Abstract
It has become apparent of late that even in tamoxifen and/or aromatase resistant breast cancers, ERα remains a bona fide therapeutic target. Not surprisingly, therefore, there has been considerable interest in developing Selective ER Degraders (SERDs), compounds that target the receptor for degradation. Currently, ICI 182,780 (ICI, fulvestrant) is the only SERD approved for the treatment of breast cancer. However, the poor pharmaceutical properties of this injectable drug and its lack of superiority over second line aromatase inhibitors in late stage breast cancer have negatively impacted its clinical use. These findings have provided the impetus to develop second generation, orally bioavailable SERDs with which quantitative turnover of ERα in tumors can be achieved. Interestingly however, the contribution of SERD activity to fulvestrant efficacy is unclear, making it difficult to define the characteristics desired of the next generation of ER antagonists. It is of significance therefore, that we have determined that the antagonist activity of ICI and its ability to induce ERα degradation are not coupled processes. Specifically, our results indicate that it is the ability of ICI to interact with ERα and to (a) competitively displace estradiol and (b) induce a conformational change in ER incompatible with transcriptional activation that are likely to be the most important pharmacological characteristics of this drug. Collectively, these data argue for a renewed emphasis on the development of high affinity, orally bioavailable pure antagonists and suggest that SERD activity though proven effective may not be required for ERα antagonism in breast cancer.
Keywords: estrogen receptor; degradation; ICI 182,780; fulvestrant; SERD
1. INTRODUCTION
The estrogen receptor (ER) α (ESR1) is expressed in the majority of breast tumors, enabling them to respond to the mitogenic actions of estrogens. Thus, therapeutic interventions targeting ER activity at the level of receptor activation (tamoxifen) or ligand synthesis (aromatase inhibitors) have a dramatic positive impact on breast cancer pathogenesis. Unfortunately, a considerable number of patients whose tumors express ERα display de novo resistance to existing ER modulators or develop resistance to these interventions over time. However, it has become apparent that even in tumors that exhibit resistance to currently available antiestrogens/aromatase inhibitors, ERα remains a bona fide therapeutic target [1–3]. A possible explanation for this apparently paradoxical finding was provided by the observation that hyperactivation of signaling pathways and processes that converge on the receptor, or its associated proteins, can result in ligand independent transcriptional activation of ERα. Of particular interest in this regard is the observation that tumors resistant to the selective estrogen receptor modulators (SERMs) such as tamoxifen frequently display increased expression of HER2 and that signaling events initiated by this receptor impinge on ERα resulting in its transcriptional activation [4, 5]. Conversely, resistance to the tyrosine kinase inhibitors trastuzumab and lapatinib have been associated with re-expression of and dependence upon ERα for growth [6]. It has also been shown in cellular models of breast cancer that treatment with aromatase inhibitors leads to a hyperactivation of the MAPK signaling pathways and that this increases cellular sensitivity to estrogens by 2–3 orders of magnitude [7]. Thus, oxysterols or other compounds with modest estrogenic activities can have profound effects on tumor growth [8, 9]. For these reasons, there is considerable interest in the potential clinical utility of an emerging class of ligands, the selective estrogen receptor degraders (SERDs) that effect a complete removal of ERα from the cell.
The only SERD currently approved for clinical use is ICI 182,780 (ICI, fulvestrant, Faslodex®). Studies performed in vitro have demonstrated that ICI is a complete antagonist/inverse agonist of ERα activity and this has been attributed to its ability to effect a quantitative turnover of the receptor [10, 11]. However, although ICI inhibits growth of breast tumor xenografts in animal models [12], this drug has not demonstrated a similar degree of success as a breast cancer intervention. In the EFFECT trial, for instance, the overall response rate was only 10% [13]. Although these findings were initially considered to indicate that SERD intervention would not be useful in breast cancer, a considerable amount of additional data has emerged to indicate that it is the pharmacological properties of this drug, rather than its mechanism of action, that limit its efficacy. Indeed, the poor bioavailability of ICI results in steady state plasma levels in the range of 6–9 ng/ml (10–14 nM) with concentrations as low as 2 nM having been measured in the tumor vicinity [14, 15]. This is in contrast to drug concentrations in the 10–1000 nM range that are routinely used to inhibit ERα signaling in vitro. Furthermore, Dowsett and colleagues have demonstrated in the pre-adjuvant setting that even in patients who demonstrate good exposure to the drug at the approved dose, less than 50% turnover of the receptor occurs, unlike the near complete ERα degradation observed in ICI-treated cells in culture [16]. Because receptor turnover is regarded as the primary mechanism by which ICI antagonizes ERα action, the observed lack of response to treatment is thought to reflect the inability of the drug to achieve quantitative turnover of the receptor in tumors.
Despite the pharmacological limitations of ICI, the potential clinical benefit of a SERD in patients whose breast cancer is resistant to endocrine therapy has led to efforts to improve responses to fulvestrant and/or to identify a new SERD with improved pharmacokinetics. This endeavor would be facilitated by an understanding of the mechanism(s) by which ICI inhibits ERα signaling and by an assessment of the relative contribution of receptor degradation to drug efficacy. It has been established that ICI induced degradation of ER involves ubiquitination of ERα and its subsequent sequestration to the nuclear matrix of the cell. From this compartment ERα is then shuttled to the 26S proteasome and degraded [11, 17]. However, studies of the impact of ICI on ER structure have demonstrated that the binding of this drug induces a conformational change in the receptor that disrupts the integrity of the primary coactivator binding surface [10, 18]. Given these results, it remains to be determined whether ICI inhibits ERα primarily through mechanisms that are extrinsic (directing receptor degradation) or intrinsic (ligand induced conformational change that blocks coactivator interaction) to the ERα-ICI complex. If ICI inhibition is intrinsic, then achieving a saturating dose of ICI or another high affinity pure antagonist of ERα would have clinical benefit in breast cancer treatment regardless of whether or not it results in receptor degradation. Therefore, we sought to define the relative contribution of pure competitive antagonism versus degradation by ICI to its antagonist efficacy.
2. MATERIALS AND METHODS
2.1. Reagents
ER ligands included 17β-estradiol (E2 – Sigma, St. Louis, MO), ICI 182,780 (ICI – Tocris, Ellisville, MO), 4-hydroxytamoxifen (4OHT – Sigma), and Lasofoxifene (Laso – a gift from Wyeth (now Pfizer, NY, NY)). Ligands were dissolved in ethanol. Antibodies used for immunoblot detection included sc-6259 (cytokeratin 18) and sc-8005 (ERα), both from Santa Cruz Biotechnology (Santa Cruz, CA). IGF1 was ordered from PeproTech (Rocky Hill, NJ), and cycloheximide (CHX) and MG132 were procured from Sigma.
2.2. Cell culture
Human mammary epithelial cells (HMECs) were obtained as an outgrowth from normal tissue obtained from a breast reducing surgery under a Duke Medical Center IRB protocol. These cells were isolated as previously described [19] and were plated for experiments on the initial passage after thawing from liquid nitrogen storage. For experiments, HMECs were plated in DFC1 media (DMEM/F12 media supplemented with EGF, Triiodothyronine, β-estradiol, insulin, hydrocortisone, ethanolamine, phosphoethanolamine, transferrin, sodium selenite, Cholera toxin, bovine pituitary extract, and 1% fetal bovine serum (FBS) (all procured from Sigma)) which was replaced 24 hours after plating with estrogen free media (phenol red free DMEM/F12 with all supplements above with the exception of β-estradiol and bovine pitutitary extract, and substitution of charcoal stripped FBS). MCF7, BT474, BT483, and MDA-MB-231 breast cancer cells were maintained in DMEM/F12 (MCF7), RPMI (BT474 and BT483) or DMEM (MDA-MB-231) media (Invitrogen, Carlsbad, CA) supplemented with 8% FBS and were plated for experiments in the same media lacking phenol red and supplemented with 8% charcoal stripped FBS (CFS). 48 hours after plating, cells were infected with recombinant adenovirus expressing human ERα using multiplicity of infection (MOI) 0–100. Cells were treated with ER ligands or growth factors as indicated immediately following infection and were harvested for immunoblot or real time quantitative PCR analysis 24 hours after treatment.
2.3. Adenovirus production
An adenoviral vector expressing human ERα was constructed by inserting the ERα cDNA into pENTR (Invitrogen) prior to shuttling into pAd-DEST-v5 using the Gateway system per manufacturer’s instructions. Adenovirus was produced and purified as previously described [20].
2.4. Transfection
MCF7 cells were plated in 6 well culture dishes at a density of 4×105 per well in phenol red free DMEM/F12 + 8% CFS, and were then transfected using Lipofectin (per manufacturer’s instructions – Invitrogen) with 500 ng per well pERE-3tata-luc (previously described [8]) and 100 ng per well of a plasmid expressing CMV-controlled β-galactosidase as an internal control. 24 hours later, cells were infected with ER adenovirus as above and treated with ER ligands. After 24 hours treatment, cells harvested for immunoblot or qRT-PCR analysis as described below or for detection of luciferase and β-galactosidase activity as previously described [8].
2.5. Immunoblot analysis
ERα expression was analyzed as previously described with minor modifications [11]. Cells were lysed in RIPA buffer (50mM Tris pH 8.0, 150mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.05% SDS, 1mM EDTA) to produce whole cell extracts (WCE) or in low detergent buffer (100mM Tris pH 8.0, 150mM NaCl, 0.5% NP-40, 0.02% SDS, 2mM EDTA) to isolate the soluble fraction of the cells. Following Bradford analysis of clarified lysates, 50 µg of protein was resolved by SDS-PAGE prior to transfer to PVDF membrane and immunoblot analysis to detect ERα (D12 SantaCruz, CA) or cytokeratin 18 (DC-10, SantaCruz) as a loading control. To analyze the insoluble fraction, pelleted debris following clarification was resuspended in Laemmli sample buffer (BioRad, Hercules, CA)and a volume equal to 50 µg of the corresponding soluble extract was resolved and detected as above.
2.6. Real time quantitative PCR analysis
Cells treated as indicated were washed in PBS prior to lysis. RNA isolation (BioRad) and reverse transcription (iScript; BioRad) were performed per kit manufacturer’s instructions. qRT-PCR of cDNA was done using iQ SYBR Green Supermix (Bio-Rad) per kit instructions and performed using the iCycler optical system with associated software (Bio-Rad). mRNA abundance was calculated using the ΔΔCT method as previously described [21]. Primer sequences are available upon request.
3. RESULTS
3.1. ICI 182,780-mediated inhibition of ERα-dependent transcriptional activity does not require proteasomal turnover of the receptor
It is generally considered that the antagonist activity of ICI 182,780 results from its ability to interact with and alter the conformation of ERα in such a way as to enable it to be recognized and degraded by the 26S proteasome. However, we have observed that ICI is a very efficient inhibitor of ERβ-mediated transcriptional activity in the absence of observable effects on receptor stability, suggesting that antagonist efficacy and degradation are not coupled processes [22]. Definition of the contribution of ERα degradation to ICI antagonist efficacy is an important issue to address, as receptor turnover in tumors is currently used as a surrogate marker for drug exposure/efficacy and considering that there is considerable interest in developing mechanism based screens for novel antiestrogens. Thus, we embarked on a study aimed at defining the relationship between ERα turnover and antagonist efficacy.
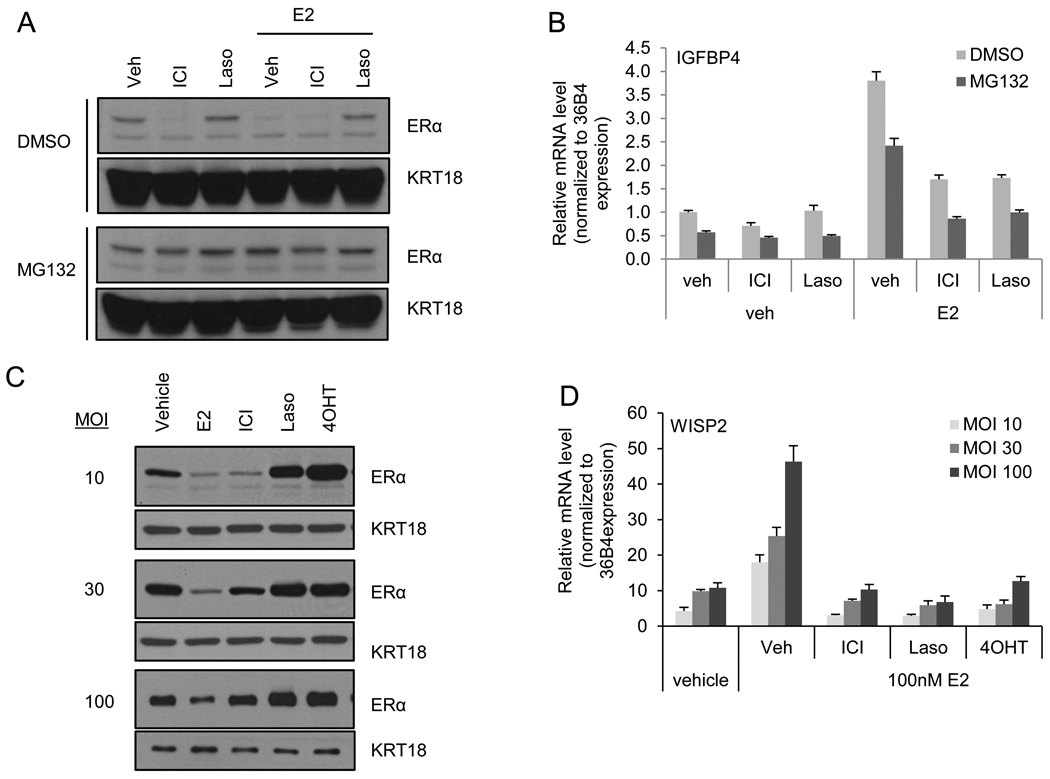
Our previous studies have indicated that both 17-β estradiol (E2) and ICI dependent degradation of ERα is a proteasome mediated process. Thus, we initially asked whether blockade of 26S proteasomal activity with the inhibitor MG132 would impact the pharmacological actions of ICI. To this end, ER-positive MCF7 breast cancer cells were treated with or without MG132 for 2 hours prior to 4 hours treatment with ICI or the high affinity competitive antagonist Lasofoxifene (Laso) in the presence or absence of E2. As anticipated, both E2 and ICI induced ER degradation in the vehicle treated cells, while MG132 blocked degradation of the receptor by either treatment. ER expression was unaffected by Laso regardless of MG132 treatment (Figure 1A). As had been reported previously MG132 treatment alone was shown to attenuate the maximal activation by E2 of ER-target gene transcription (Figure 1B). However, the efficacy of ICI as an ERα antagonist was not affected by MG132 indicating that ICI induced degradation may be dispensable for inhibition of ER by this pure antagonist. These results highlight the need to define the contributions of ERα turnover versus competitive receptor antagonism in determining the antagonist efficacy of ICI.
Figure 1. ICI inhibits ERα activation in despite apparent lack of ERα degradation.
A–B) MCF7 cells were treated 2 hours with DMSO or MG132 (30 µM) prior to treatment for 4 hours with vehicle (Veh), ICI 182,780 (ICI – 100 nM), or lasofoxifene (Laso – 100 nM) in the presence or absence of estradiol (E2 – 1 nM). A) Expression of ERα and loading control cytokeratin (KRT) 18 was detected by immunoblot analysis of whole cell extracts (WCE). B) ERα activation of target gene IGFBP4 was analyzed by real time quantitative PCR (qRT-PCR) analysis of samples treated in parallel with those in A. C–D) ER-negative HMECs were infected with an adenovirally expressed ERα followed by treatment for 24 hours with ER ligands: vehicle, E2 (100 nM), ICI (1 µM), Laso (1 µM), or 4-hydroxy tamoxifen (4OHT – 1µM). C) ERα and loading control cytokeratin (KRT) 18 levels were detected by immunoblot analysis of infected HMEC whole cell extracts. D) ERα activation of target gene WISP2 was analyzed by qRT-PCR of samples infected and treated in parallel with those in C. mRNA expression was normalized to similarly detected housekeeping gene 36B4 using the ΔΔCT method [21]. Data are representative of 3 independent experiments.
We and others have determined that ICI exhibits an IC50 for ER transactivation in the nanomolar range, a concentration that overlaps with that required to initiate receptor degradation. Thus, it has been difficult to separate competitive antagonism by ICI from its ability to induce receptor turnover in cells expressing endogenous levels of receptor. In an effort to separate these processes we asked whether the degradation pathway that mediates ICI-dependent ER degradation could be saturated by receptor overexpression. As a first step in these studies we examined the impact of ICI on ERα transcriptional activity and turnover in cells engineered to express increasing levels of receptor protein. Since breast cancer cells differentially express the coactivator AIB1 and this has a profound impact on ERα stability [23, 24], we performed our initial analysis in ERα-negative human mammary epithelial cells (HMECs), which exhibit several fold lower expression of AIB1 (data not shown). HMECs were infected with increasing amounts of an adenovirus expressing human ERα (MOI – 10, 30, and 100) and were treated for 24 hours with E2, ICI, Laso, or 4-hydroxytamoxifen (4OHT) as indicated. ERα expression levels in whole cell extracts of harvested cells were analyzed by western immunoblot while ERα transcriptional activity was determined by real time quantitative PCR (qRT-PCR) analysis of WISP2 mRNA expression. At low MOI (10), resulting in ERα expression levels comparable to that observed in MCF7 cells (not shown), ERα was efficiently degraded by ICI treatment and, as expected, was stabilized by Laso and 4OHT (Figure 1C). In cells infected and treated in parallel, E2 mediated induction of WISP2 was efficiently inhibited by ICI (Figure 1D). At increasing MOI of 30 and 100, , ERα degradation by ICI became saturated, for ERα levels remained high in the HMECs even after 24 hours ICI treatment with comparable levels observed in ICI and vehicle treated samples (Figure 1C). In the parallel analysis of ERα transcriptional activity we noticed that ICI treatment at the high MOI still retained the ability to inhibit ERα transactivation of WISP2 even though receptor protein levels were not significantly affected (Figure 1D). Similarly Laso and 4OHT, although stabilizing ERα, also effectively inhibited WISP2 expression. Taken together these data indicate that in this reconstituted model system, ligand induced degradation of ERα can be saturated, yet efficient antagonism of ERα activity was maintained. While ICI does facilitate ERα degradation, this activity does not appear to be required for its antagonist activity. Furthermore, the saturable nature of the degradation machinery indicates that differences in the expression of components of the degradation machinery in tumors may impact the ability to achieve turnover of the receptor in all tumors.
3.2. ICI-mediated degradation of ERα is saturable in transformed cells
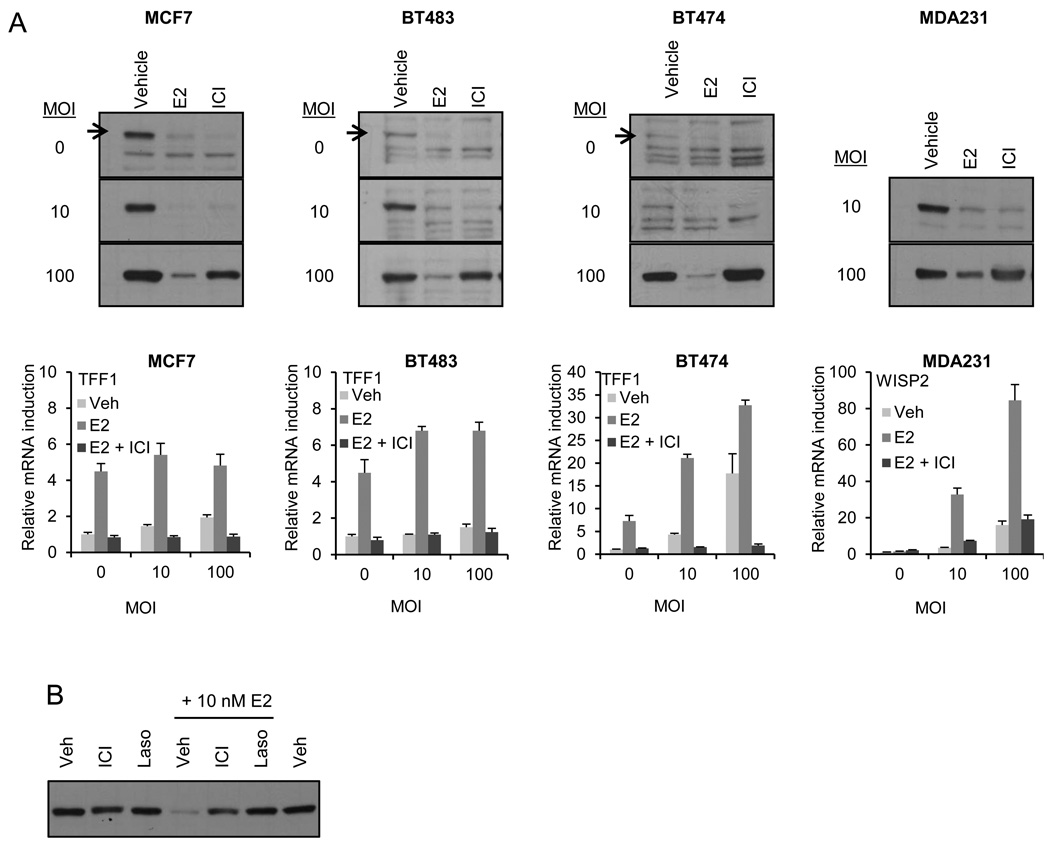
We next asked whether a similar profile was apparent in transformed breast cells. To this end, we evaluated E2 and ICI-induced ERα degradation in breast cancer cell models in which ERα is expressed at different levels: high (MCF7), low (BT483 and BT474) or absent/undetectable (MDA MB 231). We also manipulated ERα expression in these same model systems and evaluated ligand regulated receptor turnover. For this analysis cells were transduced with an increasing MOI (0–100) of adenovirus encoding ERα followed by 24 hours treatment with E2 (100 nM) or ICI (1 uM), and ERα levels in whole cell extracts were analyzed by immunoblot. In all of these cell lines, ICI induced efficient degradation of endogenously expressed ERα while receptor accumulated in ICI treated cells when high levels of ERα (MOI 100) were expressed (Figure 2A). We conclude that, independent of cellular context, there exists a threshold over which ICI dependent degradation of ERα is saturated and receptor accumulates. Whether or not this threshold is ever met under any physiological or pathological conditions remains to be determined. However, as observed in the HMECs, E2 dependent induction of the endogenous ER target genes TFF1 or WISP2 is inhibited by ICI in a manner that is not significantly influenced by the expression level of the receptor (Figure 2A). Similar results were obtained when the antagonist activity of ICI was assessed on an ERE-3 tata-luciferase reporter in transfected cells expressing different level of ERα (Suppl. Figure 2A–C).
Figure 2. ICI dependent ERα degradation is saturable in breast cancer cell lines.
A) ER positive (MCF7, BT483, and BT474) and negative (MDA-MB-231) cells were infected with increasing MOI of ERα adenovirus prior to 24 hours treatment with E2 (100 nM) or ICI (1µM). ER levels were analyzed by immunoblotting of whole cell extracts (WCE). Corresponding immunoblots of loading control KRT18 are featured in Suppl. Figure1. Expression of ER target genes TFF1 or WISP2 in cells infected in parallel and treated 24 hours with E2 (10 nM) in the presence or absence of ICI (1 µM) was analyzed by qRT-PCR as in Figure 1 (illustrated below the corresponding immunoblots). B) MCF7 cells were infected with MOI of 100 ER adenovirus and treated with Veh, ICI, or Laso (1 µM) in the presence or absence of E2 (10 nM) for 24 hours prior to immunoblot analysis of ERα. Data are representative of at least 3 independent experiments.
We also considered the possibility that the overexpressed ERα was resistant to ICI mediated turnover as it was in some way misfolded and unable to bind ligands. Our results appear to address this issue in that treatment of MCF7 cells with E2 (10 nM) resulted in near complete degradation of overexpressed ERα and co-treatment with ICI (1 µM) was able to quantitatively reverse E2 dependent degradation of its receptor (Figure 2B). Thus, despite overexpression it is possible to exchange ER-bound E2 with ICI and protect the receptor from degradation. Finally, efficient degradation of the overexpressed ER was observed in cells treated with a range of E2 concenrations (1–100 nM) but not with ICI treatment (0.1–1 µM) despite robust inhibition of ER activity at these same doses (Suppl. Figure 2D and 2E). Taken together the data from experiments performed in both normal and breast cancer cells indicate that ICI mediated inhibition of ERα is separable from its ability to effect ERα degradation.
3.3. Overexpression of ERα results in alterations in ICI-mediated receptor compartmentalization
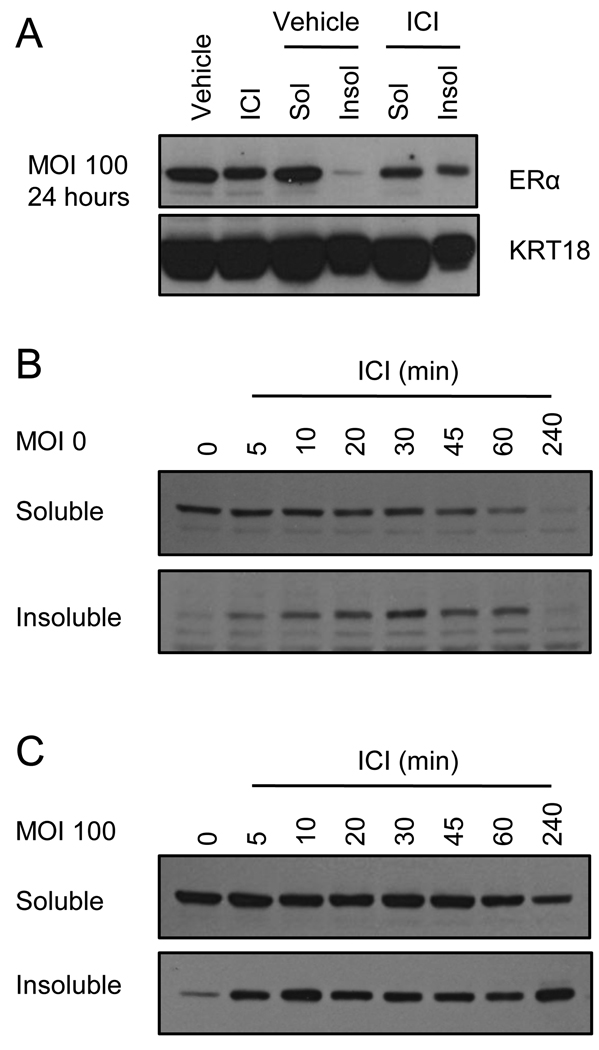
We previously demonstrated that E2 mediated turnover of ERα requires it to be delivered to DNA and that the receptor be engaged in transcriptional activation [17]. In contrast, ICI mediated receptor degradation involves the partitioning of the receptor from the soluble, cytoplasmic compartment of the cell to a highly insoluble fraction where it is shuttled to the proteasome [11]. This is an important biochemical distinction as it suggests that the inhibitory actions of ICI may relate not only to receptor degradation but to the ability of this compound to facilitate sequestration of the receptor in the insoluble/cell matrix fraction.
To define the impact of overexpression on the partitioning of the ERα/ICI complex we collected the soluble and insoluble fractions in lysates from MCF7 cells transduced with the ERα expressing adenovirus (MOI 0 or 100) that had been treated with ICI for 24 hours. Parallel samples were either analyzed as whole cell extract (RIPA buffer as above) or extracted using a low detergent buffer to release the soluble fraction of the receptor. The insoluble debris was then further extracted to release the fraction of receptor associated with either DNA or the insoluble nuclear matrix. Equal amounts of the soluble extract, or an equivalent volume of the insoluble fraction, was analyzed by immunoblot for expression of ERα (Figure 3A). Comparison of vehicle and ICI treated whole cell extracts from the infected cells indicated that similar amounts of ERα remained in the cells after 24 hours of ICI treatment. However, what was particularly evident was that, although ICI treatment resulted in the partitioning of some receptor to the insoluble fraction of the cell, a considerable amount of the receptor remained in the soluble fraction (Figure 3A). Similar results were observed using adenoviral infection of BT483 cells (suppl. Figure 3). We also analyzed the kinetics of ICI mediated ERα localization and degradation under conditions of overexpression. Specifically, MCF7 cells were infected with 0 or 100 MOI of ERα adenovirus and then 24 hours post-infection were treated with ICI for short time points (0, 5, 10, 20, 30, 45, 60, or 240 minutes) (Figure 3B and C). ERα associated with soluble or insoluble fractions following these treatments was analyzed as in Figure 3A. In the uninfected cells, translocation of ERα to the insoluble matrix was detectable after just 5 minutes of ICI treatment, with ERα levels in the insoluble fraction peaking at 30 minutes. Over the course of treatment, ERα levels in the soluble fraction steadily decline as the receptor is translocated to the insoluble fraction and then degraded until by 4 hours of treatment ERα is minimally detected in either soluble or insoluble fraction, indicating near complete degradation of the endogenous receptor (Figure 3B). In the infected cells, however, ERα is likewise detectable in the insoluble fraction following just 5 minutes treatment with ICI, but over the course of 4 hours treatment, ERα is detected in the both soluble and insoluble fractions, with a significant portion remaining in the soluble fraction even after 4 hours of treatment (Figure 3C). Thus, although ICI effects a quantitative inhibition of transcription even under conditions of overexpression (see Figure 2), this activity does not track with either receptor turnover or the partitioning of the receptor to the insoluble nuclear matrix.
Figure 3. Persisting ICI-occupied ERα is present in both soluble and insoluble fractions of cell lysate.
A) Parallel wells of MCF7 cells were infected with adenovirus expressing ER (MOI 100) and treated with vehicle or ICI (1 µM) for 24 hours. Upon harvest, one well per treatment was processed as whole cell extract while the other was fractionated into low-detergent soluble and insoluble fractions. 50 µg of WCE or soluble extract, or volume equal to soluble extract, were analyzed by immunoblotting of ERα or KRT18 loading control. B–C) MCF7 cells were infected in parallel with (A) with MOI 0 (B) or 100 (C) ERα expressing adenovirus for 24 hours prior to short treatments (0, 5, 10, 20, 30, 45, 60, or 240 minutes) with ICI (1 µM). Cells were fractionated and analyzed as in (A). KRT18 expression is depicted in Suppl. Figure 3B. Data are representative of 3 independent experiments.
3.4. The inhibitory actions of ICI on receptor action can be reversed by competitive displacement with 17-β estradiol
It has been shown that ICI binding induces a conformational change in ERα that exposes hydrophobic patches on the surface of the receptor ligand binding domain that likely results in partitioning to the insoluble nuclear matrix, at which point it is subjected to ubiquitin-mediated proteasome dependent turnover [10, 11, 18, 25, 26]. It has been assumed that the sequestration of ERα in the nuclear matrix is an irreversible process and that independent of turnover this activity contributes to the antagonist efficacy of the compound [26, 27]. However, the results presented above indicate that, when overexpressed, a significant amount of ERα does not make it to the nuclear matrix and is turned over slowly. The presence of ERα in the soluble fraction of the cell led us to question whether or not, independent of its ultimate location, the receptor was irreversibly inhibited by ICI binding, or whether it could be activated following displacement of the antagonist with E2. The ability to competitively displace ICI with E2 and achieve transcriptional activation would suggest that its ability to function simply as a high affinity competitive antagonist is an important component of its pharmacological activity. To address this question, we designed a series of experiments to ascertain whether competitive displacement of ICI from ERα with E2 was sufficient to convert the receptor to a transcriptionally active form.
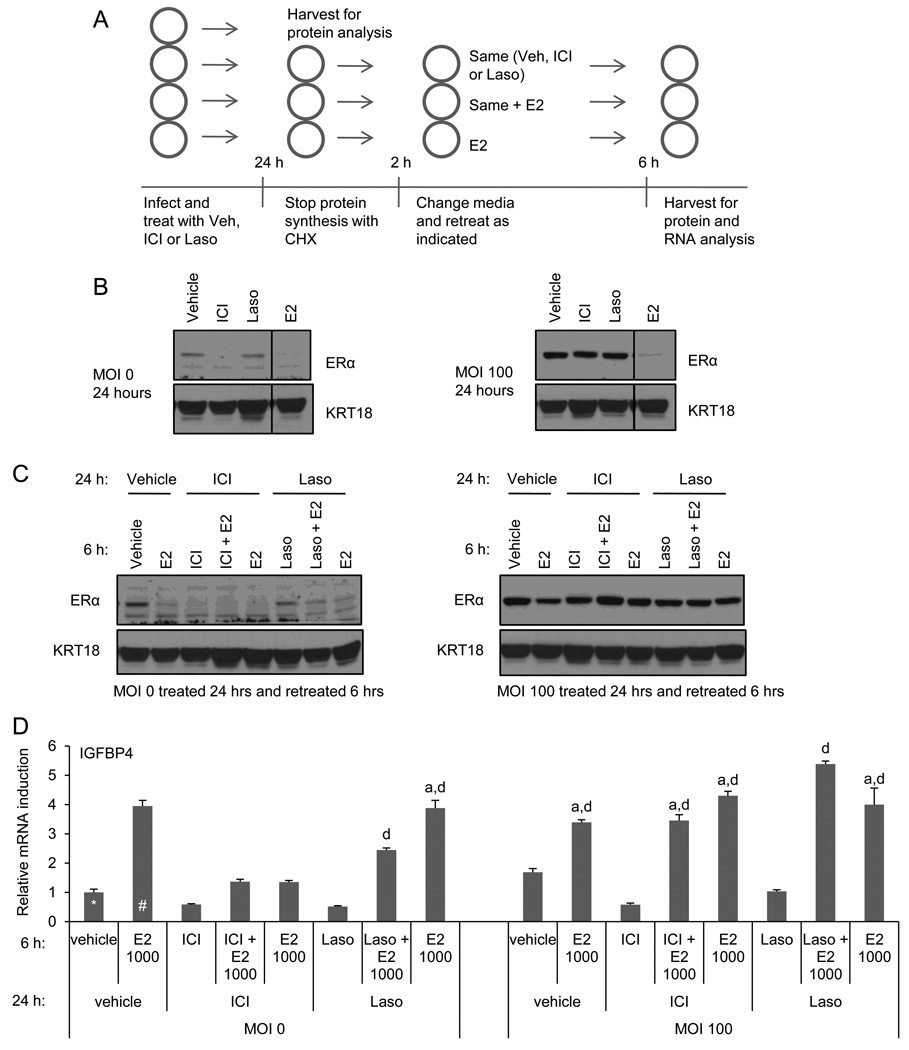
MCF7 cells were infected with 0 or 100 MOI of adenovirus expressing ERα followed by treatment with ICI or unrelated high affinity antagonist Laso (1µM) or vehicle. After 24 hours treatment, one well per treatment was harvested to assess ER expression and to confirm that ICI degradation was saturated in the infected cells (see schematic in Figure 4A). Cycloheximide (CHX) was added to the remaining wells to arrest protein synthesis; this effectively isolated the pool of remaining ERα to allow us to analyze receptor reactivation without the complication of new receptor synthesis. Following 2 hours CHX treatment, media was replaced and cells were retreated with the original treatment (vehicle, ICI or Laso) in the presence or absence of equimolar E2 (1µM) or with E2 alone (see Figure 4A). After 6 hours additional treatment, ERα expression and activity were analyzed by immunoblot of whole cell extracts and qRT-PCR, respectively.
Figure 4. ICI dependent ER inhibition can be reversed with E2.
A) Experimental schematic of B–D. MCF7 cells were infected with 0 or 100 MOI ER adenovirus and treated for 24 hours with vehicle, ICI, or Laso (1µM). Cells were harvested (B) or treated 2 hrs with CHX prior to retreatment with or without ICI or Laso in the presence or absence of E2 (1 µM). Cells were then harvested and ER expression (C) and activity (D) were analyzed by immunoblot or qRT-PCR analysis of WCE or RNA, respectively. mRNA expression was normalized as in Figure 1. Data are representative of 3 independent experiments. Statistical significance in (D) was calculated by one-way ANOVA followed by Newman-Keuls multiple comparison test. a indicates p > 0.05 as compared to the estradiol treated uninfected control (denoted #). d indicates p < 0.001 as compared the vehicle treated uninfected control (denoted *).
Analysis of ERα expression in the samples treated for 24 hours with the different ligands confirmed that endogenous ERα expression is quantitatively downregulated in the uninfected cells by both E2 and ICI but not Laso. This experiment also confirmed that the overexpressed ERα is not significantly down regulated by ICI but is still subjected to E2 mediated turnover (Figure 4B). Following CHX treatment and retreatment with ligands, ERα was minimally detected in the uninfected cells that were initially treated with ICI, regardless of the treatment regimen. In the uninfected cells initially treated with Laso, retreatment with E2 alone or together with Laso resulted in ERα degradation, demonstrating ligand exchange with E2 (Figure 4C). In the infected cells, CHX treatment followed by retreatment with either ICI or Laso, alone or together with E2, does not result in further change in ERα expression. The fact that the ER level observed in the infected cells initially treated with ICI and retreated with ICI remains comparable to the associated vehicle treated control (rather than displaying increased receptor degradation) indicates that incomplete degradation of ERα is not due to incomplete saturation of the receptor by the 24-hour original treatment (Figure 4C).
We next examined the ability of E2 to reverse the inhibition of ER-target gene expression observed in ICI or Laso treated cells under the conditions described in Figure 4C. As expected, ICI or Laso treatment inhibited both the basal and E2 stimulated expression of IGFBP4 mRNA (Figure 4D). Overexpression of ERα alone resulted in increased basal expression of IGFBP4 although this expression was further induced by the addition of E2. However, although ICI can inhibit the expression of IGFBP4 under the same conditions, adding excess E2 can reverse this inhibition. We conclude, therefore, that 1) ICI occupied receptor is available for ligand exchange and can be activated by E2, 2) ICI binding and the subsequent change in ERα conformation is reversible, and 3) that simple competitive antagonism is an important aspect of ICI pharmacology. These data indicate for the first time that the high affinity with which ICI binds to ERα and the ensuing change in receptor conformation are sufficient to inhibit ERα activity through competitive antagonism independent of degradation of ERα.
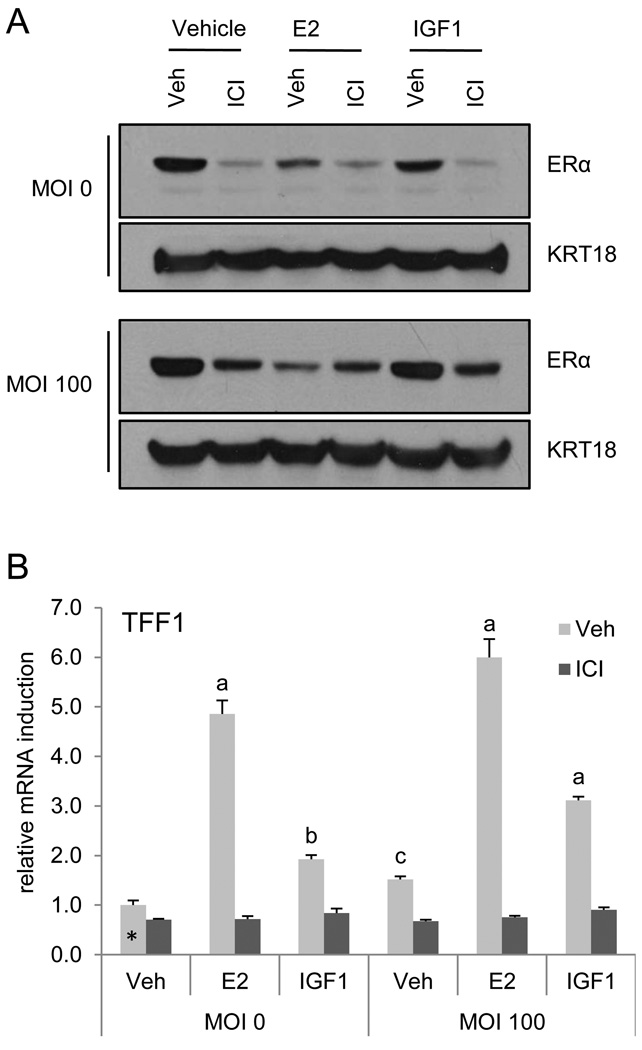
3.5. ICI inhibits growth factor receptor dependent activation of ERα independent of receptor degradation
Of particular significance to human breast cancer is the observation that activation of growth factor receptors at the membrane, such as HER2 and IGF1, results in phosphorylation and subsequent transcriptional activation of ERα [4, 28, 29]. Furthermore, it has been shown that elevation in ERα expression is a common hallmark of breast cancers that acquire resistance to growth factor receptor inhibitors such as trastuzumab and lapatanib [6]. In addition, resistance to aromatase inhibitors is thought to result in part from an upregulation of signaling pathways (i.e. MAPK) that ultimately lead to receptor activation [1, 30]. For this reason there has been a high level of interest in developing ligands, such as SERDs, that result in ERα degradation. Given these observations and our studies which demonstrate that ICI mediated degradation of ERα can be uncoupled from antagonism, we asked whether degradation was required for the inhibition of ER activation initiated by growth factor signaling. To address this issue, ERα was adenovirally expressed in MCF7 cells (0 or 100 MOI) and ERα levels and activation following treatment with estradiol or IGF1 in the presence or absence of ICI was analyzed by immunoblot or qRT-PCR using duplicate samples. E2, ICI, and to a lesser extent IGF1 treatment resulted in ERα degradation in uninfected cells, while only E2 treatment initiated degradation of the overexpressed receptor (Figure 5A). As anticipated, ICI inhibited TFF1 induction by E2 or IGF1 in the uninfected cells (Figure 5B). Despite the persistence of the receptor in the cells overexpressing ERα (Figure 5A), ICI also inhibited ERα transactivation of TFF1 in response to either E2 or IGF1. This indicates that ERα degradation is equally dispensable for ICI inhibition of growth factor receptor initiated activation of ERα (Figure 5B). These results question the need for SERD activity to achieve a useful inhibition of ligand independent activation of ERα.
Figure 5. ICI inhibits agonist-independent activation of ERα by growth factor receptor signaling.
MCF7 cells were infected with ERα expressing adenovirus (MOI = 0 or 100) prior to 16 hours treatment with ICI (1 µM) in the presence or absence of E2 (10 nM) or IGF1 (100 ng/ml). ERα and KRT18 expression and ER activation were analyzed in duplicate samples by either immunoblotting (A) or real time qRT-PCR (B), respectively. mRNA expression was normalized as in Figure 1. Statistical significance was calculated as in Figure 4. a, b, and c indicate p < 0.001, p < 0.01, and p < 0.05, respectively, as compared to the uninfected vehicle treated control (denoted *).
4. DISCUSSION
Treatment of breast cancer by targeting ERα signaling either at the level of hormone synthesis (aromatase inhibitors) or receptor activity (SERMs) has been the mainstay of endocrine treatment for ER positive tumors. However, the inhibitory activity of these drugs can be bypassed by upregulation of signaling pathways that impinge upon ER and augment the partial agonist activity of SERMs or enable ligand independent activation of the receptor. While tumors acquire resistance to endocrine therapy, ERα expression is generally retained and ER signaling remains growth stimulatory. Together these findings have provided the rationale for the development of molecules with SERD-like activity that would have clinical utility as a second or third line breast cancer treatment. Unfortunately, the poor pharmacological/pharmaceutical properties of the first clinically available SERD, fulvestrant (ICI), has been an impediment to the testing of this hypothesis. It has not yet been possible to establish a clear relationship between steady state serum levels of ICI and ERα expression. Furthermore, a sequential tumor biopsy study has indicated that even after long-term treatment with ICI at the originally approved dose, ERα is still present at approximately 50% of the original baseline [16]. It is unclear, therefore, whether the lack of turnover of ERα represents poor exposure of the tumor to the drug or its failure to effect a turnover of the receptor in the tumor. The results of the recently reported CONFIRM trial indicate that use of a high dose regimen of fulvestrant (ICI) (500 mg as compared the originally approved 250 mg) resulted in improved disease free survival as well as time to progression in patients with metastatic breast cancer [31]. It remains to be determined if this higher dose resulted in improved turnover of the receptor. Regardless, it is important to determine whether or not ERα turnover is required for the inhibitory activity of this compound, whether receptor level is a useful surrogate marker for drug efficacy, and whether the search for SERDs, as opposed to high affinity competitive antagonists, is justified. To address this issue, we have in this study used cell-based models of ERα action to determine the contribution of receptor turnover to ICI antagonist efficacy.
The ERα antagonist activity of ICI is generally thought to result from a ligand induced conformational change in the receptor and its subsequent partitioning to an insoluble matrix bound fraction where it is targeted for proteasomal degradation. This mechanism implies that antagonist efficacy is complex involving at least three different activities: (a) competitive antagonism, (b) physical removal of ER from the functional pool of receptors and (c) receptor degradation. Whereas the first two processes are intrinsic to the receptor-ligand complex, the last step is likely to be extrinsic and dependent on the relative expression level and activity of E3 ligases and other components of the degradation machinery. Our findings reveal that ICI dependent degradation of ERα is a saturable process, in HMECs and in several cellular models of breast cancer, allowing us to define the relative contribution(s) of intrinsic and extrinsic processes to the overall antagonist activity of ICI. While we find that ICI degradation of ERα is indeed an extrinsic activity whose efficiency varies between cell models, the efficacy of ICI as an inhibitor of ERα transcriptional activity was similar in all cells and under saturating conditions was not influenced by ERα expression level. Notable in this regard was the observation that ICI dependent sequestration and proteasomal degradation of ERα could be saturated by overexpression without altering the inhibitory actions of ICI on transcription. Furthermore, although ICI binding partially denatures the receptor, it is significant that ERα can be reactivated by displacing the bound drug with estradiol. From a practical point of view, these data suggest that competitive inhibition of ERα, and not degradation, is the primary mechanism by which ICI manifests its antagonist activity. However, the ability of estradiol to displace ICI from ERα and enable it to activate transcription is particularly intriguing as we have in the past demonstrated that the interaction of this drug with ER induces a ubiquitination pattern that is distinct from that observed in the presence of estradiol [17]. Whereas it is possible that, under the conditions of receptor overexpression that we employed, stoichiometric ubiquitination was not achieved, it is also possible that upon binding estradiol there is a change in the ubiquitination (or other post translational modifications) status of the receptor. Finally, it is also possible that the ICI mediated changes in the post-translational modifications of ERα are maintained and have no impact on receptor activity or that the impact of these modifications is not apparent in our transcriptional assays. These are important studies that have implications with respect to the pharmacological actions of these compounds that are currently been investigated in the laboratory.
The data we present in this study suggest that ERα degradation, or typical “SERD” activity, may not be the best measure of ICI efficacy. These findings bear significance with respect to the design of next generation SERDs or ERα antagonists for use in the treatment of breast cancer. It has been generally assumed that efficacy as a breast cancer intervention will directly correlate with the efficiency with which receptor degradation is induced - an activity that would be influenced by proteins and processes extrinsic to the ER-SERD complex. Indeed our observation that the efficiency of ICI induced ERα degradation in cells is influenced by receptor expression level would certainly support a mechanism in which degradation is a process controlled by extrinsic factors. However, notwithstanding the limitations of the in vitro assays we have used in this study, it is unclear whether receptor turnover is required to inhibit ER signaling in advanced tumors. While we would expect that initiation of ER degradation may indeed prove indispensible to ER antagonism for some compounds currently being developed as breast cancer therapeutics, our data would suggest a significant effort also be made to identify and evaluate high affinity competitive antagonists in this setting as well. Our findings suggest that an orally bioavailable, high affinity antagonist that does not display partial agonist activity would likely be effective in the treatment setting where ICI is currently used.
Supplementary Material
MCF7 (A), BT483 (B), BT474 (C) and MDA231 (D) cells were infected with increasing MOI of ERα adenovirus prior to 24 hours treatment with E2 (100 nM) or ICI (1µM). ERα (Figure 2) and KRT18 (A–D) expression were analyzed by immunoblotting of whole cell extracts. Data are representative of at least 3 independent experiments.
A–C) MCF7 cells were transfected with pERE-3tata-luc 24 hours prior to infection with ER adenovirus (MOI 0 or 100) followed by 24 hours treatment with Veh, ICI (1 uM) or E2 (100 nM). A) Expression of ERα and KRT 18 in WCE was detected by immunoblot. B–C) MCF7 cells transfected and infected as in A were treated with Veh or ICI (1 µM) in the presence or absence of E2 (10 nM). B) mRNA expression of target genes TFF1 and IGFBP, as well as luciferase, was detected by qRT-PCR as in Figure 1. C) Luciferase activity in WCE was analyzed through detection of bioluminescence. D–E) MCF7 cells were infected with MOI 0 or 100 of ERα adenovirus prior to 24 hours treatment with E2 (1, 10 or 100 nM) or ICI (0.1 or 1 µM). D) ERα and KRT 18 expression were analyzed by blotting of whole cell extracts. E) Expression of TFF1 was analyzed by qRT-PCR of samples infected in parallel with A and treated with Veh or E2 (1 or 10 nM) in the presence or absence of ICI (0.1 or 1 µM). mRNA expression was normalized as in Figure 1.
A) BT483 cells were infected with adenovirus expressing ER (MOI 0, 10 or 100) and treated with vehicle, E2 (100 nM) or ICI (1 µM) for 24 hours. Upon harvest, cells were fractionated into low-salt/low-detergent soluble and insoluble fractions. 50 µg of soluble fraction or volume equal of insoluble fraction were analyzed by immunoblotting of ERα or KRT18 loading control. B) MCF7 cells were infected in parallel with (A) with MOI 0 (B) or 100 (C) ERα expressing adenovirus for 24 hours prior to short treatments (0, 5, 10, 20, 30, 45, 60, or 240 minutes) with ICI (1 µM). Cells were fractionated and expression of ERα (Figure 3) or KRT18 (B) was analyzed by immunoblot. Data are representative of 3 independent experiments.
ACKNOWLEDGEMENTS
Supporting funding provided by: DK048807 (DPM) CA084955 (JRM)
The authors gratefully acknowledge the assistance and expertise of Gudrun Huper in isolation, maintenance and plating of HMECs. The authors are also grateful to Dr. John Norris for insightful discussions and assisting with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary: The authors have nothing to disclose.
Contributor Information
Suzanne E. Wardell, Email: suzanne.wardell@duke.edu.
Jeffrey R. Marks, Email: jeffrey.marks@duke.edu.
Donald P. McDonnell, Email: donald.mcdonnell@duke.edu.
REFERENCES
- 1.Masri S, Phung S, Wang K, Wu X, Yuan Y, Wagman L, et al. Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res. 2008;68:4910–4918. doi: 10.1158/0008-5472.CAN-08-0303. [DOI] [PubMed] [Google Scholar]
- 2.Dodwell D, Wardley A, Johnston S. Postmenopausal advanced breast cancer: options for therapy after tamoxifen and aromatase inhibitors. Breast. 2006;15:584–594. doi: 10.1016/j.breast.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Robertson JF, Come SE, Jones SE, Beex L, Kaufmann M, Makris A, et al. Endocrine treatment options for advanced breast cancer-the role of fulvestrant. Eur J Cancer. 2005;41:346–356. doi: 10.1016/j.ejca.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Gee J, Robertson J, Gutteridge E, Ellis I, Pinder S, Rubini M, et al. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signaling and oestrogen receptor activity in clinical breast cancer. Endocrine-Related Cancer. 2005;12:S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 5.Shou J, Massarweh S, Osborne C, Wakeling A, Schiff R. Mechanisms of tamoxifen resistance: Increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 6.Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue W, Fan P, Wang J, Li Y, Santen R. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–110. doi: 10.1016/j.jsbmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato H, Nishida S, Tomoyori H, Sato M, Ikeda I, Imaizumi K. Oxysterol regulation of estrogen receptor alpha-mediated gene expression in a transcriptional activation assay system using HeLa cells. Biosci, Biotechnol, Biochem. 2004;68:1790–1793. doi: 10.1271/bbb.68.1790. [DOI] [PubMed] [Google Scholar]
- 10.Wijayaratne A, Nagel S, Paige L, Christensen D, Norris J, Fowlkes D, et al. Comparative analysis of mechanistic difference among antiestrogens. Endocrinology. 1999;140:5828–5840. doi: 10.1210/endo.140.12.7164. [DOI] [PubMed] [Google Scholar]
- 11.Wittmann B, Sherk A, McDonnell D. Definition of functionally important mechanistic differences among selective estrogen receptor down-regulators. Cancer Res. 2007;67:9549–9560. doi: 10.1158/0008-5472.CAN-07-1590. [DOI] [PubMed] [Google Scholar]
- 12.Creighton CJ, Massarweh S, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, et al. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer Res. 2008;68:7493–7501. doi: 10.1158/0008-5472.CAN-08-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of Fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 14.Robertson JFR. Fulvestrant (Faslodex)-How to make a good drug better. Oncologist. 2007;12:774–784. doi: 10.1634/theoncologist.12-7-774. [DOI] [PubMed] [Google Scholar]
- 15.Wakeling A, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 16.Robertson J, Nicholson R, Bundred N, Anderson E, Rayter Z, Dowsett M, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res. 2001;61:6739–6746. [PubMed] [Google Scholar]
- 17.Wijayaratne A, McDonnell D. The human estrogen receptor-α is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem. 2001;276:35684–35692. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- 18.Pike ACW, Brzozowski AM, Walton J, Hubbard RE, Thorsell A-G, Li Y-L, et al. Structural insights into the mode of action of a pure antiestrogen. Structure. 2001;9:145–153. doi: 10.1016/s0969-2126(01)00568-8. [DOI] [PubMed] [Google Scholar]
- 19.Band V, Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proceedings of the National Academy of Sciences. 1989;86:1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaillard S, Grasfeder LL, Haeffele CL, Lobenhofer EK, Chu T-M, Wolfinger R, et al. Receptor-selective coactivators as tools to define the biology of specific receptor-coactivator pairs. Mol Cell. 2006;24:797–803. doi: 10.1016/j.molcel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.DuSell C. Department of Pharmacology and Cancer Biology. Durham, North Carolina: Duke; 2009. The molecular pharmacology of endogenous and therapeutic estrogen receptor modulators in the breast and skeleton; p. 216. [Google Scholar]
- 23.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan X-Y, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 24.Shao W, Keeton E, McDonnell D, Brown M. Coactivator AIB1 links estrogen receptor transcriptional activity and stability. Proceedings of the National Academy of Sciences. 2004;101:11599–11604. doi: 10.1073/pnas.0402997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan M, Rickert EL, Chen L, Aftab SA, Nephew KP, Weatherman RV. Characterization of molecular and structural determinants of selective estrogen receptor downregulators. Breast Cancer Res Treat. 2007;103:37–44. doi: 10.1007/s10549-006-9353-2. [DOI] [PubMed] [Google Scholar]
- 26.Long X, Nephew K. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-α. J Biol Chem. 2006;281:9607–9615. doi: 10.1074/jbc.M510809200. [DOI] [PubMed] [Google Scholar]
- 27.Lipfert L, Fisher J, Wei N, Scafonas A, Su Q, Yudkovitz J, et al. Antagonist-induced, activation function-2-independent estrogen receptor alpha phosphorylation. Mol Endocrinol. 2006;20:516–533. doi: 10.1210/me.2005-0190. [DOI] [PubMed] [Google Scholar]
- 28.Fujimoto N, Katzenellenbogen BS. Alteration in the agonist/antagonist balance of antiestrogens by activation of protein kinase A signaling pathways in breast cancer cells: antiestrogen selectivity and promoter dependence. Mol Endo. 1994;8:296–304. doi: 10.1210/mend.8.3.7517003. [DOI] [PubMed] [Google Scholar]
- 29.Yue W, Wang J-P, Conaway M, Masamura S, Li Y, Santen RJ. Activation of the MAPK pathway enhances sensitivity of MCF-7 breast cancer cells to the mitogenic effect of estradiol. Endocrinology. 2002;143:3221–3229. doi: 10.1210/en.2002-220186. [DOI] [PubMed] [Google Scholar]
- 30.Santen R, Jeng M-H, Wang J-P, Song R, Masamura S, McPherson R, et al. Adaptive hypersensitivity to estradiol: Potential mechanism for secondary hormonal responses in breast cancer patients. J Steroid Biochem Molec Biol. 2001;79:115–125. doi: 10.1016/s0960-0760(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 31.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko I, Khasanov R, et al. Results of the CONFIRM Phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor positive advanced breast cancer. J Clin Oncol. 2010;28 doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MCF7 (A), BT483 (B), BT474 (C) and MDA231 (D) cells were infected with increasing MOI of ERα adenovirus prior to 24 hours treatment with E2 (100 nM) or ICI (1µM). ERα (Figure 2) and KRT18 (A–D) expression were analyzed by immunoblotting of whole cell extracts. Data are representative of at least 3 independent experiments.
A–C) MCF7 cells were transfected with pERE-3tata-luc 24 hours prior to infection with ER adenovirus (MOI 0 or 100) followed by 24 hours treatment with Veh, ICI (1 uM) or E2 (100 nM). A) Expression of ERα and KRT 18 in WCE was detected by immunoblot. B–C) MCF7 cells transfected and infected as in A were treated with Veh or ICI (1 µM) in the presence or absence of E2 (10 nM). B) mRNA expression of target genes TFF1 and IGFBP, as well as luciferase, was detected by qRT-PCR as in Figure 1. C) Luciferase activity in WCE was analyzed through detection of bioluminescence. D–E) MCF7 cells were infected with MOI 0 or 100 of ERα adenovirus prior to 24 hours treatment with E2 (1, 10 or 100 nM) or ICI (0.1 or 1 µM). D) ERα and KRT 18 expression were analyzed by blotting of whole cell extracts. E) Expression of TFF1 was analyzed by qRT-PCR of samples infected in parallel with A and treated with Veh or E2 (1 or 10 nM) in the presence or absence of ICI (0.1 or 1 µM). mRNA expression was normalized as in Figure 1.
A) BT483 cells were infected with adenovirus expressing ER (MOI 0, 10 or 100) and treated with vehicle, E2 (100 nM) or ICI (1 µM) for 24 hours. Upon harvest, cells were fractionated into low-salt/low-detergent soluble and insoluble fractions. 50 µg of soluble fraction or volume equal of insoluble fraction were analyzed by immunoblotting of ERα or KRT18 loading control. B) MCF7 cells were infected in parallel with (A) with MOI 0 (B) or 100 (C) ERα expressing adenovirus for 24 hours prior to short treatments (0, 5, 10, 20, 30, 45, 60, or 240 minutes) with ICI (1 µM). Cells were fractionated and expression of ERα (Figure 3) or KRT18 (B) was analyzed by immunoblot. Data are representative of 3 independent experiments.