Abstract
This paper describes the design, construction and characterization of the first anti-HIV drug delivery system that is triggered to release its contents in the presence of human semen. Microgel particles were synthesized with a crosslinker containing a peptide substrate for the seminal serine protease prostate specific antigen (PSA) and were loaded with the HIV-1 entry inhibitor sodium poly(styrene-4-sulfonate) (pSS). The particles were composed of N-2-hydroxyproplymethacrylamide and bis-methacrylamide functionalized peptides based on the PSA substrates GISSFYSSK and GISSQYSSK. Exposure to human seminal plasma (HSP) degraded the microgel network and triggered the release of the entrapped antiviral polymer. Particles with the crosslinker composed of the substrate GISSFYSSK showed 17 times faster degradation in seminal plasma than that of the crosslinker composed of GISSQYSSK. The microgel particles containing 1 mol% GISSFYSSK peptide crosslinker showed complete degradation in 30 hours in the presence of HSP at 37 °C and pSS released from the microgels within 30 minutes reached a concentration of 10 µg/mL, equivalent to the published IC90 for pSS. The released pSS inactivated HIV-1 in the presence of HSP. The solid phase synthesis of the crosslinkers, preparation of the particles by inverse microemulsion polymerization, HSP-triggered release of pSS and inactivation of HIV-1 studies are described.
Keywords: microbicide, HIV, microgel, triggered release, semen, prostate specific antigen
1. Introduction
With more than 25 million infected individuals worldwide (UNAID/WHO, 2007), the HIV pandemic is one of the most significant health problems facing mankind. Notably, women globally comprise nearly half of the infected population and as high as 75% in some sub-populations (UNAID/WHO, 2007). In parts of Africa, women aged 15–24 are 2.5 times more likely to become infected than their male counterparts (Nyindo, 2005; Ramjee et al., 2006; Whitmore et al., 2005). A woman’s increased susceptibility in part stems from the fact that current methods of preventing HIV infection (Chakraborty et al., 2001; Nyindo, 2005) – abstinence, condoms, and monogamy – are often outside a woman’s control and women may be more physiologically susceptible to HIV transmission than men (Gray et al., 2001; Shattock and Moore, 2003). Women-controlled prophylactic methods called microbicides are being developed that inhibit one or more of the early steps of male-to-female transmission of HIV (Shattock and Moore, 2003; Stone, 2002). However, the close of several microbicide Phase III clinical trials suggests that further research efforts targeting development of efficacious microbicide drug delivery systems are required (Honey, 2007; Ramjee et al., 2007).
The development of effective microbicides inevitably derives from knowledge regarding the initial factors involved in HIV-1 entry and transmission at genital tissue surfaces (Shattock and Moore, 2003). The first step involves convective or diffusive transport of a virion or infected monocyte out of semen into the vaginal intraepithelial tissue (Geonnotti and Katz, 2006). Recent work by Hladik et al. has demonstrated that upon first encountering the outer vaginal tissue HIV-1 immediately entered both CD4+ T cells and Langerhans cells. They attributed the remarkable efficiency of viral infection to a high number of intravaginal epithelium CD4+ T cells that expressed CCR5 (77%) as well as expression of CD4 in Langerhans cells (54%) and CCR5 (52%) (Hladik et al., 2007). Their results strongly link the ability to prevent entry into these susceptible cells with preventing HIV infection. Other in vitro experiments have also demonstrated that the use of entry inhibitors can block the interactions between CD4 and CCR5 receptors on the target CD4+ immune cells and gp120 on the viral envelope (Ketas et al., 2007),(Hu et al., 2004; Veazey et al., 2005). Anionic polymers that bind to the positively charged regions of the HIV envelope surface protein gp120 can therefore sterically inactivate the virus and inhibit the early steps involved in HIV infection.
Critical gaps remain in the development of vaginal drug delivery systems that complement the HIV inactivation mechanism of antiretroviral agents. Some antiretrovirals such as HIV-replication inhibitors and agents that block the receptors on the target CD4+ cells should be delivered to the vaginal tissue using sustained delivery systems such that inhibitory concentrations of these agents are established in the target cells before exposure to the virus. However, entry inhibitors targeting the viral envelope would be better suited for burst release at the onslaught of viral exposure such that supra-therapeutic concentration of the drug is achieved immediately after exposure to the virus. If the burst release of viral envelope inhibitors is triggered by semen—the carrier of virus in the male-to-female transmission— there is a potential to deactivate the virus prior to exposure and penetration of the susceptible vaginal tissue.
We designed a drug delivery system capable of providing triggered release of HIV envelope inhibitors upon exposure to semen. The design consisted of an anionic antiviral polymer sequestered in microgel particles that can be incorporated into vaginal formulations and degrade when exposed to semen. Additionally, sequestering viral envelope inhibitors in microgel particles could provide several benefits. First, the viral envelope inhibitors are entrapped in microgels and separated from other potential active agents in the microbicide formulation, which will likely prevent negative drug-drug and drug-excipient interactions during storage. Secondly, semen-triggered microgel particles will retain the viral envelope inhibitors and prevent diffusion of the drug until it is needed to inactivate virions. Finally, using semen as a trigger for rapid release (Gupta et al., 2007) of supra-therapeutic concentrations of viral envelope inhibitors into semen may improve efficacy of HIV inactivation. The work presented here investigates the development of microgel particles capable of triggered release of viral envelope inhibitors in the presence of semen.
To accomplish this we have harnessed prostate specific antigen (PSA), a serine protease present in semen as the enzymatic trigger for semen-responsive drug release from these microgel particles. PSA, also known as seminogelase or human kallikrein III (EC-Number 3.4.21.77), is a 30 kD serine protease found in seminal plasma at a concentration of 0.4 to 3 g/L (Wang et al., 1998). The primary role of PSA involves degradation of seminogelin, the predominant skype1357protein component of the seminal coagulum that forms after ejaculation and is digested by PSA presumably to permit sperm motility (Balk et al., 2003; Lilja, 2003; Peter et al., 1998). Significant detailed investigations into PSA substrates with optimized subsite occupancy have been developed for PSA activated prodrug constructs for use in systemic treatment of prostate cancer (Denmeade et al., 2003; Denmeade et al., 1997; Denmeade et al., 1998) and have provided us templates for the design of PSA degradable bis-methacrylamide-derivatized peptide crosslinkers. We have synthesized two peptide crosslinkers using orthogonal solid phase peptide synthesis techniques and incorporated them into poly(2-hydroxypropyl methacrylamide) (pHPMA) based microgel particles (Figure 1). The degradation rate of these particles in the presence of human seminal plasma (HSP) was investigated, as well as the HSP triggered release rate of entrapped poly(styrene-4-sulfonate) (pSS), an anionic polymer entry inhibitor, and its resulting antiviral activity (Neurath et al., 2006).
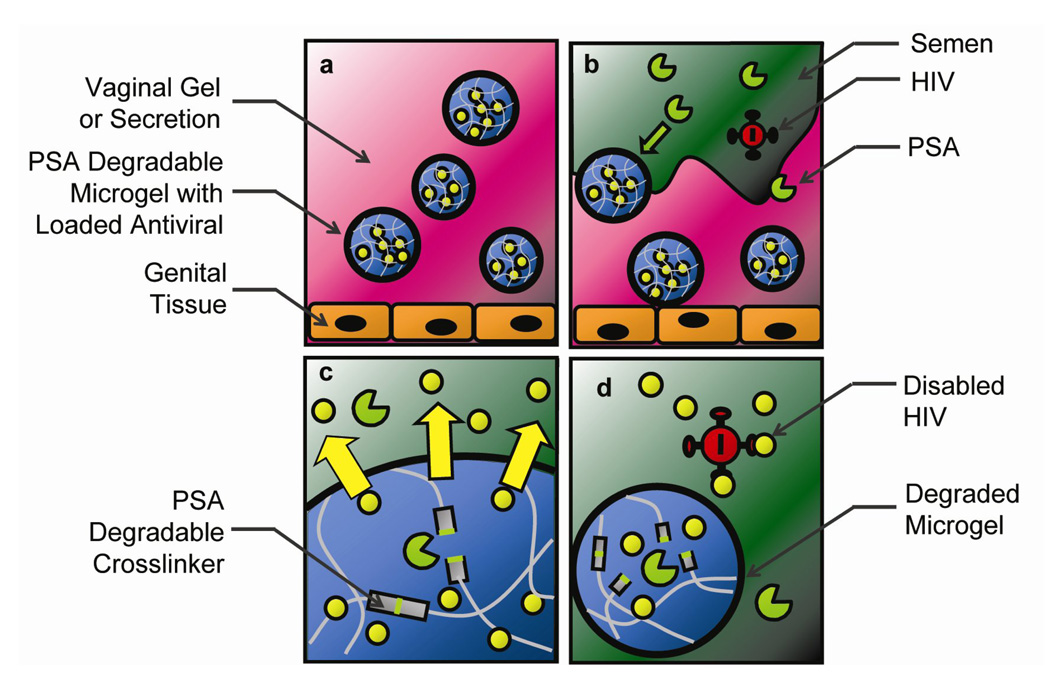
Figure 1.
Schematic of semen-triggered release from PSA degradable microgel particles as ‘smart’ microbicide drug delivery vehicles. a) Microgel particles incorporated into a vaginal gel formulation and topically applied to the vaginal epithelium. b) After exposure to semen, the viral carrier, PSA diffuses into the gel and c) begins cleaving degradable peptide-derivatized crosslinkers resulting in triggered release of viral envelope inhibitors into semen. d) HIV is thereby inactivated prior to contact with vaginal tissue and susceptible cells.
2. Materials and Methods
2.1 Materials
Methacrylic acid, ammonium persulfate (APS), N,N,N′,N′-tetramethylethylenediamine (TEMED), diisopropylcarbodiimide (DIC), diisopropylethylamine (DIPEA), fluorescein-isothiocyanate (FITC), piperidine, Span 80 and Tween 80 were purchased from Sigma-Aldrich (Milwaukee, WI). Fmoc protected amino acids, Fmoc-Lys(Alloc)-OH was obtained from Bachem (King of Prussia, PA). All other Fmoc protected amino acids and N-hydroxybenzotriazole (HOBt) were purchased from Novabiochem (San Diego, CA). N-2-hydroxypropylemthacrylamide (HPMA) was prepared according to a reported procedure (Rejmanova et al., 1977) and used after characterizing by 1H NMR for structure and purity. 2-Aminopropylmethacrylamide (APMA) was purchased from Polysciences (Warrington, PA). The 2-chlorotrityl resin at a loading of 1.5 mmol –Cl/g was obtained from CBL Patras (Patras, Greece). HSP, free of spermatazoa, was provided by the Andrology clinic of University of Utah Hospital. Colorimetric PSA substrate, Suc-RPY-pNA, was purchased from Anaspec (San Jose, CA). Trifluoroacetic acid (TFA) was purchased from Acros (Morris Plains, NJ) and was distilled in vacuo at 82°C. The polymerization inhibitor bis(t-octyl)-1,2-hydroquinone was kindly provided to us by Dr. Pavla Kopecek (Dept. of Pharmaceutics and Pharmaceutical Chemistry, University of Utah). All other reagents, solvents and chemicals were of the reagent grade and used as received without further purification unless otherwise noted.
2.2 Peptide crosslinker synthesis
The PSA specific peptide crosslinker was prepared on a 1.5 mmole scale using Fmoc/HOBt/DIC chemistry on chlorotrityl resin. The PSA substrate peptide sequence, GISSFYSSK, and GISSQYSSK were prepared with terminal vinyl groups as shown in Figure 2. On 1 g of polystyrene chlorotrityl resin (1.5 mmole –Cl/g), Fmoc-Lys(Alloc)-OH (2.04 g, 4.5 mmole) was coupled first using DIPEA (1.16 g, 9 mmole) in 5 mL of dry DMF. After the coupling, the column containing the resin was washed 3X with 25 mL DMF thoroughly and the Fmoc group was deprotected with ~2.5 M or 20% v/v piperidine in 12 mL of DMF. To build the peptide sequence, three equivalents of the corresponding amino acids were coupled with HOBt/DIC chemistry followed by a DMF wash. Fmoc deprotection was accomplished with 12 mL 20% v/v piperidine in DMF for deprotection of the Fmoc group. After completion of the sequence, a solid supported diamine was created by selectively deprotecting the C-terminal Alloc and N-terminal Fmoc groups. Palladium catalyst and borane dimethylamine complex was used to deprotect the Alloc group as described elsewhere (Albericio, 2000). Upon deprotection, vinyl groups were added to the two amines at each end of the sequence by reacting methacrylic acid (0.48 g, 5.58 mmole, containing MEHQ (hydroquinone monomethyl ether) as stabilizer) with the resin for 6 hr at laboratory temperature with shaking in the presence of HOBt (0.85 g, 5.58 mmole) and DIC (0.70 g, 5.58 mmole) as coupling agents. The resin was then washed completely with DMF, followed by DCM, and dried under vacuum for an hour. The solid phase resin was pre-treated with a scavenger solution of triethylsilane:DCM (18 mL, 1:9, v/v) containing 4 mM of the polymerization inhibitor, bis(t-octyl)-1,2 hydroquinone for 15 min and then 18 mL volume of distilled TFA was added and shaken for 5 hr at laboratory temperature to cleave the fully deprotected peptide crosslinker from the resin. The resin was filtered and the filtrate concentrated in vacuo. The resulting peptide was then triturated with 10 volumes of cold diethyl ether (0 °C) and the ether was decanted. The trituration was carried out 7 times (each time with 10 volumes of cold ether) and the precipitate was dried under vacuum. The peptide crosslinkers were analyzed by ESI MS (Sciex API-III, Concord, Ontario) and HPLC. The final yield of the peptide crosslinker GISSFYSSK was 39% calculated from the reported resin loading. The peptide crosslinker GISSFYSSK: purity by HPLC, 71% MS calculated: MW=1111.2, M+1=1112.2, M+2=556.6. Observed: M+1=1112.4, M+2=556.3. The peptide crosslinker GISSQYSSK: purity by HPLC 80%, MS calculated: MW=1091.51 Observed: M−1=1090.47, HPLC analysis condition: gradient changing from 90% water and 10% acetonitrile to 100% acetonitrile in 20 min (TFA was added as 0.1% (v/v) in both water and acetonitrile).
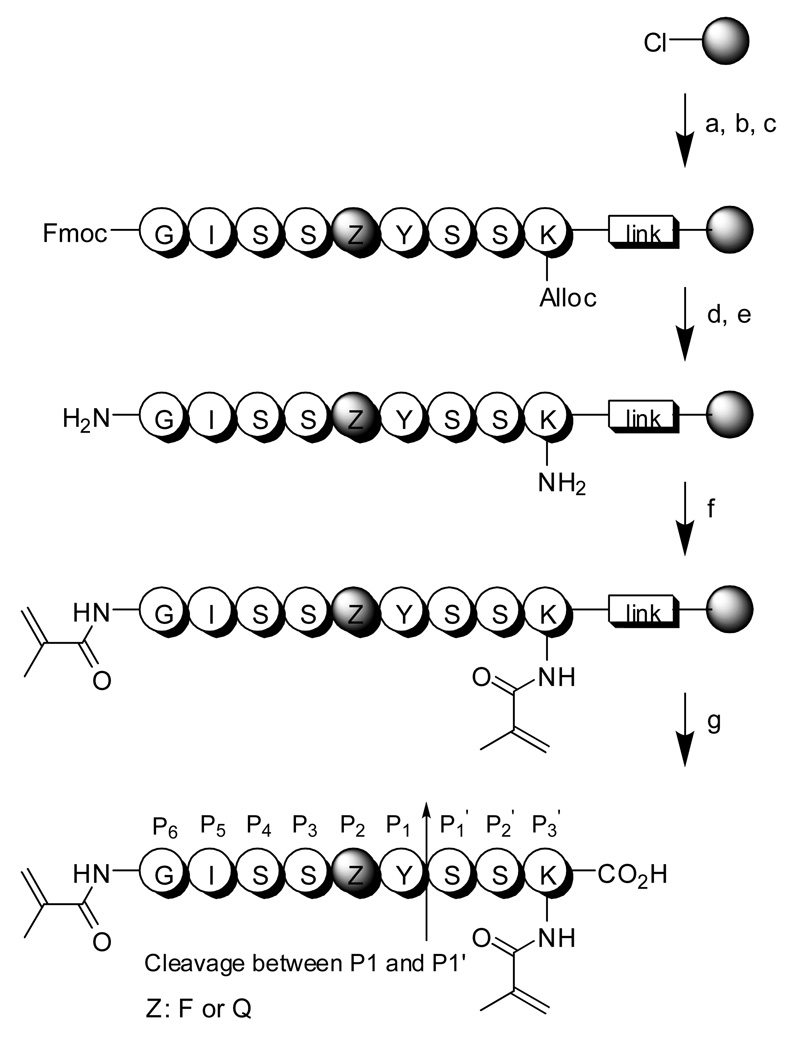
Figure 2.
Peptide crosslinker synthesis. Reagents and experimental condition: a) 3 eq. Lys(Alloc)-Fmoc, 6 eq. DIPEA, DMF, twice; b) 20% piperidine/DMF, twice; c) subsequent coupling with 3 eq. amino acids, HOBt, DIC, DMF; d) Pd catalyst, borane complex; e) 20% piperidine/DMF, twice; f) 6 eq. methacrylic acid, HOBt, DIC, DMF; g) TES:DCM (10:90), TFA:DCM(50:50), 10 eq. cold ether (see experimental section for details). PSA cleaves between Y (P1) and S (P1').
2.3 Preparation of PSA degradable microgel particles
Microgel particles were prepared using inverse microemulsion polymerization following an analogous process reported elsewhere (Goh et al., 2004). In brief, poly(HPMA-co-APMA-co-peptide crosslinker) microgel particles were prepared using a 96:3:1 molar monomer feed ratio. HPMA (25 mg, 0.7 mmole) and APMA (1 mg, 0.022 mmole) were dissolved in 87 µL of a DMSO + 200 mM aqueous NaCl solution (1:1 v/v) mixture in a PCR tube. The peptide crosslinker (GISSFYSSK, 2.0 mg, 0.073 mmole or GISSQYSSK, 2.0 mg, 0.073 mmole) is then added to the above solution and dissolved. TEMED (2.4 mg, 0.02 mmole) was added to the mixture and was sonicated at laboratory temperature for 1 minute to dissolve all of the components. Next, 10 µL of an APS solution ([APS] = 0.26 M, 0.6 mg, 0.026 mmole) prepared in 200 mM NaCl solution was added rapidly to initiate the free radical polymerization. The aqueous mixture of DMSO, monomers and initiator was then immediately added to 2 mL of previously stirring hexane solution containing 3 wt% of 3:1 weight ratio of Span 80 and Tween 80 in 4 mL-glass vial on magnetic stir plate. This container was sealed with septa and purged with N2 for 3 min and stirred for overnight at 1200 rpm. After reacting overnight, the aqueous phase formed polydispersed microgel particles that were washed with 1 mL of hexane 5 times and 1 mL of acetone once to remove surfactants and other impurities. Washing was accomplished by a sequence of vortexing in the solvent followed by centrifugation (16000g, Galaxy 16D, VWR International, West Chester, PA). Finally, the microgel particles were dried overnight under vacuum and the final yield was 74% (20.3 mg).
2.4 Characterization of Microgel Particle Size Distribution
Microgel particles were suspended in DI water and imaged by optical microscopy using an Olympus IX-70 microscope equipped with a 20× objective and digital camera. Particle size analysis was performed on a total of 120 particles from 11 randomly selected fields of view using ImageJ software.
2.5 Determination of PSA activity in HSP
The HSP obtained from the University of Utah Andrology clinic was centrifuged (10 min at 16000 g) to remove any traces of sperm cells and stored at −80 °C until use. Every seminal plasma sample was tested for its PSA activity before use according to the procedure below. Briefly, a 1 mM stock solution of the colorimetric PSA substrate, MeO-Suc-RPY-pNA (S2586, DiaPharma, West Chester, OH), in 125 mM PBS (pH 7.7) buffer was prepared and stored at −20 °C. A known volume (50 µL) of the substrate solution was mixed with 50 µL of HSP which had been diluted 10X with 125 mM PBS (pH 7.7). The mixture was placed in the clear-bottom 96-well plate and OD change at 405 nm was monitored at 37 °C using a plate reader (Spectra Max M2, Molecular Devices, Sunnyvale, CA). The PSA activity of HSP was determined as 6.60 ± 0.78 (ΔOD×103/min) for all samples used in these studies. The variation in the activity was not considered significant in the degradation experiments carried out in this study. HSP is inactivated after heating at 70 °C for 30 min. The activity loss of heat treated HSP was confirmed using the colorimetric PSA assay described above which showed no detectable cleavage of the colorimetric substrate. Heat inactivated HSP was used as a control for some experiments (see below).
2.6 HSP-triggered microgel particle degradation
The degradation of microparticles by HSP was monitored by microscopy (Axiovert 100TV, Zeiss, Germany) over time on a temperature controlled stage. Initially, the microgel particles with the PSA-degradable crosslinker were fully swelled in PBS buffer (125 mM, pH 7.7) for 24 hr followed by separation using a bench top centrifuge (16000g, Galaxy 16D, VWR International, West Chester, PA). These swollen microgel particles were resuspended with HSP and the particle suspension solution was placed in a sealed glass microscope slide chamber. One drop of the HSP-microgel particle mixture was placed in the chamber and the chamber was sealed with a cover glass slide. Avoiding trapped bubbles between the glass slides, the edge of the cover glass slide was sealed with silica sealant. The glass slide chamber was placed on the heating stage at 37 °C. Swelling of particles followed by degradation was monitored under microscope using a 100X oil immersion objective and micrograph images of microgel particles (indicating the change in the volume) were collected over a 4 hr time period. The images were analyzed using Adobe Photoshop™ and pixel sizes were calibrated using a stage micrometer to determine the number of pixels/µm for each imaging session.
2.7 Preparation of sodium poly(styrene-4-sulfonate) (pSS)
Free radical polymerization of styrene 4-sulfonic acid sodium salt monomer (0.5 g, 2.42 mmole) was performed using 17.2 wt.% monomer in nitrogen saturated distilled water with ammonium persulfate (2.5 mg, 0.011 mmole) at 60 °C for 24 h. The resulting viscous solution was kept at −80°C for 10 minutes to stop the polymerization reaction and then kept at RT for 15 minutes. To the obtained viscous solution, 1 ml of water was added and mixed well. This solution was added to about 30 mL of methanol (under stirring) to precipitate out the polymer. The polymer was further washed with methanol several times to remove any unreacted monomer followed by drying under vacuum.
2.8 Preparation of FITC labeled sodium poly(styrene-4-sulfonate) (FITC-pSS)
Free radical polymerizations were performed using 99.5:0.5 molar ratio of styrene sulfonic acid sodium salt with FITC-MA. Polymerizations were performed using 16.8 % w/w monomers in nitrogen saturated distilled water with ammonium persulfate (0.07 mol%) at 60 °C for 24 h. After the polymerization, the solution was kept at −80°C for 10 minutes to stop the polymerization reaction and then kept at RT for 15 minutes. The resulting solution was precipitated in methanol (7 mL) to precipitate out the polymer. The polymer was further washed with methanol 4 times to remove any unreacted monomers followed by drying under vacuum. Absolute molecular weight distributions were determined for pSS and FITC-pSS by GPC equipped with Agilent 1100 series HPLC pump (Agilent Technologies Inc., Santa Clara, CA), differential refractive index detector (BIDNDC, Brookhaven Instruments Corporation, Holtsville, NY) and multi-angle light scattering detector (BI-MwA molecular weight analyzer, Brookhaven Instruments Corporation, Holtsville, NY) in DDI water containing 50mM NaCl. A mixed bed PL aquagel-OH water column (Polymer Laboratories, Varian Inc., Amherst, MA) was used. Polyethylene glycol standards (Polymer Laboratories, Varian Inc., Amherst, MA) of Mp 126,500 and 791,500 g mol−1 were used for calibration.
2.9 Preparation of pSS loaded PSA degradable microgels
The hydrogel microparticles were prepared using an inverse microemulsion polymerization method following a similar procedure reported elsewhere (Goh et al., 2004). Poly(HPMA-co-peptide crosslinker) hydrogel microparticles were prepared in 98.5:1.5 molar monomer ratio. HPMA (320 mg, 2.23 mmole) and peptide crosslinker (GISSFYSSK, 36 mg, 0.032mmole) were dissolved in 0.530 mL of DMSO followed by the addition of 0.530 mL of water containing FITC-pSS+pSS. The FITC-pSS+pSS solution was made by dissolving 40 mg of FITC-pSS and 80 mg of pSS in 3 mL of water and 0.530 mL of this solution was used. To the solution of DMF and monomers was added 40 µL of TEMED. This mixture was vortexed and sonicated for a few minutes to completely dissolve HPMA and crosslinker. To this, APS (5.2 mg, 0.0056 mmole) was added and then mixed well by vortexing. To this aqueous monomer and pSS mixture, 2 mL of surfactant solution (3 wt % of surfactant (3:1 v/v Span 80:Tween 80) in hexane) was added, mixed thoroughly and immediately added to a surfactant solution (15 mL) previously under stirring in a 20-mL glass vial on a magnetic stir plate. The container was sealed with a rubber septum, purged with N2 for 3 min and stirred for overnight at 1200 rpm. After overnight reaction, the microgel particles were washed with hexane 5 times and acetone once to remove surfactants and other impurities through vortexing and centrifugation (1620 g, Eppendorf centrifuge 5810, Brinkman Instruments Inc., Westbury, NY). Then, the microgel particles were dried overnight under vacuum (250 mg, Yield = 70%).
The hydrogel microparticles (containing entrapped pSS+FITC-pSS) were thoroughly washed with PBS (DPBS, pH 7.4, Cambrex Biosciences) to remove any surface bound pSS+FITC-pSS. The PBS solution was replaced every 24 hours for 11 days and the fluorescence of the supernatant solution was monitored using a fluorescence plate reader (Spectramax M2, Molecular Devices, Sunnyvale, CA) to determine the point at which all of the surface absorbed FITC labeled pSS had stopped leaching out of the gel network. The hydrogel microparticles without pSS+pSS-FITC were also soaked in PBS for 24 hr once. The particles were centrifuged under 2500 rpm for 20 min. After removing the supernatant, wet particles were frozen and lyophilized to get dry microparticles.
2.10 In vitro release kinetics of pSS-FITC+pSS from microgel particles
The in vitro pSS release profile was obtained from release kinetics experiments carried out with microparticle concentrations of 15 and 66 mg/mL over 24 hours. 4.5 mg of dry microgel particles were weighed into a PCR tube (Eppendorf, 1.5-mL capacity) and 0.3 mL of pre-warmed (at 37 °C for 30 minutes in a water bath) HSP was added. The microgel particles were mixed well; the tube was closed and kept in a 37 °C (± 1.0) water bath (Shel Lab, Sheldon Manufacturing Inc., Cornelius, OR, Model 1214, purchased from VWR international, West Chester, PA). At each time point, an aliquot of about 30 µL of the semen microparticle mixture was taken out and transferred into another smaller PCR tube (200 µL capacity) and centrifuged (16000 g, Galaxy 16D, VWR International, West Chester, PA). 10 µL of supernatant was separated out and transferred into another PCR tube (100 µL capacity). Sampling was continued and at each time point 10 µL of supernatant was separated out. Samples for a total of seven time points (30 min, 1, 2, 4, 8, 12 and 24h) were collected and the experiment was conducted simultaneously in triplicate and samples generated accordingly. The entire experiment control was carried out again using PBS instead of semen.
All the supernatant samples collected were diluted with 90 µL of PBS and mixed well. From this mixture (of 100 µL), 90 µL was transferred into a 96-well opaque plate-reader plate and the fluorescence (excitation 480nm, emission 520nm) was measured. The pSS release (pSS+FITC-pSS) was determined using a calibration curve generated in PBS. The calibration curve obtained by measuring the fluorescence for 90 µL (in triplicates) of different concentrations (0.001 mg/mL to 1.0 mg/mL) of pSS+FITC-pSS dissolved in PBS.
2.11 Viral inactivation by pSS released from microgel particles
The viral inactivation as a function of time for semen triggered release of pSS from microgel particles was studied. Similar to the in vitro release kinetics experiments, another in vitro release kinetics experiment was carried out at a microparticle concentration of 66 mg/mL. 91.4 mg (91.4 ± 0.3 mg, N = 3) of dry microgel particles were weighed into a PCR tube (Eppendorf, 1.5-mL capacity) and 1.39 mL of semen was added, mixed and kept in a 37 °C (± 1.0) water bath. At each time point, an aliquot of about 300 µL of the HSP + microparticle mixture was taken out and transferred into two Air-fuge insert tubes (250-µL capacity, Beckman) and centrifuged (28 kPa pressure, 120000 g, Airfuge (with A100 rotor) Beckman Coulter, CA). 50 µL of supernatant was separated out and transferred a PCR tube (200 µL capacity). A total of 4 time points (20, 60, 140 and 270 min) were taken. Similarly, a placebo control experiment was carried out by weighing 66.4 mg (66.4 ± 0.3mg, N = 3) microgel particles without pSS in a PCR tube (Eppendorf, 1.5-mL capacity), 1 mL of semen was added, mixed and incubated in a 37 °C (± 1.0) water bath. For this control experiment 50 µL of supernatant was collected at one time point (270 min). All experiments were performed in triplicate.
2.12 HIV-1 HeLa-CD4-LTR-β-Gal EntryAssay in the Presence of 50% HSP
Twenty-four hours prior to compound and virus addition, 100 µL of HeLa-CD4-LTR-β-Galactosidase cells at a density of 1×104 cells/well in DMEM containing 10% FBS were plated in 96-well flat bottomed plates and incubated overnight at 37 °C/5% CO2. Following overnight incubation, media was removed and 50 µL of each sample was added to the wells. Fifty microliters (50 µL) of seminal plasma containing release material from the degraded microparticles was added to the virus and 50 µL of media was added to the cell control wells. Plates were incubated for approximately 15 minutes at 37 °C while virus (100X concentrated HIV-1IIIB) was prepared at a pre-determined titer for infection. Once prepared 50 µL of virus was added to the wells containing compound and to the virus control wells. The final in-well concentration of seminal plasma for all samples was 50%. Plates were incubated for 2 hours at 37°C/5% CO2. Following incubation, the monolayers were washed three times with 150 µL of room temperature RPMI-1640 media without additives. Wo-hundred microliters (200 µL) of 10% complete DMEM was added to all wells and the plates were incubated at 37°C/5% CO2 for 48 hours. Following incubation, the plates were visually observed for cellular toxicity prior to evaluating the cultures for β-galactosidase production using a chemiluminescent substrate (Gal-Screen, Applied Biosystems).
3. Results
3.1 Synthesis of PSA degradable Crosslinker
The synthesis of PSA-degradable crosslinkers QY and FY were made using PS-Wang resin with batch phase Fmoc/tBu chain assembly performed via DIC/HOBt protocols. Briefly, the resin-bound fully protected peptides were subjected to Pd-catalyzed Alloc deprotection of the C-terminus ε-amine of lysine, followed by Fmoc deprotection of the N terminal amine residue (Figure 2). We observed that the conditions for allyl deprotection of the P3'-ε amine resulted in Fmoc deprotection of the terminal amine residue. This did not pose difficulties for our synthetic scheme, and each batch underwent sequential piperidine Fmoc deprotection to ensure complete removal of the protecting group prior to acylation. The diamine was then acrylated to afford the solid-supported side-chain-protected crosslinker. Prior to cleavage, beads were exposed to a 9:1 DCM:triethylsilane solution for 10 min prior to addition of TFA for a 1:1 dilution. We found that the free radical scavenger bis(t-octyl)-1,2 hydroquinone added to the cleavage inhibits polymerization of the deprotected crosslinker during acidlysis. The crude peptide isolated was triturated in cold (0 °C) diethyl ether to afford the products in a yield of 39%.
3.2 Microgel particle preparation
The purified crosslinkers QY and FY were incorporated into poly(N-2-hydroxypropylmethacrylamide) (pHPMA) based microgel particles using inverse microemulsion polymerization (Goh et al., 2004). As previously reported, the crosslinker molar percentage, concentration of bulk monomer, polymerization solvent and stirring rate play roles in the resulting physical characteristics of the hydrogel particles (Goh et al., 2004). A range of crosslinker concentrations were explored, however polymerization attempts with a crosslinker feed ratio lower than 1 mol % resulted in low yields and were not further analyzed. The optimal polymerization conditions used to prepare hydrogels 1 and 2 were comprised of 97.5:1:1.5 mole ratio HPMA:crosslinker:APMA feed ratio. Combined monomers were dissolved in an aqueous solvent system 1:1 DMSO:200 mM NaClaq. DMSO was required due to insolubility of peptide crosslinker in aqueous solution alone. The resulting total monomer concentration was 24 wt%. The aqueous phase was then added to 20 times the volume of hexane containing 3 wt% surfactant and stirred overnight at 1200 rpm.
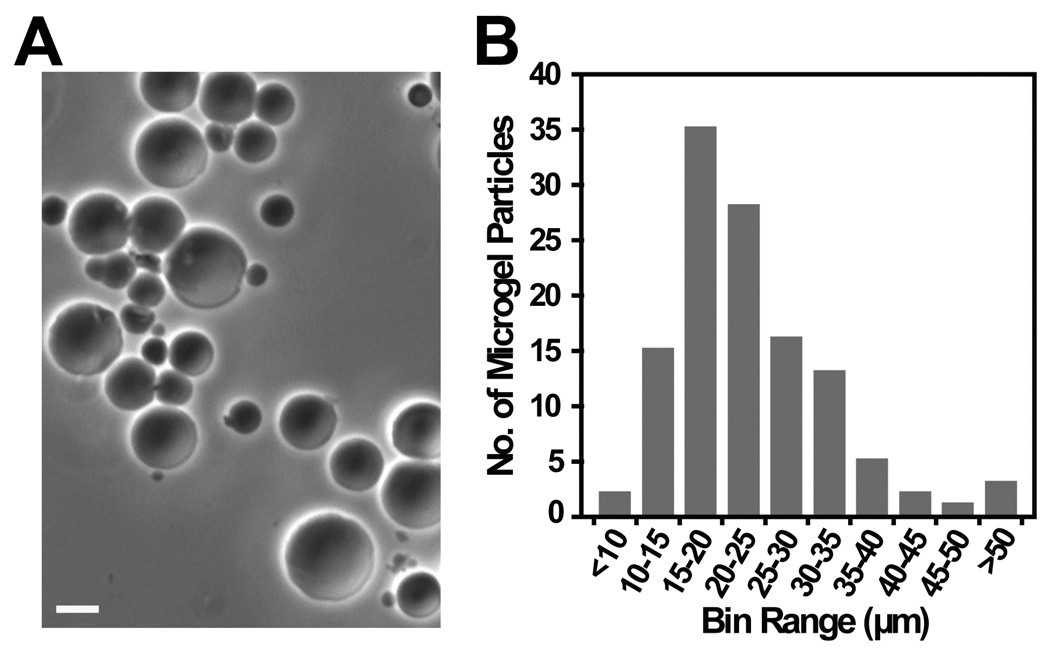
Figure 3A shows the optical micrograph of the spherical particles obtained from the polymerization of HPMA, APMA and the peptide crosslinker QY. The mean (± SD) diameter of the microgel particles was 23 ± 9 µm, with a range of 9 to 53 µm (Figure 3B).
Figure 3.
A) Optical micrograph of the microgel particles in DI water prepared by the polymerization of HPMA, APMA and peptide crosslinker (97.5:1.5:1 mol%), GISSQYSSK (QY); Scale bar = 10 µm. B) Bar graph depicting size distribution of microgel particles.
3.3 HSP degradation of microgel particles
The degradation rate of microgel particles created with a 1 mol % crosslink density of either the FY or QY were determined by monitoring the change in volume of these particles upon exposure to HSP. The microgel particles were initially rehydrated in PBS to reach an equilibrium swelling volume. They were then suspended in HSP, followed by with PBS to prevent particle aggregation and sealed in a microscope glass slide chamber maintained at 37°C. Images taken of the FY crosslinked gels revealed that the gel to sol transition occurred in four hours, after which distinct microgel particles could no longer be visualized. Comparatively, 70 hours were required to reach a similar transition with the QY crosslinked particles (data not shown) and microgels treated with heat-inactivated HSP did not show any change in their swelling which suggested that the swelling of microgel particles was specifically triggered by HSP. Ultimately, modification at the P2 position in the PSA substrate resulted in a 17-fold increase in the degradation rate of the microgels. Microgel particles containing the FY crosslinker were therefore chosen for further characterization.
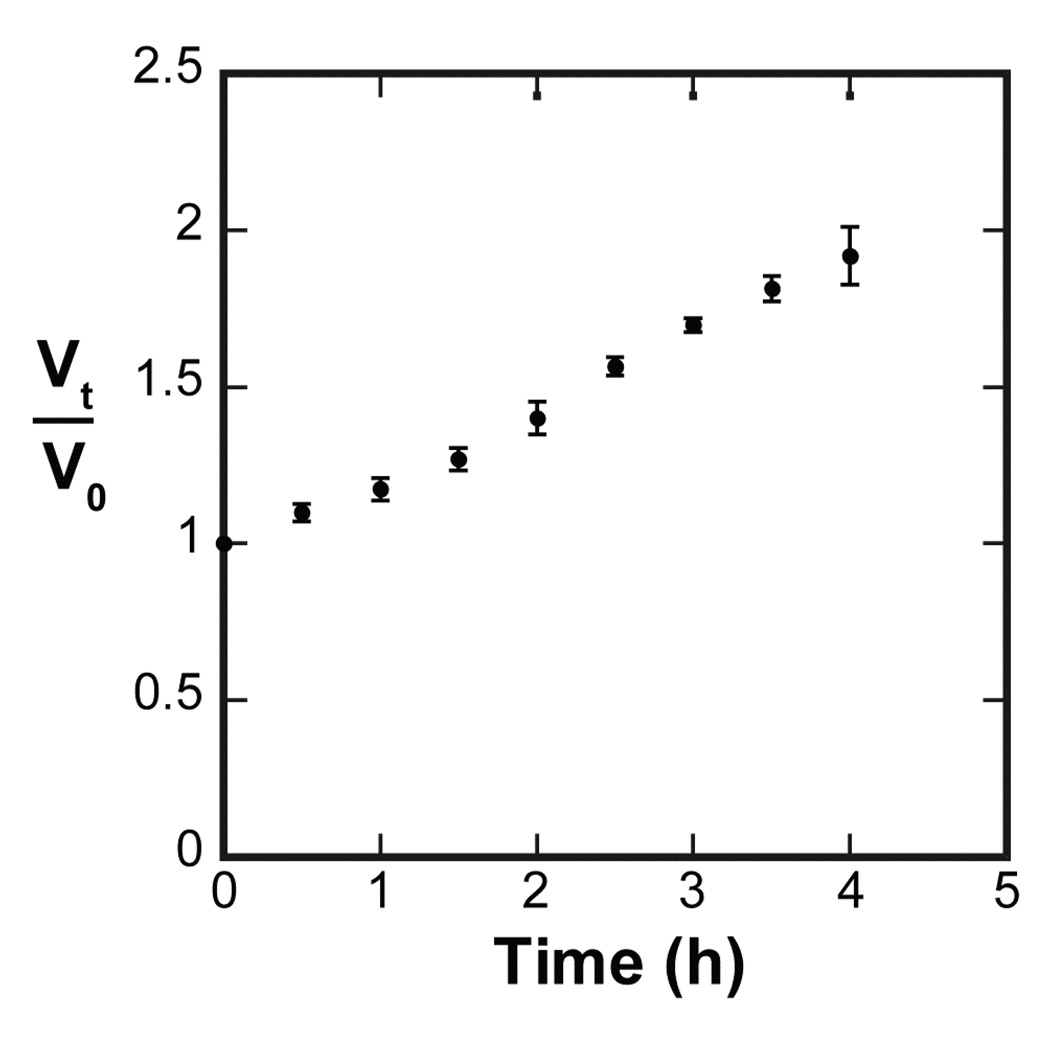
In addition to the microscopic observation of the microgel degradation, the change in particle volume was analyzed. The swelling ratio of FY particles’ volume compared to their initial volume (V/V0) over time was determined and plotted against time (Figure 4). The result shows that the microgel particles swelled to 1.8 times their initial volume (V/V0 ≈ 1.8) after four hours at which point they were no longer visible via optical microscopy.
Figure 4.
Normalized volume change of PSA degradable microgel particles in PBS (FY crosslinker) diluted HSP over time. After four hours microgels were no longer distinguishable via microscopy. N=3, mean ± SD.
3.4 Semen-triggered release of pSS and HIV inactivation
pSS was prepared by free radical polymerization of styrene-4-sulfonic acid sodium salt monomer in water. The corresponding FITC labeled pSS was also synthesized for fluorescent characterization of pSS release from microgel particles. FITC-acrylamide was synthesized by amidation of FITC with APMA. This purified monomer was then copolymerized with styrene-4-sulfonic acid sodium salt under free radical polymerization with a feed ratio of 0.5:95.5. The average molecular weights of each polymer were characterized by gel permeation chromatography using a multi-angle light scattering detector. The Mn, Mw and Mp of pSS were 665, 698 and 675 kDa respectively. The Mn, Mw and Mp of FITC-pSS were 732, 756 and 799 kDa respectively.
Microgel particles were prepared using an inverse microemulsion polymerization method similar to that previously described using HPMA and the FY crosslinker in a feed ratio of 98.5:1.5. Monomers were initially dissolved in DMSO to which an aqueous solution of containing a 1:2 ratio of FITC-pSS:pSS was added. The resulting aqueous phase was added to 16 times the volume of hexane and stirred overnight at 12000 rpm. Particles were thoroughly washed to remove surface bound FITC-pSS/pSS. Control microgel particles were prepared without FITC-pSS/pSS in the aqueous phase.
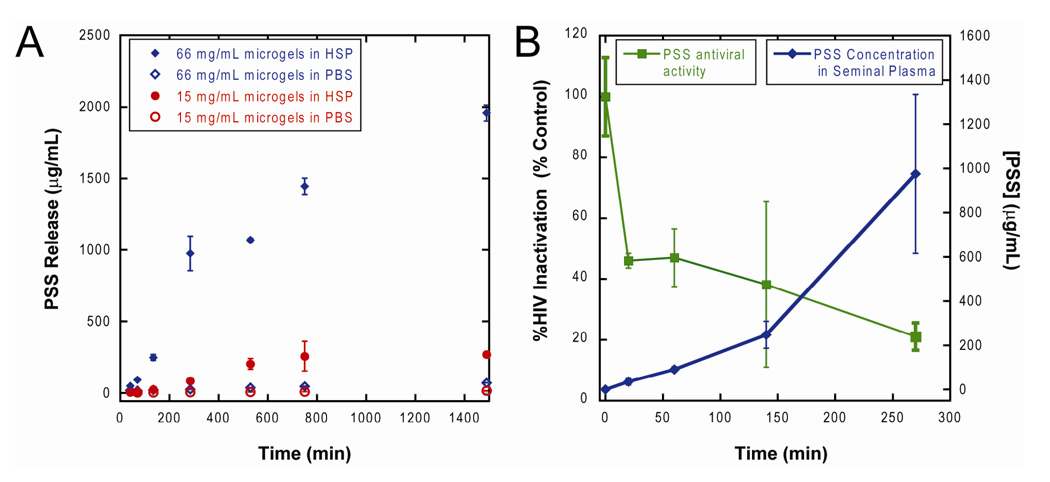
The in vitro pSS release profile was obtained from release kinetics experiments carried out on two particle concentrations, 15 mg/mL and 66 mg/mL, of lyophilized microgels. Microgels were brought to equilibrium swelling volume by incubating in PBS and were then washed extensively to remove any loosely entrained pSS. The microgel particles were then exposed to 100% HSP or PBS. At varying time points (0.5, 1, 2, 4, 8, 12 and 24 hours), samples were removed from the homogenized mixture, centrifuged and supernatant removed. The supernatant was analyzed for fluorescent intensity and compared to a calibration curve based on FITC-pSS to determine the amount of pSS present at each time point. As shown in Figure 5a, pSS was released from the microgel particles only when in the presence of HSP. After 30 min exposure to HSP, microgel particles at 66 mg/mL released approximately 50 µg/mL of pSS, a concentration that is 5-fold higher than the reported IC90 for pSS against HIV-1 Bal (Neurath et al., 2006). This amount of pSS released from the 66 mg/mL microgel particles was also sufficient to reduce HIV-1IIIB viral activity by almost 60%, whereas longer exposures to HSP resulted in enough pSS release to inactivate up to 80% viral activity (Figure 5b).
Figure 5.
Release of pSS and viral inactivation by semen-triggered release of pSS from PSA degradable microgels with GISSFYSSK crosslinker. A) Release as a function of time in HSP and PBS at two particle concentrations. B) Comparison of pSS release and antiviral activity of the sample in HSP. Mass of dry microgel sample was 66 mg/mL. Microgels were swollen in PBS buffer, isolated and exposed to HSP or PBS for control. 100% HIV-1IIIb viral activity (t=0 min) was determined from viral titer in HSP alone all samples. N=3, mean ± s.e.m. Note that the pSS released in the first 30 minutes from 66 mg/mL microgel particles reduced the HIV-1IIIB viral activity by more than 50% and continued to inactivate up to 80% of virus in 270 min. Controls of microgel particles alone without semen showed no reduction of infectivity.
4. Discussion
Our design of PSA degradable bis-methacrylamide-derivatized peptide crosslinkers involved adaptation from peptide sequences investigated as pro-drugs or markers for prostate cancer and the recently reported solid phase synthesis of peptide crosslinkers by Moss et al. (Moss et al., 2006). Work performed by Denmeade et al. initially studied a range of peptide sequences to determine cleavage rate as well as specificity (Denmeade et al., 2003; Denmeade et al., 1997; Denmeade et al., 1998). The fastest cleaving sequence was KGISSQY↓fluoroprobe which cleaved with a kcat/Km of 270 M−1s−1 after the P1 tyrosine residue (Denmeade et al., 1997). Further exploration by Coombs et al. revealed that the preferred subsite occupancy for peptide cleavage required serine residues in the P1' and P2' positions as well as phenylalanine in the P2 position (Coombs et al., 1998). The fastest cleaving sequence discovered in this study was GISSFY↓SSTEERLW with a kcat/Km of 2200 M−1s−1. Several considerations mandated attention prior to adaptation of these two sequences for development as crosslinkers. First, we wanted to keep the P and P' residue lengths the same for each crosslinker. For ease of synthesis, eight amino acid residues for the PSA sequence were utilized beginning with P6. Second, we construed that installation of the acrylamide functionalities was easiest performed by acryloylation of the terminal amine residue at the P6 residue and affixing an addition P3'-ε-N-acryloylysine residue. Finally, the choice of orthogonal protecting groups were required to perform acryloylation of the appended P3' lysine residue without affecting tBu side chain protecting groups.
The orthogonal Alloc protecting group (Thieriet et al., 1997) was chosen to allow for synthesis of a bis-methacrylamide peptide sequence performed on solid phase support with acid and base protecting groups. This synthesis scheme was similar to that reported by Moss et al. (Moss et al., 2006). Ultimately, the two bis-methacrylamide peptide crosslinkers GISSQ↓YSSK (QY) and GISSF↓YSSK (FY) were synthesized to investigate the impact on degradation rate of either a Q or F residue in the P2 position. Clearly these modifications imposed some potential reduction or increase in PSA activity towards our substrate but based on ease of synthesis were required for the work investigated.
It should be noted that the size distribution of the particles was found to be relatively broad, with most microgel particles ranging between 10 and 35 µm in size. Narrow size distribution is difficult to achieve with this polymerization method, as supported by other reports (Chen et al., 2003; Goh et al., 2004) for similar particle preparation.
Sulfonated anionic polymers such as sodium poly(naphthalene sulfonate), carrageenan and pSS, bind to HIV envelope protein gp120 at cationic regions of the V3-loop (Fletcher et al., 2006; Neurath et al., 2002). We chose pSS as our model compound for this study because of its known antiviral activity and its ease of synthesis (Neurath et al., 2006). Current formulations of pSS expose vaginal tissue to the polymeric drug immediately upon application and may increase susceptibility for inflammation, potentially increasing a woman’s chance of contracting HIV (Catalone et al., 2005; Lard-Whiteford, 2004; Ratterree et al., 2005; Warrier et al., 2004). It is therefore vital to investigate delivery vehicles that offer the ability to reduce tissue exposure to drug while dispensing an efficacious dose at the time of viral attack. Semen-triggered release of entry inhibitors imparts this capability. We thus sought to characterize the in vitro semen-triggered release of pSS trapped in a PSA degradable hydrogel network and correlate the dose-release rate to antiviral activity.
It is likely that the microgel particles will be under equilibrium swelling state after incorporation in a vaginal semi-solid gel dosage form. We performed extensive washing of the particles in PBS before performing the release studies in HSP so that an insignificant amount of pSS was released during incubation in PBS. In a clinical application this initial release could provide a protective background dose, followed by a burst after interaction of the microgel particles with semen. After washing, pSS was released from the microgel particles only in the presence of HSP. The IC90 of pSS against HIV-1Bal has recently been reported to be ~10 µg/mL in semen (Neurath et al., 2006). We exceeded this level in less than 30 minutes in our system at 66 µg/mL of the microgel particles. The pSS released in the first 30 minutes from 66 mg/mL microgel particles also reduced the HIV-1 IIIB viral activity by nearly 60%.
Ideally we aim to achieve a release of active that exceeds the IC90 several times in the first few seconds of exposure to semen. Using this crosslinker-drug combination does not achieve a supra-therapeutic drug concentration in the first seconds after exposure to semen. This accumulation of activity is clearly displayed in Figure 5b where 100% inhibition of HIV-1IIIb is not achieved at the concentration of microgels we studied. We note that our HIV-1 inhibition assay appears to be less sensitive to pSS in semen than results obtained in other groups (Neurath et al., 2006). This could be due to differences in our semen samples and those studied by others. We were limited in the loading of pSS in the system by the viscosity and solubility of the pSS and were limited to ~70 mg/mL microgel particles by the viscosity of this microgel particle suspension when mixed with HSP in our studies. However, if we were able to achieve similar loading of a more active viral envelope inhibitor such as cyanovirin-N (Boyd et al., 1997; Tsai et al., 2004), our release rates indicate that this system could obtain significant and rapid inhibition of viral activity upon exposure to proteases in semen. Furthermore, the kinetics of release can, in principle, be made more rapid by incorporation of peptide crosslinkers with a greater kcat/Km as recently described (Matsumura et al., 2005; Rehault et al., 2002).
5. Conclusion
We have synthesized for the first time a drug delivery system designed to release an anti-HIV drug in a burst release profile when in contact with enzymes in semen. To accomplish this, we developed new methodology for the synthesis of peptide crosslinkers on solid phase. These PSA labile crosslinkers were incorporated in microgel polymeric constructs with and without antiviral agents. Beads constructed with the 1 mol% crosslinker composed of the sequence GISSFYSSK displayed significant degradation of the microgels over a four hour period in the presence of HSP. pSS of 700 kDa Mp was incorporated in beads of this composition and released a pSS concentration in less than 30 minutes of HSP exposure that was sufficient to reduce viral activity by 60%. Future work will investigate increasing the release rate by modifying crosslinker structure. We will also study the use of other entry inhibitor molecules and develop particulate systems for co-delivery that allow for triggered burst release of HIV entry inhibitors and sustained release of intracellular inhibitors.
Acknowledgements
This research was supported by a grant from The National Institutes of Health (NIH 1 R21 AI062445-01) to PFK, (NIH 5R21AI079772-02) to RWB. and an IGERT fellowship to JIJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albericio F. Orthogonal protecting groups for N(alpha)-amino and C-terminal carboxyl functions in solid-phase peptide synthesis. Biopolymers. 2000;55:123–139. doi: 10.1002/1097-0282(2000)55:2<123::AID-BIP30>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21:383–391. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, Shoemaker Rh, O'Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, Buckheit RWJ, Nara Pl, Pannell LK, Sowder RC, Henderson LE. Discovery of Cyanovirin-N, a Novel Human Immunodefiency Virus - Inactivating Protein that Binds Viral Surface Envelope Glycoprotein gp120: Potential Applications to Micbrocide Development. Antimicrobial Agents and Chemotherapy. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalone BJ, Miller SR, Ferguson ML, Malamud D, Kish-Catalone T, Thakkar NJ, Krebs FC, Howett MK, Wigdahl B. Toxicity, inflammation, and anti-human immunodeficiency virus type 1 activity following exposure to chemical moieties of C31G. Biomed Pharmacother. 2005;59:430–437. doi: 10.1016/j.biopha.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Chakraborty H, Sen PK, Helms RW, Vernazza PL, Fiscus SA, Eron JJ, Patterson BK, Coombs RW, Krieger JN, Cohen MS. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. Aids. 2001;15:621–627. doi: 10.1097/00002030-200103300-00012. [DOI] [PubMed] [Google Scholar]
- Chen X, Yu Z-T, Chen J-S, Yang B. In situ hydrothermal preparation of CdS/polymer composite particles with cadmium-containing polymer latexes. Materials Letters. 2003;58:384–386. [Google Scholar]
- Coombs GS, Bergstrom RC, Pellequer JL, Baker SI, Navre M, Smith MM, Tainer JA, Madison EL, Corey DR. Substrate specificity of prostate-specific antigen (PSA) Chem Biol. 1998;5:475–488. doi: 10.1016/s1074-5521(98)90004-7. [DOI] [PubMed] [Google Scholar]
- Denmeade SR, Jakobsen CM, Janssen S, Khan SR, Garrett ES, Lilja H, Christensen SB, Isaacs JT. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J Natl Cancer Inst. 2003;95:990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]
- Denmeade SR, Lou W, Lovgren J, Malm J, Lilja H, Isaacs JT. Specific and efficient peptide substrates for assaying the proteolytic activity of prostate-specific antigen. Cancer Res. 1997;57:4924–4930. [PMC free article] [PubMed] [Google Scholar]
- Denmeade SR, Nagy A, Gao J, Lilja H, Schally AV, Isaacs JT. Enzymic activation of a doxorubicin-peptide prodrug by prostate-specific antigen. Cancer Research. 1998;58:2537–2540. [PubMed] [Google Scholar]
- Fletcher PS, Wallace GS, Mesquita PM, Shattock RJ. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology. 2006;3:46. doi: 10.1186/1742-4690-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geonnotti AR, Katz DF. Dynamics of HIV neutralization by a microbicide formulation layer: biophysical fundamentals and transport theory. Biophys J. 2006;91:2121–2130. doi: 10.1529/biophysj.106.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh SL, Murthy N, Xu M, Frechet JM. Cross-linked microparticles as carriers for the delivery of plasmid DNA for vaccine development. Bioconjug Chem. 2004;15:467–474. doi: 10.1021/bc034159n. [DOI] [PubMed] [Google Scholar]
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- Gupta KM, Barnes SR, Tangaro RA, Roberts MC, Owen DH, Katz DF, Kiser PF. Temperature and pH sensitive hydrogels: An approach towards smart semen-triggered vaginal microbicidal vehicles. J Pharm Sci. 2007;96:670–681. doi: 10.1002/jps.20752. [DOI] [PubMed] [Google Scholar]
- Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey K. Microbicide trial screeches to a halt. The Journal of clinical investigation. 2007;117:1116. doi: 10.1172/JCI32291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Frank I, Williams V, Santos JJ, Watts P, Griffin GE, Moore JP, Pope M, Shattock RJ. Blockade of Attachment and Fusion Receptors Inhibits HIV-1 Infection of Human Cervical Tissue. J. Exp. Med. 2004;199:1065–1075. doi: 10.1084/jem.20022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketas TJ, Schader SM, Zurita J, Teo E, Polonis V, Lu M, Klasse PJ, Moore JP. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology. 2007;364:431–440. doi: 10.1016/j.virol.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Lard-Whiteford SL. Recommendations for the preclinical development of topical microbicides for prevention of HIV transmission: An update. J Acquir Immune Defic Syndr. 2004;36:541–552. doi: 10.1097/00126334-200405010-00001. [DOI] [PubMed] [Google Scholar]
- Lilja H. Biology of prostate-specific antigen. Urology. 2003;62:27–33. doi: 10.1016/s0090-4295(03)00775-1. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Bhatt AS, Andress D, Clegg N, Takayama TK, Craik CS, Nelson PS. Substrates of the prostate-specific serine protease prostase/KLK4 defined by positional-scanning peptide libraries. Prostate (New York, NY, United States) 2005;62:1–13. doi: 10.1002/pros.20101. [DOI] [PubMed] [Google Scholar]
- Moss JA, Stokols S, Hixon MS, Ashley FT, Chang JY, Janda KD. Solid-phase synthesis and kinetic characterization of fluorogenic enzyme-degradable hydrogel cross-linkers. Biomacromolecules. 2006;7:1011–1016. doi: 10.1021/bm051001s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath AR, Strick N, Li YY. Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides. BMC Infect Dis. 2002;2:27. doi: 10.1186/1471-2334-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath AR, Strick N, Li YY. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect Dis. 2006;6:150. doi: 10.1186/1471-2334-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyindo M. Complementary factors contributing to the rapid spread of HIV-I in sub-Saharan Africa: a review. East Afr Med J. 2005;82:40–46. doi: 10.4314/eamj.v82i1.9293. [DOI] [PubMed] [Google Scholar]
- Peter A, Lilja H, Lundwall A, Malm J. Semenogelin I and semenogelin II, the major gel-forming proteins in human semen, are substrates for transglutaminase. Eur J Biochem. 1998;252:216–221. doi: 10.1046/j.1432-1327.1998.2520216.x. [DOI] [PubMed] [Google Scholar]
- Ramjee G, Govinden R, Morar NS, Mbewu A. South Africa's experience of the closure of the cellulose sulphate microbicide trial. PLoS medicine. 2007;4:e235. doi: 10.1371/journal.pmed.0040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjee G, Shattock R, Delany S, McGowan I, Morar N, Gottemoeller M. Microbicides 2006 conference. AIDS Res Ther. 2006;3:25. doi: 10.1186/1742-6405-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratterree M, Gettie A, Williams V, Malenbaum S, Neurath AR, Cheng-Mayer C, Blanchard J. Safety and distribution of cellulose acetate 1,2-benzenedicarboxylate (CAP), a candidate anti-HIV microbicide in rhesus macaques. Aids. 2005;19:1595–1599. doi: 10.1097/01.aids.0000185990.16477.47. [DOI] [PubMed] [Google Scholar]
- Rehault S, Brillard-Bourdet M, Bourgeois L, Frenette G, Juliano L, Gauthier F, Moreau T. Design of new and sensitive fluorogenic substrates for human kallikrein hK3 (prostate-specific antigen) derived from semenogelin sequences. Biochim Biophys Acta. 2002;1596:55–62. doi: 10.1016/s0167-4838(02)00204-2. [DOI] [PubMed] [Google Scholar]
- Rejmanova P, Labsky J, Kopecek J. Aminolyses of monomeric and polymeric 4-nitrophenyl esters of N-methacryloylamino acids. Makromolekulare Chemie. 1977;178:2159–2168. [Google Scholar]
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nature Reviews Microbiology. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- Stone A. Microbicides: A new approach to preventing HIV and other sexually transmitted infections. Nature Reviews Drug Discovery. 2002;1:977–985. doi: 10.1038/nrd959. [DOI] [PubMed] [Google Scholar]
- Thieriet N, Alsina J, Giralt E, Guibe F, Albericio F. Use of Alloc-amino acids in solid-phase peptide synthesis. Tandem deprotection-coupling reactions using neutral conditions. Tetrahedron Letters. 1997;38:7275–7278. [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20:11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- UNAID/WHO. United Nations; 2007. AIDS Epidemic Update. [Google Scholar]
- Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Rittenhouse HG, Wolfert RL, Lynne CM, Brackett NL. PSA concentrations in seminal plasma. Clin Chem. 1998;44:895–896. [PubMed] [Google Scholar]
- Warrier BK, Kostoryz E, Lee CH. Biocompatibility of components of a female controlled drug delivery system. J Biomed Mater Res. 2004;71A:209–216. doi: 10.1002/jbm.a.30111. [DOI] [PubMed] [Google Scholar]
- Whitmore SK, Satcher AJ, Hu S. Epidemiology of HIV/AIDS among non-Hispanic black women in the United States. J Natl Med Assoc. 2005;97:19S–24S. [PMC free article] [PubMed] [Google Scholar]