for two weeks in four right-handed chronically aphasic patients. Significant improvements in naming were observed, which persisted for at least 8 months following completion of stimulation (Martin et al., 2004; Naeser et al., 2005a). We have replicated these results and demonstrated that stimulation of the right pars triangularis also results in persistent improvements in spontaneous elicited speech (Hamilton et al., 2010). Naeser and colleagues have also recently reported on the case of a patient with chronic nonfluent aphasia and sleep apnea who experienced substantial gains in language ability when 1 Hz rTMS of the right pars triangularis was paired with continuous positive airway pressure (CPAP) (Naeser et al., 2010a).
Table 1.
rTMS Studies in Aphasia
| Study | N | Lesion Location | Time Since Stroke | Aphasia Type(s) | Location(s) Stimulated | Stimulation Parameters | Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|
| Naeser et al., 2005a | 4 | Large left frontotemporal cortical/subcortical lesions (n=3); frontotemporal subcortical lesion with cortical sparing (n=1) | 5–11 years | Broca’s (n=2); anomic/conduction (n=1); global (n=1) | Right pars triangularis | 1 Hz rTMS; 90/MT; 10 daily sessions over two weeks | Boston Naming lest (BNI), Boston DiagnosticAphasia Examination (BDAE); Snodgrass and Vanderwart (S&V) picture naming | Improved accuracy and speeded reaction time for S&V items after 10 TMS sessions; improvement on BNT and Animal and Tool/Implement subtests of BDAE 2 and 8 months after stimulation |
| Naeser et al., 2005b* | 1 | Left frontotemporal subcortical lesion with cortical sparing | 6.5 years | Global | Best cortical region of interest (ROI) determined by stimulation of multiple left hemisphere targets; Right pars triangularis selected | 1 Hz rTMS; 90/MT; 10 daily sessions over two weeks | BNT, BDAE, Cognitive Linguistic Quick Test (CLQT) | Improvements in BNT and Animal and Tool/Implement subtests of BDAE as above; further improvement on BDAE and CLQT in speech therapy 1 year following stimulation |
| Martin et al., 2009 | 2 | Subjects 1&2: Large left frontotemporal cortical/subcortical lesions; Subject 2: Additional involvement of fibers under SMA, Wernicke’s area, and posterior middle frontal gyrus | 1.5 and 10 years | Nonfluent | ROI determined by stimulation of multiple targets; Right pars triangularis selected | 1 Hz rTMS; 90/MT; 10 daily sessions over two weeks | BNT, BDAE, Cookie Theft Picture Description, fMRI during overt naming task | Subject 1: Improvement on BNT, BDAE, and Cookie Theft; new perilesional left frontal activation on fMRI 16 months post-TMS; Subject 2: No significant language improvement or fMRI changes |
| Naeser et al., 2010 | 1 | Left temporal cortical/subcortical lesion; minor involvement of left inferior frontal gyrus | 2 years | Nonfluent | ROI determined by stimulation of multiple targets; Right pars triangularis selected | 1 Hz rTMS; 90/MT; 10 daily sessions over two weeks; patient started on CPAP | BDAE, BNT | Increased phrase length, auditory comprehension, and naming persisting 3 months, 6 months, and 2.4 years after stimulation |
| Hamilton et al., 2010 | 1 | Large left frontotemporal cortical/subcortical lesion | 7 years | Broca’s | ROI determined by stimulation of multiple targets; Right pars triangularis selected | 1 Hz rTMS; 90/MT; 10 daily sessions over two weeks | Western Aphasia Battery (WAB), BDAE, Cookie Theft Picture Description | Improved object and action naming and Cookie Theft. Persistent benefits at 2,6, and 10 months |
| Kakuda et al., 2010a | 4 | Left frontotemporal cortical lesion (n=2); frontal cortical lesion (n=1); left putamen (n=1) | 5–28 years | Motor-dominant (impaired speech). | Site determined by fMRI activation during naming task: left frontal (n=2) and right frontal (n=2). | 1 Hz rTMS; 90/MT; 10 sessions over six days | Japanese versions of WAB, Standard Language Test of Aphasia (SLTA), and Supplementary tests of SLTA (SLTA-ST) | Presumed improvement in WAB, SLTA, and SLTA-ST (no statistical analyses) |
| Kakuda et al., 2010b | 2 | Left MCA territory lesions | 7 months and 8 months | Sensory-dominant (impaired comprehension) | Wernicke’s area | 1 Hz rTMS; 90/MT; 10 sessions over six days; daily language therapy | Token test, Japanese SLTA | Presumed improvement in scores on Token test and some subtests of SLTA that persisted at 3 months (no statistical analyses). |
| Barwood et al., 2010 | 12 | Left MCA territory cortical/subcortical lesions | 2–6 years | Nonfluent | Right pars triangularis; subjects received real (n=6) or sham (n=6) stimulation | 1 Hz rTMS; 90/MT; 10 daily sessions over 10 days | BDAE, Cookie Theft, BNT, S&V. | Improved naming and picture description among subjects receiving real TMS but not sham; persistent effects 2 months after real stimulation. |
| Weiduschat et al., 2010 | 10 | Left posterior temporoparietal lesions (n=4);frontal cortical/subcortical lesions (n=3); subcortical lesions (n=2); entire left MCA territory (n=1) | 16 weeks | Wernicke’s (n=5); Broca’s (n=2); global (n=1); amnestic (n=1) | Right pars triangularis (study group, n=5), vertex (control control group, n=5) | 1 Hz rTMS; 90/MT; 10 daily sessions over two weeks; Speech and language therapy after each TMS session | Aachen Aphasia Test (AAT), PET activation during verb generation task | Improved AAT scores 2 weeks after stimulation in study patients but not control group; PET activation toward right hemisphere in control but not in treatment group 2 weeks after stimulation compared to baseline; no relationship between laterality shift and clinical improvement. |
Naeser et al., 2005b describes in further detail a patient previously reported in Naeser et al., 2005a
One major limitation in prior studies employing rTMS in chronic aphasia has been the small number of subjects reported. Encouragingly, our results and those of Naeser and colleagues were recently further replicated by Barwood and colleagues (2010), who studied a cohort of 12 subjects with chronic aphasia (six real stimulation; six sham) and found that 1-Hz rTMS (20 minutes; 10 sessions over 10 days) administered to the right pars triangularis resulted in significant improvements in picture naming, spontaneous elicited speech, and auditory comprehension in the real rTMS group compared to the sham group. These benefits were observed two months following discontinuation of stimulation. In another recent study, Weiduschat and colleagues (2010) extended earlier findings by applying 1 Hz rTMS (20 minutes; 10 sessions over two weeks) to the right pars triangularis of six patients with subacute aphasia (mean period after stroke = 50 days). Four similar patients received only sham stimulation. Stimulated subjects improved significantly on the Aachen Aphasia test, while patients receiving sham did not. While such studies lend further support to the notion that low-frequency rTMS of the right pars triangularis can facilitate recovery in patients with aphasia, additional investigations that replicate and extend these results in even larger cohorts of patients will be crucial in order to convincingly demonstrate the reliability of this technique.
Not all patients with chronic nonfluent aphasia appear to benefit from low-frequency rTMS of the pars triangularis. In a recent small case series, Martin and colleagues (2009) contrasted findings in two aphasic subjects, one of whom showed improvement after receiving rTMS and one of whom did not. The authors emphasized differences in the distribution of the subjects’ lesions. The subject who responded poorly to rTMS had a lesion that extended beyond the inferior frontal gyrus to encompass dorsal regions of the left motor and premotor cortex, deep white matter near the left supplementary motor area, and the posterior portion of the middle frontal gyrus, a region previously implicated as having an important role in naming ability (Duffau et al., 2005). These structures were spared in the subject who responded well. The subject who responded to rTMS of the right pars triangularis also showed increased fMRI activity in left supplementary motor area (SMA) during a naming task 16 months after receiving rTMS compared to his earlier neuroimaging studies. This change in activation was not seen in the patient who responded poorly to stimulation. These data suggest that differences in lesion anatomy may strongly modulate the functional and behavioral consequences of intervention with rTMS.
Not all investigations using TMS in chronic aphasia have solely targeted the right hemisphere. Hypothesizing that inhibitory interhemispheric connections may have deleterious effects on recovering language networks in either hemisphere, Kakuda and colleagues (2010a) recently applied 1 Hz rTMS (20 minutes; 10 sessions over six days) to sites that were contralateral to those found to be most activated by fMRI during a repetition task. Stimulating the right frontal lobe in two patients and the left frontal lobe in two others, they observed modest benefits in measures of spontaneous speech, repetition, writing, and naming that lasted at least four weeks (Kakuda, Abo, Kaito, Watanabe, & Senoo, 2010a). In another recent study, Kakuda and colleagues (2010b) found that 1 Hz TMS (20 minutes; 10 sessions over six days followed by weekly sessions for three months) administered to Wernicke’s area in the left hemisphere resulted in improvement on a Token Test and several subtests of the Standard Language Test of Aphasia (SLTA; a Japanese language instrument) in two patients with chronic fluent aphasia (Kakuda, Abo, Uruma, Kaito, & Watanabe, 2010b). Unfortunately, both studies reported by Kakuda and colleagues were limited in that neither demonstrated that the gains in performance made by subjects were statistically significant and neither employed a control condition to ensure that patients’ behavioral changes were specifically attributable to TMS.
Data from tDCS studies are limited but encouraging (See Table 2). Monti and colleagues (2008) explored the immediate effects tDCS in patients with chronic aphasia by applying anodal, cathodal and sham stimulation (2 mA, 10 minutes) over the left frontotemporal cortex of eight aphasic patients who had suffered ischemic strokes. In their first experiment, four subjects underwent a single session of cathodal tDCS and a single sham tDCS session separated by at least one week; the four other subjects underwent anodal tDCS and sham sessions. Subjects performed a 20-item list picture naming task immediately prior to and after completion of stimulation with no additional behavioral or language training prior to or during the task. Reaction time and accuracy on a picture-naming task was observed before and immediately after stimulation (Monti et al., 2008). Cathodal tDCS improved accuracy on the naming task by 34%, whereas anodal and sham stimulation had no effect. In a second experiment, stimulation over an occipital control site elicited no effects, supporting the conclusion that the influence of cathodal tDCS was site- and polarity-specific. These results suggest that a single 10-minute tDCS application is able to induce an immediate improvement in naming, although the duration of this benefit was not explored. The authors argue that cathodal stimulation may down-regulate overactive inhibitory cortical interneurons in the lesioned hemisphere, ultimately giving rise to increased activity and function in the damaged left hemisphere. In a more recent study, Baker and colleagues (2010) found that anodal tDCS (1mA, 20 minutes for 5 days) to the left frontal lobe resulted in improvements in naming accuracy among 10 patients with left hemisphere strokes and chronic aphasia (Baker, Rorden, & Fridriksson, 2010). In this study, administration of tDCS was paired with a concurrent anomia treatment consisting of a picture-naming task and the benefit observed persisted for at least one week following administration of stimulation. In another recent study by Fiori and colleagues (2010), five daily sessions of anodal stimulation (20 minutes, 1 mA) over Wernicke’s area in the left hemisphere paired with intensive language training resulted in improved accuracy on a picture-naming task in three patients with chronic nonfluent aphasia (Fiori et al., 2010). In two of these patients, benefits were shown to persist for at least three weeks. One notable difference between the study by Monti and colleagues (2008) and later investigations is the polarity of the electrode (anode or cathode) associated with behavioral benefits. Other differences in the execution of these studies, including the number of sessions employed and the presence or absence of concurrent behavioral treatment may have contributed to different results. Nonetheless, these reported differences in the polarity-specific effects of tDCS complicates our understanding of the neurophysiologic and behavioral effects of tDCS in aphasia, and indicates the need for additional investigations.
Table 2.
tDCS Studies in Aphasia
| Study | N | N Lesion Location | Time Since Stroke | Aphasia Type(s) | Location(s) Stimulated | Stimulation Parameters | Outcome Measure | Results |
|---|---|---|---|---|---|---|---|---|
| Monti et al., 2008 | 8 | Left frontal cortical/subcortical lesions (n=3); frontoparietal cortical/subcortical lesions (n=2); 8 frontotemporoparietal cortical/subcortical lesions (n=2); frontoparietal subcortical lesion | 2–8 years | Broca’s aphasia (n=4); global aphasia (n=4) | Broca’s area; occipital lobe control site | Single session of anodal, cathodal, and sham tDCS over Broca’s area at 1 mA for 20 min; cathodal and sham tDCS applied to occipital lobe in separate experiment | Picture naming | Improved picture naming accuracy immediately after cathodal tDCS of Broca’s area but not anodal, sham, or occipital tDCS |
| Fiori et al., 2010 | 3 | Left frontoparietal subcortical lesion (n=1); frontoparietal 3 cortical/subcortical lesion (n=1); large frontotemporoparietal cortical/subcortical lesion (n=1) | ~2–6 years | Mild (n=1), moderate (n=1), and severe (n=1) nonfluent aphasia | Wernicke’s area | 5 consecutive daily sessions of anodal (1 mA for 20 min) or sham tDCS paired with language therapy | Picture naming | Improved naming accuracy and reaction time after 5 days of anodal but not sham tDCS. In 2 subjects, improvements persisted at 1 and 3 weeks after stimulation |
| Baker et al., 2010 | 10 | Left temporoparietal lesions (n=4); frontotemporal lesions (n=3); frontotemporoparietal lesion (n=1); 10 temporoparietooccipital lesion (n=1); Lesion of entire MCA territory, medial frontal lobe, and basal ganglia (n=1) | ~1–20 years | Anomic aphasia (n=6), Broca’s aphasia (n=4) | tDCS localization guided to left hemisphere targets by fMRI activation during an overt naming task: premotor cortex (n=5), dorsolateral prefrontal cortex (n=2), anterior prefrontal cortex (n=1), pars triangularis (n=1), pars opercularis (n=1) | 5 consecutive daily sessions of anodal (1 mA for 20 min) or sham tDCS paired with anomia therapy | Picture naming | Improved picture naming after anodal tDCS compared to sham stimulation; persistent benefits 1 week after treatment |
To date, findings from the use of TMS and tDCS to treat chronic aphasia have largely been interpreted as supporting the model of interhemispheric inhibition, on the presumption that either facilitating activity in lesioned or perilesional areas or decreasing activity in inhibitory contralesional areas allows for improved language function (Fregni & Pascual-Leone, 2007). However, this model cannot easily account for all TMS and tDCS findings in patients with chronic aphasia. One important issue in this regard is the possible topographic specificity of rTMS. Almost all prior studies that have demonstrated a beneficial effect of right hemisphere stimulation have involved the administration of TMS specifically to the pars triangularis (Barwood et al., 2010; Hamilton et al., 2010; Martin et al., 2009; M. A. Naeser et al., 2005b; M. A. Naeser et al., 2005a; Naeser et al., 2010b; Weiduschat et al., 2010). Naeser and colleagues and we have employed an approach that involves stimulating various sites in the right inferior frontal gyrus as well as the right motor cortex, in order to determine whether there is a specific site that responds best to TMS. Both our preliminary data and that of Naeser and colleagues suggest that TMS-induced improvements in naming are often associated with stimulation of the pars triangularis, but not with stimulation of other nearby right hemisphere sites (Hamilton et al., 2010; Naeser et al., 2010b). Although more data are needed to support this finding conclusively, we believe it is unlikely in the setting of large left hemisphere lesions, that the inhibitory transcallosal connections between left and right hemisphere regions would be so specific as to account for differences in performance that are linked to a single site in the right hemisphere.
An alternative explanation for these findings is that the right hemisphere may contribute to language function in chronic aphasic patients, but not always efficiently. By this account, TMS applied to different right perisylvian regions in patients may differentially affect specific components of a remodeled language network. In some cases, inhibitory stimulation of a right-hemisphere target might increase the overall function of the language network by decreasing the contribution of a dysfunctional element in that network. Our own ALE meta-analysis findings suggest that the pars triangularis is activated in a homotopic manner but is not homologous in its function compared to sites in the left hemisphere language network in normal individuals (Turkeltaub et al., Submitted). In other words, activity in this site is unlikely to contribute efficiently to the operation of reorganized language networks in the right or left hemisphere. Extending this reasoning further, inefficient neural activity in the right pars triangularis may contribute deleterious noise to the operation of reorganized language circuits. Thus inhibition of this site may result in beneficial suppression of a cortical region that would otherwise have an adverse effect on performance.
The notion that noninvasive brain stimulation improves the functionality of an inefficiently reorganized language network fits one aspect of the data that is not readily explained by other hypotheses, namely the finding that language function improves over the course of months following stimulation (Martin et al., 2004; Naeser et al., 2005a; Naeser et al., 2010b). This improvement may reflect further enhancement of connection strengths in improved networks over time as they are reinforced by their continued use in the service of language processing (Bi & Poo, 2001; Spatz, 1996).
Limitations and challenges of noninvasive brain stimulation in aphasia
While mounting evidence suggests that noninvasive brain stimulation may be a useful adjunctive treatment for patients with aphasia after stroke, both TMS and tDCS have limitations that must be considered. One important caveat regarding noninvasive brain stimulation techniques is their limited spatial resolution and the difficulty of knowing precisely which region or regions of the brain are being affected. These concerns are especially applicable to tDCS, which employs relatively large electrodes (typically 5×7 or 5×5 cm) for stimulation. Evidence from computer modeling studies also suggests that the distribution of current in the brain associated with tDCS can be quite diffuse, and that regions of maximal stimulation can be unpredictable, varying with factors like reference electrode size and position (Bikson, Datta, & Elwassif, 2009). While the spatial resolution of TMS is understood to be considerably higher than that of tDCS, evidence suggests that the degree of spatial resolution required to target specific cortical sites such as the pars triangularis is achieved more readily when rTMS is used in conjunction with image-guided navigation techniques (Julkunen et al., 2009), which are not employed by many investigators currently using TMS. Moreover, predictions about neurophysiologic effects of brain stimulation are further complicated in stroke patients by the presence of lesions of varying size and distribution (Wagner et al., 2006).
Another important limitation of noninvasive brain stimulation techniques in aphasia is that current understanding of their neurophysiologic effects and their impact on behavior remains incomplete. For example, while low frequency rTMS is often presumed to have inhibitory effects and high frequency rTMS to have excitatory effects on cortical activity and related behaviors, considerable interindividual variability in these effects has been observed (Gangitano et al., 2002). Perplexingly, some studies that have employed TMS and tDCS in patients with aphasia have reported results contrary to what would have been predicted based on the findings of other investigators. For instance, recent tDCS studies have reported improvement on language performance measures in aphasic patients receiving stimulation of opposite polarities—either cathodal (Monti et al., 2008) or anodal (Baker et al., 2010) —to the left frontal lobe. Thus, while a growing body of evidence suggests that noninvasive brain stimulation techniques may be useful for facilitating aphasia recovery, specific inferences about the anatomic or functional mechanisms of TMS and tDCS in patients with aphasia must still be viewed with some caution until more data has been reported.
A hierarchical model of aphasia recovery
Varying accounts of post-stroke language recovery are not mutually exclusive. Given the weight of evidence supporting both left- and right-hemisphere models of aphasia recovery, it is most likely the case that the recovery process is a dynamic one that involves a variety of plastic changes in both hemispheres. To that end, it has been argued (Heiss & Thiel, 2006) that a hierarchical combination of changes is likely to occur in patients recovering language function after stroke.
According to this hierarchical model, when lesions of the left hemisphere are very small or do not affect critical left hemisphere language centers, complete or near-complete language recovery can often be achieved by restoration of normal patterns of activation in left hemisphere language networks. When lesions of the left hemisphere damage important language centers, perilesional regions of the left hemisphere may be recruited to subserve language function, often leading to good recovery (Karbe et al., 1998b; Karbe et al., 1998a; Miura et al., 1999; Warburton et al., 1999). However, when left hemisphere networks are more severely impaired, the right hemisphere appears to be capable of assuming some language functions, by employing homotopic regions in ways that may mirror some aspects of language processing in the left hemisphere (Basso et al., 1989; Buckner et al., 1996; Gold & Kertesz, 2000; Ohyama et al., 1996; Rosen et al., 2000; Warburton et al., 1999; Weiller et al., 1995). This right hemisphere recruitment for language may be facilitated by the release of interhemispheric inhibition from the damaged left hemisphere. While right hemisphere recruitment for language tasks may contribute to overall language recovery in severely affected patients, the remodeled language network in these patients is likely inefficient compared to premorbid intact left hemisphere perisylvian regions. This is in part because networks in the nondominant right hemisphere may be intrinsically less adept at language processing compared to their dominant left hemisphere counterparts due to genetic predisposition, developmental factors, neuroplastic changes that occur during language learning, or any combination thereof. However, increased recruitment of right hemisphere networks may also be inefficient because they may prevent activation of more efficient left hemisphere language networks via transcallosal inhibition (Belin et al., 1996; Martin et al., 2004; Rosen et al., 2000; Shimizu et al., 2002). In short, the hierarchical model of effective aphasia recovery can be summarized as follows: 1) Best recovery is achieved when left hemisphere language networks recover normal function, 2) good recovery is achieved when perilesional left hemisphere areas compensate for damaged left hemisphere language regions, and 3) limited recovery is achieved when the right hemisphere is inefficiently recruited for language tasks.
As discussed above there also appears to be a temporal component to the distribution of right- and left-sided language function after stroke (Saur et al., 2006). In the context of acute and subacute lesions of the left hemisphere language network there appears to be greater tendency for reallocation of language function to right hemisphere perisylvian circuits, particularly among patients with extensive left hemisphere injury. Over time there is, for a number of patients at least, diminished recruitment of right hemisphere structures for language tasks. Eventually, for some patients with chronic aphasia, significant language recovery is associated with redistribution of language processing back to left hemisphere perisylvian areas.
Intervention with noninvasive brain stimulation may work in several different ways. To date, most therapeutic stimulation studies have employed inhibitory stimulation of right hemisphere structures. This approach may modulate both right and left hemisphere components of chronically reorganized language networks in ways that allow them to function more efficiently. The effect of stimulation in the right hemisphere may be to down-regulate local inhibition of right hemisphere regions engaged in language related tasks. Concurrently, inhibitory stimulation of intact contralesional cortical areas may facilitate increased recruitment of perilesional regions of the left hemisphere into reorganized language networks by diminishing the impact of transcallosal inhibitory inputs to those areas. Finally, although it has been proposed that the effects of noninvasive brain stimulation are specific with respect to their effect on reorganized language networks, it may be the case that the changes in language performance observed after brain stimulation may relate to alterations in cerebral function that are less focal and that may affect a variety of neural functions in ways that have not yet been described. Further investigations will be critical to further clarifying the impact of noninvasive brain stimulation on different mechanisms of aphasia recovery.
Future Directions
Noninvasive brain stimulation provides a potentially promising set of tools for understanding and enhancing aphasia recovery. Future investigations involving noninvasive brain stimulation may be able to further characterize the roles of the left and right hemispheres in aphasia recovery by employing a variety of experimental manipulations. For example, noninvasive brain stimulation techniques could be paired with behavioral techniques that are believed to facilitate right hemisphere involvement in language tasks (Crosson et al., 2007; Schlaug, Marchina, & Norton, 2009). Other investigations may explore the degree to which reorganized language networks in the right hemisphere share functional homology with perisylvian language circuits in the left hemisphere. Administration of therapeutic rTMS to different regions in the right hemisphere could result in manipulation of specific linguistic processes, further elucidating structure-function relationships in reorganized language networks. Additional noninvasive stimulation studies could further characterize temporal aspects of language recovery by stimulating the right and left hemispheres at different timepoints relative to stroke onset. Finally, one important caveat to the current body of data on brain stimulation, as used to enhance language recovery, is that studies to date have largely focused on patients with frontal lobe lesions and nonfluent aphasia. Future studies will need to explore the effects of brain stimulation across a range of aphasia types and in a variety of lesion locations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert ML, Sparks RW, Helm NA. Melodic intonation therapy for aphasia. Arch Neurol. 1973;29:130–131. doi: 10.1001/archneur.1973.00490260074018. [DOI] [PubMed] [Google Scholar]
- Alexander MP. Aphasia: Clinical and anatomical aspects. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. New York: McGraw-Hill; 1997. pp. 133–149. [Google Scholar]
- Andoh J, Martinot JL. Interhemispheric compensation: A hypothesis of TMS-induced effects of language-related areas. European Psychiatry. 2008;23:281–288. doi: 10.1016/j.eurpsy.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Paulus W. External modulation of visual perception in humans. Neuroreport. 2001;12:3553–3555. doi: 10.1097/00001756-200111160-00036. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Karhu J, Ilmoniemi RJ. Transcranial magnetic stimulation as a tool for cognitive studies. Scand J Psychol. 2001;42(3):297–305. doi: 10.1111/1467-9450.00239. [DOI] [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. stroke. 2010;41:1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow T. On the case of double cerebral hemiplegia, with cerebral symmetrical lesions. Brit Med J. 1877;2:103–104. doi: 10.1136/bmj.2.865.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, O’Sullivan JD, et al. Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. Eur J Neurol. 2010 doi: 10.1111/j.1468-1331.2010.03284.x. [DOI] [PubMed] [Google Scholar]
- Basso A, Gardelli M, Grassi MP, Mariotti M. The role of the right hemisphere in recovery from aphasia: Two case studies. Cortex. 1989;25:555–566. doi: 10.1016/s0010-9452(89)80017-6. [DOI] [PubMed] [Google Scholar]
- Basso A, Marangolo P. Cognitive rehabilitation: the emperor’s new clothes? Neuropsychol Rehab. 2000;10:219–229. [Google Scholar]
- Belin P, Van Eeckhout P, Zilbovicious M, Remy P, Francois C, Guillaume S, et al. Recovery from nonfluent aphasia after melodic intonation therapy: A PET study. Neurology. 1996;47:1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb’s postulate revisited. Annu Rev Neurosci. 2001;24:139–66. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiol. 2009;120(6):1033–1034. doi: 10.1016/j.clinph.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Corbetta M, Schatz J, Raichle ME, Petersen SE. Preserved speech abilities and compensation following prefrontal damage. Proc Nat Acad Sci. 1996;93:1249–1253. doi: 10.1073/pnas.93.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch CM, Kleiser R, Seitz RJ. Post-lesional cerebral reorganisation: evidence from functional neuroimaging and transcranial magnetic stimulation. J Physiol Paris. 2006;99:437–454. doi: 10.1016/j.jphysparis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Cherney LH, Small SL. Task-dependent changes in brain activation following therapy for nonfluent aphasia: discussion of two individual cases. J Int Neuropsychol Soc. 2006;12:828–842. doi: 10.1017/S1355617706061017. [DOI] [PubMed] [Google Scholar]
- Cornelissen K, Laine M, Tarkiainen A, Järvensivu T, Martin N, Salmelin R. Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci. 2003;15:441–461. doi: 10.1162/089892903321593153. [DOI] [PubMed] [Google Scholar]
- Coslett HB. Spatial influences on motor and language function. Neuropsychologia. 1999;37:695–706. doi: 10.1016/s0028-3932(98)00116-x. [DOI] [PubMed] [Google Scholar]
- Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, Chang YL, et al. Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol Rev. 2007;17:157–177. doi: 10.1007/s11065-007-9024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Moore AB, McGregor KM, Chang YL, Benjamin M, Gopinath K, et al. Regional changes in word-production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain and Lang. 2009;111:73–85. doi: 10.1016/j.bandl.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Watkins KE. Stimulating language: insights from TMS. Brain. 2007;130(Pt 3):610–622. doi: 10.1093/brain/awl331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128(Pt 4):797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Elkana O, Frost R, Kramer U, Ben-Bashat D, Hendler T, Schmidt D, et al. Cerebral reorganization as a function of linguistic recovery in children: An fMRI study. Cortex. 2011;47(2):202–216. doi: 10.1016/j.cortex.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Finger S, Buckner RL, Buckingham H. Does the right hemisphere take over after damage to Broca’s area? The Barlow case of 1877 and its history. Brain and Lang. 2003;85:385–395. doi: 10.1016/s0093-934x(03)00060-9. [DOI] [PubMed] [Google Scholar]
- Fiori V, Coccia M, Marinelli CV, Vecchi V, Bonifazi S, Ceravolo MG, et al. Transcranial Direct Current Stimulation Improves Word Retrieval in Healthy and Nonfluent Aphasic Subjects. J Cogn Neurosci. 2010;20(8):1415–22. doi: 10.1162/jocn.2010.21579. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16(14):1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Gainotti G. The riddle of the right hemisphere’s contribution to the recovery of language. Eur J Disord Commun. 1993;28:227–246. doi: 10.3109/13682829309060038. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Valero-Cabre A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone A. Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol. 2002;113(8):1249–1257. doi: 10.1016/s1388-2457(02)00109-8. [DOI] [PubMed] [Google Scholar]
- Gold BT, Kertesz A. Right hemisphere semantic processing of visual words in an aphasic patient: An fMRI study. Brain & Lang. 2000;73:456–465. doi: 10.1006/brln.2000.2317. [DOI] [PubMed] [Google Scholar]
- Gowers WR. Lectures on the diagnosis of diseases of the brain. London: Churchill; 1887. [Google Scholar]
- Hamilton RH, Sanders L, Benson J, Faseyitan O, Norise C, Naeser M, et al. Stimulating Conversation: Enhancement of Elicited Propositional Speech in a Patient with Chronic Nonfluent Aphasia Following Transcranial Magnetic Stimulation. Brain and Lang. 2010:45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Hillis AE. Aphasia: Progress in the last quarter of a century. Neurology. 2007;69:200–213. doi: 10.1212/01.wnl.0000265600.69385.6f. [DOI] [PubMed] [Google Scholar]
- Horn SD, DeJong G, Smout RJ, Gassaway J, James R, Conroy B. Stroke rehabilitation patients, practice, and outcomes: is earlier and more aggressive therapy better? Arch Phys Med Rehabil. 2005;86 (12 Suppl 2):S101–S114. doi: 10.1016/j.apmr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Flöel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(Pt 3):490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Praamstra P. Magnetc stimulation reveals the distribution of language in normal population. Nature Neurosci. 2002;5:613–614. doi: 10.1038/nn0702-613. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Saisanen L, Danner N, Niskanen E, Hukkanen T, Mervaala E, et al. Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. Neuroimage. 2009;44(3):790–795. doi: 10.1016/j.neuroimage.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Kaito N, Watanabe M, Senoo A. Functional MRI-based therapeutic rTMS strategy for aphasic stroke patients: a case series pilot study. Int J Neurosci. 2010a;120(1):60–66. doi: 10.3109/00207450903445628. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Uruma G, Kaito N, Watanabe M. Low-frequency rTMS with language therapy over a 3-month period for sensory-dominant aphasia: case series of two post-stroke Japanese patients. Brain Inj. 2010b;24(9):1113–1117. doi: 10.3109/02699052.2010.494587. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Naeser MA, Martin PI, Ho M, Wang Y, Baker E, et al. Horizontal portion of arcuate fasciculus fibers track to pars opercularis, not pars triangularis, in right and left hemispheres: a DTI study. Neuroimage. 2010;52:436–444. doi: 10.1016/j.neuroimage.2010.04.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbe H, Thiel A, Weber-Luxenburger G. Reorganization of the cerebral cortex in post-stroke aphasia studied with positron emission tomography. Neurology. 1998a;50:A321. [Google Scholar]
- Karbe H, Thiel A, Weber-Luxenburger G, Herholz K, Josef K, Heiss WD. Brain plasticity in poststroke aphasia: What is the contribution of the right hemisphere? Brain Lang. 1998b;64:215–230. doi: 10.1006/brln.1998.1961. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Harlock W, Coates R. Computer tomographic localization, lesion size, and prognosis in aphasia and nonverbal impairment. Brain Lang. 1979;8:34–50. doi: 10.1016/0093-934x(79)90038-5. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The minor cerebral hemisphere as a source of aphasic speech. Arch of Neurol. 1971;25:302–306. doi: 10.1001/archneur.1971.00490040028003. [DOI] [PubMed] [Google Scholar]
- Knecht S, Flöel A, Dräger B, Breitenstein C, Sommer J, Henningsen H, et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat Neurosci. 2002;5:695–699. doi: 10.1038/nn868. [DOI] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004;156:439–443. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- Laska AC, Hellblom A, Murray V, Kahan T, Von Arbin M. Aphasia in acute stroke and relation to outcome. J Intern Med. 2001;249:413–422. doi: 10.1046/j.1365-2796.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- Lazar RM, Speizer AE, Festa JR, Krakauer JW, Marshall RS. Variability in language recovery after first-time stroke. J Neurol Neurosurg Psychiatry. 2008;79:530–534. doi: 10.1136/jnnp.2007.122457. [DOI] [PubMed] [Google Scholar]
- Lendrem W, Lincoln NB. Spontaneous recovery of language in patients with aphasia between 4 and 34 weeks after stroke. J Neurol Neurosurg Psychiatry. 1985;48:743–748. doi: 10.1136/jnnp.48.8.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Pascual-Leone A. Transcranial magnetic stimulation: studying motor neurophysiology of psychiatric disorders. Psychopharmacology (Berl) 2003;168(4):359–376. doi: 10.1007/s00213-002-1216-x. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Treglia E, Kaplan E, Baker EH, et al. Research with transcranial magnetic stimulation in the treatment of aphasia. Curr Neurol Neurosci Rep. 2009;9(6):451–458. doi: 10.1007/s11910-009-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Theoret H, Maria Tormos J, Nicholas M, Kurland J, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Seminars in Speech and Language. 2004;25:181–191. doi: 10.1055/s-2004-825654. [DOI] [PubMed] [Google Scholar]
- McNeil MR, Pratt SR. Defining aphasia: Some theoretical and clinical implications of operating from a formal definition. Aphasiology. 2001;15:900–911. [Google Scholar]
- Miura K, Nakamura Y, Miura F, Yamada I, Takahashi M, Yoshikawa A, et al. Functional magnetic resonance imaging to word generation task in a patient with Broca’s aphasia. JNeurol. 1999;246:939–942. doi: 10.1007/s004150050486. [DOI] [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, et al. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79:451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain. 1999;122 (Pt 9):1781–1790. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Lundgren K, Klein R, Kaplan J, Treglia E, et al. Improved language in a chronic nonfluent aphasia patient after treatment with CPAP and TMS. Cogn Behav Neurol. 2010a;23(1):29–38. doi: 10.1097/WNN.0b013e3181bf2d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, et al. Improved naming after TMS treatments in a chronic, global aphasia patient-case report. Neurocase. 2005b;11(3):182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain and Language. 2005a;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Treglia E, Ho M, Kaplan E, Bashir S, et al. Research with rTMS in the treatment of aphasia. Restorative Neurology and Neuroscience. 2010b;28:511–529. doi: 10.3233/RNN-2010-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Theoret H, Kobayashi M. Modulation of cortical areas with repetitive transcranial magnetic stimulation to improve naming in nonfluent aphasia. Presented at the 8th International Conference on Functional Mapping of the Human Brain.2002. [Google Scholar]
- Nicholas ML, Helm-Estabrooks N, Ward-Lonergan J, Morgan AR. Evolution of severe aphasia in the first two years post onset. Arch Phys Med Rehabil. 1993;74:830–836. doi: 10.1016/0003-9993(93)90009-y. [DOI] [PubMed] [Google Scholar]
- Nickels L. Improving word finding: practices makes (closer to) perfect? Aphasiology. 2002;16:1047–1060. [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Friel KM. Cortical plasticity after stroke: implications for rehabilitation. Rev Neurol. 1999;155:713–717. [PubMed] [Google Scholar]
- Ohyama M, Senda M, Kitamura S, Ishii K, Mishina M, Terashi A. Role of the nondominant hemisphere and undamaged area during word repetition in poststroke aphasics: a PET activation study. Stroke. 1996;27:807–903. doi: 10.1161/01.str.27.5.897. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, et al. Single-trial fMRI Shows Contralesional Activity Linked to Overt Naming Errors in Chronic Aphasic Patients. J Cogn Neurosci. 2010;22:1299–1318. doi: 10.1162/jocn.2009.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RR. The efficacy of treatment for aphasic persons: a meta-analysis. Brain Lang. 1994;47:582–608. doi: 10.1006/brln.1994.1060. [DOI] [PubMed] [Google Scholar]
- Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res. 1995;41:172–187. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- Robey RR, Schultz MC, Crawford AB, Sinner CA. Single-subject clinical-outcome research: designs, data, effect sizes, and analyses. Aphasiology. 1999;16:445–473. [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;26:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006;129(Pt 6):1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann NY Acad Sci. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Sparks R, Helm N, Albert M. Aphasia rehabilitation resulting from melodic intonation therapy. Cortex. 1974;10:303–316. doi: 10.1016/s0010-9452(74)80024-9. [DOI] [PubMed] [Google Scholar]
- Spatz HC. Hebb’s concept of synaptic plasticity and neuronal cell assemblies. Behav Brain Res. 1996;78:3–7. doi: 10.1016/0166-4328(95)00221-9. [DOI] [PubMed] [Google Scholar]
- Thiel A, Habedank B, Herholz K, Kessler J, Winhuisen L, Haupt WF, et al. From the left to the right: How the brain compensates progressive loss of language function. Brain and Language. 2006;98:57–65. doi: 10.1016/j.bandl.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Thiel A, Herholz K, Koyuncu A, Ghaemi M, Kracht LW, Habedank B, et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann Neurology. 2001;50:620–629. doi: 10.1002/ana.1253. [DOI] [PubMed] [Google Scholar]
- Thompson CK. Neuroplasticity: Evidence from aphasia. J Commun Disord. 2000;33:357–366. doi: 10.1016/s0021-9924(00)00031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Shapiro LP, Ballard KJ, Jacobs BJ, Schneider SS, Tait ME. Training and generalized production of wh- and NP movement structures in agrammatic speakers. J Speech Lang Hear Res. 1997;41:228–244. doi: 10.1044/jslhr.4002.228. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Messing S, Norise C, Hamilton RH. Meta -analysis reveals consistent activation patterns in aphasic patients. (Submitted) [Google Scholar]
- Vargha-Khadem F, Carr LJ, Isaacs E, Brett E, Adams C, Mishkin M. Onset of speech after left hemispherectomy in a nine-year-old boy. Brain. 1997;120:159–182. doi: 10.1093/brain/120.1.159. [DOI] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Eden U, Ramos-Estebanez C, Grodzinsky A, Zahn M, et al. Transcranial magnetic stimulation and stroke: a computer-based human model study. Neuroimage. 2006;30(3):857–870. doi: 10.1016/j.neuroimage.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial magnetic stimulation : a neurochronometrics of mind. Cambridge, Mass: MIT Press; 2003. [Google Scholar]
- Warburton E, Price CJ, Swinburn K, Wise RJ. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neuros Psychiatry. 1999;66:155–161. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiduschat N, Thiel A, Rubi-Fessen I, Hartmann A, Kessler J, Merl P, et al. Effects of Repetitive Transcranial Magnetic Stimulation in Aphasic Stroke: A Randomized Controlled Pilot Study. Stroke. 2010 doi: 10.1161/STROKEAHA.110.597864. [DOI] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntjes M, Huber W, Muller S, Bier D, et al. Recovery from Wernicke’s aphasia: A positron emission tomographic study. Ann Neurology. 1995;37:723732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. The right inferior frontal gyrus and poststroke aphasia: a follow-up investigation. Stroke. 2007;38:1286–1292. doi: 10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]