Summary
CD5 is a scavenger-like receptor expressed in association with the antigen-specific receptors on T and B-1a lymphocytes. Recent studies reveal a broader biology for CD5 that includes its role as regulator of cell death and as a receptor for pathogen associated molecular patterns, in addition to its previously described function as an inhibitory receptor. These findings shed new light into the mechanistic role of CD5 in leukemias and effector cells to exogenous (infectious) or endogenous (autoimmune, tumoral) antigens. The newly identified properties make this receptor a potential candidate to be targeted for therapeutic intervention as well as immune modulation. This review describes the current knowledge on the function of CD5 as an immunomodulatory receptor both in health and disease.
Introduction
The CD5 lymphocyte receptor (also named T1, Tp67 in humans or Lyt-1 in mouse) was one of the first surface markers used to identify T cells, and was later found to be expressed by leukemic B cells and a subset of normal mature B-cells (named B-1a B cells) that secrete natural poly-reactive antibodies [1]. Structurally, CD5 is a member of the ancient and highly conserved superfamily of protein receptors named SRCR (for scavenger receptor cysteine-rich)[2]. The extracellular region of CD5 is composed of 3 type B SRCR domains (D1, D2, and D3) each one containing 8 cysteines and encoded by a single exon (in contrast to type A, which contain 6 cysteines and are encoded by two or more exons)[3]. Other group B of members of the SRCR superfamily expressed on lymphocytes also include CD6, T19/WC1, CD163, DMBT1/gp340 and Spα/AIM (revised in [3]).
The nature of the endogenous CD5 ligand/s (CD5L) is still an open question. Several counter-receptors, including CD72, gp35–40, gp150, gp200, the framework region of IgVH and the CD5 itself [3,4], have been reported. However, most are insufficiently characterized and none have been independently verified. Thus, the physiological role of CD5 ligation remains an active area of investigation.
In both T-cells and B-cells, CD5 associates with their antigen-specific receptor complexes and modulates their signals both qualitatively and quantitatively (reviewed in [5,6]). The expression levels of CD5 on T-cells reflects the strength of antigen receptor signaling, which reciprocally tunes the threshold of the response. CD5 is primarily considered to be an inhibitory receptor, a view that needs to be revised based on accumulating new evidence. This review will focus on the current understanding of the different physiological properties of CD5 with respect to immunity and inflammation.
CD5 as a modulator of antigen receptor signaling
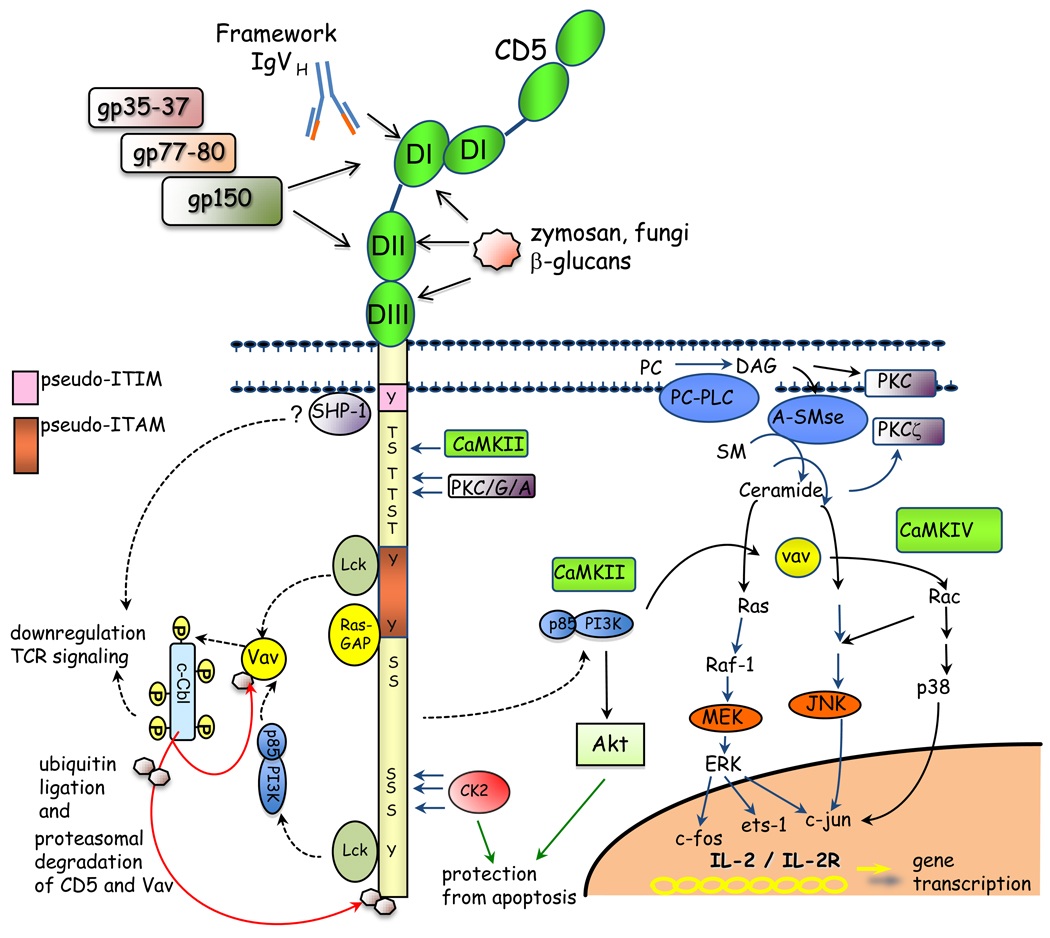
Initially, CD5 was considered a co-stimulatory molecule of T cell activation, but its function as a regulatory receptor became apparent following the development of the CD5 knock-out mouse (reviewed in [5]). Although the molecular mechanism of CD5-dependent negative signaling remains unresolved, independent studies implicate activation of negative regulators that include SHP-1, Ras-GAP, c-Cbl and CK2 [7–9]) (Figure 1).
Figure 1. Extracellular and intracellular interactions mediated by CD5 with endogenous and/or exogenous ligands.
For simplicity, crosstalk signalling pathways activated through the TCR are not shown. Binding domains described for each putative ligand are depicted: gp35–37, gp77–80, gp150 (DI and DII); Framework IgVH and CD5 (DI); zymosan (β glucans) (DI, DII and DIII). The pseudo ITAM domain is shaded in orange. Intracellular signalling molecules that associate with CD5 cytoplasmic tail are: Lck, PI3K, CaMKII, PKC – activation molecules; C-Cbl, RAS-GAP, SHP-1 – negative regulators; CK2 – prosurvival molecule. The downstream effectors activated by CD5 are shown. The association of SHP-1 to membrane proximal tyrosine of CD5 is controversial. CK2 and Akt mediate CD5-dependent survival in T lymphocytes.
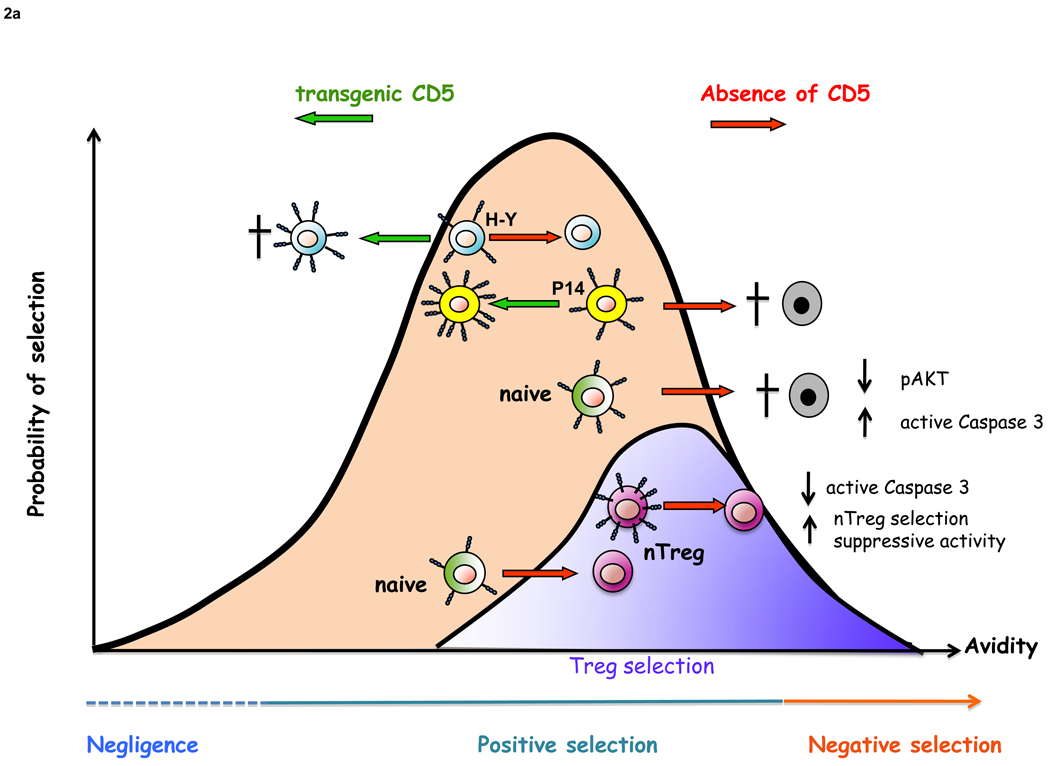
T-cells, by altering the expression levels of CD5, tune the threshold of TCR response. The level of CD5 expression is directly proportional to the avidity of antigen-specific receptors on T-cells and plays a crucial role during T cell development [10] (Figure 2a). B-cells can also modulate CD5 expression levels by a mechanism that involves the use of alternate exon 1 [11]. Depending on the exon 1 usage for transcription, CD5 can be expressed as a cell surface form or as a truncated form that is retained in the cytoplasm and their expression varies inversely with each other. By regulating the level of a DNA methyl transferase, B-cells can modulate between the expression of the two forms. Down-modulation of the cell surface form leads to the lowering of threshold of BCR signaling. Intriguingly, chronic lymphocytic leukemias (CLLs) express predominantly the cell surface form, a characteristic that might provide them a survival advantage (discussed further below).
Figure 2. CD5 T and B cell development and homeostasis.
a) CD5 acts as a negative regulator of TCR signaling, modulating the threshold of positive and negative selection. The absence of CD5 leads to enhanced negative selection of thymocytes with high affinity TCRs, such as P14, but does not affect selection of low affinity TCRs (H–Y). On the other hand, transgenic expression of CD5 decreases positive selection of H–Y due to enhanced death by neglect, but does not affect positive selection of P14 thymocytes. The absence of CD5 leads to the novo generation of nTregs, due to lowering threshold for activation, in parallel, leads to increased negative selection of naïve thymocytes, which are unable to phosphorylate Akt in response to CD3 plus CD28 crosslinking. Adapted from Maloy KJ and Powrie F, Nat. Immunol. (2001); 9: 816–822 and Azzam HS et al. J. Immunol (2001); 166:5464–72.
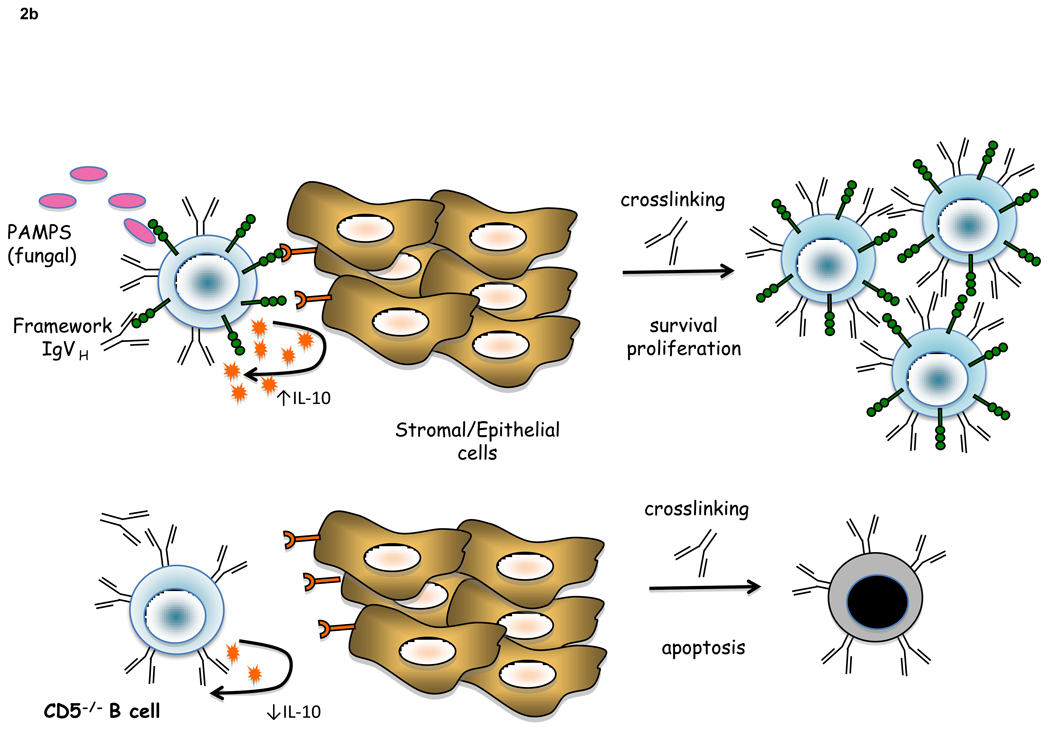
b) CD5 can interact with PAMPs and/or other CD5 ligands to trigger IL-10 secretion from BCR-crosslinked CD5+ B cells, acting as an autocrine factor to promote peritoneal CD5+ B cell survival and proliferation. In the absence of CD5, peritoneal B cell homeostasis is altered due to a decrease in IL-10 production, leading to increased apoptosis upon BCR crosslinking.
Role of CD5 in lymphocyte survival
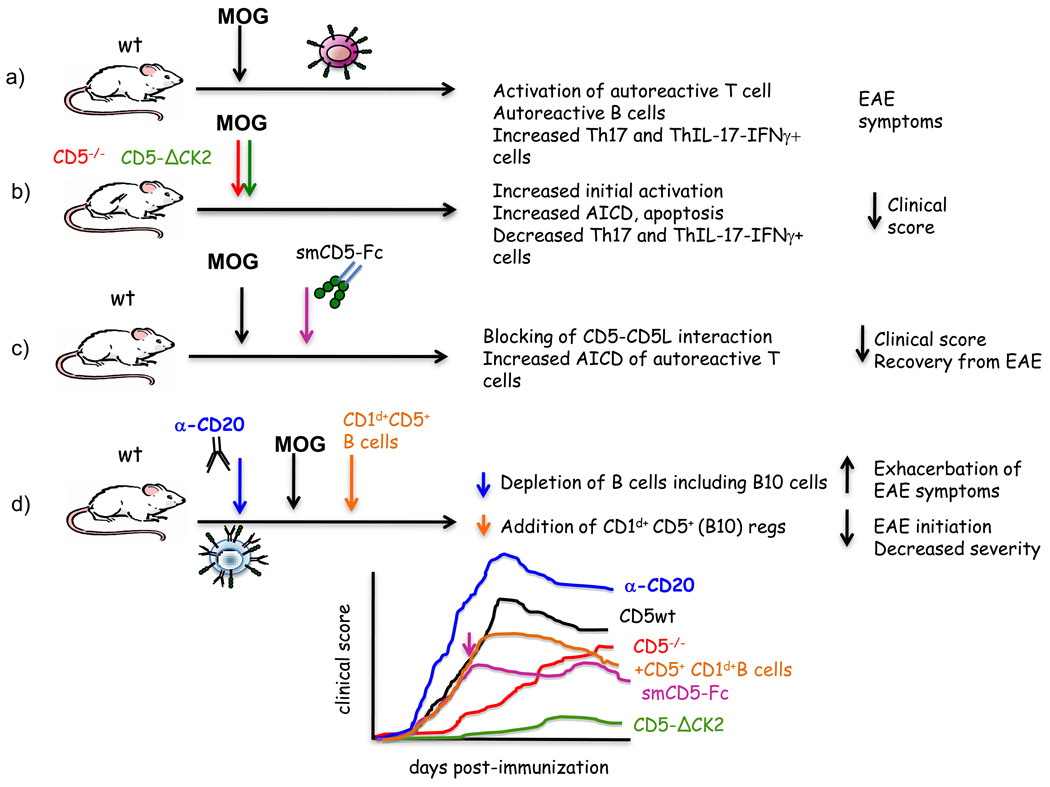
CD5 is rapidly emerging as an important regulator of T-cell and B-1a cell survival. This discovery explains why loss of regulation in T-cells and B-1a cells in CD5−/− mice does not lead to autoimmunity. The first evidence that CD5 has a pro-survival role came from the observation that CD5−/− mice develop attenuated experimental autoimmune encephalomyelitis (EAE) [12] (Figure 3). The attenuated EAE was associated with inability of antigen-reactive T-cells to persist following hyperproliferation. A subsequent study showed that CD5 expression levels on human tumor antigen specific CD8 CTL inversely correlated with susceptibility to autologous tumor-mediated activation induced cell death (AICD) [13]. CD5 promoted survival of CTL by down-regulating FasL and leading to inhibition of caspase 8 activity. Remarkably, tumor-infiltrating lymphocytes (TILs) with lower expression of CD5 exhibited the greatest anti-tumor activity [14]. Cytokines such as IL-7 and IL-21, independently and synergistically down modulate CD5 expression in CTLs by upregulating the expression of the E protein family transcription factor, E47 [15]. Such cytokine-signaling dependent down modulation of CD5 leads to increased responsiveness of CD8 T-cells to antigen and provides a mechanism by which CTLs can develop adaptive ability to increase their anti-tumor activity. E47 and the IL-4 induced transcription factor, GATA-3, are transcriptional repressors of CD5 [16,17]. These studies suggest that CTLs can balance survival and cytolytic activity by regulating CD5 expression levels, a property regulated by local cytokine milieu. Similarly, CD5 up-regulation has been shown to protect autoreactive CD4 T-cells from Fas-mediated death [18].
Figure 3. Immunomodulatory role of CD5 in EAE.
a) Immunization with MOG results in the activation of specific autoreactive T cells leading to autoimmunity and disease symptoms. b) CD5−/− mice and CD5-ΔCK2 mutant mice exhibit attenuated EAE as a result of enhanced activation induced cell death (AICD), demonstrating a role for CD5 in T lymphocyte protection from apoptosis. In CD5−/− enhanced AICD is preceded by T-cell hyperactivation as a consequence of absence of negative regulation. In CD5-ΔCK2 mutant mice, low disease severity is also associated decreased numbers of Th cells expressing both IFN-γ and IL-17. c) Treatment of EAE mice with AdmCD5Fc (soluble mouse CD5Fc recombinant adenovirus) results in reduced clinical score and recovery from EAE, due increased AICD of autoreactive clones. d) CD5+ B10 regulatory cells play a role in the control of EAE. B cell depletion (anti-CD20 treatment) leads to exacerbation of EAE symptoms, while transfer of the CD1d+ CD5+ (B10) regulatory subpopulation results in a delayed initiation and reduced severity of the disease. The bottom graph represents a composite picture of EAE severity in the experimental paradigms described in a–d.
The molecular basis underlying CD5-mediated T-cell survival has not yet been fully elucidated. However, one mechanism could be that CD5 promotes survival through activation of CK2. Transgenic reconstitution of CD5−/− mice with wild-type CD5, but not CK2-binding deficient mutant CD5, restores resistance to AICD [9]. The result reveals that CD5 regulates activation and survival through independent domains. However, overlapping functions cannot be excluded. CK2 can promote survival by inducing expression of pro-survival molecules such as Bcl-2 and Bcl-xl, or by inhibiting proteins associated with apoptotic pathways, such as caspases and Bid. An alternate pro-survival mechanism is through the activation of Akt, as seen in thymocytes (Figure 2a) [19]. The PI3K/Akt signaling pathway is critical for thymocyte survival and thymocyte activation leads to recruitment of PI3K to CD5 [20](Figure 1).
In CD5 expressing B cells, survival is associated with CD5-dependent IL-10 production [21] (Figure 2b). CD5 provides viability signals to B cell chronic lymphocytic leukemias (B-CLL) by a mechanism that involves PKC [22]. Phosphorylation of CD5 in certain B cell lymphomas correlated with malignancy [23]. Interestingly, CD5 is constitutively phosphorylated on tyrosines in B-CLL, suggesting chronic stimulation in vivo through the BCR rather than through CD5 and therefore a pro-survival mechanism independent of the CD5-CK2 axis. Intriguingly, a recent report has linked a single nucleotide polymorphism (SNP), coding for a conservative amino acid change (V447A) C-terminal to the ITAM-like motif of CD5, with progression-free survival in B-CLL patients [24]. The functional consequences of this CD5 polymorphism in B-CLL cells remain to be explored. B-1a cells are selected and maintained in the peritoneum by self-antigen [25]. The expression of CD5 might function to promote survival and maintain homeostasis of B-1a cells that are susceptible to AICD due to continuous stimulation of the BCR (Figure 2b).
Role of CD5 in tolerance
CD25 and FoxP3 expression define nTregs, however, it should be noted that high levels of CD5 is also a constitutive characteristic of both nTreg and iTregs [19,26]. This suggests that CD5 may regulate the generation and/or function of these cells (Figure 2a). In fact, CD5−/− mice have increased numbers of thymus-derived Treg cells both in the thymus and in the periphery [19]. This report demonstrated that in the absence of CD5, enhanced TCR signaling favoured de novo selection of nTregs but in parallel, induced deletion of naïve thymocytes (see below), leading to an enrichment of nTregs. Interestingly, CD5 −/− nTregs appeared to be more resistant to apoptosis than wild type (wt) nTregs. Moreover, CD5−/− Tregs showed enhanced ability to suppress in vitro proliferation of anti-CD3 stimulated CD4+CD25− naive T lymphocytes. Accordingly, the severity of dextran sulfate sodium (DSS)-induced colitis in CD5−/− mice was lesser than in wt, arguing in favour of an inhibitory role of CD5 in the suppressive function of CD4+ CD25+ Tregs [27]. These observations suggest that CD5-dependent regulation of Treg activity is linked to control of T-cell activation.
Upregulation of CD5 expression is associated with the development of antigen-specific unresponsiveness in T-cells [28,29] and B cells [30]. A direct causal relationship between upregulation of CD5 expression and antigen-specific anergy has been best demonstrated in a study where dendritic-cells (DC) were selectively targeted to present a self-reactive myelin peptide to T-cells, in vivo [31]. In that study, immature DC targeted with myelin oligodendrocyte glycoprotein (MOG) peptide rendered mice resistant to EAE to a subsequent immunization with the myelin peptide. The self-reactive T-cells had elevated expression of CD5 and exhibited antigen-specific unresponsiveness, but were not anergic to direct stimulation of the antigen-receptor in vitro. Remarkably, this type of antigen-specific unresponsiveness could not be generated in CD5−/− mice, demonstrating a direct role for CD5 in this form of induced T-cell tolerance. The molecular mechanism of this “novel” form of tolerance remains to be elucidated. However, it might be speculated that such a mechanism might be active for the generation of antigen-specific induced Treg cells [32].
The CD5-CK2 axis may have implications in the development of autoimmune diseases. Severity and incidence of EAE, the mouse model of human multiple sclerosis (MS), in CD5-CK2 binding deficient mice (CD5-ΔCK2) was dramatically lower than wild-type and CD5−/− mice [9] (Figure 3). The resistance to development of disease was associated with decreased survival of effector cells as well as attenuated generation of a Th effector population that expressed both IFN-γ and IL-17.
High CD5 surface expression is also a hallmark of some B cells with regulatory function, now defined as B10 (for IL-10 producing B cells)[33]. These B10 cells, display a unique CD5+CD1dhi phenotype, and represent 1–2% of splenic B cells and 7%–8% of peritoneal B cells in wt mice but are not normally detectable in blood or lymph nodes. IL-10 producing B cells have an important role in controlling mouse models of autoimmune diseases, such as EAE [34], collagen-induced arthritis (CIA) [35], inflammatory bowel disease (IBD) [36], contact hypersensivity (CHS) [33] and SLE [37]. TLR signals are necessary for maturation and expansion of B10 cells [38] (Figure 2b). This observation is consistent with an earlier study showing that TLR-signaling in B cells suppressed inflammatory Th1 and Th17 responses and promoted recovery from EAE [39]. Although it is not established that CD5 is necessary for the generation of B10 cells, the link between CD5, IL-10 and survival is striking [21]. In favor of a physiological role for CD5 in Breg homeostasis, fewer CD1dhiIL-10+ B-cells are present in CD5 deficient mice and in a newly developed knock-in mouse expressing CD5 lacking the ability to bind and activate CK2 (CD5ΔCK2BD) (Cashman, Fenutria and Raman; unpublished data). For detection of IL-10 cells were stimulated with PMA and ionomycin in the presence of brefeldin A before intracellular cytokine staining. In the CD5ΔCK2BD mouse, co-expression of CD5 with CD1d and IL-10 was used to identify B10 B-cells cells. Similarly, blocking of CD5-CD5L interaction, by administration of soluble hCD5 or in shCD5tg mice was shown to reduce 10 numbers of B1a and B10 cells (Fenutria R and Lozano F, unpublished data).
Breg represent a heterogenous cell population and includes subsets that do not express CD5. A recent study demonstrated the existence of a human IL-10 producing CD5−CD24hiCD27+ B cell subset that functionally parallels the mouse B10 cell [40]. CD5−, but IL-10 producing regulatory B-cells that probably developed from B2 B-cells have been reported in other studies with important regulatory function in attenuating rheumatoid arthritis and inflammatory bowel disease [36,41,42].
CD5 as a receptor for pathogen associated molecular patterns (PAMPs)
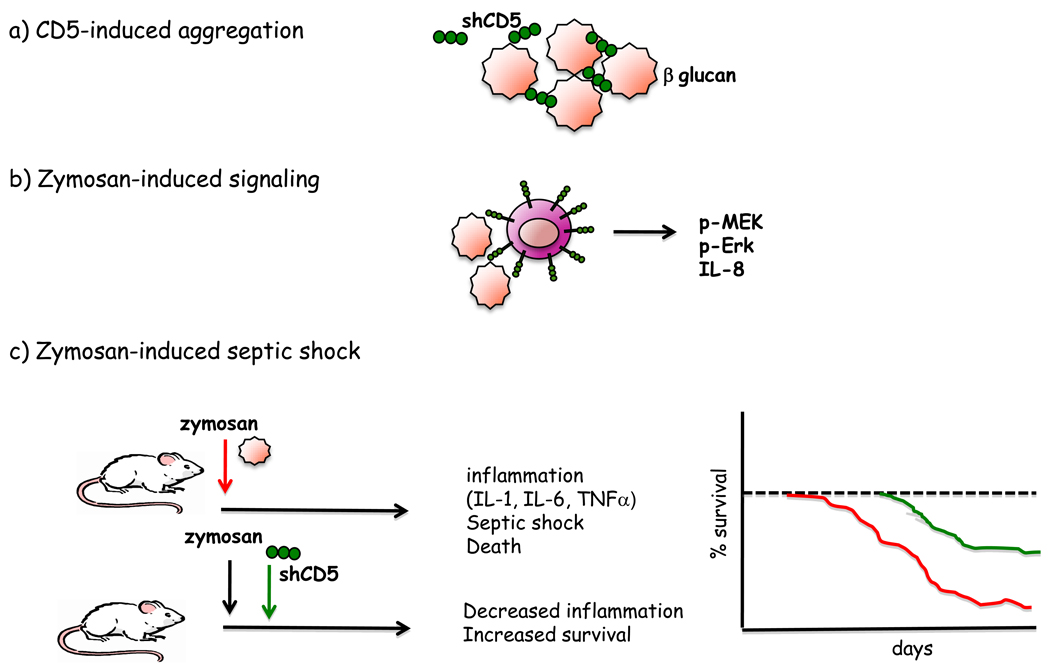
Recognition of pathogen-associated molecular patterns (PAMPs) by pathogen recognition receptors (PRRs) on immune associated cells is critical for host defense against pathogens. CD5 functions as PRR for the recognition of certain PAMPS present on fungal but not bacterial surfaces [43]. Both membrane-bound and soluble recombinant human CD5 (rshCD5) can bind to the β-glucan-rich particle zymosan as well to different soluble β-glucans expressed on fungi, but not to bacterial cell wall components. RshCD5 bound and induced aggregation of a diverse group of both saprophytic and pathogenic fungal cells, including Saccharomyces cerevisiae, Schizosaccharomyces pombe, Aspergillus fumigatus, Candida albicans, and Criptococcus neoformans, (Figure 4a); and this property also applied to any of the three individual SRCR domains of CD5. The Kd of this interaction was similar in magnitude (at nM range) to that reported for Dectin-1, the main β-glucan receptor present on myeloid cells [44]. Zymosan, following binding to membrane-bound CD5 expressed on lymphoid (Jurkat 2G5) and non-lymphoid (Cos-7) transfectants, induced signaling events that included MAP kinase activation and IL-8 production, respectively [43] (Figure 4b). Interestingly rshCD5 abolished the IFN-γ release of purified peripheral T cells stimulated with anti-CD3 mAb plus zymosan.
Figure 4. Immunomodulatory role of CD5 in the response against fungal infections.
a) Soluble CD5 can interact with β-glucans present on cell walls from saprophytic and pathogenic fungi leading to cell aggregation. b) Zymosan can interact with surface CD5 expressed on T cells and lead to intracellular signaling (p-MEK, p-Erk) and IL-8 secretion. c) Zymosan-induced septic shock can be prevented by soluble CD5, leading to a reduced inflammatory process and increased mice survival.
This finding establishes CD5 as a receptor for PAMPs, a property exhibited by several other members of highly conserved group of SRCR protein receptors. These include CD6 (a protein structurally and functionally homologous to CD5), SR-AI/II, MARCO, DMBT1/gp340/salivary agglutinin, CD163 and Spα/AIM. Interestingly, CD6 interacts with PAMPs broadly distributed among bacterial species (such as LPS, PGN and LTA) but absent in fungi [45]. These data suggests that CD5 and CD6 may complement each other in the recognition of pathogens; while CD5 is well adapted to interact with fungal associated PAMPs, CD6 has evolved to recognize bacterial ones. It has been hypothesized that recognition of PAMPs by these lymphoid scavenger receptors, particularly CD5, may result in the optimization of anti-infectious responses while minimizing the activation of auto-reactive cell clones [46]. In the presence of microbial products binding to CD5, it would increase the threshold for TCR or BCR signaling from auto-reactive lymphocytes likely activated during infectious processes. On the contrary, similar binding of CD5 by microbial components would allow the preferential activation of those lymphocytes with the highest reactive TCR o BCR, able to overcome the negative signals of CD5 [46]. However, extralymphoid expression of CD5 has also been reported, namely macrophages [47] and DC [48], and this might influence the innate arm of the immune responses.
Immunomodulatory role of soluble CD5 in inflammation
Soluble CD5 (sCD5) arising from proteolytic cleavage is detectable in human serum [49]. At steady state, sCD5 levels are very low with discrete increased levels in some autoimmune and inflammatory disorders [50–52]. The biological significance of endogenous sCD5 is unknown. However, it could function as a decoy receptor blocking the interaction of cell-surface CD5 with its endogenous ligand/s and thereby attenuating CD5 engagement-dependent activities such as survival and perhaps suppressor/regulatory activity. In fact, expression of chimeric CD5-Fc in mice leads to apoptosis of activated T-cells in super-antigen Staphylococcus enterotoxin B treated mice by counteracting CD5-dependent survival signals [12]. CD5-Fc treatment in mice also attenuated EAE in mice after initiation of disease (Figure 3c) as well as development of a murine model antibody-mediated membranous glomerulonephritis [53]. Similarly, recombinant soluble hCD5 treatment on mice in a zymosan-induced septic shock model, resulted in attenuated production of pro-inflammatory cytokines, reduction of leukocyte infiltration into the peritoneum and liver, and significant increase in mice survival, compared to untreated mice [43] (Figure 4c). These studies provide the emerging rationale for considering sCD5 as decoy receptor for the treatment of autoimmune diseases, fungi induced septic shock and B-CLL.
Concluding remarks
The evidence provided in this review supports the role of CD5 as a crucial immunomodulator both under homeostatic and inflammatory conditions. The limited success of the early anti-CD5-based immunotherapy studies can now be reinterpreted in light of the recent information regarding elevated expression of CD5 in regulatory cell subsets: CD5-mediated cell depletion may lead to enhanced immune response and pathological consequences, as CD5 is overexpressed on Treg [19] and Breg cells (B10, Cd1dhi CD5+) [33]. In contrast to anti-CD5 therapy, targeting CD5 biology using sCD5 is likely to be therapeutically beneficial to treat inflammation, autoimmunity and perhaps leukemias without inducing immunodepletion.
An important issue to be answered is the functional consequence of CD5 crosslinking on Tregs and Bregs. Can CD5 act similarly to other PRR such as TLRs to modulate Treg/Breg function? Several lines of evidence indicate that, early in infection, activation through the TCR in the presence of simultaneous ligation of TLRs [54–56] allow the expansion/recruitment of both effector (Teff) and regulatory (Tregs) T cells while diminishing the suppressive activity of the latter. This would lead to preferential expansion and activation of newly activated specific Teff, thus favoring the control of the infection. After the pathogen has been eliminated, Treg that have been previously expanded/recruited recover their suppressive capacity, and contribute to the immune system homeostasis. Since scavenger-like receptors, such as CD5, also sense the presence of PAMPs and are expressed at different densities on Teff and Treg, it would be interesting to explore the homeostatic role of them during early and late phases of antigen-specific T cell responses.
Acknowledgements
GS was recipient of a fellowship from PASPA (DGAPA, UNAM) and from PIV2009, AGAUR, Generalitat of Catalonia. Work in the laboratory of GS is supported by grants from Conacyt (#79573) and DGAPA, UNAM (PAPIIT IN228205). Research in the laboratory of CR is supported by grants from the NIH (AI1076562) and NMSS (RG3891). Work by FL is supported by grants from the Spanish Ministry of Education (SAF2007-62197 and SAF2010-19717), Generalitat of Catalonia (2009/SGR/252), and the Spanish Research Network on Infectious Diseases (RD06/0008/1013).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Resnick D, Pearson A, Krieger M. The SRCR superfamily: a family reminiscent of the Ig superfamily. Trends Biochem Sci. 1994;19:5–8. doi: 10.1016/0968-0004(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 3.Sarrias MR, Gronlund J, Padilla O, Madsen J, Holmskov U, Lozano F. The Scavenger Receptor Cysteine-Rich (SRCR) domain: an ancient and highly conserved protein module of the innate immune system. Crit Rev Immunol. 2004;24:1–37. doi: 10.1615/critrevimmunol.v24.i1.10. [DOI] [PubMed] [Google Scholar]
- 4.Brown MH, Lacey E. A ligand for CD5 is CD5. J Immunol. 2010;185:6068–6074. doi: 10.4049/jimmunol.0903823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozano F, Simarro M, Calvo J, Vila JM, Padilla O, Bowen MA, Campbell KS. CD5 signal transduction: positive or negative modulation of antigen receptor signaling. Crit Rev Immunol. 2000;20:347–358. [PubMed] [Google Scholar]
- 6.Raman C. CD5, an important regulator of lymphocyte selection and immune tolerance. Immunol Res. 2002;26:255–263. doi: 10.1385/IR:26:1-3:255. [DOI] [PubMed] [Google Scholar]
- 7.Dennehy KM, Broszeit R, Ferris WF, Beyers AD. Thymocyte activation induces the association of the proto-oncoprotein c-cbl and ras GTPase-activating protein with CD5. Eur J Immunol. 1998;28:1617–1625. doi: 10.1002/(SICI)1521-4141(199805)28:05<1617::AID-IMMU1617>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Demydenko D. c-Cbl mediated ubiquitylation and regulation of cell surface exposure of CD5. Biochem Biophys Res Commun. 2010;392:500–504. doi: 10.1016/j.bbrc.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 9. Axtell RC, Xu L, Barnum SR, Raman C. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J Immunol. 2006;177:8542–8549. doi: 10.4049/jimmunol.177.12.8542. **This paper identifies a role for the CD5-CK2 axis as a regulator of TCR signaling and as a pro-survival pathway in activated T lymphocytes. The study was also the first to show an association between Th cells producing both IFN-γ and IL-17 (dual producers) and pathogenecity in autoimmune diseases.
- 10.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 11. Garaud S, Le Dantec C, Berthou C, Lydyard PM, Youinou P, Renaudineau Y. Selection of the alternative exon 1 from the cd5 gene down-regulates membrane level of the protein in B lymphocytes. J Immunol. 2008;181:2010–2018. doi: 10.4049/jimmunol.181.3.2010. **This paper shows that B-cells can modulate the cell surface expression of CD5 by switching the use of exon 1to alternate between membrane form and a truncated cytoplasmic form. The study has implications for regulating B-cell responses and survival.
- 12. Axtell RC, Webb MS, Barnum SR, Raman C. Cutting edge: critical role for CD5 in experimental autoimmune encephalomyelitis: inhibition of engagement reverses disease in mice. J Immunol. 2004;173:2928–2932. doi: 10.4049/jimmunol.173.5.2928. **This paper provides the first demonstration that CD5 has a pro-survival role in activated T-lymphocytes that is CD5-engagement dependent. This finding reinforces the value of targeting CD5 for treatment of autoimmune diseases and perhaps CLL.
- 13. Friedlein G, El Hage F, Vergnon I, Richon C, Saulnier P, Lecluse Y, Caignard A, Boumsell L, Bismuth G, Chouaib S, et al. Human CD5 protects circulating tumor antigen-specific CTL from tumor-mediated activation-induced cell death. J Immunol. 2007;178:6821–6827. doi: 10.4049/jimmunol.178.11.6821. *This paper demonstrated that expression of CD5 protects CTLs from autologous tumor-dependent activation induced cell death (AICD) through a mechanism that involves downregulation of FasL expression and subsequent inhibition of caspase 8.
- 14. Dorothee G, Vergnon I, El Hage F, Le Maux Chansac B, Ferrand V, Lecluse Y, Opolon P, Chouaib S, Bismuth G, Mami-Chouaib F. In situ sensory adaptation of tumor-infiltrating T lymphocytes to peptide-MHC levels elicits strong antitumor reactivity. J Immunol. 2005;174:6888–6897. doi: 10.4049/jimmunol.174.11.6888. **This study showed that identical T-cells clones isolated from different locations respond differently to peptide-MHC stimulation by modulating CD5 expression. This strenghtens the concept that CD5 expression is dynamically regulated and it is a mechanism utilized by T-cells to actively adapt and regulate their responses.
- 15. Gagnon J, Chen XL, Forand-Boulerice M, Leblanc C, Raman C, Ramanathan S, Ilangumaran S. Increased antigen responsiveness of naive CD8 T cells exposed to IL-7 and IL-21 is associated with decreased CD5 expression. Immunol Cell Biol. 2010;88:451–460. doi: 10.1038/icb.2009.109. *This study is the first to demonstrate a molecular mechanism by which CTLs can modulate CD5 expression to adaptively enhance responsiveness
- 16.Yang Y, Contag CH, Felsher D, Shachaf CM, Cao Y, Herzenberg LA, Herzenberg LA, Tung JW. The E47 transcription factor negatively regulates CD5 expression during thymocyte development. Proc Natl Acad Sci U S A. 2004;101:3898–3902. doi: 10.1073/pnas.0308764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling KW, van Hamburg JP, de Bruijn MJ, Kurek D, Dingjan GM, Hendriks RW. GATA3 controls the expression of CD5 and the T cell receptor during CD4 T cell lineage development. Eur J Immunol. 2007;37:1043–1052. doi: 10.1002/eji.200636485. [DOI] [PubMed] [Google Scholar]
- 18.Ryan KR, McCue D, Anderton SM. Fas-mediated death and sensory adaptation limit the pathogenic potential of autoreactive T cells after strong antigenic stimulation. J Leukoc Biol. 2005;78:43–50. doi: 10.1189/jlb.0205059. [DOI] [PubMed] [Google Scholar]
- 19. Ordonez-Rueda D, Lozano F, Sarukhan A, Raman C, Garcia-Zepeda EA, Soldevila G. Increased numbers of thymic and peripheral CD4(+) CD25(+)Foxp3(+) cells in the absence of CD5 signaling. Eur J Immunol. 2009;39:2233–2247. doi: 10.1002/eji.200839053. **This paper demonstrates that CD5 regulates the generation of nTregs and acts as a pro-survival molecule in developing thymocytes protecting them from apoptosis through a mechanism that involves the phosphorylation of Akt.
- 20.Dennehy KM, Broszeit R, Garnett D, Durrheim GA, Spruyt LL, Beyers AD. Thymocyte activation induces the association of phosphatidylinositol 3-kinase and pp120 with CD5. Eur J Immunol. 1997;27:679–686. doi: 10.1002/eji.1830270316. [DOI] [PubMed] [Google Scholar]
- 21.Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood. 2002;100:4537–4543. doi: 10.1182/blood-2002-05-1525. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Chacon G, Vargas JA, Jorda J, Morado M, Rosado S, Martin-Donaire T, Losada-Fernandez I, Rebolleda N, Perez-Aciego P. CD5 provides viability signals to B cells from a subset of B-CLL patients by a mechanism that involves PKC. Leuk Res. 2007;31:183–193. doi: 10.1016/j.leukres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Gary-Gouy H, Sainz-Perez A, Marteau JB, Marfaing-Koka A, Delic J, Merle-Beral H, Galanaud P, Dalloul A. Natural phosphorylation of CD5 in chronic lymphocytic leukemia B cells and analysis of CD5-regulated genes in a B cell line suggest a role for CD5 in malignant phenotype. J Immunol. 2007;179:4335–4344. doi: 10.4049/jimmunol.179.7.4335. [DOI] [PubMed] [Google Scholar]
- 24.Sellick GS, Wade R, Richards S, Oscier DG, Catovsky D, Houlston RS. Scan of 977 nonsynonymous SNPs in CLL4 trial patients for the identification of genetic variants influencing prognosis. Blood. 2008;111:1625–1633. doi: 10.1182/blood-2007-08-110130. [DOI] [PubMed] [Google Scholar]
- 25.Rowley B, Tang L, Shinton S, Hayakawa K, Hardy RR. Autoreactive B-1 B cells: constraints on natural autoantibody B cell antigen receptors. J Autoimmun. 2007;29:236–245. doi: 10.1016/j.jaut.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuniyasu Y, Takahashi T, Itoh M, Shimizu J, Toda G, Sakaguchi S. Naturally anergic and suppressive CD25(+)CD4(+) T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int Immunol. 2000;12:1145–1155. doi: 10.1093/intimm/12.8.1145. [DOI] [PubMed] [Google Scholar]
- 27. Dasu T, Qualls JE, Tuna H, Raman C, Cohen DA, Bondada S. CD5 plays an inhibitory role in the suppressive function of murine CD4(+) CD25(+) T(reg) cells. Immunol Lett. 2008;119:103–113. doi: 10.1016/j.imlet.2008.05.008. *This paper demonstrates that CD5 regulates the supressive function of nTregs,showing that CD5−/− Tregs are more potent in inhibiting T cell proliferation in vitro and controling DSS-induced colitis in vivo.
- 28.Stamou P, de Jersey J, Carmignac D, Mamalaki C, Kioussis D, Stockinger B. Chronic exposure to low levels of antigen in the periphery causes reversible functional impairment correlating with changes in CD5 levels in monoclonal CD8 T cells. J Immunol. 2003;171:1278–1284. doi: 10.4049/jimmunol.171.3.1278. [DOI] [PubMed] [Google Scholar]
- 29.Frommer F, Heinen TJ, Wunderlich FT, Yogev N, Buch T, Roers A, Bettelli E, Muller W, Anderton SM, Waisman A. Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. J Immunol. 2008;181:5748–5759. doi: 10.4049/jimmunol.181.8.5748. [DOI] [PubMed] [Google Scholar]
- 30.Hippen KL, Tze LE, Behrens TW. CD5 maintains tolerance in anergic B cells. J Exp Med. 2000;191:883–890. doi: 10.1084/jem.191.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. **This paper describes a new mechanism for induced peripheral T cell tolerance that is associated with upregulation of CD5 expression. Importantly, this property also requires CD5 expression; antigen-specific T-cells in mice genetically unable to express CD5 remain non-tolerant. The study also demonstrates the requirement of DCs in this process.
- 32.Yamazaki S, Steinman RM. Dendritic cells as controllers of antigen-specific Foxp3+ regulatory T cells. J Dermatol Sci. 2009;54:69–75. doi: 10.1016/j.jdermsci.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. **This paper identifies a unique subpopulation of CD1dhiCD5+ regulatory B cells, which exert their suppressive function through the production of IL-10 and are able to negatively regulate antigen-specific T cell-dependent inflammation during CHS responses in vivo.
- 34.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, Sato S, Tedder TF, Fujimoto M. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4809. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 40.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol. 2010;22:761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 43. Vera J, Fenutria R, Canadas O, Figueras M, Mota R, Sarrias MR, Williams DL, Casals C, Yelamos J, Lozano F. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc Natl Acad Sci U S A. 2009;106:1506–1511. doi: 10.1073/pnas.0805846106. **This paper shows for the first time that the SRCR domains in CD5 specifically bind fungal components (β glucans), inducing fungal cell aggregation. Furthermore, soluble CD5 prevents septic shock-like syndrome induced by the β-glucan-rich particle zymosan in mice, supporting the therapeutic potential of soluble CD5 in fungal sepsis.
- 44.Adams EL, Rice PJ, Graves B, Ensley HE, Yu H, Brown GD, Gordon S, Monteiro MA, Papp-Szabo E, Lowman DW, et al. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J Pharmacol Exp Ther. 2008;325:115–123. doi: 10.1124/jpet.107.133124. [DOI] [PubMed] [Google Scholar]
- 45.Sarrias MR, Farnos M, Mota R, Sanchez-Barbero F, Ibanez A, Gimferrer I, Vera J, Fenutria R, Casals C, Yelamos J, et al. CD6 binds to pathogen-associated molecular patterns and protects from LPS-induced septic shock. Proc Natl Acad Sci U S A. 2007;104:11724–11729. doi: 10.1073/pnas.0702815104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenz LL. CD5 sweetens lymphocyte responses. Proc Natl Acad Sci U S A. 2009;106:1303–1304. doi: 10.1073/pnas.0812579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borrello MA, Palis J, Phipps RP. The relationship of CD5+ B lymphocytes to macrophages: insights from normal biphenotypic B/macrophage cells. Int Rev Immunol. 2001;20:137–155. doi: 10.3109/08830180109056727. [DOI] [PubMed] [Google Scholar]
- 48.De Bernardis F, Lucciarini R, Boccanera M, Amantini C, Arancia S, Morrone S, Mosca M, Cassone A, Santoni G. Phenotypic and functional characterization of vaginal dendritic cells in a rat model of Candida albicans vaginitis. Infect Immun. 2006;74:4282–4294. doi: 10.1128/IAI.01714-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calvo J, Places L, Espinosa G, Padilla O, Vila JM, Villamor N, Ingelmo M, Gallart T, Vives J, Font J, et al. Identification of a natural soluble form of human CD5. Tissue Antigens. 1999;54:128–137. doi: 10.1034/j.1399-0039.1999.540203.x. [DOI] [PubMed] [Google Scholar]
- 50.Jamin C, Magadur G, Lamour A, Mackenzie L, Lydyard P, Katsikis P, Youinou P. Cell-free CD5 in patients with rheumatic diseases. Immunol Lett. 1992;31:79–83. doi: 10.1016/0165-2478(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 51.Ramos-Casals M, Font J, Garcia-Carrasco M, Calvo J, Places L, Padilla O, Cervera R, Bowen MA, Lozano F, Ingelmo M. High circulating levels of soluble scavenger receptors (sCD5 and sCD6) in patients with primary Sjogren's syndrome. Rheumatology (Oxford) 2001;40:1056–1059. doi: 10.1093/rheumatology/40.9.1056. [DOI] [PubMed] [Google Scholar]
- 52.Noh GW, Lee KY. Circulating soluble CD5 in atopic dermatitis. Mol Cells. 1998;8:618–622. [PubMed] [Google Scholar]
- 53.Biancone L, Bowen MA, Lim A, Aruffo A, Andres G, Stamenkovic I. Identification of a novel inducible cell-surface ligand of CD5 on activated lymphocytes. J Exp Med. 1996;184:811–819. doi: 10.1084/jem.184.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 56.Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005;175:8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]