Abstract
Background & Aims
Iron deficiency and iron overload affect over a billion people, worldwide. Dietary iron absorption in the small intestine is required for systemic iron homeostasis. Ferroportin (FPN) is the only characterized, mammalian, basolateral iron exporter. Despite the importance of FPN in maintaining iron homeostasis, its in vivo mechanisms of regulation are unclear.
Methods
Systemic iron homeostasis was assessed in mice with intestine-specific disruption of genes encoding the von Hippel-Lindau tumor suppressor protein (Vhl), hypoxia-inducible factor (HIF)-1α, HIF-2α, and aryl hydrocarbon nuclear translocator (ARNT).
Results
We observed biphasic regulation of Fpn during iron deficiency. Fpn was rapidly induced under conditions of low iron, which required the transcription factor HIF-2α. Targeted disruption of HIF-2α in the intestine inhibited Fpn induction in mice with low iron, through loss of transcriptional activation. Analysis of the Fpn promoter and in vivo chromatin immunoprecipitation assays demonstrated that HIF-2α directly binds to the Fpn promoter and induces its expression, indicating a mechanism of transcriptional regulation of Fpn following changes in systemic levels of iron. During chronic iron deficiency, FPN protein levels also increased, via increased stability through a HIF-2α-independent pathway.
Conclusion
In mice, expression of the gene that encodes Fpn and its protein levels are regulated by distinct pathways to provide a rapid and sustained response to acute and chronic iron deficiency. Therapies that target FPN might be developed for patients with iron-related disorders..
Keywords: ChIP assay, Hepcidin, diet, metabolism
Introduction
Dietary iron is absorbed in the small intestine and utilized mainly in the production of red blood cells (RBC). In an iron-deficient state, iron is mobilized by increasing its dietary absorption from the small intestine. This is accomplished by an adaptive increase in duodenal cytochrome b (DcytB), an apical ferric reductase that reduces dietary ferric iron (Fe3+) to ferrous iron (Fe2+). Ferrous iron is transported into the enterocyte by the divalent metal transporter-1 (DMT-1, also known as Nramp2, SLC11a2 and DCT1)1–5. Iron is exported from the enterocyte into circulation by the only known mammalian iron exporter ferroportin (FPN, also known as SLC40A1)6–9. DMT-1 and FPN are expressed in cells that are critical in maintaining iron homeostasis, mainly macrophages, hepatocytes and enterocytes. FPN is post-transcriptionally regulated through an iron-regulated hepatic peptide hormone, hepcidin10–14. Under normal physiological iron levels, hepcidin is highly expressed and secreted from the liver into the bloodstream. Hepcidin binds to FPN leading to an increase in degradation15,16. When serum iron levels are low, hepcidin is potently repressed correlating with an increase in FPN expression12,13. Hepcidin signaling is the best-characterized pathway shown to regulate FPN during changes in systemic iron requirements. Hepcidin promotes endocytosis and degradation of FPN in HEK293, HeLa and macrophages15,17,18. However, intestinal-derived cell lines are not responsive to hepcidin-induced FPN downregulation19,20. Furthermore, short-term hepcidin treatment did not have an effect on duodenal iron transport, whereas multiple treatments for 72 hours significantly decreased duodenal iron transport21. These data suggest that other mechanisms may be critical in the intestine to regulate FPN expression. Iron deprivation can induce intestinal FPN mRNA through an unknown mechanism9. Recently, other transition metals such as zinc have been shown to induce FPN mRNA through direct binding of the metal transcription factor-1 on the promoter of FPN22. However, this pathway is not involved in the iron-dependent increase in FPN mRNA. Moreover, the relative contribution that FPN mRNA and protein stability play in the total change in intestinal FPN protein expression following an increase in systemic iron requirements are not clear.
In contrast to FPN, regulation of DMT-1 and DcytB following changes in iron levels have been recently characterized. The transcription factor, hypoxia-inducible factor (HIF)-2α regulates expression of DMT-1 and DcytB by binding to consensus HIF response elements (HREs) on their respective promoters. In addition, HIF-2α was shown to be critical in regulating DMT-1 and DcytB expression during iron deficiency in vivo23,24. In the present study, whole genome microarray analysis was performed in the small intestines of iron-deprived wild-type and HIF-2α intestine-specific knockout mice in order to identify additional roles HIF-2α may play in regulating systemic iron homeostasis. Surprisingly, over 95% of the most enriched gene expression changes following low-iron diet were mediated via a HIF-2α-dependent pathway. HIF-2α was shown to be the critical regulator of FPN mRNA following changes in systemic iron requirements. The data establishes a novel bimodal mechanism for acute and long-term regulation of FPN. The increase in FPN transcription via HIF-2α is essential for acute regulation of iron export, whereas a chronic increase in systemic iron requirements is regulated through protein stability via a HIF2α-independent mechanism.
Methods
Luciferase assay
The mouse FPN1B luciferase reporter plasmid and the HRE mutant promoters were constructed by cloning the upstream regions as previously described by Zhang et al.25 into the pGL3-basic vector (Promega, Madison WI) using primers listed in Supplementary Table 1. The luciferase reporters were co-transfected with HIF-2α expression vector or empty vector into Caco-2 and HCT116 cells with Fugene 6 transfection reagent (Roche, Indianapolis, IN). Standard dual luciferase assay was used and normalized to a co-transfected control reporter (Promega).
Animals and diets
VhlF/F, VhlΔIE, ArntF/F, ArntΔIE and VhlΔIE/Hif-1αΔIE mice were previously described24, 26–29. For disruption of HIF-2α, Hif-2αF/F mice were crossed with mice harboring the Cre recombinase under control of the villin promoter and backcrossed to C57BL/6 for five generations. For the HIF-2α and VHL double knockout, VhlF/F/Hif-2αF/F mice hemizygous for villin-cre were mated to each other. The mice were used between the ages of 6–8 weeks old for all experiments. The mice were housed in temperature and light-controlled rooms, and were given water and pelleted LabDiet 5001chow ad libitum (PMI Nutrition, St. Louis, MO). In the low-iron studies, mice were given iron-replete AIN93G diet containing 350 ppm of iron or iron-deficient AIN93G diet (less than 5 ppm of iron) for 2-weeks or 8-weeks (Dyets, Bethlehem, PA). All animal studies were carried out in accordance with Institute of Laboratory Animal Resources guidelines and approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Histological analysis
Small intestines and livers were processed in paraffin. Macroscopic assessment was performed on routine hematoxylin and eosin or Perl’s iron stained sections.
RNA analysis
RNA was extracted from mucosal scrapings of the duodenum or from whole livers and kidneys and analyzed by qPCR as previously described 24. Primer sequences are listed in Supplementary Table 1.
Western blot analysis
For detection of HIF-2α, nuclear proteins were isolated using the NE-PER nuclear extraction kit (Thermo Scientific, Rockford, IL). For detection of FPN and DcytB, scraped duodenal mucosa were dounce homogenized and passed through a 27-gauge needle several times in lysis buffer (5mM Tris ph 8.0, 2mM EDTA, 250mM of sucrose and protease inhibitor). The nuclei were pelleted and the supernatant was centrifuged at 100,000 × g for 45 min. The resulting membrane pellet was resuspended in storage buffer (75mM Tris Ph8.0, 12.5mM MgCl2, 5mM EDTA and protease inhibitor). Membrane extracts were mixed with 2X sample buffer and incubated at 65 C° for 5 min, for HIF-2α and RAN detection the nuclear extracts were boiled for 5 min. The proteins were separated and transferred to nitrocellulose membranes using standard Western blotting techniques. The membranes were incubated with antibodies against HIF-2α (Novus Biologicals, Littleton, CO), FPN and DcytB (Alpha Diagnostic International, San Antonio, TX); the signals obtained were normalized to RAN (Santa Cruz Biotechnology Inc, Santa Cruz, CA) for nuclear extracts and to Coomassie staining for total membrane proteins. For assessment of hepcidin-induced FPN degradation, intestine-derived Caco-2 and HCT116 cells and the hepcidin-responsive HeLa and Hek293 cells were transfected with green fluorescent protein-tagged FPN (FPN-GFP). The plasmid construct is previously described30 and was a kind gift from Dr. William Griffiths. 48 hours post-transfection the cells were treated with 1 μg/mL of hepcidin (Bachem, Torrance, CA) for 8 hours. Cells were lysed using RIPA buffer and FPN-GFP was detected using an antibody for GFP and the blot was normalized to GAPDH (Santa Cruz).
cDNA microarray analysis
cDNA microarrays were performed as previously described 24. Briefly, RNA was extracted from duodenal mucosal scrapings from Hif2aF/Fand Hif2aΔIE mice on control or low-iron diet for 2-weeks. Labeled cDNA was hybridized to whole mouse genome 60-mer oligonucleotide microarray (Agilent Technologies, Santa Clara, CA).
Serum iron and hematological analysis
Routine erythrocyte and serum iron analysis was performed as previously described 24. Tissue iron was quantified by the Diagnostic Center for Population and Animal Health at Michigan State University.
Chromatin immunoprecipitation (ChIP) assay
Duodenal epithelium scrapings from VhlF/F and VhlΔIE mice were crosslinked in 1% formaldehyde in 1X PBS for 20 min. Nuclei were isolated and lysed in an SDS lysis buffer (50mM Tris-HCl pH 8.1, 10 mM, EDTA, 1% SDS, and protease inhibitors). Sheared soluble chromatin was immunoprecipitated with primary antibody for HIF-2α (Novus Biologicals). Decrosslinked samples were incubated with RNase A and proteinase K and 1 μL of purified DNA was used for PCR or qPCR with primers listed in Supplementary Table 2.
Data analysis
Results are expressed as mean ± S.D. P values were calculated by Independent t-test and ANOVA, Dunnett’s t-test. p < 0.05 was considered significant.
Results
HIF-2α is a major regulator of iron-responsive genes in the intestine
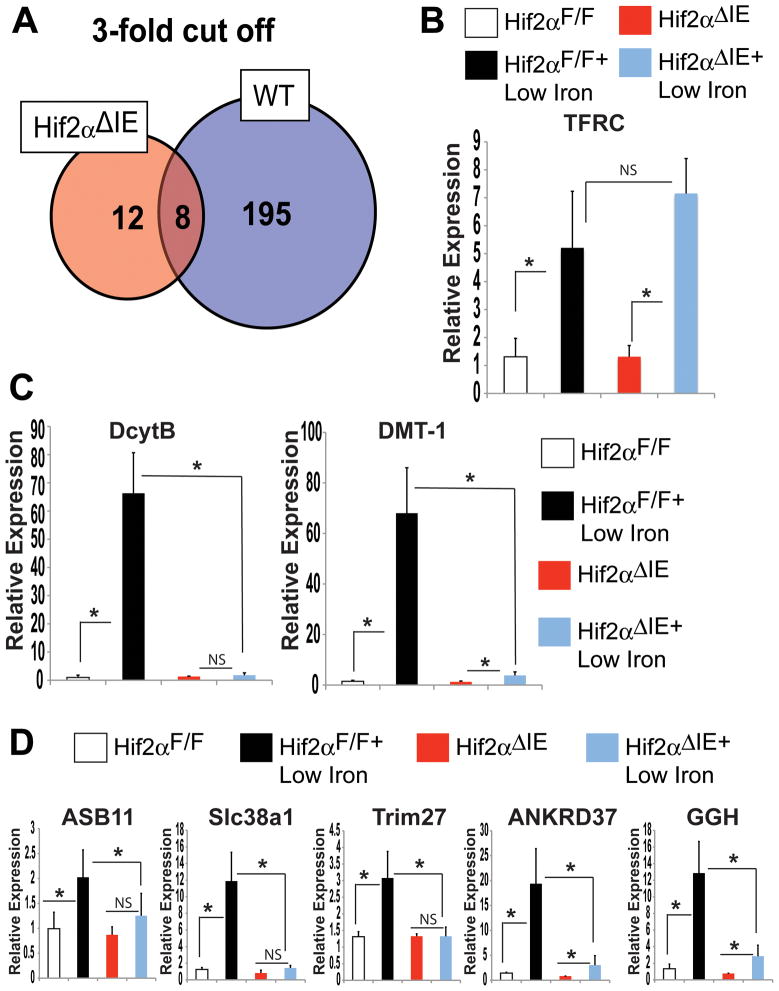
Intestinal HIF-2α overexpressing mice (VhlΔIE) generated by an intestine-specific disruption of the E3 ubiquitin recognition protein of HIF-1α and HIF-2α, the von Hippel-Lindau tumor suppressor protein (Vhl)24,27,31,32. VhlΔIE mice demonstrated a systemic dysregulation of iron homeostasis24. These data suggest that HIF-2α may regulate genes other than DMT-1 and DcytB to control iron homeostasis. To better characterize the systemic response mediated by intestinal HIF-2α, mice were generated that lacked HIF-2α specifically in the intestine (Hif-2αΔIE) (Supplemental Figure 1). Duodenal gene expression profiles of Hif-2αΔIE and wild-type mice on 2-week low-iron diet were compared. By analyzing the most enriched changes (genes induced or repressed over 3-fold), 187 out of 195 genes (95%) were regulated in a HIF-2α-dependent manner (Figure 1A). To demonstrate that low-iron diet was working properly in wild-type and Hif-2αΔIE mice, a well-characterized iron-responsive gene was assessed. Transferrin receptor (TFRC), a gene known to be induced by low-iron diet was increased to the same level in Hif-2αΔIE mice and wild-type littermates (Figure 1B). In addition, both Hif-2αΔIE mice and wild-type littermates had a robust repression of liver hepcidin mRNA expression (Supplemental Figure 2A). The iron-responsive HIF-2α target genes DcytB and DMT-1, were increased in the small intestine of wild-type mice following low-iron diet, but not elevated in Hif-2αΔIE mice (Figure 1C). Expression of several genes that were significantly altered as assessed by microarray analysis were confirmed by qPCR. Ankyrin repeat domain 37 (ANKRD37), an iron-responsive gene containing putative HIF response elements in the promoter33,34 was significantly induced following low-iron diet in a HIF-2α-dependent manner (Figure 1D). In addition, the data identified novel iron responsive genes; ankyrin repeat and SOCS box-containing 11 (ASB11), solute carrier family 38, member 1 (Slc38a1), tripartite motif-containing 27 (Trim27), and gamma glutamyl hydrolase (GGH) (Gene list containing expression changes of 2-fold or more are in Supplementary Table 2). Indeed, the microarray data correlated well with the qPCR quantification, demonstrating a central role for HIF-2α in mediating the intestinal response following iron deprivation.
Figure 1. HIF-2α is critical in the regulation of iron responsive genes.
(A) Global gene expression profiling was assessed in duodenal RNAs isolated from Hif-2αΔIE or Hif-2αF/F mice receiving a low-iron diet for 2-weeks. (B) qPCR analysis of TFRC, (C) DMT-1 and DcytB and (D) selected genes from microarray results in duodenal epithelial cells from Hif-2αΔIE or Hif-2αF/F mice receiving a low-iron diet for 2-weeks. Expression was normalized to β-actin and each bar represents the mean value ± S.D. (*)=P<.05.
Iron-induced FPN transcription is dependent on HIF-2α
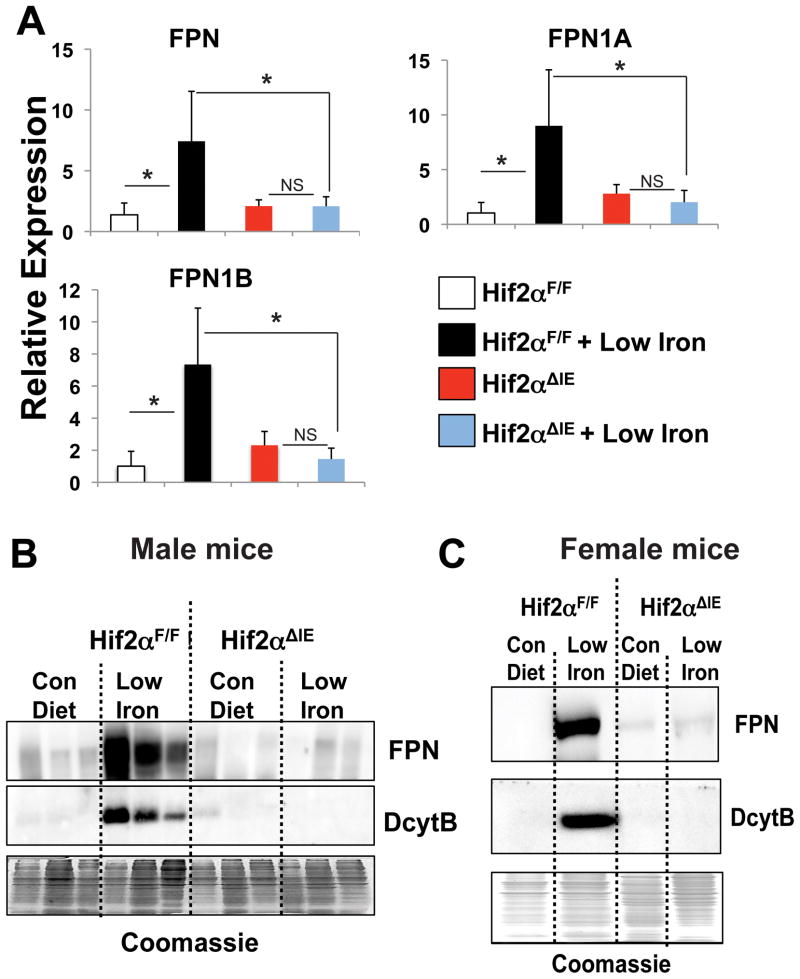
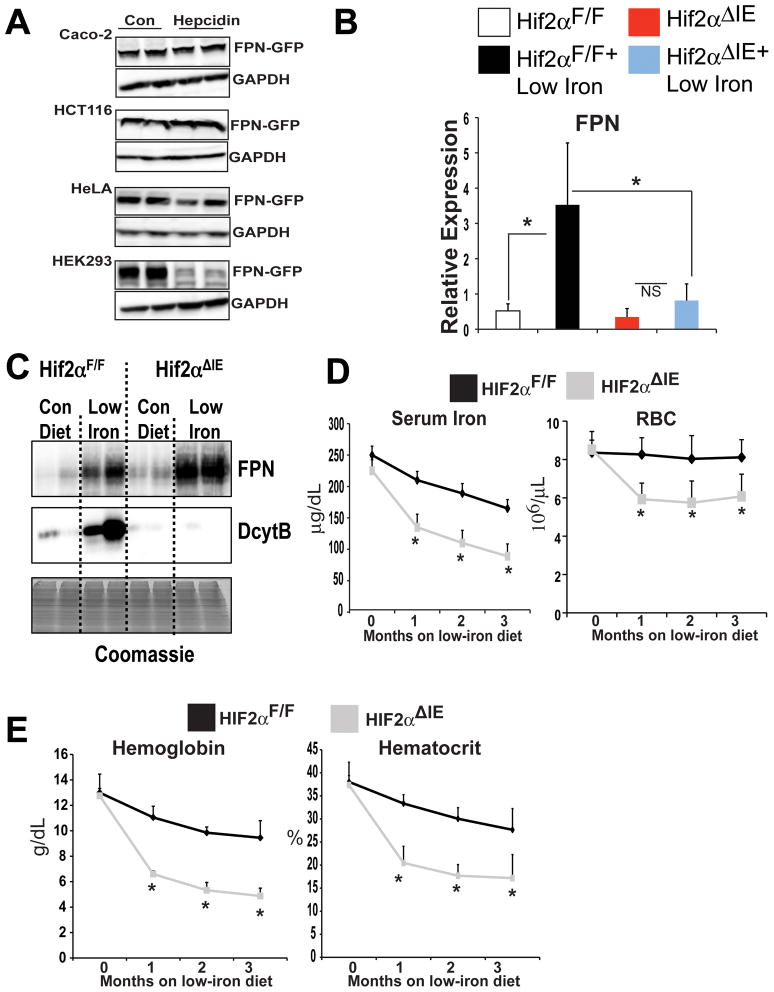
Through microarray analysis, FPN mRNA was shown to be significantly induced in a HIF-2α-dependent manner following 2-weeks of a low-iron diet treatment. Recently, a study revealed that FPN exists as two isoforms, FPN1A and FPN1B, and that both are induced following low-iron treatment25. To confirm the microarray results and determine the specific FPN isoform regulated by HIF-2α, qPCR was performed using primers to detect total FPN and FPN1A and FPN1B isoforms. Total FPN, FPN1A, and FPN1B mRNAs were significantly increased following 2-weeks of low-iron diet treatment in wild-type mice; no increase was found in the Hif-2αΔIE mice (Figure 2A). FPN is regulated by transcriptional as well as post-transcriptional mechanisms, we sought to delineate the relative contributions that mRNA expression and protein stability play in regulating total FPN expression in the intestine. Hif-2αΔIE mice completely lose their ability to induce FPN mRNA expression following a low-iron diet treatment (Figure 2A), and therefore are an ideal model to address this question. Membrane proteins were isolated from Hif-2αΔIE and wild-type mice following 2-week low-iron diet treatment and Western blot analysis was performed. An induction of FPN expression was observed in wild-type mice. In addition, a significant increase was observed in the iron responsive ferric reductase, DcytB. In the Hif-2αΔIE mice, as expected, the increase in DcytB following 2-week low-iron treatment was completely abolished (Figure 2B and C). Interestingly, 2-week low-iron diet treatment did not increase membrane expression of FPN in the Hif-2αΔIE male (Figure 2B) and female (Figure 2C) mice. These data demonstrate that following 2-week low-iron diet treatment, FPN induction requires intact HIF-2α signaling. Furthermore, the data suggest that the increase in FPN is dependent on the iron induced transcriptional response of FPN rather than an increase in protein stability.
Figure 2. HIF-2α signaling activates FPN expression during iron deficiency.
(A) qPCR analysis of FPN, FPN1A, and FPN1B in duodenal epithelial cells from Hif-2αΔIE or Hif-2αF/F mice receiving a low-iron diet for 2-weeks. Expression was normalized to β-actin and each bar represents the mean value ± S.D. (*)=P<.05. (B and C) Western blot analysis of FPN and DcytB in membrane extracts from duodenal epithelial cells isolated from (B) male and (C) female Hif-2αΔIE or Hif-2αF/F mice receiving a low-iron diet for 2-weeks. Expression was normalized to Coomassie stained gels.
Disruption of VHL in the small intestine induces FPN expression
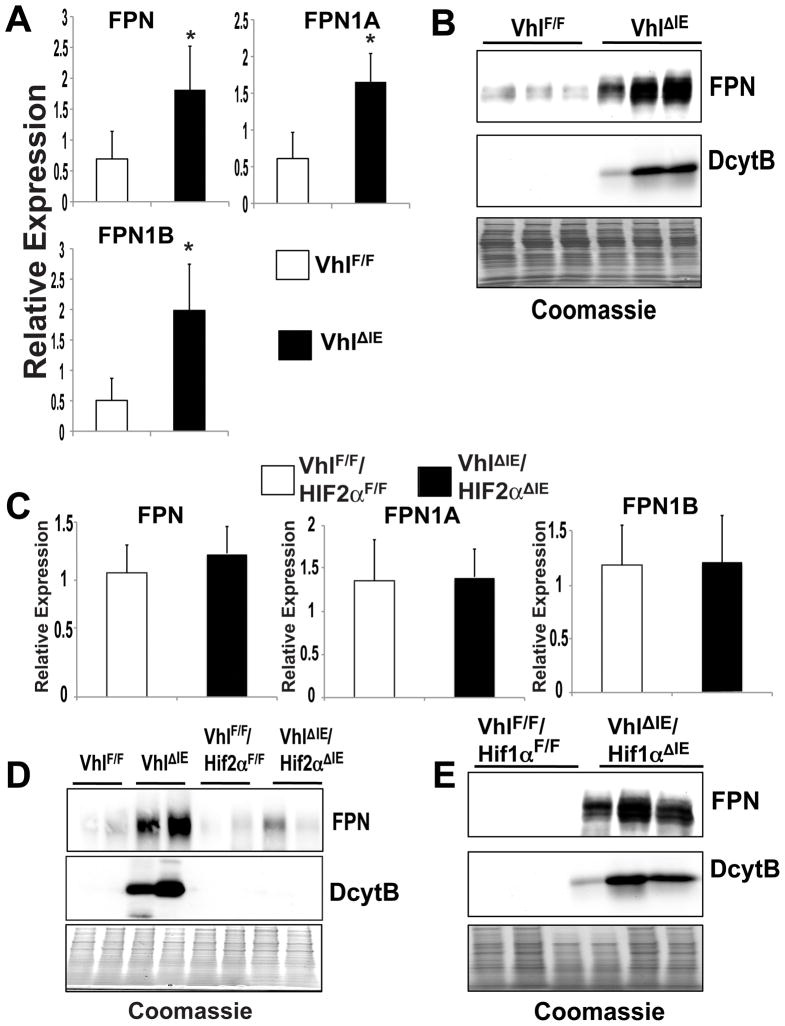
To investigate the role of intestinal HIF-2α overexpression on FPN regulation, duodenal FPN mRNA and protein expression were assessed in the VhlΔIE and littermate control mice. Our previous study did not demonstrate that FPN was significantly induced in the VhlΔIE mice, although there was a trend toward increased expression24. However, using more specific primers that detect total FPN, FPN1A, and FPN1B, a significant induction of intestinal FPN expression was observed in the VhlΔIE mice compared to littermate control VhlF/F mice (Figure 3A). Next, to assess FPN protein expression, duodenal membrane extracts were prepared from VhlΔIE and wild-type mice. A robust induction of FPN protein was observed in duodenal extracts from VhlΔIE mice compared to VhlF/F mice (Figure 3B). To assess the influence of the HIF-dependent pathways on FPN expression in the small intestine, mice with a double disruption of VHL and HIF-1α or VHL and HIF-2α were generated. Disruption of VHL and HIF-1α inhibits HIF-1 heterocomplexes, but not HIF-2 heterocomplexes, whereas disruption of VHL and HIF-2α prevents the formation of functional HIF-2. Disruption of both VHL and HIF-2α (VhlΔIE/Hif-2αΔIE) completely abrogated the induction of FPN mRNA, whereas disruption of both VHL and HIF-1α (VhlΔIE/Hif-1αΔIE) had no effect on FPN mRNA as compared to VhlΔIE mice (Figure 3C and data not shown). Consistent with the gene expression data, the increase in FPN protein expression observed in VhlΔIE duodenal membrane extracts was abrogated in the VhlΔIE/Hif-2αΔIE mice, whereas FPN protein expression was significantly induced in the VhlΔIE/Hif-1αΔIE mice compared to littermate controls (Figure 3D and E). These data further demonstrate that HIF-2α regulates FPN expression.
Figure 3. Intestine-specific disruption of VHL increases FPN expression through a HIF-2α-dependent mechanism.
(A) qPCR measuring FPN, FPN1A, and FPN1B expression in duodenal epithelium from VhlΔIE or VhlF/F mice. Expression was normalized to β-actin and each bar represents the mean value ± S.D. (*)=P<.05. (B) Western blot analysis measuring FPN and DcytB expression in duodenal epithelium membrane extracts from VhlΔIE or VhlF/F mice. Expression was normalized to Coomassie stained gels (C) qPCR measuring FPN, FPN1A, and FPN1B expression in duodenal epithelium from VhlΔIE/Hif-2αΔIE double knockout mice or littermate controls. Expression was normalized to β-actin and each bar represents the mean value ± S.D. (*)=P<.05. (D and E) Western analysis measuring FPN expression in duodenal epithelium membrane extracts from (D) VhlΔIE/Hif-2αΔIE double knockout mice or (E) VhlΔIE/Hif-1αΔIE double knockout mice and littermate controls. Expression was normalized to Coomassie stained gels
HIF-2α regulates FPN1B promoter in the duodenum by directly binding to the regulatory regions in vivo
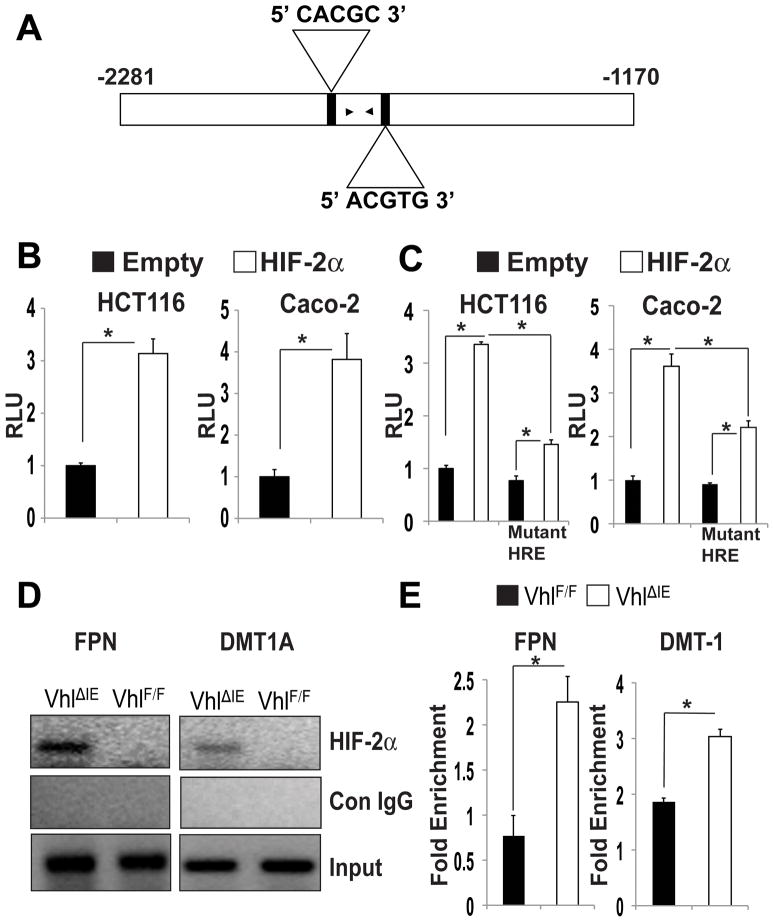
Duodenal epithelial cells use the FPN1B promoter25 and the upstream sequence from the transcription initiation site of FPN1B contained two putative HIF response elements (HRE) (Figure 4A). The FPN1B promoter containing the putative HREs was analyzed by transient transfection using a luciferase reporter construct (Figure 4B). Co-transfection with a mammalian expression plasmid for HIF-2α strongly increased the luciferase activity in intestine-derived Caco-2 and HCT116 cells compared to empty vector (Figure 4B). Mutating the putative HREs inhibited the HIF-2α-mediated induction, thus confirming that these sites were functional HREs (Figure 4C). Next, to demonstrate that HIF-2α could directly bind the FPN1B promoter, in vivo ChIP assays were performed in duodenal samples from VhlF/F or VhlΔIE mice. HIF-2α was immunoprecipitated from crosslinked soluble chromatin isolated from VhlF/F or VhlΔIE mice. Using PCR primers in close proximity to both HREs, a significant increase in HIF-2α promoter occupancy was observed at the FPN1B HREs in the VhlΔIE mice compared to the VhlF/F mice (Figure 4D). As a control, the previously characterized HREs of DMT-1 were assessed and an increase in HIF-2α binding was observed in the VhlΔIE mice compared to the VhlF/F mice. The promoter occupancy was quantitated using qPCR ChIP analysis, and this data demonstrates that HIF-2α is able to bind the FPN1B regulatory region in vivo similarly to what is observed on the DMT-1 promoter region (Figure 4E).
Figure 4. HIF-2α directly binds to the endogenous promoter of FPN.
(A) Schematic diagram of FPN1B promoter illustrating the HREs in the regulatory region. The upstream regions are numbered in relation to the translation initiation site. The black arrowheads correspond to primers used for ChIP analysis. (B and C) Luciferase-reporter constructs under the control of the regulatory region of the mouse (B) FPN1B (C) or the FPN1B promoter with mutated HREs. Caco-2 and HCT116 cells were transiently transfected with the luciferase construct, and co-transfected with empty vector or HIF-2α expression plasmids. Standard dual luciferase assays were performed on cell extracts as described in the Materials and Methods. Each bar represents the mean value ± S.D. (*)=P<.05. (D and E) In vivo ChIP assays on duodenal extracts from VhlF/F and VhlΔIE mice using primers amplifying HREs of the FPN1B and DMT-1 promoter and detected by (D) ethidium stained agarose gel or (E) quantitated by qPCR and the data is expressed as fold enrichment over control IgG and normalized to input. Each bar represents the mean value ± S.D. (*)=P<.05.
Increased expression of FPN following chronic treatment with low-iron is not HIF-2α-dependent
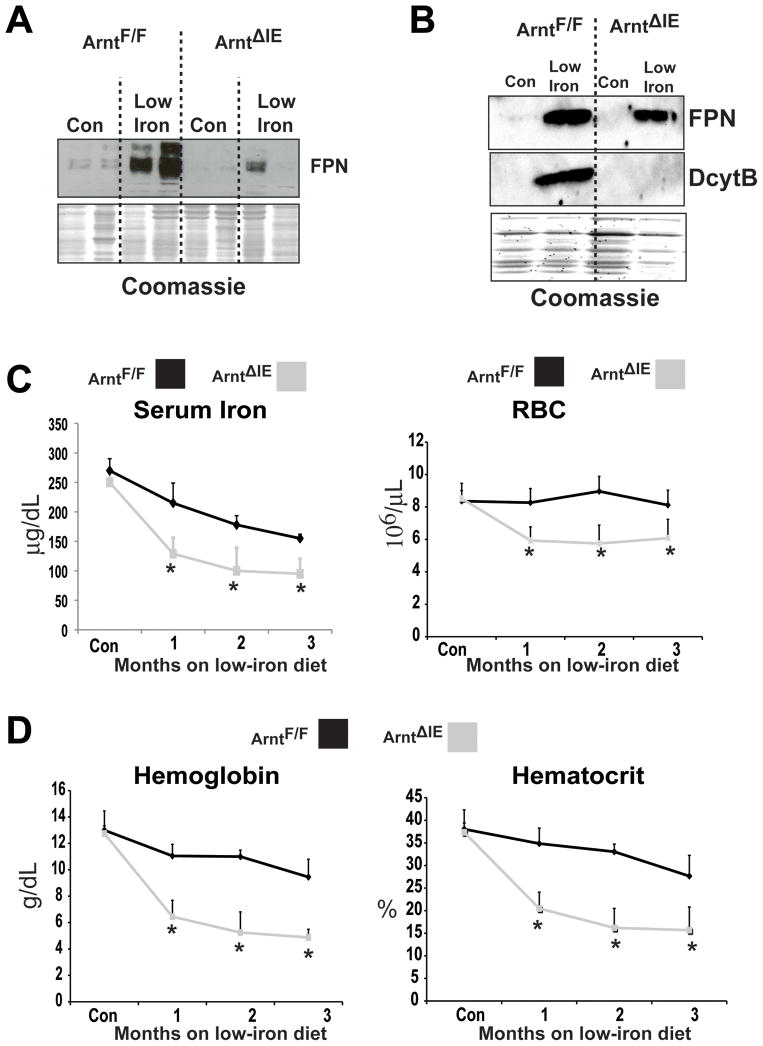
Together, the data suggest that FPN regulation in the intestine is independent of hepcidin, and mainly controlled by HIF-2α through transcriptional regulation. This conclusion is in agreement with studies assessing FPN expression in intestine-derived cell lines. In Caco-2 and HCT116 cells hepcidin had no inhibitory effect on FPN protein expression, whereas in Hek293 and HeLa cells hepcidin significantly inhibited FPN protein expression (Figure 5A and 19,20). However, there are a number of studies that demonstrate the importance of hepcidin-induced FPN degradation in regulating iron homeostasis35,36. In mouse models, hepcidin deficiency is linked to hemochromatosis and increased expression of hepcidin is associated with anemia10,11,14. Moreover, mice that have decreased hepcidin levels, demonstrate an increase in duodenal FPN protein expression37. To understand these discrepancies, HIF-2α-dependent regulation of FPN expression was examined following long-term iron deprivation. Consistent with the 2-week low-iron diet treatment, wild-type mice under an 8-week low-iron diet treatment, significantly induced FPN, FPN1A, and FPN1B mRNA expression; no increase was noted in 8-week low-iron diet treated Hif-2αΔIE mice (Figure 5B, and data not shown). In addition, increases in DMT-1 and DcytB following long-term low-iron treatment were HIF-2α dependent (Supplemental Figure 3A). However, FPN protein expression was induced following 8-week low-iron diet treatment in both wild-type and Hif-2αΔIE mice (Figure 5C). These data correlated well with hematological and serum iron parameters. The Hif-2αΔIE mice were highly susceptible to iron-deficiency-induced anemia. The most significant drop in iron levels, RBCs, hematocrit, and hemoglobin in Hif-2αΔIE mice were observed following a 1-month low-iron diet treatment (Figure 5D–E). The RBC and serum iron levels, as well as hematocrit and hemoglobin measurements stabilized following 2- and 3-month low-iron diet treatment. To further confirm the role of HIF-2α in the biphasic regulation of FPN, mice with an intestine-specific disruption of ARNT were assessed. ARNT is an obligate heterodimer partner for HIF-1α and HIF-2α, and therefore inactivation of ARNT ablates both HIF-1α and HIF-2α function38. The intestine-specific ARNT knockout mice (ArntΔIE) were fed low-iron diet for 2-weeks and FPN protein expression was measured. Similar to that observed in the Hif-2αΔIE mice, inactivation of ARNT inhibited FPN induction in response to 2-week iron starvation (Figure 6A). In addition, The ArntΔIE mice demonstrated a similar response to the Hif-2αΔIE mice on long-term low-iron diet. FPN protein expression was induced following 8-week low-iron diet treatment of both wild-type and ArntΔIE mice (Figure 6B). The ArntΔIE mice were susceptible to iron deficiency-induced anemia, where the most significant drop in iron levels, RBCs, hematocrit, and hemoglobin in ArntΔIE mice was observed following a 1-month low-iron treatment (Figure 6C–D). This response was not due to a dysregulation of the hepcidin-FPN axis since liver hepcidin expression was significantly inhibited in both Hif-2αΔIE and ArntΔIE mice following acute and chronic low-iron diet treatment (Supplemental Figure 2A and data not shown). Together, these studies demonstrate that distinct pathways are activated that regulate intestinal FPN expression during acute and chronic iron-deficient states.
Figure 5. Long-term low-iron diet treatment increases FPN expression independent of HIF-2α.
(A) Intestine-derived Caco-2 and HCT116 and the hepcidin-responsive HeLa and HEK293 cells were transfected with a mammalian expression construct for human FPN tagged with green fluorescent protein (FPN-GFP). Following transfection the cells were treated with 1ug/mL of hepcidin for 8 hours and FPN-GFP was detected by Western blot analysis. Expression was normalized to GAPDH. (B) qPCR analysis measuring FPN mRNA expression in duodenal epithelial cells in Hif-2αΔIE or Hif-2αF/F mice receiving low-iron diet for 8 weeks. Expression was normalized to β-actin and each bar represents the mean value ± S.D. (*)=P<.05. (C) Western blot analysis measuring FPN expression from duodenal epithelial cells in Hif-2αΔIE or Hif-2αF/F mice receiving low-iron diet for 8 weeks. (D) Serum iron analysis and red blood cell (RBC) count from Hif-2αΔIE or Hif-2αF/F mice receiving low-iron diet for 1, 2 or 3 months. (E) hematocrit and hemoglobin analysis from Hif-2αΔIE or Hif-2αF/F mice receiving low-iron diet for 1, 2 or 3 months. Each time point represents the mean value ± S.D. (*)=P<.05.
Figure 6. Low-iron diet treatment increases FPN expression by an Arnt- dependent and independent mechanism.
Western blot analysis measuring FPN expression from duodenal epithelial cells in ArntΔIE or ArntF/F mice receiving low-iron diet for (A) 2-weeks or (B) 8-weeks. (C) Serum iron analysis and red blood cell (RBC) count from ArntΔIE or ArntF/F mice receiving low-iron diet for 1, 2, or 3 months. (E) hematocrit and hemoglobin analysis from ArntΔIE or ArntF/F mice receiving low-iron diet for 1, 2, or 3 months. Each time point represents the mean value ± S.D. (*)=P<.05.
Discussion
Iron supplementation is the most widely used therapy for iron deficiency. However, a significant number of anemic patients are refractory to iron39. Moreover, the main treatment for hemochromatosis is phlebotomy. Since several rounds of treatment are required, this is a slow-acting treatment option40. Therefore, alternative treatment strategies are needed in iron refractory anemia and hemochromatosis. FPN provides an optimal target in iron-related disorders. Hemochromatosis and several other iron-related disorders are due to dysregulation of FPN expression41–43 and conditional disruption of FPN in the intestine demonstrates its central role in regulating systemic iron homeostasis8. Despite the importance of FPN in maintaining iron homeostasis, the mechanism for its induction in the intestine following an increase in systemic iron requirements was hitherto unclear. The present data demonstrates a novel role for HIF-2α in iron homeostasis by directly regulating FPN during acute increases in systemic iron requirements. This conclusion is based on the following data: (i) Intestinal ablation of HIF-2α leads to loss of the adaptive induction of intestinal FPN gene expression following 2-week low-iron diet treatment. (ii) VhlΔIE mice demonstrate an increase in FPN mRNA and protein expression. (iii) HIF-2α activates the FPN1B regulatory region in two different intestinal epithelial cell lines. (iv) ChIP assays using chromatin isolated from the duodenum of VhlΔIE mice demonstrate direct binding of HIF-2α to the promoter in vivo. (v) Lastly, long-term iron deprivation leads to an increase in intestinal FPN protein expression in wild-type and Hif-2αΔIE mice.
The present study provides a novel model for FPN regulation. Acute decreases in dietary iron activate intestinal HIF-2α signaling, which stimulates an adaptive increase in apical iron transport through DMT-1 and DcytB and mobilizes iron into circulation through an increase in basolateral iron export via FPN. Moreover, the data in the present work suggests that the acute adaptive induction of FPN is due to the transcriptional increase of FPN mRNA. However, during chronic states of low-iron, FPN is activated independent of HIF-2α signaling. FPN protein is highly induced independent of mRNA induction. This biphasic model of FPN regulation allows for rapid as well as sustained response to increase systemic iron requirements. Currently, the mechanism by which FPN is regulated following chronic iron starvation is unknown. However, measuring kidney erythropoietin levels in 2-week and 8-week low-iron treated mice suggest that both erythropoiesis or whole body hypoxia are not activated (Supplemental Figure 3B). Transgenic mice that overexpress hepcidin or mice that are disrupted for hepcidin demonstrate dramatic changes in intestinal FPN expression37,44. Therefore, it is possible that effects of hepcidin on intestinal FPN are delayed or hepcidin does not directly regulate intestinal FPN expression. During the preparation of this manuscript it was demonstrated that in Caco-2 cells and in duodenal segments hepcidin did not decrease FPN expression, but directly decreased DMT-1 protein expression45. Therefore, it is possible that chronic changes in hepcidin could modulate intestinal FPN indirectly through changes in DMT-1 expression.
Hepcidin expression in the liver is significantly lower in the Hif-2αΔIE mice compared to Hif-2αF/F mice on the control matched AIN93G diet supplemented with 350 ppm of iron (Supplemental Figure 2A). This data is consistent with a previous publication using an independent intestine-specific HIF-2α knockout mouse line23. However, a significant change in liver hepcidin was not observed in Hif-2αΔIE mice on the standard grain-based chow (Supplemental Figure 2B). This may be due to the source of iron, as the controlled match AIN93G diet contains iron from a ferric (Fe3+) source, whereas 5001 diet the iron is from a ferrous (Fe2+) source. Therefore, iron in the grain-based diet is significantly easier to absorb since it does not need to be reduced. Lower basal levels of hepcidin on control AIN93G diet correlated with a slight increase in basal FPN protein expression observed in Hif-2αΔIE mice compared to the Hif-2αF/F mice following 8-weeks on the control AIN93G diet. This increase was not observed in Hif-2αΔIE mice on 2-weeks of control AIN93G diet. These data further provide evidence that the effect of hepcidin on intestinal FPN levels may be delayed, consistent with the biphasic regulation of FPN proposed in this study. Interestingly, the significant decrease in liver hepcidin levels in the Hif-2αΔIE mice, did not correlate to an increase liver iron staining, further demonstrating the importance of HIF-2α in intestinal iron absorption (Supplemental Figure 2C).
The present work established that the FPN1B isoform is directly regulated by HIF-2α. The FPN1B transcript encodes FPN with an identical open reading frame as FPN1A. FPN1B lacks the iron response element and is not repressed under iron-deficient conditions, and therefore it likely contributes to FPN protein expression in the duodenums of iron-deficient mice25. In assessing the regulatory region, two HREs were identified and mutating their sequences inhibited FPN1B promoter induction following HIF-2α overexpression. However, the HIF-2α activity on the FPN1B promoter was not completely lost following mutation of the HREs, suggesting that other ancillary HRE-like sequences are involved in FPN1B promoter regulation. Interestingly, FPN1A mRNA is also increased after low-iron treatment in a HIF-2α dependent manner. However, no consensus HRE was found within this promoter area. The current focus is to understand the precise mechanism by which FPN1A and FPN1B are induced by HIF-2α and assess the contributions of FPN1A and FPN1B to total FPN expression.
In conclusion, FPN is a pivotal transporter in regulating iron homeostasis. The current study provides a unique and novel mechanism to control FPN expression independent of hepcidin signaling. These findings may lead to novel HIF-2α-based therapies in hemochromatosis and iron deficiency.
Supplementary Material
Acknowledgments
Grant support: This study was supported by grants from the National Institutes of Health (CA148828) and The University of Michigan Gastrointestinal Peptide Center to Y.M.S.
Abbreviations
- ANKRD37
ankyrin repeat domain 37
- ARNT
aryl hydrocarbon nuclear translocator
- ASB11
ankyrin repeat and SOCS box-containing 11
- ChIP
chromatin immunoprecipitation
- DcytB
duodenal cytochrome b
- DMT-1
divalent metal transporter-1
- FPN
ferroportin
- GFP
green fluorescent protein
- GGH
gamma glutamyl hydrolase
- HIF
hypoxia inducible factor
- HRE
HIF response element
- ppm
parts per million
- qPCR
quantitative RT-PCR
- RBC
red blood cell
- Slc38a1
solute carrier family 38, member 1
- TFRC
transferring receptor
- Trim27
tripartite motif-containing 27
- Vhl
von Hippel-Lindau tumor suppressor protein
Footnotes
Disclosures: The authors have no conflict of interest to disclose.
Author contributions: Study concept and design; MT, AQ, EA, FG, YS. Acquisition of data; MT, AQ, EA, TM, AM, YS. Analysis and interpretation of data; MT, AQ, EA, TM, AM, YS. Drafting of the manuscript; YS. Critical revision of the manuscript for important intellectual content; MT, AQ, EA, AM, FG, YS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fleming MD, Trenor CC, 3rd, Su MA, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–6. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 2.Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–8. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 3.Latunde-Dada GO, Simpson RJ, McKie AT. Duodenal cytochrome B expression stimulates iron uptake by human intestinal epithelial cells. J Nutr. 2008;138:991–5. doi: 10.1093/jn/138.6.991. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie B, Garrick MD. Iron Imports. II. Iron uptake at the apical membrane in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G981–6. doi: 10.1152/ajpgi.00363.2005. [DOI] [PubMed] [Google Scholar]
- 5.McKie AT, Barrow D, Latunde-Dada GO, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–9. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- 6.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–12. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 7.Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–81. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 8.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 9.McKie AT, Marciani P, Rolfs A, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 10.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–5. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596–601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–9. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 14.Lesbordes-Brion JC, Viatte L, Bennoun M, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108:1402–5. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- 15.De Domenico I, Lo E, Ward DM, et al. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci U S A. 2009;106:3800–5. doi: 10.1073/pnas.0900453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Domenico I, Ward DM, Langelier C, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–78. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 18.Knutson MD, Oukka M, Koss LM, et al. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–8. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaston T, Chung B, Mascarenhas M, et al. Evidence for differential effects of hepcidin in macrophages and intestinal epithelial cells. Gut. 2008;57:374–82. doi: 10.1136/gut.2007.131722. [DOI] [PubMed] [Google Scholar]
- 20.Mena NP, Esparza A, Tapia V, et al. Hepcidin inhibits apical iron uptake in intestinal cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G192–8. doi: 10.1152/ajpgi.00122.2007. [DOI] [PubMed] [Google Scholar]
- 21.Laftah AH, Ramesh B, Simpson RJ, et al. Effect of hepcidin on intestinal iron absorption in mice. Blood. 2004;103:3940–4. doi: 10.1182/blood-2003-03-0953. [DOI] [PubMed] [Google Scholar]
- 22.Troadec MB, Ward DM, Lo E, et al. Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood. doi: 10.1182/blood-2010-04-278614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastrogiannaki M, Matak P, Keith B, et al. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–66. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah YM, Matsubara T, Ito S, et al. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–64. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang DL, Hughes RM, Ollivierre-Wilson H, et al. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009;9:461–73. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito S, Chen C, Satoh J, et al. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest. 2007;117:1940–50. doi: 10.1172/JCI31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah YM, Ito S, Morimura K, et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–48. 2048 e1–3. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomita S, Ueno M, Sakamoto M, et al. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol. 2003;23:6739–49. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haase VH, Glickman JN, Socolovsky M, et al. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–8. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths WJ, Mayr R, McFarlane I, et al. Clinical presentation and molecular pathophysiology of autosomal dominant hemochromatosis caused by a novel ferroportin mutation. Hepatology. 51:788–95. doi: 10.1002/hep.23377. [DOI] [PubMed] [Google Scholar]
- 31.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 32.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 33.Benita Y, Kikuchi H, Smith AD, et al. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37:4587–602. doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Z, Gulec S, Collins JF. Cross-Species Comparison of Gene Expression Profiles Reveals Induction of Hypoxia Inducible Factor Responsive Genes in Iron Deprived Intestinal Epithelial Cells. Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganz T, Nemeth E. Hepcidin and Disorders of Iron Metabolism. Annu Rev Med. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 36.Papanikolaou G, Tzilianos M, Christakis JI, et al. Hepcidin in iron overload disorders. Blood. 2005;105:4103–5. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viatte L, Lesbordes-Brion JC, Lou DQ, et al. Deregulation of proteins involved in iron metabolism in hepcidin-deficient mice. Blood. 2005;105:4861–4. doi: 10.1182/blood-2004-12-4608. [DOI] [PubMed] [Google Scholar]
- 38.Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu A, Kaneshiro M, Kaunitz JD. Evaluation and treatment of iron deficiency anemia: a gastroenterological perspective. Dig Dis Sci. 55:548–59. doi: 10.1007/s10620-009-1108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mair SM, Weiss G. New pharmacological concepts for the treatment of iron overload disorders. Curr Med Chem. 2009;16:576–90. doi: 10.2174/092986709787458434. [DOI] [PubMed] [Google Scholar]
- 41.Montosi G, Donovan A, Totaro A, et al. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest. 2001;108:619–23. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Njajou OT, Vaessen N, Joosse M, et al. A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat Genet. 2001;28:213–4. doi: 10.1038/90038. [DOI] [PubMed] [Google Scholar]
- 43.Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis. 2004;32:131–8. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Viatte L, Nicolas G, Lou DQ, et al. Chronic hepcidin induction causes hyposideremia and alters the pattern of cellular iron accumulation in hemochromatotic mice. Blood. 2006;107:2952–8. doi: 10.1182/blood-2005-10-4071. [DOI] [PubMed] [Google Scholar]
- 45.Brasse-Lagnel C, Karim Z, Letteron P, et al. Intestinal DMT1 cotransporter is downregulated by hepcidin via proteasome-internalization and degradation. Gastroenterology. doi: 10.1053/j.gastro.2010.12.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.