Abstract
Several studies in non-human primates have shown that neurons in the dorsolateral prefrontal cortex have activity that persists throughout the delay period in delayed matching to sample tasks, and age-related changes in the microcolumnar organization of the prefrontal cortex are significantly correlated with age-related declines in cognition. Activity that persists beyond the presentation of a stimulus could mediate working memory processes, and disruption of those processes could account for memory deficits that often accompany the aging process. These potential memory and aging mechanisms are being systematically examined with eyeblink conditioning paradigms in non-primate mammalian animal models including the rabbit. The trace version of the conditioning paradigm is a particularly good system to explore declarative memory since humans do not acquire trace conditioning if they are unable to become cognitively aware of the association between a conditioning tone and an airpuff to the eye. This conditioning paradigm has been used to show that the hippocampus and cerebellum interact functionally since both conditioned responses and conditioned hippocampal pyramidal neuron activity are abolished following lesions of the cerebellar nuclei and since hippocampal lesions prevent or abolish trace conditioned blinks. However, since there are no direct connections between the hippocampal formation and the cerebellum, and since the hippocampus is not necessary for trace conditioning after a period of consolidation has elapsed, we and others have been examining the prefrontal cortex for its role in forebrain-dependent trace eyeblink conditioning. This review examines some of the literature which suggests that the prefrontal cortex serves to orchestrate a neuronal network that interacts with the cerebellum to mediate adaptively timed conditioned responses.
Keywords: prefrontal cortex, blink, trace conditioning, hippocampus, cerebellum, caudate, pons, red nucleus
Introduction
Several studies in non-human primates have shown that neurons in the dorsolateral prefrontal cortex (dlPFC) have activity that persists throughout the delay period in delayed matching to sample tasks (Bodner, Kroger & Fuster, 1996; Fuster, 1973, 1990, 1991; Wallis & Miller, 2003; Funahashi, 2006). Activity related to oculomotor behavior, especially memory guided saccades, has also been recorded from neurons of the dlPFC (Pierrot-Deseilligny et al, 2003, 2004), and age-related changes in the microcolumnar organization of area 46 of the dlPFC in rhesus monkeys is significantly correlated with age-related declines in cognition (Cruz, Roe, Urbanc, Cabral, Stanley & Rosene, 2004). Activity that persists beyond the presentation of a stimulus could mediate working memory processes, and disruption of these processes could account for memory deficits that often accompany the aging process. These potential memory and aging mechanisms are being systematically examined with eyeblink conditioning (EBC) paradigms in non-primate mammalian animal models including the rabbit, rat, and mouse (Takehara-Nishiuchi & McNaughton, 2008; Kalmbach et al., 2009; Oswald, Maddox & Powell, 2008; Woodruff-Pak & Disterhoft, 2008; Tseng et al., 2004; Weiss et al., 1999). This paradigm offers excellent control procedures and has a wealth of data available for understanding the basics of delay conditioning (Thompson & Steinmetz, 2009; Thompson, 1986; Weiss & Disterhoft, 1996), a forebrain independent version of the paradigm that does not include a stimulus free interval between the conditioning and unconditioned stimuli. However, the trace version of the conditioning paradigm is likely to be a good system to explore declarative memory since humans do not acquire trace conditioning if they are unable to become consciously aware of the association between a conditioning tone and an airpuff to the eye (Manns, Clark, Squire, 2000; Knuttinen et al., 2001).
Essential Circuitry for Blink Conditioning
The cerebellum, brainstem, and thalamus are essential for acquisition and retention of delay EBC (Thompson, 1986; Christian & Thompson, 2005; Halverson & Freeman, 2006), and several forebrain sites including the hippocampus (Moyer et al., 1990; Solomon et al., 1986; Kim, Clark & Thompson, 1995; Tseng et al., 2004; Weiss, Bouwmeester, Power & Disterhoft, 1999), prefrontal cortex (Weible et al. 2000; Takehara, Kawahara & Kirino, 2003), caudate nucleus (Flores & Disterhoft, 2009) and primary sensory cortex (Galvez, Weiss, Weible & Disterhoft, 2006; Galvez, Weible & Disterhoft, 2007) are required for trace, but not delay EBC when the interval between the end of the conditioning stimulus (CS) and onset of the unconditioned stimulus (US) is greater than a critical duration (500 ms for rabbits), or when emotional arousal is low (Oswald et al., 2009), such as when an airpuff is used as the unconditioned stimulus instead of a periorbital shock. However, how and where the association between forebrain and cerebellum occurs for trace EBC is still being actively investigated. An important clue to the role of the forebrain in trace conditioning is that lesions of any of these forebrain areas result in conditioned responses (CRs) with short onset latencies and low amplitude rather than the stereotypical CR of rabbits that peak at about the time of US onset. This result suggests that different regions of the PFC may both potentiate the pontine nucleus and inhibit short latency responses that might otherwise have been evoked.
Given that previously conditioned rabbits recover CRs with extended training after lesions of the PFC (Oswald et al., 2010), parts of the PFC may be involved in planning rather than executing consolidated responses as suggested by Goldman-Rakic (1995). We have been using multiple single-neuron recordings, lesions, and anatomical tract tracing to test the hypothesis that prefrontal cortex (PFC) and associated forebrain regions interact to potentiate cortico-pontine projections to the cerebellum such that the effective timing of parallel fiber and climbing fiber inputs to Purkinje cells will be within the temporal limits for long-term depression (LTD) to occur (Weiss et al., 2006). LTD of the synapses at parallel-fiber and Purkinje cell junctions would make the deep cerebellar nuclei more excitable and increase activation to the red nucleus and motor nuclei to mediate CRs. These timing relations between the CS and US are critical for well-timed adaptive responses to occur. In the case of eyeblink conditioning, a properly timed response will protect the cornea whereas a poorly timed response will leave the cornea susceptible to the noxious qualities of the unconditioned stimulus. In fact, a common result of lesions to the hippocampus, caudate, sensory cortex and prefrontal cortex is that short latency conditioned responses are evoked. Those responses are not timed well enough to fully protect the cornea, and the data suggest that proper orchestration of all the involved brain nuclei may be required to produce a CR that is protective.
Prefrontal Cortex, Cerebellum, and Hippocampus
Interactions of the hippocampus and cerebellum during trace EBC have been clearly demonstrated by the abolition or prevention of conditioned hippocampal pyramidal neuron activity following lesions of the cerebellar nuclei (Clark et al., 1984; Sears & Steinmetz, 1990; Sears, Logue & Steinmetz, 1996; Ryou et al., 1998) and by prevention or abolition of trace conditioned blinks following hippocampal lesions (Moyer et al., 1990; Solomon et al., 1986; Kim et al., 1995). These reciprocal lesion effects of the hippocampus and cerebellum are convincing evidence of important interactions between the two regions. However, the nature of the interaction is challenging to define since there are no direct connections from one region to the other. Several years ago we proposed a neural circuit that could functionally connect the two regions via the frontal cortex and pons (Weiss & Disterhoft, 1996). We have since expanded and modified that circuit (Weiss et al., 2006) based on our in vivo electrophysiological (Weible et al., 2003; Yoon et al., 2010) and anatomical (Weible, Weiss & Disterhoft, 2006) studies of the cingulate gyrus. The additional parts of the revised circuit include an attentional role for the caudal anterior cingulate gyrus (cAC), maintenance of the CR by the rostral AC, and involvement of the basal ganglia (especially the caudate nucleus) and the sensory cortex as sites within a neural network that represent the behavioral significance of the CS.
Interactions between the prefrontal cortex and hippocampus are also important for memory (Witter, 2003), but connections between these two regions are mostly to the ventral / temporal hippocampus, and this part of the hippocampus is less involved than the dorsal hippocampus during trace EBC (Weible et al., 2006). An alternative explanation for hippocampal-prefrontal interactions might be that both structures receive common cholinergic facilitation during conditioning such that the two circuits work together in parallel rather than in series (Hasselmo & Sarter, 2010). These cholinergic circuits can facilitate persistent spiking in neurons, and they are affected by aging as the levels of acetylcholine tend to decrease. These circuits can facilitate EBC when cholinesterase inhibitors or cholinergic agonists are provided (Kronforst-Collins et al., 1997; Weiss et al., 2000) and they can retard learning when cholinergic antagonists are given (Kaneko & Thompson, 1997).
Over time, as the CS-US association is consolidated, structures mediating the CR appear to reorganize such that the hippocampus becomes less important and the prefrontal cortex becomes more important. The first hint for this, other than the preserved remote memory for H.M. (Scoville & Milner, 1957), came from Kim and Fanselow (1992) who found that lesions of the hippocampus impaired fear conditioning if done one day, but not seven or more days after fear conditioning. A similar effect was found later for eyeblink conditioning when hippocampal lesions done one or thirty days after conditioning were compared (Kim, Clark & Thompson, 1995). Kirino’s laboratory then showed with aspiration lesions that the medial prefrontal cortex (anterior cingulate and prelimbic) in rats becomes more involved than the hippocampus when examined four weeks after conditioning occurred (Takehara, Kawahara, Kirino, 2003). These data clearly indicate a reorganization of the circuitry mediating memory and the behavioral significance of the conditioning stimulus.
Why would the cerebellum require forebrain mediated input when the trace interval exceeds a minimum duration (300–500 ms in rabbits)? We proposed (Weiss & Disterhoft, 1996) that forebrain and hippocampal circuitry are needed to “potentiate” the effect of the CS at the pontine nuclei on its way to the cerebellum where the CS and US are integrated within the cerebellar cortex and deep nuclei. This mechanism would effectively lengthen the duration of a short duration CS such that it overlaps with the signal from the unconditioned stimulus. The mechanism underlying CS-US associations during delay conditioning may be the loss (Connor et al., 2009) or long term depression (LTD) of parallel fiber–Purkinje cell synapses (Karachot, Kado, Ito, 1994), or of mossy fiber-deep cerebellar nuclear synapses (Zhang & Linden, 2006), which are both critically dependent on the relative timing of the two inputs. In support of an LTD based mechanism are data from mutant mice that are deficient in LTD in cerebellar cortex, i.e., they have impaired delay eyeblink conditioning (Aiba et al., 1994). Also, a transgenic mouse (L7-PKCI) with Purkinje cell specific inhibition of protein kinase C has impaired adaptation of the vestibular-ocular reflex (De Zeeuw et al., 1998), another form of motor learning that is often studied to examine plasticity mechanisms in the cerebellum (Raymond, Lisberger & Mauk, 1996). Lastly, we found using fMRI that there was deactivation in the area of HVI (with an accompanying activation in the area of IPA) during delay EBC in the rabbit (Miller et al., 2003). These results suggest that LTD may mediate cerebellar dependent motor learning when the stimuli are appropriately timed, and forebrain inputs to the cerebellum (via the pontine nuclei) may help to optimize the effects of the CS and US for LTD to occur when the timing of the two stimuli is less than optimal.
Frontal Cortex & Cerebellum
Although the hippocampus is necessary for acquisition of trace EBC according to lesion studies (Moyer et al., 1990; Solomon et al., 1986; Weiss, Boumeester, et al., 1999; Tseng et al., 2004), it is only needed transiently (Kim et al., 1995; Takehara, Kawahara, Kirino, 2003). How and where the trace interval is encoded is still unknown. As we suggested previously (Weiss & Disterhoft, 1996), the frontal cortex and sensory cortex are likely sites (among others) for more permanent plasticity. The dlPFC is an obvious part of frontal cortex to examine during trace EBC, especially since its expansion occurs in parallel with expansion of the lateral cerebellum across phylogeny (Ramnani, 2006). Although rabbits do not have a granular PFC (Rose & Woolsey, 1948), as do primates, the medial part of the rabbit PFC is thought to be the homologue of the primate dlPFC based on projections from the dorsomedial thalamus and dopaminergic neurons. The PFC receives input from the cerebellum via the dorsomedial (MD) thalamus (the VL thalamus also receives input from the cerebellum but does not appear to be related to conditioned hippocampal activity (Sears, Logue & Steinmetz, 1996)). Elegant transneuronal tracing studies in nonhuman primates from Peter Strick’s laboratory revealed differences between cerebello-cortical loops that might mediate motor activity and cognition (Kelly & Strick, 2000; Kelly & Strick, 2003; Hoshi et al., 2005), and which could explain the differential activation of conditioned hippocampal activity related to the VL and MD thalamus. They found that the arm area of MI (which should be similar for eye related regions) is reciprocally linked to discrete areas in cerebellar hemispheric lobules IV–VI, whereas area 46 of the dlPFC is reciprocally linked with Crus II of the cerebellar cortex. This result is consistent with the involvement of HVI and IPA for delay and trace conditioning, and the specificity of Crus II, MD thalamus, and dlPFC for forebrain dependent trace conditioning.
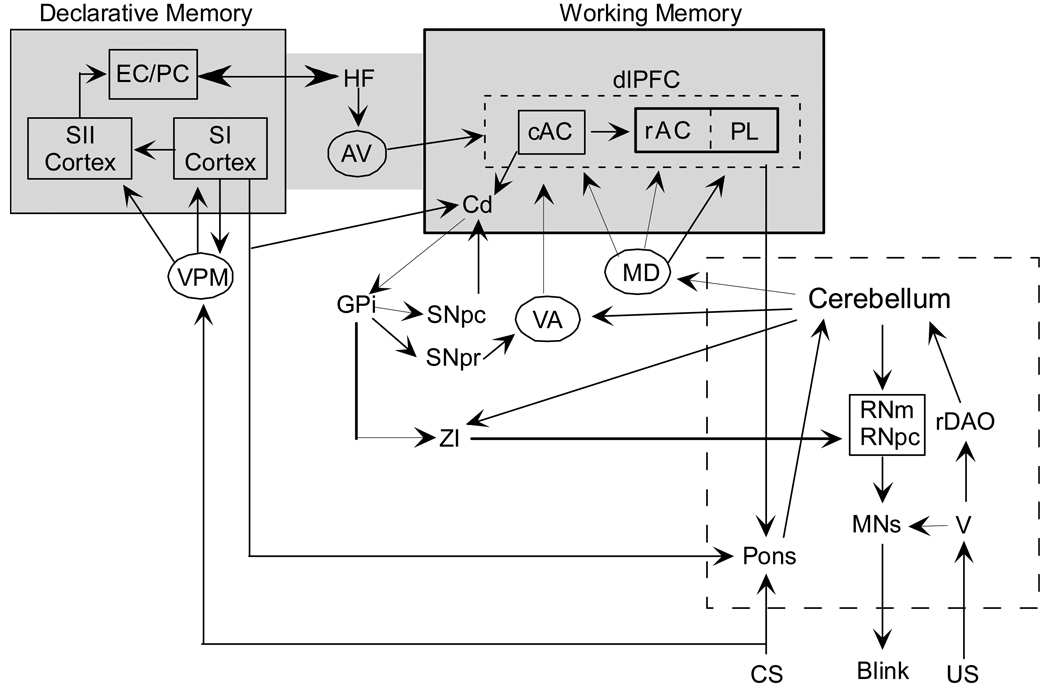
An example of the functional impact of these connections was shown by Pasupathy and Miller (2005) while recording from the caudate and areas 9 and 46 of the PFC in monkeys trained to make reversals of newly acquired memory guided saccades to one of two potential targets. They found that caudate neurons exhibited a rapid and robust change in firing rate and directional selectivity for the saccade after the reversal as compared to neurons from the PFC (those neurons exhibited a slower shift in directional selectivity). This result suggests that the striatum may play a teaching role for the cortex so that it can modulate the pontine nuclei (indirectly) or thalamo-cortical afferents to affect the cerebellum. These data support the hypothesis that the MD thalamus and the rabbit homologue of the primate dlPFC are likely to be critically involved in mediating trace EBC. A summary diagram of the interconnections hypothesized to mediate prefrontal-cerebellar interactions during trace EBC is shown in Figure 1.
Figure 1.
A circuit diagram showing connections between the forebrain and cerebellum for whisker-signaled trace eyeblink conditioning. For tone-signalled conditioning the medial geniculate nucleus and auditory cortex would replace the VPM and somatosensory cortex. The pontine nuclei are indicated as the critical node between the forebrain and the cerebellum, and the thalamic nuclei are shown as the interface between the cerebellum and the different cortical regions. We have also hypothesized which parts of the circuit are more related to declarative memory and which parts are more related to working memory. The hippocampus is shown at an intersection of the two processes. The circuitry for these two processes are not yet confirmed, and may overlap. SI and SII: primary and secondary somatosensory cortex; VPM: ventral posterior medial thalamus; cAC: caudal anterior cingulate; rAC: rostral anterior cingulate; PL: prelimbic cortex; dlPFC: dorsolateral prefrontal cortex; AV: anteroventral thalamus; VA: ventral anterior thalamus; MD: dorsomedial thalamus; HF: hippocampal formation; BG: basal ganglia; RNm: magnocellular red nucleus; rDAO: rostral dorsal accessory olive; MNs: motor neurons; V: trigeminal nucleus; EC/PC: entorhinal / perirhinal cortex; ZI zona incerta; RNpc parvicellular red nucleus..
Prefrontal Cortex and Eyeblink conditioning
Some studies of the cAC in relation to trace EBC in rabbits suggest that the cAC may be the homologue of the primate dlPFC. Injections of WGA-HRP into the cAC reveal reciprocal connections with the MD thalamus (Weible, Weiss & Disterhoft, 2007), and lesions of the cAC prevent acquisition of trace EBC (Weible et al., 2000; Oswald et al., 2006). However, recordings of single neurons within the cAC revealed changes early in the trial sequence, i.e., during the first block of 10 CS-US pairings that soon returned to baseline levels of activity with continued training (Weible et al., 2003). These data suggest that one role for cAC neurons is to change the behavioral salience of stimuli that were originally neutral, and hence mediate attention during the conditioning process. Our study with WGA-HRP tract tracing support the assertion of an attentional role, i.e., retrograde labeled neurons were found in the horizontal limb of the diagonal band of Broca(HDB) and the nucleus basalis magnocellularis (NBM) that are components of the basal forebrain cholinergic system. The prelimbic cortex is also sensitive to the salient nature of the conditioning stimulus. Powell, Maxwell and Penny (1996) used long delay conditioning (1.1 – 1.25 sec tone paired with a coterminating US) and found that neurons in this region were much more responsive to an eye-shock than an airpuff to the eye. This suggests that the prefrontal cortex may require cholinergic input from the NBM when an airpuff is used as the US (Kaneko & Thompson, 1997) in order to increase the activity of its neurons and amplify the significance of the US.
The functional circuit we propose suggests that the cAC mediates attention during initial acquisition of trace CRs and that the rAC and prelimbic areas mediate a more permanent role in orchestrating neural activity that bridges the trace interval. The observations of Gilmartin and McEchron (2005) are also consistent with this proposal. They reported neural models of trace fear conditioning in the rAC, i.e. they found 30% of prelimbic neurons in the rat with persistent activation during the trace interval. This percentage might be even higher with trace EBC since the trace interval is considerably shorter, and the eyeblink conditioned behavior is more tightly coupled in time to the US than is the freezing response during fear conditioning.
So, could the AC still be the homologue of the primate dlPFC? A comparison of recording sites from the Weible et al. (2003) study with the location of WGA-HRP labeled cells following injections into the rabbit MD thalamus (Buchanan et al., 1989) suggest that the latter were in the rostral AC (rAC) instead of the cAC. Our early study of the rAC found that pretraining lesions of the rAC did not impair acquisition of trace EBC (Kronforst-Collins & Disterhoft, 1998). However, our recent preliminary results from simultaneous recordings of the prelimbic cortex (Brodmann area 32) and the caudal and rostral anterior cingulate gyrus (Brodmann area 24) are consistent with a shift of activity from the caudal to the rostral portion of the anterior cingulate as the rabbits acquire the trace CR (Yoon, Disterhoft & Weiss, 2010). Thus, the possibility still exists that consolidation of trace EBC requires the rAC. Donald Powell’s laboratory also has several lines of data relevant to this puzzle (Simon, et al., 2005; Powell et al., 2001), but their results are difficult to interpret in terms of consolidated memory since they made permanent lesions (electrolytic and ibotenic acid) and only waited 1–7 days after behavioral criterion was achieved to make the lesions. However, post-training lesions of the rat mPFC (including the caudal and rostral AC and the PL region) have been reported to significantly impair the percentage of adaptively timed trace CRs when the lesions were done four weeks after the rats achieved a criterion of 60% CRs (Takehara, Kawahara & Kirino, 2003).
The AC (or “dlPFC”) could affect the hippocampus via demonstrated projections to the thalamus, striatum or claustrum (Weible, Weiss & Disterhoft, 2007) which could then loop back to the hippocampus via multisynaptic pathways. The cAC could also affect the cerebellum directly via projections to the pontine nuclei which we have shown with the histochemical detection of transported WGA-HRP. We have also demonstrated the cAC-pontine projection with the transport of MnCl2 which is visualized with magnetic resonance imaging (MRI, unpublished observations). The latter projections may be to either the same pontine cells that relay CS information to the cerebellum during delay conditioning, or to a separate population of pontine neurons (“trace pontine cells”) that then project on to the “delay pontine cells” (Kalmbach et al., 2009). Kalmbach et al. (2010) used stimulation of nearby groups of fibers in the middle cerebellar peduncle at the base of lobule HVI to show that stimulating one group during the CS and the other during the CS and trace intervals leads to the expression of CRs. More experiments, especially recording experiments, are needed to understand the role of the forebrain-cerebellar interactions and the source of different mossy fibers that mediate trace conditioning.
The Thalamus as a Bridge between the Prefrontal Cortex and Cerebellum
The cAC, and sensory cortex (Knowlton, Thompson & Thompson, 1993), can affect the pontine nuclei directly, and hence the cerebellum, but cooperative interactions of the cortex and cerebellum require feedback from one region to the other. The thalamic nuclei, especially the anterior-ventral (AV) and ventral-anterior (VA) nuclei, are situated to provide such feedback (Aggleton et al., 2010). We previously proposed (1996) that the VA nucleus would provide feedback from the cerebellum to the PFC, and that the AV thalamus would provide feedback from the hippocampus (via the subiculum) to the PFC.
The ventral posterior medial (VPM) thalamus is part of the lemniscal somatosensory system and is interesting in light of a report from Halverson and Freeman (2006) who found that lesions of the medial division of the medial geniculate (auditory thalamus) severely impaired acquisition of delay EBC in rats. The VPM thalamus and somatosensory cortex are comparable to the medial geniculate nucleus and the auditory cortex. Our preliminary recordings from the whisker barrels (SI cortex) and barreloids (VPM thalamus) indicate that significant processing is occurring in the cortex during whisker-signalled trace EBC (Ward, Flores, Weiss, Disterhoft, 2010).
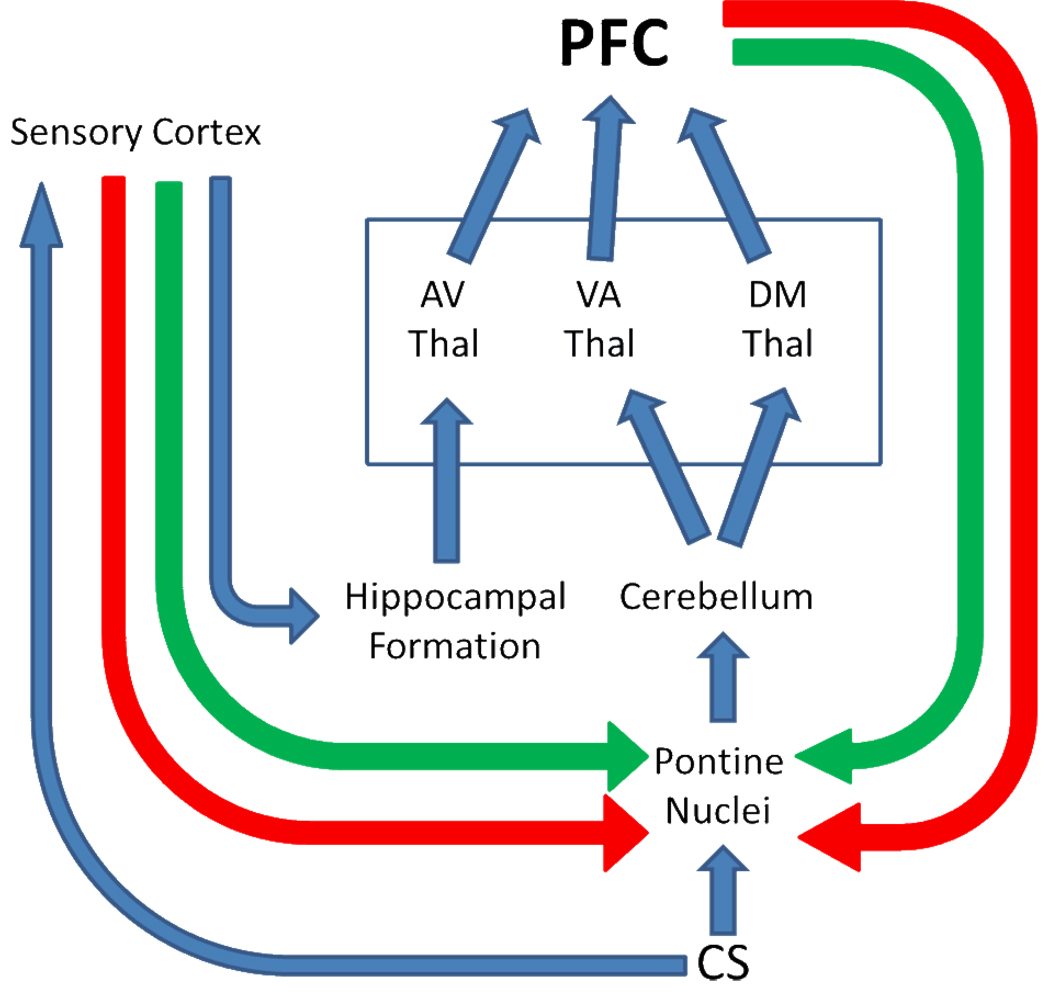
The dorsomedial (MD) thalamus is interesting because it receives input from the cerebellum (Stanton, 1980; Yamamoto et al., 1992; Middleton & Strick, 1994), and projects to most of the medial wall of the cortex rostral to the corpus callosum (Benjamin, Jackson & Golden, 1978) which is involved in working memory (Markowitsch, 1982). A summary of these connections that highlights the position of the thalamic nuclei is shown in Figure 2. The MD thalamus also projects to the dlPFC which has neurons that appear to mediate a memory trace during delayed matching to sample tasks in primates. Reciprocal connections presumably exist, since cooling the dlPFC diminishes spontaneous firing of MD neurons (Alexander & Fuster, 1973). In regards to EBC, knife cuts that disconnect the MD thalamus from the PFC retard acquisition of trace 0 (delay) conditioning with a periorbital shock US (Buchanan & Powell, 1988), as do lesions of the MD thalamus (Buchanan, 1994; Powell & Churchell, 2002). We assume the effect would be more severe with a forebrain-dependent trace paradigm, especially since monkeys were impaired on a delayed response task when their prefrontal cortex was temporarily inactivated by cooling (Alexander & Fuster, 1973; Bauer & Fuster, 1976). A comparison of the MD and AV “cognitive” thalamic nuclei with the VA “somatomotor” thalamus, in relation to the activity in the dlPFC, should help to understand better the role of the thalamic nuclei in consolidation of trace EBC.
Figure 2.
This figure highlights the influence of different thalamic nuclei for prefrontal control of pontine input to the cerebellum. The red and green arrows indicate that the prefrontal cortex and sensory cortex may both inhibit a tendency for short latency responses and facilitate input from the CS to help achieve optimal timing for long term depression of parallel fiber- Purkinje cell synapses within the cerebellar cortex.
The role of the AV thalamus is especially interesting given its position between the hippocampus and the frontal cortex, and in light of the memory impairment associated with Korsakoff’s syndrome where the mamillary bodies and AV thalamus degenerate due to a thiamine deficiency. The amnestic subtype of these patients have impaired delay EBC (McGlinchey-Berroth et al., 1995) in addition to impaired declarative memory, and Harding et al. (2000) determined that the amnesia is specific to those patients with additional neuronal loss in the anterior thalamic nucleus. The AV thalamus can be seen in Figure 1 as an interface between the hippocampal formation and the prefrontal cortex.
The role of the VA thalamus is intriguing since it receives basal ganglia output from the globus pallidus and substantia nigra pars reticulata (SNpr), and since lesions of the SN retard acquisition of EBC (using tone and periorbital shock) in a trace 0 (delay) paradigm (Kao & Powell, 1988).
Lastly, although the posterior thalamic nucleus (PO) has not been examined with eyeblink conditioning, it has been examined during a tone discrimination learning task in rats. This region exhibits large learning related changes in firing rates early in the trial sequence of a tone discriminative learning task for food reinforcement (Disterhoft & Olds, 1972). This increase may be due to presynaptic inhibition of inhibitory zona incerta afferents into the medial PO (Masri et al., 2006), a function that can be regulated by cholinergic tegmental neurons (Trageser et al., 2006). Alternatively, feedback from the SI cortex may be regulating activity in the PO since suppressing activity in the cortex suppresses the normal response to single whisker stimulation (Diamond, Armstrong-James, Budway & Ebner, 1992). A small percentage of the POm neurons also respond to noxious stimuli (Chiaia, Rhoades, Fish & Killackey, 1991), and receive projections from SI and SII. Changes in the responsiveness of neurons coding for noxious stimuli may account for learning that occurs even though the US is still delivered, as in the case of conditioning with a periorbital shock US. In this situation the subject does not avoid or even escape the shock, but if neurons coding for pain are functionally inhibited, the CR may mediate a reward (perhaps by changes in the dopamine system) to further facilitate conditioning. Although this is a relatively large list of thalamic nuclei to examine, an advantage of a paradigm like EBC is that data recorded from any of the areas mentioned can be integrated into a common framework since all subjects are treated the same and their behavior can be aligned to similar performance levels across animals.
Striatal Contributions to Trace Eyeblink Conditioning
The rabbit caudate nucleus, as in other mammalian species, receives projections from layer five of most cortical regions (Carman, Cowan & Powell, 1963) and from dopaminergic neurons of the substantia nigra pars compacta. The main efferent targets of the caudate are the globus pallidus and the substantia nigra pars reticulata which then projects to the VA thalamus (see Figure 1). In addition, the globus pallidus projects to the zona incerta and a parvicellular region of the red nucleus that then projects to the facial motor nucleus (Pong, Horn & Gibson, 2008). This parvicellular region of red nucleus has been examined in the cat, which also has a nictitating membrane, and has been found to receive projections from dorsal regions of the contralateral dentate nucleus and the adjacent region of the interpositus nucleus (Pong et al., 2008). These regions of the deep cerebellar nuclei also mediate conditioned blinks.
Lesions of the rabbit substantia nigra retard acquisition of EBC when tone and periorbital shock are paired in a trace 0 (delay) paradigm (Kao & Powell, 1988), and lesions of the caudate nucleus impair delay conditioned responses (Powell et al., 1978) and the timing of trace conditioned responses such that short latency responses are evoked and the cornea is not protected by the nictitating membrane at the time of airpuff onset (Flores & Disterhoft, 2009).
Responses of rabbit striatal neurons during delay EBC (White et al., 1994) were found to be similar to those of the hippocampus during delay EBC, i.e., responses to the US were found early in training and responses during the CR occurred later in training. Graybiel and colleagues (Blazquez et al., 2002) examined tonically active interneurons (TANs) in the monkey striatum during a variety of tasks, including delay EBC with a tone CS, and found that the population of responses was tightly coupled to the probability that a stimulus would evoke a CR. The percent of responding cells increased from 11–92% as the percent of CRs increased to a plateau level, and then decreased from 100% responders to 40% during extinction training. During trace EBC in the rabbit, Flores and Disterhoft (2009) found that presumptive medium spiny neurons were most responsive to the conditioning stimulus early in training and that tonically active neurons became responsive during the trace interval after learning had occurred. The results from these various experiments on the basal ganglia suggest an important role for the striatum in EBC, perhaps as a mediator of information flow from prefrontal association cortex to rostral motor areas that are involved in “cognitive” motor control (McFarland & Haber, 2002), and the output from the globus pallidus is positioned to modulate feedback activity from the cerebellum via the zona incerta and RNpc..
Pontine Nuclei As Targets of Prefrontal Cortical Neurons
The pontine nuclei are a critical integrative node from the cortex to the cerebellum. Several studies indicate that the lateral pontine nuclei (LPN) are part of the CS pathway mediating delay EBC (Knowlton & Thompson, 1988; Berger & Bassett 1992; Cartford et al., 1997; Clark et al., 1997; Bao, Chen & Thompson, 2000), including the finding that electrical stimulation of the LPN serves as an effective CS (Steinmetz et al. 1989; Castro-Alamancos & Borrell 1993; Tracy & Steinmetz 1998). However, the LPN is believed to be a relay for CS information rather than a site of plasticity during delay EBC (Tracy et al. 1998). The role of the pontine nuclei during trace conditioning may be quite different, and neurons here are positioned to provide an extended expression of the CS.
Auditory and visual responsive regions of the pons have been examined for EBC, and we have been using stimulation of the vibrissae as a CS for the last few years to take advantage of the relatively well understood barrel system for analyzing processing of the CS (Das, Weiss & Disterhoft, 2001; Galvez et al., 2006). The somatosensory system, including whisker related cortex, also provides significant, if not more input to the pons than do the auditory and visual systems. Leergaard et al. (2004) found that whisker-related projections from SI and MI are largely segregated as are cortical and subcortical pathways from the basal ganglia and cerebellum. Furthermore, SI contributes significantly more corticopontine projections than MI, and the SI/SII projections to the pons exhibit more overlap than do the SI/SII projections to the striatum. These structural differences indicate a larger capacity for integration of information within the same sensory modality in the pontocerebellar system compared to the basal ganglia which has more widely overlapping projections. This is further evidence supporting the hypothesis that sensory integration could be occurring in the cortex (and striatum) and then project to the cerebellum via the pontine nuclei.
We proposed that direct projections from the cAC, and perhaps other regions of the PFC, to the LPN (Weible et al. 2006) may be important to the conditioning of auditory stimuli, given the proposed role for the LPN as a relay for auditory-CS information, and we expect the same for somatosensory stimuli (whisker vibration). The relay could be direct, i.e., to the same pontine cells that project to the cerebellar cortex and deep nucleus, or the cAC could be projecting to one or more populations of pontine cells that then project onto the cells that ultimately project into the cerebellum (Kalmbach, Ohyama, Kreider & Mauk, 2006). More experiments are needed to answer these questions.
In summary, forebrain-dependent trace EBC in the awake rabbit provides an excellent model for testing the hypothesis that prefrontal-cerebellar interactions provide a mechanism for a relatively brief CS to span the trace interval and potentiate input to the cerebellum to enable it to associate the CS and US effectively and generate a conditioned response. We propose that the rabbit homologue of the primate dlPFC orchestrates the thalamic nuclei, sensory cortex, and basal ganglia to produce an adaptively timed CR. The PFC is thus situated to orchestrate consolidation and retrieval within the neuronal network that enables neural activity to span the trace interval.
Acknowledgments
Funding: NINDS R01NS059879 (CW), NIMH R01MH47340 (JFD), Kirschstein-NRSA 5T32AG020506 (TY)
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
REFERENCES
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79(2):377–388. [PubMed] [Google Scholar]
- Aggleton JP, O'Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31(12):2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. PMID: 20550571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Fuster JM. Effects of cooling prefrontal cortex on cell firing in the nucleus medialis dorsalis. Brain Res. 1973;61:93–105. doi: 10.1016/0006-8993(73)90518-0. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Thompson RF. Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behav Neurosci. 2000;114(2):254–261. doi: 10.1037//0735-7044.114.2.254. [DOI] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. J Comp Physiol Psychol. 1976;90(3):293–302. doi: 10.1037/h0087996. [DOI] [PubMed] [Google Scholar]
- Blazquez PM, Fujii N, Kojima J, Graybiel AM. A network representation of response probability in the striatum. Neuron. 2002;33(6):973–982. doi: 10.1016/s0896-6273(02)00627-x. [DOI] [PubMed] [Google Scholar]
- Berger TW, Bassett JL. System properties of the hippocampus. In: Gormezano I, Wasserman EA, editors. Learning and memory: The behavioral and biological substrates. Erlbaum; 1992. [Google Scholar]
- Bodner M, Kroger J, Fuster JM. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport. 1996;7(12):1905–1908. doi: 10.1097/00001756-199608120-00006. PMID: 8905689. [DOI] [PubMed] [Google Scholar]
- Buchanan SL. Mediodorsal thalamic lesions impair acquisition of an eyeblink avoidance response in rabbits. Behav Brain Res. 1994;65(2):173–179. doi: 10.1016/0166-4328(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Buchanan SL, Penney J, Tebbutt D, Powell DA. Lesions of the mediodorsal nucleus of the thalamus and classical eyeblink conditioning under less-than-optimal stimulus conditions: role of partial reinforcement and interstimulus interval. Behav Neurosci. 1997;111(5):1075–1085. doi: 10.1037//0735-7044.111.5.1075. [DOI] [PubMed] [Google Scholar]
- Buchanan SL, Powell DA. Parasagittal thalamic knife cuts retard Pavlovian eyeblink conditioning and abolish the tachycardiac component of the heart rate conditioned response. Brain Res Bull. 1988;21(5):723–729. doi: 10.1016/0361-9230(88)90038-x. [DOI] [PubMed] [Google Scholar]
- Buchanan SL, Powell DA, Thompson RH. Prefrontal projections to the medial nuclei of the dorsal thalamus in the rabbit. Neurosci Lett. 1989;106(1–2):55–59. doi: 10.1016/0304-3940(89)90201-2. PMID: 2479892. [DOI] [PubMed] [Google Scholar]
- Carman JB, Cowan WM, Powell TP. The organization of cortico-striate connexions in the rabbit. Brain. 1963;86:525–562. doi: 10.1093/brain/86.3.525. [DOI] [PubMed] [Google Scholar]
- Cartford MC, Gohl EB, Singson M, Lavond DG. The effects of reversible inactivation of the red nucleus on learning-related and auditory-evoked unit activity in the pontine nuclei of classically conditioned rabbits. Learning and Mem. 1997;3(6):519–531. doi: 10.1101/lm.3.6.519. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Borrell J. Active avoidance behavior using pontine nucleus stimulation as a conditioned stimulus in the rat. Behav Brain Res. 1993;55(1):109–112. doi: 10.1016/0166-4328(93)90013-g. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Rhoades RW, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat: II. Morphological and functional properties of medial ventral posterior nucleus and posterior nucleus neurons. J Comp Neurol. 1991;314(2):217–236. doi: 10.1002/cne.903140203. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Long-term storage of an associative memory trace in the cerebellum. Behav Neurosci. 2005;119(2):526–537. doi: 10.1037/0735-7044.119.2.526. PMID: 15839799. [DOI] [PubMed] [Google Scholar]
- Clark GA, McCormick DA, Lavond DG, Thompson RF. Effects of lesions of the cerebellar nuclei on conditioned behavioral and hippocampal neuronal responses. Brain Res. 1984;291:125–136. doi: 10.1016/0006-8993(84)90658-9. [DOI] [PubMed] [Google Scholar]
- Clark RE, Gohl EB, Lavond DG. The learning-related activity that develops in the pontine nuclei during classical eye-blink conditioning is dependent on the interpositus nucleus. Learn Mem. 1997;3(6):532–544. doi: 10.1101/lm.3.6.532. [DOI] [PubMed] [Google Scholar]
- Connor S, Bloomfield J, LeBoutillier JC, Thompson RF, Petit TL, Weeks AC. Eyeblink conditioning leads to fewer synapses in the rabbit cerebellar cortex. Behav Neurosci. 2009;123(4):856–862. doi: 10.1037/a0016370. PMID: 19634946. [DOI] [PubMed] [Google Scholar]
- Cruz L, Roe DL, Urbanc B, Cabral H, Stanley HE, Rosene DL. Age-related reduction in microcolumnar structure in area 46 of the rhesus monkey correlates with behavioral decline. Proc Natl Acad Sci U S A. 2004;101(45):15846–15851. doi: 10.1073/pnas.0407002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Weiss C, Disterhoft JF. Eyeblink conditioning in the rabbit (Oryctolagus cuniculus) with stimulation of the mystacial vibrissae as a conditioned stimulus. Behav. Neurosci. 2001;115:731–736. doi: 10.1037//0735-7044.115.3.731. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hansel C, Bian F, Koekkoek SK, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20(3):495–508. doi: 10.1016/s0896-6273(00)80990-3. PMID: 9539124. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Budway MJ, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J Comp Neurol. 1992;319(1):66–84. doi: 10.1002/cne.903190108. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci U S A. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Olds J. Differential development of conditioned unit changes in thalamus and cortex of rat. J Neurophysiol. 1972;35(5):665–679. doi: 10.1152/jn.1972.35.5.665. [DOI] [PubMed] [Google Scholar]
- Flores LC, Disterhoft JF. Caudate nucleus is critically involved in trace eyeblink conditioning. J Neurosci. 2009;29(46):14511–14520. doi: 10.1523/JNEUROSCI.3119-09.2009. PMID: 19923285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2006;139(1):251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol. 1973;36(1):61–78. doi: 10.1152/jn.1973.36.1.61. PMID: 4196203. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Prefrontal cortex and the bridging of temporal gaps in the perception-action cycle. Ann N Y Acad Sci. 1990a;608:318–329. doi: 10.1111/j.1749-6632.1990.tb48901.x. discussion 330-6. Review. PMID: 2127512. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Behavioral electrophysiology of the prefrontal cortex of the primate. Prog Brain Res. 1990b;85:313–323. discussion 323–4. Review. PMID: 2094902. [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex and its relation to behavior. Prog Brain Res. 1991;87:201–211. doi: 10.1016/s0079-6123(08)63053-8. Review. PMID: 1907745. [DOI] [PubMed] [Google Scholar]
- Galvez R, Weible AP, Disterhoft JF. Cortical barrel lesions impair whisker-CS trace eyeblink conditioning. Learn Mem. 2007;14(1):94–100. doi: 10.1101/lm.418407. PMID:17272654; PMCID: PMC1838550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Weiss C, Weible AP, Disterhoft JF. Vibrissa-signaled eyeblink conditioning induces somatosensory cortical plasticity. J Neurosci. 2006;26(22):6062–6068. doi: 10.1523/JNEUROSCI.5582-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119(6):1496–1510. doi: 10.1037/0735-7044.119.6.1496. PMID: 16420154. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann. N.Y. Acad. Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic nuclei are necessary for eyeblink conditioning. Behav Neurosci. 2006;120(4):880–887. doi: 10.1037/0735-7044.120.4.880. PMID:16893294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123(Pt 1):141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharm Rev. 2010:1–22. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8(11):1491–1493. doi: 10.1038/nn1544. PMID: 16205719. [DOI] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Mauk MD. Trace eyelid conditioning is mediated by cerebellar learning driven input from forebrain. SNS Abs. 2006:667.22. [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn Mem. 2009;16(1):86–95. doi: 10.1101/lm.1178309. PMID: 19144967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Mauk MD. Temporal patterns of inputs to cerebellum necessary and sufficient for trace eyelid conditioning. J Neurophysiol. 2010;104(2):627–640. doi: 10.1152/jn.00169.2010. PMID: 20484534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Thompson RF. Disruption of trace conditioning of the nictitating membrane response in rabbits by central cholinergic blockade. Psychopharmacology. 1997;131(2):161–166. doi: 10.1007/s002130050279. [DOI] [PubMed] [Google Scholar]
- Kao KT, Powell DA. Lesions of the substantia nigra retard Pavlovian eye-blink but not heart rate conditioning in the rabbit. Behav Neurosci. 1988;102(4):515–525. doi: 10.1037//0735-7044.102.4.515. [DOI] [PubMed] [Google Scholar]
- Karachot L, Kado RT, Ito M. Stimulus parameters for induction of long-term depression in in vitro rat Purkinje cells. Neurosci Res. 1994;21(2):161–168. doi: 10.1016/0168-0102(94)90158-9. PMID: 7724067. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23(23):8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods. 2000;103(1):63–71. doi: 10.1016/s0165-0270(00)00296-x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Thompson RF. Microinjections of local anesthetic into the pontine nuclei reduce the amplitude of the classically conditioned eyelid response. Physiol Behav. 1988;43(6):855–857. doi: 10.1016/0031-9384(88)90389-7. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Thompson JK, Thompson RF. Projections from the auditory cortex to the pontine nuclei in the rabbit. Behav Brain Res. 1993;56(1):23–30. doi: 10.1016/0166-4328(93)90019-m. [DOI] [PubMed] [Google Scholar]
- Knuttinen MG, Power JM, Preston AR, Disterhoft JF. Awareness in classical differential eyeblink conditioning in young and aging humans. Behav Neurosci. 2001;115(4):747–757. doi: 10.1037//0735-7044.115.4.747. PMID: 11508714. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the Caudal Area of Rabbit Medial Prefrontal Cortex Impair Trace Eyeblink Conditioning. Neurobiology of Learning and Memory. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Moriearty PL, Schmidt B, Disterhoft JF. Metrifonate improves associative learning and retention in aging rabbits. Behav Neurosci. 1997;111(5):1031–1040. doi: 10.1037//0735-7044.111.5.1031. PMID: 9383522. [DOI] [PubMed] [Google Scholar]
- Leergaard TB, Alloway KD, Pham TA, Bolstad I, Hoffer ZS, Pettersen C, Bjaalie JG. Three-dimensional topography of corticopontine projections from rat sensorimotor cortex: comparisons with corticostriatal projections reveal diverse integrative organization. J Comp Neurol. 2004;478(3):306–322. doi: 10.1002/cne.20289. [DOI] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Awareness predicts the magnitude of single-cue trace eyeblink conditioning. Hippocampus. 2000;10(2):181–186. doi: 10.1002/(SICI)1098-1063(2000)10:2<181::AID-HIPO7>3.0.CO;2-V. PMID: 10791840. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Thalamic mediodorsal nucleus and memory: a critical evaluation of studies in animals and man. Neurosci Biobehav Rev. 1982;6(3):351–380. doi: 10.1016/0149-7634(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Masri R, Trageser JC, Bezdudnaya T, Li Y, Keller A. Cholinergic Regulation of the Posterior Medial Thalamic Nucleus. J Neurophysiol. 2006 doi: 10.1152/jn.00476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22(18):8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Cermak LS, Carrillo MC, Armfield S, Gabrieli JD, Disterhoft JF. Impaired delay eyeblink conditioning in amnesic Korsakoff's patients and recovered alcoholics. Alcohol Clin Exp Res. 1995;19(5):1127–1132. doi: 10.1111/j.1530-0277.1995.tb01590.x. [DOI] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Carrillo MC, Gabrieli JD, Brawn CM, Disterhoft JF. Impaired trace eyeblink conditioning in bilateral, medial-temporal lobe amnesia. Behav Neurosci. 1997;111(5):873–882. doi: 10.1037//0735-7044.111.5.873. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Chen NK, Li L, Tom B, Weiss C, Disterhoft JF, Wyrwicz AM. fMRI of the conscious rabbit during unilateral classical eyeblink conditioning reveals bilateral cerebellar activation. J Neurosci. 2003;23(37):11753–11758. doi: 10.1523/JNEUROSCI.23-37-11753.2003. PMID: 14684877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Oswald BB, Maddox SA, Powell DA. Prefrontal control of trace eyeblink conditioning in rabbits: role in retrieval of the CR? Behav Neurosci. 2008;122(4):841–848. doi: 10.1037/0735-7044.122.4.841. PMID: 18729637. [DOI] [PubMed] [Google Scholar]
- Oswald BB, Maddox SA, Tisdale N, Powell DA. Encoding and retrieval are differentially processed by the anterior cingulate and prelimbic cortices: a study based on trace eyeblink conditioning in the rabbit. Neurobiol Learn Mem. 2010;93(1):37–45. doi: 10.1016/j.nlm.2009.08.001. PMID: 19682591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald B, Knuckley B, Mahan K, Sanders C, Powell DA. Prefrontal control of trace versus delay eyeblink conditioning: role of the unconditioned stimulus in rabbits (Oryctolagus cuniculus) Behav Neurosci. 2006;120(5):1033–1042. doi: 10.1037/0735-7044.120.5.1033. PMID: 17014255. [DOI] [PubMed] [Google Scholar]
- Oswald BB, Knuckley B, Mahan K, Sanders C, Powell DA. Prefrontal control of trace eyeblink conditioning in rabbits (Oryctolagus cuniculus) II: effects of type of unconditioned stimulus (airpuff vs. periorbital shock) and unconditioned stimulus intensity. Physiol Behav. 2009;96(1):67–72. doi: 10.1016/j.physbeh.2008.08.013. PMID: 18793661. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433(7028):873–876. doi: 10.1038/nature03287. PMID: 15729344. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126(Pt 6):1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17(1):17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Pong M, Horn KM, Gibson AR. Pathways for control of face and neck musculature by the basal ganglia and cerebellum. Brain Res Rev. 2008;58(2):249–264. doi: 10.1016/j.brainresrev.2007.11.006. PMID: 18199482. [DOI] [PubMed] [Google Scholar]
- Powell DA, Churchwell J. Mediodorsal thalamic lesions impair trace eyeblink conditioning in the rabbit. Learn Mem. 2002;9(1):10–17. doi: 10.1101/lm.45302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Mankowski D, Buchanan S. Concomitant heart rate and corneoretinal potential conditioning in the rabbit (Oryctolagus cuniculus): effects of caudate lesions. Physiol Behav. 1978;20(2):143–150. doi: 10.1016/0031-9384(78)90066-5. [DOI] [PubMed] [Google Scholar]
- Powell DA, Maxwell B, Penney J. Neuronal activity in the medial prefrontal cortex during Pavlovian eyeblink and nictitating membrane conditioning. J. Neurosci. 1996;16(19):6296–6306. doi: 10.1523/JNEUROSCI.16-19-06296.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system:anatomy and function. Nature Rev. Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272(5265):1126–1131. doi: 10.1126/science.272.5265.1126. PMID: 8638157. [DOI] [PubMed] [Google Scholar]
- Rose JE, Woolsey CN. Structure and relations of limbic cortex and anterior thalamic nuclei in rabbit and cat. J Comp Neurol. 1948;89(3):279–347. doi: 10.1002/cne.900890307. PMID: 18103781. [DOI] [PubMed] [Google Scholar]
- Ryou JW, Cho SY, Kim HT. Lesion of the cerebellar interpositus nucleus or the red nucleus affects classically conditioned neuronal activity in the hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22(1):169–185. doi: 10.1016/s0278-5846(97)00187-5. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Logue SF, Steinmetz JE. Involvement of the ventrolateral thalamic nucleus in rabbit classical eyeblink conditioning. Behav Brain Res. 1996;74(1–2):105–117. doi: 10.1016/0166-4328(96)00171-4. PMID: 8851919. [DOI] [PubMed] [Google Scholar]
- Sears LL, Steinmetz JE. Acquisition of classically conditioned-related activity in the hippocampus is affected by lesions of the cerebellar interpositus nucleus. Behav Neurosci. 1990;104:681–692. doi: 10.1037//0735-7044.104.5.681. [DOI] [PubMed] [Google Scholar]
- Simon B, Knuckley B, Churchwell J, Powell DA. Post-training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J Neurosci. 2005;25(46):10740–10746. doi: 10.1523/JNEUROSCI.3003-05.2005. PMID: 16291947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Stanton GB. Topographical organization of ascending cerebellar projections from the dentate and interposed nuclei in Macaca mulatta: an anterograde degeneration study. J Comp Neurol. 1980;190(4):699–731. doi: 10.1002/cne.901900406. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3(3):225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23(30):9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. PMID: 14586019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science. 2008;322(5903):960–963. doi: 10.1126/science.1161299. PMID: 18988855. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162(3):732–755. doi: 10.1016/j.neuroscience.2009.01.041. PMID: 19409234. [DOI] [PubMed] [Google Scholar]
- Thompson RF. The neurobiology of learning and memory. Science. 1986;233(4767):941–947. doi: 10.1126/science.3738519. PMID: 3738519. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Thompson JK, Krupa DJ, Thompson RF. Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behav Neurosci. 1998;112(2):267–285. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]
- Trageser JC, Burke KA, Masri R, Li Y, Sellers L, Keller A. State-dependent gating of sensory inputs by zona incerta. J Neurophysiol. 2006;96(3):1456–1463. doi: 10.1152/jn.00423.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14:58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18(7):2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. PMID: 14622240. [DOI] [PubMed] [Google Scholar]
- Ward RL, Flores LC, Weiss C, Disterhoft JF. Thalamic and cortical barrel activity during acquisition of whisker-signaled trace eyeblink conditioning in rabbits. Society for Neuroscience Abs. 2010 [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav Neurosci. 2000;114(6):1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weible AP, O'reilly JA, Weiss C, Disterhoft JF. Comparisons of dorsal and ventral hippocampus cornu ammonis region 1 pyramidal neuron activity during trace eye-blink conditioning in the rabbit. Neuroscience. 2006;141(3):1123–1137. doi: 10.1016/j.neuroscience.2006.04.065. PMID: 16753261. [DOI] [PubMed] [Google Scholar]
- Weible A, Weiss C, Disterhoft JF. Activity profiles of single neurons in supragenual cingulate cortex during trace eyeblink conditioning in the rabbit. J Neurophysiol. 2003;90:599–561. doi: 10.1152/jn.01097.2002. [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Afferent and efferent connections of the caudal anterior cingulate cortex in rabbit: A proposed circuit mediating initial acquisition of the trace eyeblink conditioned reflex. Neuroscience. 2007;145(1):288–302. doi: 10.1016/j.neuroscience.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Weiss C, Disterhoft JF. Eyeblink conditioning, motor control, and the analysis of limbic-cerebellar interactions. Behavioral and Brain Sciences. 1996;19(3):479–481. [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal Lesions Prevent Trace Eyeblink Conditioning in the Freely Moving Rat. Behav. Brain Res. 1999;99(2):123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Weiss C, Knuttinen M-G, Power JM, Patel RI, O’Connor MS, Disterhoft JF. Trace eyeblink conditioning in the freely moving rat: Optimizing the conditioning parameters. Behav. Neurosci. 1999;113(5):1–6. doi: 10.1037//0735-7044.113.5.1100. [DOI] [PubMed] [Google Scholar]
- Weiss C, Preston AR, Oh MM, Schwarz RD, Welty D, Disterhoft JF. The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons. J Neurosci. 2000;20(2):783–790. doi: 10.1523/JNEUROSCI.20-02-00783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Weible AP, Galvez R, Disterhoft JF. Forebrain-cerebellar interactions during learning. CellScience reviews. 2006;3(2):1–31. [PMC free article] [PubMed] [Google Scholar]
- White IM, Miller DP, White W, Dike GL, Rebec GV, Steinmetz JE. Neuronal activity in rabbit neostriatum during classical eyelid conditioning. Exp Brain Res. 1994;99(2):179–190. doi: 10.1007/BF00239585. [DOI] [PubMed] [Google Scholar]
- Witter MP. Organization of cortico-hippocampal networks in rats related to learning and memory. Int. Cong. Series. 2003;1250:131–145. [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends Neurosci. 2008;31(2):105–112. doi: 10.1016/j.tins.2007.11.006. PMID: 18199490. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yoshida K, Yoshikawa H, Kishimoto Y, Oka H. The medial dorsal nucleus is one of the thalamic relays of the cerebellocerebral responses to the frontal association cortex in the monkey: horseradish peroxidase and fluorescent dye double staining study. Brain Res. 1992;579(2):315–320. doi: 10.1016/0006-8993(92)90067-j. [DOI] [PubMed] [Google Scholar]
- Yoon T, Disterhoft JF, Weiss C. Activity of single neurons in the rabbit prefrontal cortex during trace eyeblink conditioning. Soc. Neurosci. Abs. 2010:807.13. [Google Scholar]
- Zhang W, Linden DJ. Long-term depression at the mossy fiber-deep cerebellar nucleus synapse. J Neurosci. 2006;26(26):6935–6944. doi: 10.1523/JNEUROSCI.0784-06.2006. PMID: 16807323. [DOI] [PMC free article] [PubMed] [Google Scholar]