Abstract
Background & Aims
High level coffee consumption has been associated with reduced progression of pre-existing liver diseases and lower risk of hepatocellular carcinoma. However, its relationship with therapy for Hepatitis C virus (HCV) infection has not been evaluated.
Methods
Patients (n=885) from the lead-in phase of the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) trial recorded coffee intake before re-treatment with peginterferon alfa-2a (180 μg/wk) and ribavirin (1000–1200 mg/day). We assessed patients for early virologic response (EVR, 2 log10 reduction in level of HCV RNA at week 12; n=466) and undetectable HCV RNA at week 20 (W20VR; n=320), week 48 (end of treatment, EOT; n=284), and week 72 (sustained virologic response, SVR; n=157).
Results
The median log10 drop from baseline to week 20 was 2.0 (interquartile range: 0.6–3.9) among non-drinkers and 4.0 (2.1–4.7) among patients that drank ≥3 cup/day of coffee (P-trend <0.0001). In unadjusted models, the odds ratios (OR) and 95% confidence intervals (CI) for drinking ≥3 cups/day vs non-drinking were 3.2 (1.9–5.3) for EVR, 3.1 (1.8–5.1) for W20VR, 3.5 (2.0–5.9) for EOT, and 2.7 (1.4–5.3) for SVR (P-trend<0.0001 for all). After adjustment for age, race/ethnicity, sex, alcohol, cirrhosis, ratio of aspartate aminotransferase:alanine aminotransferase, the IL28B polymorphism rs12979860, dose reduction of peginterferon, and other covariates, the OR (95% CI) for EVR was 2.0 (1.1–3.6; P-trend = 0.004); for W20VR was 2.1 (1.1–3.9; p-trend=0.005); for EOT was 2.4 (1.3–4.6; P-trend=0.001), and for SVR was 1.8 (0.8–3.9; P-trend=0.034).
Conclusion
High-level consumption of coffee (more than 3 cups per day) is an independent predictor of improved virologic response to peginterferon plus ribavirin in patients with Hepatitis C.
Keywords: Liver fibrosis, diet, risk factor, caffeine
Introduction
Approximately, 70–80% of individuals exposed to Hepatitis C (HCV) become chronically infected.1 Worldwide, these individuals are estimated to number between 130 and 170 million.2 Treatment with peginterferon and ribavirin resolves chronic hepatitis C in about half of patients.3, 4 However, those who fail or are unable to tolerate treatment have few current treatment options.
A number of factors affect response to therapy,5 including African-American race,6–8 presence of cirrhosis,8 baseline aspartate aminotransferase (AST)- alanine aminotransferase (ALT) ratio,8 baseline serum HCV level,8 insulin resistance,9, 10 particular single nucleotide polymorphisms (SNPs), including rs12979860 or rs8099917 near IL28B,11–15 genotype 1 of HCV,8, 16, 17 and patients’ ability to tolerate full doses of peginterferon during treatment.18
Coffee drinking has been associated with several aspects of liver health, including concentrations of the liver enzymes ALT, AST, and gamma-glutamyltransferase,19–24 progression of pre-existing liver disease,25 and hepatocellular carcinoma.26, 27 It is not known whether coffee affects spontaneous HCV clearance, or among chronically infected individuals, patients’ response to HCV therapy.28
Therefore, we investigated the association between coffee intake and virologic response to peginterferon plus ribavirin treatment in the lead-in phase of the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) trial of patients with baseline fibrosis or cirrhosis who had failed previous interferon therapy.29
Materials and Methods
Patient population
As previously described,8, 18, 29, 30 the lead-in phase of HALT-C enrolled 1145 Hepatitis C positive patients who had an Ishak fibrosis score ≥3, had failed previous interferon treatment, and had no evidence of hepatic decompensation or hepatocellular carcinoma. During lead-in, patients received 180 μg per week of peginterferon alfa-2a and 1000 mg/day ribavirin for those weighing ≤75 kg and 1200 mg/day for those weighing >75 kg. Patients with declining neutrophil, platelet, hemoglobin counts, or other adverse effects were managed by dose reduction of peginterferon and or ribavirin.18 The amount of medication taken by each patient over the first twenty weeks was expressed as a proportion of the original prescribed dose. The study protocol was approved by the institutional review board of each participating institution, and written consent was obtained from all patients.
Assessment of coffee and tea consumption
At the beginning of the lead-in-phase, patients completed a previously validated31, 32 Block 98.2 food frequency questionnaire (FFQ; Nutrition Quest, Berkeley, CA). Patients reported typical intake of 110 food items over the past year, using nine frequency categories ranging from “never” to “every day” and four categories of portion size (1 cup, 2 cups, 3–4 cups, and 5+ cups). One question assessed coffee intake and did not distinguish decaffeinated from caffeinated coffee. A second question assessed tea intake and did not distinguish black from green tea. Patients failing lead-in therapy entered the randomized phase and completed a second Block FFQ approximately a year after beginning the randomized phase.
For analysis, we created categorical variables of coffee (never, >0 to <1, ≥1 to <3, and ≥3 cups/day) and tea intake (never, >0 to <1, ≥1 to <2, and ≥2 cups/day). We excluded 259 patients who did not complete a FFQ and one patient with extreme caloric intake (more than two interquartile ranges from the median), leaving 885 patients for the current analysis. Patients completing the FFQ were similar to those who did not, other than being more typically Caucasian (76.2% vs. 65.3%, p=0.034) and having a lower baseline AST/ALT ratio (median=0.78 vs. 0.82, p=0.0056).
Assessment of outcomes
Serum samples obtained from all subjects enrolled in the HALT-C Trial were tested in real time at the University of Washington Virology Laboratory with both the quantitative Roche COBAS® Amplicor HCV Monitor Test, v. 2.0 assay [lower limit of detection (LLOD) 600 IU/mL] and, if negative, by the Roche COBAS ® Amplicor HCV Test, v. 2.0 assay (Roche Molecular Systems, Branchburg, NJ) with LLOD 100 IU/mL as previously described.8, 33 HCV genotypes were determined with the INNO-LiPA HCV II kit (Siemens Medical Solutions Diagnostics, Tarrytown, NY). Serum HCV RNA level was assessed at baseline, along with week 12, week 20, and week 48 of treatment. Early virologic response (EVR) was defined as a ≥2-log10 decline in serum HCV RNA level at week 12. Week 20 virologic response was defined as the absence of detectable serum HCV RNA (<100 IU/mL) at week 20. Week 20, as opposed to the traditional week 24, was chosen in order to provide sufficient time to identify non-responders for randomization into the main HALT-C trial. Patients with undetectable virus at week 20 continued to receive peginterferon plus ribavirin treatment for an additional 28 weeks (48 weeks total) at which point treatment was stopped. Sustained virologic response was defined as the absence of detectable serum HCV RNA at week 72, 24 weeks after the end of treatment. For analysis, we set undetectable viral levels at the detection limit (100, i.e. 2 log10, IU/mL).
Statistical analysis
All tests were two-sided and an alpha of <0.05 was considered to be statistically significant. Analyses were performed with SAS, release 9.2 (SAS Institute, Cary, NC). We tabulated baseline behavioral and clinical, demographic, and genetic features by categories of coffee intake. The Jonckheere-Terpstra test for trend for continuous variables and the Mantel-Haenszel test for trend for categorical variables were used to assess variation across categories of coffee intake. Variation across categories of race/ethnicity was assessed by the Pearson Chi-square test. Associations between coffee and tea intake with virologic response were determined using logistic regression. Linear trend tests were performed by assigning participants the median intake for their categories and entering that term as a continuous variable in the regression models. We present results from unadjusted crude models, along with models adjusted for continuous baseline age, AST/ALT ratio, log HCV RNA level, hemoglobin, neutrophils, platelets, and categories of sex, race/ethnicity, alcohol use at baseline, cirrhosis, HCV genotype 1, previous use of ribavirin, dose reduction of peginterferon during the first 20 weeks of treatment, and rs12979860 genotype. Additional adjustment for Short Form-36 (SF-36)34 general health, physical function, or vitality quality of life scores, pack-years of cigarettes, rs8099917 genotype, dose reduction of ribavirin during the first 20 weeks of treatment, body mass index, the homeostatic model assessment score of insulin resistance (HOMA2), total serum cholesterol, high-density lipoprotein cholesterol (HDL), or triglycerides had no appreciable effect on risk estimates for virologic response (data not shown). Additionally, we performed propensity score analysis35 in order to better balance possible confounders between coffee drinkers and non-drinkers. We created a propensity score for coffee intake using the following covariates: age (continuous), sex, race/ethnicity (Caucasian, African American, Hispanic, Other), alcohol use (current, former, and never), cirrhosis at baseline, genotype 1, AST/ALT ratio (continuous), log HCV RNA level at baseline (continuous), previous use of ribavirin, hemoglobin (continuous), neutrophils (continuous), platelets (continuous), categories of peginterferon medication dose during first 20 weeks of treatment (≥98%–100%, ≥80%–<98%, ≥60%–<80%, and <60%), and rs12979860 genotype (TT, CT, CC). We then adjusted risk estimates for coffee with virologic response for quintiles of the propensity score using indicator variables. Risk estimates were also calculated across strata of propensity score quintiles.
We investigated possible interactions between coffee intake and a number of clinical and behavioral features by examining the association between coffee and virologic response by stratum of each clinical and behavioral feature. We formally tested for effect modification by including an interaction term between each stratifying variable and continuous coffee intake in the model.
Results
Of the 885 patients who began full-dose peginterferon and ribavirin therapy, 85% drank coffee and 14.9% of patients drank ≥3 cups per day. At baseline, those consuming higher quantities of coffee were more likely to be Caucasian, drink alcohol and smoke cigarettes, have the CC genotype of rs12979860 (near IL28B), have higher hemoglobin, neutrophils, platelets, and total cholesterol, less likely to have cirrhosis at baseline, and have lower serum AST/ALT and HOMA2 score of insulin resistance (p<0.05 for all; Table 1). Whereas 50.4% of non-coffee drinkers tolerated the full dose of peginterferon alfa-2a during treatment, 60.6% of ≥3 cups per day coffee drinkers tolerated the full dose (p=0.0015). Among determinants of peginterferon dose reduction, 58% were due to low neutrophils and 22.6 % were due to low platelets. During treatment, coffee drinkers were less likely to have a dose reduction due to either low neutrophils (p=0.016) or platelets (p=0.059). The relationships between coffee and clinical and demographic variables were generally similar in analyses restricted to Caucasians (n=674), although we noted one difference. The association for coffee with rs8099917 genotype became statistically significant (p=0.001).
Table 1.
Association of coffee intake with baseline variables
| Variables | Coffee consumption | ||||
|---|---|---|---|---|---|
| Non-drinkers | > 0 to <1 cups/day | ≥ 1 to <3 cups/day | ≥ 3 cups/day | P for trend* | |
| Number in study | 133 (15.0) | 253 (28.6) | 367 (41.5) | 132 (14.9) | |
| Coffee intake (cups/day), Median (IQR) | 0 | 0.16 (0.03–0.5) | 2 (1–2) | 3.5 (3.5–3.5) | |
| Age, years, Median (IQR) | 48 (45–53) | 49 (46–55) | 49 (46–54) | 49 (46–53) | 0.47 |
| Gender, female, No. (%) | 38 (28.6) | 74 (29.3) | 102 (27.8) | 30 (22.7) | 0.22 |
| Race/ethnicity | |||||
| Caucasian, No. (%) | 96 (72.2) | 163 (64.4) | 296 (80.7) | 119 (90.2) | <0.0001 |
| African American, No. (%) | 24 (18.1) | 53 (21.0) | 35 (9.5) | 2 (1.5) | |
| Hispanic, No. (%) | 7 (5.3) | 32 (12.7) | 28 (7.6) | 7 (5.3) | |
| Other, No. (%) | 6 (4.5) | 5 (2.0) | 8 (2.2) | 4 (3.0) | |
| Current alcohol drinker,† No. (%) | 19 (14.4) | 38 (15.0) | 82 (22.5) | 28 (21.2) | <0.0001 |
| Pack years of cigarettes, Median (IQR) | 2.7 (0–14.0) | 3.0 (0–14.5) | 10.5 (1.2–25.0) | 20.8 (4.3–34.8) | <0.0001 |
| Baseline Homa2 score,† Median (IQR) | 4.7 (3.0–8.5) | 4.2 (2.8–6.6) | 4.0 (2.7–6.4) | 3.7 (2.2–5.6) | 0.001 |
| Serum total cholesterol (mg/dL),† Median (IQR) | 162 (143–185) | 169 (146–190) | 174 (158–196) | 176 (152–202) | <0.0001 |
| Serum HDL cholesterol (mg/dL),† Median (IQR) | 40 (32–49) | 43 (35–52) | 41 (34–51) | 37 (32–46) | 0.14 |
| Serum triglyceride (mg/dL), Median (IQR) | 118 (76–182) | 102 (78–138) | 109 (75–166) | 108 (79–161) | 0.62 |
| General Health SF-36 score, Median (IQR) | 62 (40–77) | 62 (47–77) | 62 (42–77) | 57 (40–77) | 0.23 |
| Physical Function SF-36 score,† Median (IQR) | 85 (60–100) | 90 (65–100) | 85 (60–100) | 85 (55–95) | 0.58 |
| Vitality SF-36 score, Median (IQR) | 60 (40–80) | 60 (40–75) | 55 (35–75) | 50 (30–70) | 0.002 |
| Cirrhosis on biopsy, No. (%) | 52 (39.1) | 109 (43.1) | 123 (33.5) | 39 (29.6) | 0.004 |
| HCV genotype 1, No. (%) | 121 (91.0) | 227 (89.7) | 333 (90.7) | 108 (81.8) | 0.05 |
| AST/ALT | 0.82 (0.66–1.04) | 0.83 (0.68–1.02) | 0.75 (0.63–0.93) | 0.70 (0.61–0.86) | <0.0001 |
| Previous use of ribavirin, yes, No. (%) | 91 (68.4) | 181 (71.5) | 256 (69.8) | 92 (69.7) | 0.86 |
| Hemoglobin, g/dL, median (IQR) | 15.1 (13.8–15.9) | 15.1 (14.0–15.8) | 15.2 (14.2–16.3) | 15.4 (14.4–16.3) | 0.004 |
| Platelets, × 1000/mm3, median (IQR) | 159 (115–208) | 154 (115–205) | 168 (127–211) | 170 (133–216.5) | 0.01 |
| Neutrophils × 1000/mm3, median (IQR) | 2.9 (2.1–3.6) | 2.7 (2.2–3.5) | 3.1 (2.4–3.9) | 3.4 (2.7–4.5) | <0.0001 |
| Peginterferon alfa-2a dose (% maximum) | |||||
| ≥ 98%–100%, No. (%) | 67 (50.4) | 106 (41.9) | 201 (54.8) | 80 (60.6) | 0.0015 |
| ≥ 80%–<98%, No. (%) | 24 (18.1) | 64 (25.3) | 72 (19.6) | 24 (18.2) | |
| ≥ 60%–<80%, No. (%) | 23 (17.3) | 37 (14.6) | 41 (11.2) | 14 (10.6) | |
| <60%, No. (%) | 19 (14.3) | 46 (18.2) | 53 (14.4) | 14 (10.6) | |
| Ribavirin dose (% maximum) | |||||
| ≥ 98%–100%, No. (%) | 67 (50.4) | 113 (44.7) | 182 (49.6) | 63 (47.7) | 0.50 |
| ≥ 80%–<98%, No. (%) | 23 (17.3) | 62 (24.5) | 77 (21.0) | 27 (20.5) | |
| ≥ 60%–<80%, No. (%) | 16 (12.0) | 38 (15.0) | 64 (17.4) | 21 (15.9) | |
| <60%, No. (%) | 27 (20.3) | 40 (15.8) | 44 (12.0) | 21 (15.9) | |
| rs12979860 genotype (IL28B):† | |||||
| TT, No. (%) | 29 (24.0) | 55 (24.6) | 59 (17.9) | 14 (12.0) | 0.0007 |
| CT, No. (%) | 69 (57.0) | 115 (51.3) | 184 (55.8) | 64 (54.7) | |
| CC, No. (%) | 23 (19.0) | 54 (24.1) | 87 (26.4) | 39 (33.3) | |
| rs8099917 genotype (IL28B):† | |||||
| TT, No. (%) | 57 (46.7) | 117 (52.2) | 166 (50.2) | 68 (58.1) | 0.078 |
| GT, No. (%) | 53 (43.4) | 90 (40.2) | 145 (43.8) | 45 (38.5) | |
| GG, No. (%) | 12 (9.8) | 17 (7.6) | 20 (6.0) | 4 (3.4) |
Abbreviations: No: Number; IQR: Interquartile range; Short form-36 (SF-36);
Mantel-Haenszel test for trend for categorical variables. Jonckheere-Terpstra test for trend for continuous variables. Chi-square test for race/ethnicity.
Data not available for all participants: Alcohol drinking available for 881 patients; Serum total cholesterol for 847; Serum HDL cholesterol for 845; HOMA2 score for 857; Physical Function SF-36 score for 884 patients; rs12979860 genotype for 792 patients; rs8099917 genotype for 794 patients.
More coffee consumption was associated with slightly higher baseline HCV RNA levels (p for trend =0.007).(Table 2) Yet with increasing coffee intake, the decline in patients’ serum HCV RNA level from baseline was greater and absolute levels of patients’ serum HCV RNA at weeks 12 and 20 were lower (Table 2). Thus, for example, the median log10 HCV RNA at week 20 was 4.6 (IQR: 2.0–5.8) for non-drinkers and 2.0 (IQR: 2.0–4.3) for those who drank ≥3 cups per day (p-trend <0.0001). Consistent results were observed for the log decrease in HCV RNA from baseline to week 12 (1.7 (IQR: 0.7–3.6) in non-drinkers vs. 3.7 (IQR: 1.8–4.2) for ≥3 cup per day drinkers; p-trend p<0.0001) and from baseline to week 20 (2.0 (IQR: 0.6–3.9) in non-drinkers vs. 4.0 (IQR: 2.1–4.7) for ≥3 cup per day drinkers; p-trend p<0.0001).
Table 2.
Association of coffee intake with log HCV RNA level
| Variables | Coffee consumption | ||||
|---|---|---|---|---|---|
| Non-drinkers | > 0 to <1 cups/day | ≥ 1 to <3 cups/day | ≥ 3 cups/day | P for trend* | |
| Number in study† | 133 (15.0) | 253 (28.6) | 367 (41.5) | 132 (14.9) | |
| Baseline Log HCV RNA level, Median (IQR) | 6.4 (6.1–6.7) | 6.4 (6.1–6.8) | 6.5 (6.1–6.8) | 6.6 (6.2–6.9) | 0.007 |
| Week 12 Log HCV RNA level, Median (IQR) | 4.3 (2.8–5.7) | 4.8 (2.8–5.7) | 3.6 (2.0–5.6) | 2.8 (2.0–4.6) | <0.0001 |
| Week 20 Log HCV RNA level, Median (IQR) | 4.6 (2.0–5.8) | 4.5 (2.0–5.9) | 2.8 (2.0–5.5) | 2.0 (2.0–4.3) | <0.0001 |
| Log decrease, baseline to week 12, Median (IQR) | 1.7 (0.7–3.6) | 1.6 (0.7–3.5) | 2.8 (0.9–4.0) | 3.7 (1.8–4.2) | <0.0001 |
| Log decrease, baseline to week 20, Median (IQR) | 2.0 (0.6–3.9) | 1.9 (0.6–3.9) | 3.2 (1.0–4.4) | 4.0 (2.1–4.7) | <0.0001 |
Abbreviation: IQR: Interquartile range;
Jonckheere-Terpstra test for trend.
Log HCV RNA level was available for 885 patients at baseline, 860 patients at week 12, and 846 patients at week 20.
Coffee drinkers were also more likely to have a virologic response according to the pre-defined endpoints (Table 3). Among non-drinkers, 45.7% had an early virologic response (≥2 log drop in their serum HCV RNA level at week 12), 26.3% had no detectable serum HCV RNA at week 20, 21.8% had no detectable serum at week 48, and 11.3% had a sustained virologic response. In contrast, the corresponding proportions for ≥3 cup per day coffee drinkers were 72.7%, 52.3%, 49.2%, and 25.8% respectively. From crude logistic regression models, patients who drank ≥3 cups per day of coffee were about three times more likely to have a virologic response at the four time-points of interest (Table 3). Ability to tolerate treatment had minimal effect on the relationship of coffee and virologic response. For example, the odds ratio for patients who drank ≥3 cups per day relative to non-drinkers for week 20 response changed slightly from 3.07 (Crude: Table 3) to 2.92 (data not in table) with control for peginterferon dose and the p-trend remained highly statistically significant (p-trend<0.0001). Multivariate adjustment for age, sex, race/ethnicity, alcohol use, cirrhosis at baseline, genotype 1, AST/ALT ratio, log HCV RNA level at baseline, previous use of ribavirin, hemoglobin, neutrophils, platelets, peginterferon medication dose during first 20 weeks of treatment, and rs12979860 genotype, attenuated associations with coffee, though associations remained significant for each virologic response endpoint (Table 3). Risk estimates using propensity score methods were similar to those from multivariate adjusted models (data not shown).
Table 3.
Association between coffee intake and virologic response:
| Coffee consumption | ||||||
|---|---|---|---|---|---|---|
| Continuous (cup/day) | Non-drinkers | > 0 to <1 cups/day | ≥ 1 to <3 cups/day | ≥ 3 cups/day | P for trend | |
| No. in study | 885 (100) | 133 (15.0) | 253 (28.6) | 367 (41.5) | 132 (14.9) | |
| W12, log drop ≥2 | 466 (54.2) | 59 (45.7) | 109 (44.7) | 205 (57.1) | 93 (72.7) | |
| Crude odds ratio (95% CI) | 1.33 (1.20–1.48) | 1.00 (ref) | 0.96 (0.62–1.47) | 1.58 (1.05–2.37) | 3.15 (1.87–5.31) | <0.0001 |
| Multivariate adjusted* | 1.21 (1.07–1.37) | 1.00 (ref) | 0.88 (0.54–1.45) | 1.26 (0.79–2.01) | 1.97 (1.08–3.61) | 0.0039 |
| No. with week 20 response | 320 (36.2) | 35 (26.3) | 73 (28.9) | 143 (39.0) | 69 (52.3) | |
| Crude odds ratio (95% CI) | 1.29 (1.18–1.42) | 1.00 (ref) | 1.14 (0.71–1.82) | 1.79 (1.15–2.77) | 3.07 (1.83–5.13) | <0.0001 |
| Multivariate adjusted* OR | 1.20 (1.07–1.36) | 1.00 (ref) | 1.03 (0.58–1.81) | 1.45 (0.86–2.45) | 2.10 (1.12–3.93) | 0.0047 |
| No. with week 48 response | 284 (32.1) | 29 (21.8) | 61 (24.1) | 129 (35.2) | 65 (49.2) | |
| Crude odds ratio (95% CI) | 1.32 (1.20–1.45) | 1.00 (ref) | 1.14 (0.69–1.88) | 1.94 (1.22–3.09) | 3.48 (2.04–5.94) | <0.0001 |
| Multivariate adjusted* OR | 1.22 (1.08–1.37) | 1.00 (ref) | 1.07 (0.59–1.94) | 1.61 (0.93–2.77) | 2.42 (1.28–4.60) | 0.0011 |
| No. with SVR | 157 (17.7) | 15 (11.3) | 32 (12.7) | 76 (20.7) | 34 (25.8) | |
| Crude odds ratio (95% CI) | 1.20 (1.08–1.34) | 1.00 (ref) | 1.14 (0.59–2.19) | 2.06 (1.14–3.72) | 2.73 (1.41–5.30) | <0.0001 |
| Multivariate adjusted* | 1.11 (0.97–1.26) | 1.00 (ref) | 1.03 (0.49–2.17) | 1.69 (0.86–3.34) | 1.80 (0.83–3.94) | 0.034 |
Abbreviation: CI, confidence interval;
Adjusted for age (continuous), sex, race/ethnicity (Caucasian, African American, Hispanic, Other), alcohol use (current, former, and never), cirrhosis at baseline, genotype 1, AST/ALT ratio (continuous), log HCV RNA level at baseline (continuous), previous use of ribavirin, hemoglobin (continuous), neutrophils (continuous), platelets (continuous), categories of peginterferon medication dose during first 20 weeks of treatment (≥98%–100%, ≥80%–<98%, ≥60%–<80%, and <60%), and rs12979860 genotype (TT, CT, CC).
In contrast to results for coffee, no effect was observed for drinking tea (p-trend =0.92, 0.96, 0.89, and 0.49 for early, week 20, week 48, and sustained virologic response, respectively).
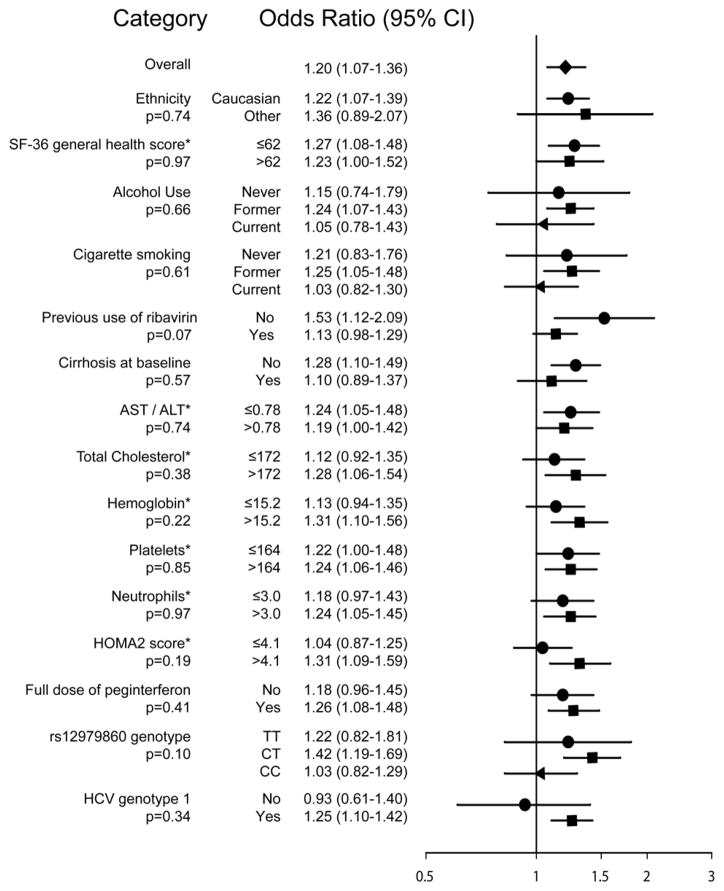
In stratified analyses, we investigated effect modification (interaction) for week 20 HCV negativity across stratum of HCV genotype, race/ethnicity, cirrhosis at baseline, baseline AST/ALT ratio, hemoglobin, neutrophils, platelets, total cholesterol, HOMA score, SF-36 general health score, dose reduction of peginterferon, alcohol use, cigarette smoking, or rs12979860 genotype. Results are presented for week 20 virologic response, but were similar for early virologic response (week 12), end of treatment response (week 48), and sustained virologic response (week 72; data not shown). Risk estimates generally appeared similar in each stratum and the p-values for interaction were all >0.05 (Figure 1). For example, of the 454 patients who tolerated full dose, 43.4% had a week 20 virologic response compared with 28.5% of the 431 patients who took less than full dose. The relative benefit of coffee on virologic response was similar in these two groups (OR 1.26 for full dose and 1.18 for lower dose) despite the absolute difference in response. The relationships between coffee and virologic responses were also very similar in analyses restricted to Caucasians. Specifically, there was a statistically significant increase in week 20 virological response per cup increase in coffee consumption among Caucasians. Associations between coffee intake and virologic response were apparent in patients with both fibrosis and cirrhosis at baseline; though stronger in those with fibrosis. Finally, risk estimates for coffee appeared stronger in patients with the less favorable IL28B rs12979860 TT or CT genotype, though again differences in risk estimates were not statistically significant.
Figure 1.
Stratified analysis of the association of baseline coffee intake with week 20 virologic response in the HALT-C trial. Odds ratios shown are for an increase in coffee consumption of one drink per day and are adjusted for age (continuous), sex, race/ethnicity (Caucasian, African American, Hispanic, Other), alcohol use (current, former, and never), cirrhosis at baseline, genotype 1, AST/ALT ratio (continuous), log HCV RNA level at baseline (continuous), previous use of ribavirin, hemoglobin (continuous), neutrophils (continuous), platelets (continuous), categories of peginterferon medication dose during first 20 weeks of treatment (≥98%–100%, ≥80%–<98%, ≥60%–<80%, and <60%), and rs12979860 genotype (TT, CT, CC). Median values were used to define cut-points for the starred characteristics. Black diamond indicates the overall point estimate. Black circles, squares, and triangles represent the point estimate for each indicated subgroup. Horizontal lines represent 95% confidence intervals (CI). The solid vertical line indicates an odds ratio of one. P values are for the interaction between coffee intake and each stratifying variable and are taken from the Wald-test for the cross-product term of each stratifying variable and continuous coffee intake.
We were unable to determine coffee intake during lead-in therapy. But for patients failing lead-in therapy, coffee intake was assessed on a second occasion, 18 months after baseline, i.e. 12 months after these patients had been randomized to low-dose peginterferon or no treatment. Median coffee intake was the same (one cup per day) at baseline and at the second time-point for patients in both randomization groups. The weighted kappa for the two assessments was 0.58 overall (p <0.0001), 0.54 in those receiving treatment (p <0.0001), and 0.63 in those receiving no-treatment (p <0.0001), indicating good agreement.
Discussion
In patients with advanced Hepatitis C related chronic liver disease in the HALT-C trial receiving peginterferon plus ribavirin treatment, ≥3 cups per day coffee drinkers were three times more likely to have a virologic response than non-drinkers. Associations were attenuated but persisted after adjustment for a wide range of behavioral, clinical, and genetic features, suggesting an effect independent of other known risk factors. In contrast to results for coffee, no effect was observed for tea drinking.
Coffee intake has been associated with lower level of liver enzymes, reduced progression of chronic liver disease,25 and reduced incidence of hepatocellular carcinoma.26, 27 Since little other data on the association of coffee drinking with virologic response is available, the herein observed association needs replication in other studies.
A number of risk factors have previously been associated with virologic response in HALT-C and in other studies,5, 8, 12, 14, 15, 18, 25 including African-American race, presence of cirrhosis, AST/ALT ratio, serum HCV RNA level, particular genotypes near the IL28B gene, and ability to tolerate full doses of peginterferon during treatment. Intriguingly, coffee was modestly associated with nearly all of these factors. African-Americans in our study tended to drink less coffee than Caucasians, and coffee drinking was associated with lower AST/ALT ratio, ability to tolerate full doses of peginterferon alfa-2a during treatment, and particular genotypes of SNPs near to the IL28B gene, which have recently been linked to virologic response.11–15 Yet, the association for coffee persisted after adjustment for these and other potential confounders and was similar across stratums of each of these risk factors, e.g. a similar effect for coffee on virologic response was observed for both those receiving a full dose of peginterferon and those having a dose reduction. These results suggest that coffee drinkers had a better response to treatment that was independent other risk factors, including higher tolerance for peginterferon treatment.
Even so, associations between coffee and features associated with virologic response raise the possibility of reverse causality, i.e. sicker patients were less likely to drink coffee and in this way, less likely to respond to treatment. But in HALT-C, if anything, patients drinking coffee reported a worse quality of life on the SF-36 quality of life questionnaire than non-drinkers. Quality of life was also not associated with virologic response. Nevertheless, as in all observational studies, we cannot exclude unmeasured or residual confounding as an explanation for our results. Observed associations could also simply be due to chance.
Coffee has more than a 1,000 compounds, any one of which could be involved in virologic response. One major constituent of coffee is caffeine. Though we could not distinguish caffeinated from decaffeinated coffee in our study, we found no association with consumption of black or green tea. Fewer individuals consumed tea in our study and tea contains less caffeine than coffee, however. It is unlikely that coffee and its constituents have a direct antiviral effect. If so, HCV RNA levels at baseline would have been expected to be lower with greater coffee consumption. In fact, baseline levels were actually higher with greater consumption (Table 2). More likely coffee would have a facilitating effect on response to peginterferon and ribavirin treatment by a mechanism yet to be understood. It is intriguing that the C allele of rs12979860 near the IL28B gene has been associated with higher baseline viral levels, lower levels of interferon stimulated-gene expression, and better treatment response.14, 36, 37 The IL28B genotype effect on virologic response may be through the JAK-STAT signaling pathway.38 Recently published results potentially link kahweol, a diterpene in coffee, to JAK-STAT signaling,39 suggesting one of many possible mechanisms for the observed association in our study.
A number of studies have linked high serum total and low-density lipoprotein (LDL) cholesterol with increased virologic response to peginterferon plus ribavirin therapy.40–42 LDL has also been recently posited to mediate, at least partly, the effect of the rs12979860 C allele.41, 43 Coffee intake was associated with higher serum total cholesterol in our study and has also been linked to higher serum total cholesterol and LDL in past observational and interventional studies.44 Adjustment for total cholesterol, however, did not affect risk estimates in the current analysis. We lacked assessment of LDL.
Alternatively, insulin resistance has been associated with poor virologic response in a number of previous studies.9, 10 Consistent with previous studies of type-II diabetes,45, 46 coffee intake was inversely associated with insulin resistance in HALT-C. Adjustment for HOMA2 score did not affect risk estimates for coffee with virologic response in the current analysis, however.
Our study has several advantages, including a large number of patients with histological staging of liver fibrosis, careful assessment of virologic response using a central virology laboratory, and comprehensive assessment of clinical and histologic features. Limitations include a lack of information on caffeine and coffee brewing methods and the assessment of coffee via self-report at a single time point. As such, we do not know patients’ coffee intake at the time of initial treatment or whether coffee consumption was maintained over the course of the lead-in phase. However, for patients failing lead-in therapy subsequently randomized to half-dose peginterferon treatment or to no treatment, coffee consumption was similar at baseline and eighteen months later (6 months after randomization). Since patients in HALT-C also had previously failed interferon therapy, it is not clear whether our results can be generalized to other patient populations, such as those with less advanced disease, those who are treatment-naïve to prior therapy, or who are being treated with newer anti-viral agents.
In summary, we observed an independent association between coffee intake and virologic response to peginterferon plus ribavirin retreatment in the lead-in phase of the HALT-C trial. Future studies are needed to replicate this finding in other populations.
Acknowledgments
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Herbert L. Bonkovsky, MD, Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Adrian M. Di Bisceglie, MD, Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Jules L. Dienstag, MD, Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, David P. Lundmark
University of Colorado Denver, School of Medicine, Aurora, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01) Gregory T. Everson, MD, Thomas Trouillot, MD, Marcelo Kugelmas, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) Timothy R. Morgan, MD, John C. Hoefs, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) William M. Lee, MD, Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Anna S. Lok, MD, Robert J. Fontana, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Richard K. Sterling, MD, MSc, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: Marc G. Ghany, MD, T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) Chihiro Morishima, MD, David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD
Inova Fairfax Hospital, Falls Church, VA: Zachary D. Goodman, MD, PhD, Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Grant support: This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc. (now Genentech), through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health. This research was also supported in part by the Intramural Research Program of the National Cancer Institute. The funding organizations had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Abbreviations
- CI
Confidence Interval
- EOT
End of treatment (week 48) virologic response
- EVR
early virologic response
- FFQ
food frequency questionnaire
- HALT-C
Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis Trial
- HDL
high-density lipoprotein
- HOMA2
homeostatic model assessment score of insulin resistance
- LDL
low-density lipoprotein
- LLOD
lower limit of detection
- OR
Odds Ratio
- SNP
Single Nucleotide Polymorphism
- SF-36
Short Form-36
- SVR
sustained virologic response
- W20VR
Week 20 virologic response
Footnotes
Author contributions: All authors contributed to the study concept and design, statistical analysis and interpretation of data, and the drafting and revision of the manuscript.
Disclosures: K. L. Lindsay was a consultant and received research support from Hoffmann-La Roche, Inc. (now Genentech), during this study and is now an employee of Tibotec, Inc. (a subsidiary of Johnson and Johnson), Titusville, NJ. Authors with nothing to disclose: N. D. Freedman, T. M. Curto, E. C. Wright, R. Sinha, and J. E. Everhart.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 (Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 4.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 5.Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response in hepatitis B and C. J Hepatol. 2008;49:634–651. doi: 10.1016/j.jhep.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Howell C, Jeffers L, Hoofnagle JH. Hepatitis C in African Americans: summary of a workshop. Gastroenterology. 2000;119:1385–1396. doi: 10.1053/gast.2000.19582. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–98. vi. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Shiffman ML, Di Bisceglie AM, Lindsay KL, et al. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Harrison SA. Insulin resistance among patients with chronic hepatitis C: etiology and impact on treatment. Clin Gastroenterol Hepatol. 2008;6:864–876. doi: 10.1016/j.cgh.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Gomez M, Del MV, Andrade RJ, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 12.Rauch A, Kutalik Z, Descombes P, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–45. 1345. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 14.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis GL, Wong JB, McHutchison JG, et al. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 17.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 18.Shiffman ML, Ghany MG, Morgan TR, et al. Impact of reducing peginterferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology. 2007;132:103–112. doi: 10.1053/j.gastro.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Arnesen E, Huseby NE, Brenn T, et al. The Tromso Heart Study: distribution of, and determinants for, gamma-glutamyltransferase in a free-living population. Scand J Clin Lab Invest. 1986;46:63–70. doi: 10.3109/00365518609086483. [DOI] [PubMed] [Google Scholar]
- 20.Casiglia E, Spolaore P, Ginocchio G, et al. Unexpected effects of coffee consumption on liver enzymes. Eur J Epidemiol. 1993;9:293–297. doi: 10.1007/BF00146266. [DOI] [PubMed] [Google Scholar]
- 21.Honjo S, Kono S, Coleman MP, et al. Coffee consumption and serum aminotransferases in middle-aged Japanese men. J Clin Epidemiol. 2001;54:823–829. doi: 10.1016/s0895-4356(01)00344-4. [DOI] [PubMed] [Google Scholar]
- 22.Klatsky AL, Morton C, Udaltsova N, et al. Coffee, cirrhosis, and transaminase enzymes. Arch Intern Med. 2006;166:1190–1195. doi: 10.1001/archinte.166.11.1190. [DOI] [PubMed] [Google Scholar]
- 23.Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Tokunaga S, Kono S, et al. Coffee consumption and decreased serum gamma-glutamyltransferase and aminotransferase activities among male alcohol drinkers. Int J Epidemiol. 1998;27:438–443. doi: 10.1093/ije/27.3.438. [DOI] [PubMed] [Google Scholar]
- 25.Freedman ND, Everhart JE, Lindsay KL, et al. Coffee intake is associated with lower rates of liver disease progression in chronic hepatitis C. Hepatology. 2009;50:1360–1369. doi: 10.1002/hep.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132:1740–1745. doi: 10.1053/j.gastro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 27.Bravi F, Bosetti C, Tavani A, et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46:430–435. doi: 10.1002/hep.21708. [DOI] [PubMed] [Google Scholar]
- 28.Purnak T, Ozaslan E. Coffee intake and chronic hepatitis C. Hepatology. 2009;50:1673. doi: 10.1002/hep.23243. [DOI] [PubMed] [Google Scholar]
- 29.Di Bisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee WM, Dienstag JL, Lindsay KL, et al. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472–492. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Block G, Woods M, Potosky A, et al. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 32.Block G, Thompson FE, Hartman AM, et al. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–693. [PubMed] [Google Scholar]
- 33.Morishima C, Chung M, Ng KW, et al. Strengths and limitations of commercial tests for hepatitis C virus RNA quantification. J Clin Microbiol. 2004;42:421–425. doi: 10.1128/JCM.42.1.421-425.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 35.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Honda M, Sakai A, Yamashita T, et al. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology. 2010;139:499–509. doi: 10.1053/j.gastro.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 37.Urban TJ, Thompson AJ, Bradrick SS, et al. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology. 2010 doi: 10.1002/hep.23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien TR. Interferon-alfa, interferon-lambda and hepatitis C. Nat Genet. 2009;41:1048–1050. doi: 10.1038/ng.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen T, Park YC, Kim SH, et al. Nuclear factor-kappaB/signal transducers and activators of transcription-1-mediated inflammatory responses in lipopolysaccharide-activated macrophages are a major inhibitory target of kahweol, a coffee diterpene. Biol Pharm Bull. 2010;33:1159–1164. doi: 10.1248/bpb.33.1159. [DOI] [PubMed] [Google Scholar]
- 40.Harrison SA, Rossaro L, Hu KQ, et al. Serum cholesterol and statin use predict virological response to peginterferon and ribavirin therapy. Hepatology. 2010;52:864–874. doi: 10.1002/hep.23787. [DOI] [PubMed] [Google Scholar]
- 41.Li JH, Lao XQ, Tillmann HL, et al. Interferon-lambda genotype and low serum low-density lipoprotein cholesterol levels in patients with chronic hepatitis C infection. Hepatology. 2010;51:1904–1911. doi: 10.1002/hep.23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramcharran D, Wahed AS, Conjeevaram HS, et al. Associations between serum lipids and hepatitis C antiviral treatment efficacy. Hepatology. 2010;52:854–863. doi: 10.1002/hep.23796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pineda JA, Caruz A, Rivero A, et al. Prediction of response to pegylated interferon plus ribavirin by IL28B gene variation in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2010;51:788–795. doi: 10.1086/656235. [DOI] [PubMed] [Google Scholar]
- 44.Jee SH, He J, Appel LJ, et al. Coffee consumption and serum lipids: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2001;153:353–362. doi: 10.1093/aje/153.4.353. [DOI] [PubMed] [Google Scholar]
- 45.Huxley R, Lee CM, Barzi F, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 46.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]