Abstract
Angiotensin II (Ang II) causes skeletal muscle wasting via an increase in muscle catabolism. To determine whether the wasting effects of Ang II were related to its ability to increase NADPH oxidase-derived reactive oxygen species (ROS) we infused wild-type C57BL/6J or p47phox−/− mice with vehicle or Ang II for 7 days. Superoxide production was increased 2.4 fold in the skeletal muscle of Ang II infused mice, and this increase was prevented in p47phox−/− mice. Apocynin treatment prevented Ang II-induced superoxide production in skeletal muscle, consistent with Ang II increasing NADPH oxidase derived ROS. Ang II induced loss of body and skeletal muscle weight in C57BL/6J mice, whereas the reduction was significantly attenuated in p47phox−/− animals. The reduction of skeletal muscle weight caused by Ang II was associated with an increase of proteasome activity, and this increase was completely prevented in the skeletal muscle of p47phox−/− mice. In conclusion, Ang II-induced skeletal muscle wasting is in part dependent on NADPH oxidase derived ROS.
Keywords: Angiotensin II, Skeletal Muscle, Oxidative Stress, NADPH oxidase, Atrophy
Introduction
The wasting effects of Ang II may be important for clinical conditions such as congestive heart failure and chronic kidney disease (for reviews see [1]). In the case of cardiac cachexia, there is an evident association between this condition and high Ang II as such patients typically display high renin activity and high aldosterone levels [1,2,3]. Ang II has been shown to reduce body weight in rodents via induction of anorexia and increased protein degradation through the ubiquitin-proteasome pathway and increased apoptosis in skeletal muscles [4,5]. Ang II has pro-oxidant and proinflammatory effects which modulate growth, migration, apoptosis and inflammation in several tissues [6,7,8]. Although elevated ROS formation has been reported in rodent models of skeletal muscle wasting, including cancer cachexia [9], disuse atrophy [10], age-related atrophy [11], atrophy induced by TNF-α [12] or diabetes [13], the potential involvement of ROS in Ang II-induced skeletal muscle wasting is unknown. The aim of this study was to determine whether the wasting effects of Ang II were related to its ability to increase ROS. We evaluated the contribution of Ang-II-induced oxidative stress to skeletal muscle wasting.
Materials and Methods
Materials
Angiotensin II was from Phoenix Pharmaceuticals, Burlingame, CA, apocynin from Calbiochem, dihydroethidium (DHE) from Invitrogen, Carlsbad, CA and polyethylene glycol-superoxide dismutase (PEG-SOD) from Sigma-Aldrich (St. Louis, MO).
Animal studies
Experiments were approved by the Institutional Animal Care and Use Committee at Tulane University. To determine the potential involvement of NADPH oxidase in muscle wasting, eight-to 12-week-old male p47phox−/− mice (B6(Cg)-Ncf1m1J/J, Jackson Laboratory, Bar Harbor, Maine) and wild type mice of same genetic background (C57Bl/6J, Jackson Laboratory) were infused subcutaneously via osmotic minipump (ALZET model 1007D; DURECT™ DURECT Corporation, Cupertino, CA ) with Ang II (1500 ng/kg/min; Ang II group) or saline (ad libitum group) for 7 days. To normalize for differences in food intake induced by Ang II [4], saline-infused mice were pair-fed, i.e., given the identical food intake as the Ang II groups (pair-fed group), as previously described [5]. Body weight/food intake were measured daily and blood pressure was taken on days 0, 2, 4 and 6 in conscious mice by tail-cuff plethysmography (Visitech Systems Inc). Mice were sacrificed by ketamine/xylazine overdose in order to collect the skeletal muscles (gastrocnemius, tibialis anterior and quadriceps). Muscle wet weights were recorded and samples snap-frozen in liquid nitrogen and stored at −80°C for further use.
Skeletal muscle superoxide levels
Gastrocnemius muscle superoxide levels were determined as previously described with minor modifications [14]. This method has been validated to detect superoxide in frozen sections in cardiac muscle [15] and aorta [16] among others. One half of the muscle was incubated in OCT and PBS (50%/50%, v/v) for 30 min at room temperature and then slow-frozen in 100% OCT on dry ice. Another half of the muscle was pre-incubated with apocynin (200 μM) in OCT/PBS for 30 min for some of the experiments. 10 μm cryosections were stained with superoxide sensitive dye DHE (2 μmol/L) in a light-protected and humidified chamber for 40 min at 37°C. 700 U/ml of polyethylene glycol-superoxide dismutase (PEG-SOD, Sigma Chemical Co., St. Louis, MO) were applied in combination with DHE to adjacent sections to normalize for background signal. Three non-overlapping images per section were analyzed with a fluorescence microscope and the signal was quantified using Image Pro Plus (Media Cybernetics).
Basal 20S proteasome activity
20S Proteasome activity was determined via an optimized protocol modified from the “20S Proteasome Assay Kit for Drug Discovery” kit (Enzo Life Sciences, BML-AK740). Activity was quantified fluormetrically following cleavage of the fluorophor 7-amino-4-methylcoumarin (AMC) from substrate over time. In short, homogenates of gastrocnemius muscles were prepared by bead beating tissue in hypotonic cell lysis buffer (25 mM HEPES, 5 mM MgCl2, 5mM EDTA, 5 mM DTT, pH 7.5) at 4°C followed by centrifugation at 14,000 rpm for 2 minutes at 4°C. Protein concentration in the supernatant was determined using the BCA Protein Assay Kit (Pierce) and 35μg of total protein was added to each assay. Assay volumes were standardized by the addition of assay buffer, and inhibitor(s) were added to the appropriate wells of black 96-well clear/flat bottom plates. The plates were then incubated at 37°C for 15 minutes to allow enzyme-inhibitor interaction, as well as to allow for adequate depletion of endogenous ATP stores. Following incubation, ATP (1mM final concentration) was added to the appropriate wells in order to determine “ATP-dependent” 20S activity, which was defined as the difference between the activities in ATP supplemented wells and wells not supplemented with exogenous ATP for the same sample. Substrates were added to all wells immediately prior to data collection and emission was measured (excitation: 360nm, emission: 460nm) in a fluorescence microplate reader. Data were recorded at 1–2 minute time intervals over 60 minutes and activity was plotted as AFU/min over the linear range of the curves.
Statistical analysis
All the data were expressed as mean±SEM. One-way or 2-way ANOVA with Bonferroni’s correction was used to compare the changes in body weight, skeletal muscle weight, blood pressure, superoxide DHE levels and proteasome activity. A value of p<0.05 was regarded as significant.
Results
Ang II-induced skeletal muscle wasting is significantly blunted in p47phox−/− mice
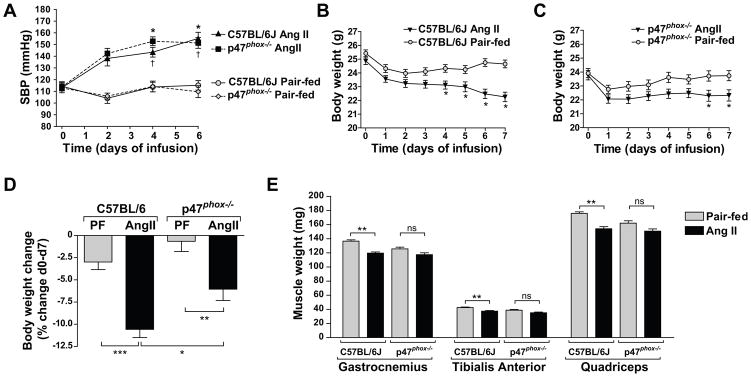
Ang II infusion increased blood pressure in C57BL/6J and p47phox−/− mice over 7 d (47.0 ± 6 mm Hg and 38 ± 6 mm Hg increase respectively, Fig 1A). We have shown that Ang II causes body weight reduction via anorexigenic and food intake-independent catabolic effects [4]. In addition to the body weight reduction due to the reduced food intake (Pair-fed control), Ang II caused a progressive and significant loss of body weight in C57BL/6J mice (10.58 ± 0.92 % decrease at 7 d compared to the initial body weight, Fig. 1B, D). This catabolic effect of Ang II was significantly suppressed in p47phox−/− mice (6.05 ± 1.27 % decrease, Fig. 1C, D). Changes in muscle weights largely paralleled those of body weights. Thus Ang II infusion in C57BL/6J mice reduced gastrocnemius, tibialis anterior and quadriceps weights when compared to the pair-fed group (12.4 %, 11.9 % and 12.3 % decrease respectively, Fig 1E). On the other hand, this effect was significantly blunted in p47phox−/− mice compared to wild-type mice (Fig 1E).
Figure 1.
Ang II increased skeletal muscle superoxide levels, role of NADPH oxidase
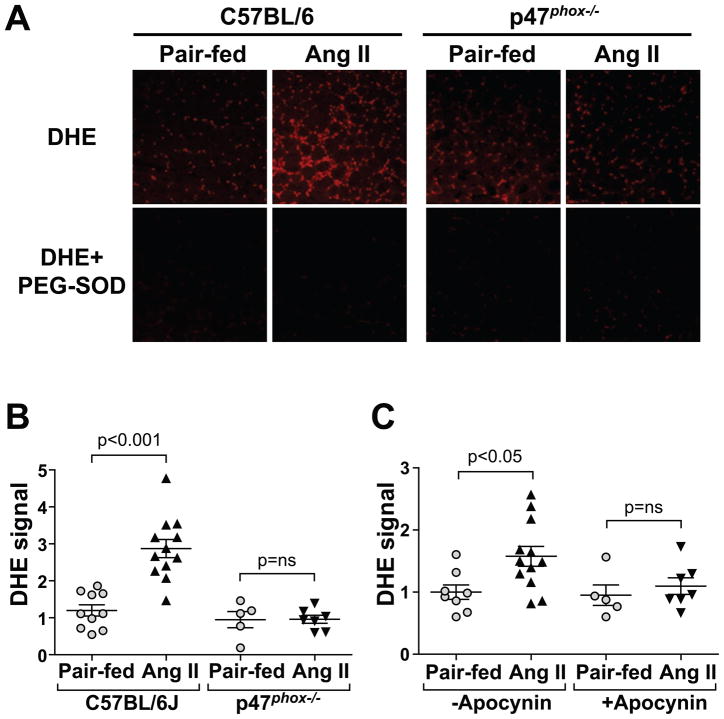
Ang II significantly increased skeletal muscle superoxide levels in C57BL/6J mice (139% increase at 7 d in gastrocnemius muscle compared to pair-fed, Fig 2A, B, and 90% increase in quadriceps muscle at 7 d, not shown). In marked contrast Ang II failed to increase superoxide levels in skeletal muscle of p47phox−/− mice (Fig. 2B) suggesting that a functional NADPH oxidase was required for Ang II induction of muscle oxidative stress. Apocynin also prevented the Ang II-induced superoxide increase, which further confirms that Ang II increases superoxide via NADPH oxidase (Fig. 2C).
Figure 2.
Ang II-induced skeletal muscle proteasome activity is significantly blunted in p47phox−/− mice
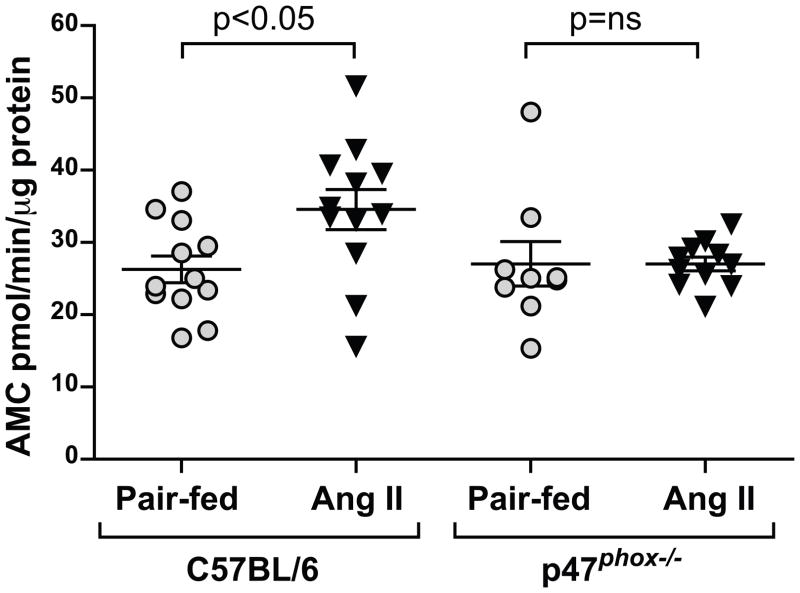
20S proteasome activity, an index of muscle protein degradation[17] was significantly increased in Ang II-infused C57BL/6J mice (32% increase compared to pair-fed controls, p< 0.05) and this increase was completely inhibited in p47phox−/− mice (Fig. 3). Taken together, our data showed that the wasting effects of Ang II in C57BL/6J mice that derive primarily from increased catabolism are markedly suppressed in the p47phox−/− background, suggesting an important role for the NADPH oxidase in the wasting effect of Ang II.
Figure 3.
DISCUSSION
We have previously demonstrated that Ang II-induced skeletal muscle wasting is related to anorexigenic and food intake-independent catabolic effects of Ang II that lead to increased protein degradation and apoptosis of muscle [4,5]. Our present study showed that the catabolic effect of Ang II is mediated, at least in part, via increased superoxides produced by NADPH oxidase.
A potential link between ROS and physiological effects of Ang II was first suggested by the demonstration that Ang II increased NAD(P)H activity and superoxide production in cultured vascular smooth muscle cells [18], and that the effect of Ang II on blood pressure was reduced by the administration of superoxide dismutase [19]. Subsequent studies provided evidence for an important role for ROS and particularly superoxide in Ang II-induced signaling, contributing to cardiac myocyte and vascular smooth muscle cell hypertrophy, endothelial dysfunction, hypertension, and insulin resistance [20,21]. However, there are very few studies on the effect of Ang II on skeletal muscle. Russell et al. demonstrated that murine C2C12 myotubes produced ROS in response to Ang II. This effect was inhibited by diphenyleneiodonium, implying the participation of NADPH oxidase, and was accompanied by increased protein degradation which was blunted by the use of antioxidants [22]. In addition, the expression of Nox2, Nox4, p22phox [23,24], p47phox and p67phox [24] subunits of the NADPH oxidase has been detected in skeletal muscle, suggesting that these NADPH oxidase subunits have a functional role in regulating skeletal muscle oxidative stress. In contrast to the results of Russell et al. our laboratory has not detected significant expression of Ang II receptors on C2C12 cells (Delafontaine, unpublished results), which is consistent with other reports [25] and with our prior studies that have indicated that the wasting effect of Ang II is mediated at least in part via intermediate cytokines such as IL-6 and serum amyloid A [26]. Furthermore, we have previously shown that Ang II markedly increases urinary corticosterone levels in the mouse and that the glucocorticoid inhibitor RU486 blunted Ang II-induced wasting [27]. Our current study demonstrates that irrespective of the downstream mediators of Ang II-induced wasting an increase in oxidative stress appears to be critically required for this effect of Ang II. Ang II-induced oxidative stress was accompanied by an increase in muscle 20S proteasome activity (Fig 3), and these effects were blocked in p47phox−/− mice. Interestingly, we did not find that the hypertensive response to Ang II was inhibited in p47phox−/− mice, in contrast to a previous report from Landmesser et al. [28] However, it is pertinent to note that Landmesser et al. used a lower dose of Ang II (486 ng/kg/min vs. 1500 ng/k/min in our study) and that the hypertensive response to Ang II in their study was only partially blocked in p47phox−/− mice. Our results indicate that the inhibition of Ang II-induced muscle atrophy in the p47phox−/− mice was independent of Ang II effects on systolic blood pressure. This is consistent with our prior data [4], which showed that the reduction in body weight induced by Ang II was not altered by normalization of blood pressure with hydralazine, but was markedly blunted by the Ang II AT1 receptor antagonist, losartan.
It is of note that our data does not exclude the possibility of Ang II-induced superoxide formation from other sources such as mitochondria. NADPH oxidase-dependent superoxide production in response to Ang II may be very important in diseases such as hypertension. Thus, vascular activation of superoxide production was attenuated in the aorta of p47phox−/− mice infused with Ang II [28]. However, this enzyme complex is not the only cellular source of superoxide that can be affected by this peptide. Ang II activates mitochondrial ROS formation in endothelial [29], vascular smooth muscle cells and in rat aorta in vivo [30]. Furthermore, there is positive feedback among enzymes producing ROS. Thus, ROS are able to directly activate NADPH oxidase to produce more ROS in vascular smooth muscle cells [31]. It is been speculated that NADPH oxidase-induced ROS could directly stimulate the mitochondria [30]. Indeed, myocardial mitochondrial ATP-sensitive potassium channels are activated by cytosolic superoxides derived from NADPH oxidase [32], and opening of these channels leads to mitochondrial ROS release [33]. Further studies are required to determine potential cross-talk mechanisms involving NADPH oxidase and mitochondria and their contribution to Ang II-dependent atrophy signaling.
Skeletal muscle wasting is a major contributor to negative outcomes in conditions such as cancer, chronic kidney disease and advanced congestive heart failure. Our findings indicate that Ang II-induced catabolic effects that lead to skeletal muscle wasting are redox-dependent and involve NADPH oxidase. The implication that superoxide formation is part of the signaling pathways contributing to skeletal muscle atrophy could be important in developing new drugs targeting muscle wasting in different conditions.
Acknowledgments
This study was supported by grants from the National Institutes of Health/National Heart, Lung and Blood Institute R01HL070241 and R01HL080682, National Institutes of Health/National Center for Research Resources P20RR018766 and National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 7K99DK083455-02. We acknowledge Christopher Blackstock, Sarah Galvez, Jane Titterington and Sumit Tiwari for their contribution to the completion of this project.
Abbreviations
- Ang II
angiotensin II
- DHE
dihydroethidium
- ROS
reactive oxygen species
- NADPH
nicotinamide adenine dinucleotide phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med. 2004;36:518–529. doi: 10.1080/07853890410017467. [DOI] [PubMed] [Google Scholar]
- 2.Adigun AQ, Ajayi AA. The effects of enalapril-digoxin-diuretic combination therapy on nutritional and anthropometric indices in chronic congestive heart failure: preliminary findings in cardiac cachexia. Eur J Heart Fail. 2001;3:359–363. doi: 10.1016/s1388-9842(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain JS. ACE inhibitor bulks up muscle. Nat Med. 2007;13:125–126. doi: 10.1038/nm0207-125. [DOI] [PubMed] [Google Scholar]
- 4.Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996;97:2509–2516. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, Delafontaine P. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology. 2001;142:1489–1496. doi: 10.1210/endo.142.4.8082. [DOI] [PubMed] [Google Scholar]
- 6.Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, Bahtyiar G, Stas S, Kumar P, Sha Q, Ferrario CM, Sowers JR. Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am J Physiol Endocrinol Metab. 2005;288:E353–359. doi: 10.1152/ajpendo.00402.2004. [DOI] [PubMed] [Google Scholar]
- 7.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 9.Gomes-Marcondes MC, Tisdale MJ. Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett. 2002;180:69–74. doi: 10.1016/s0304-3835(02)00006-x. [DOI] [PubMed] [Google Scholar]
- 10.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 11.Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, 2nd, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996;15:1753–1765. [PMC free article] [PubMed] [Google Scholar]
- 13.Dorenkamp M, Riad A, Stiehl S, Spillmann F, Westermann D, Du J, Pauschinger M, Noutsias M, Adams V, Schultheiss HP, Tschope C. Protection against oxidative stress in diabetic rats: role of angiotensin AT(1) receptor and beta 1-adrenoceptor antagonism. Eur J Pharmacol. 2005;520:179–187. doi: 10.1016/j.ejphar.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 15.Gross ER, LaDisa JF, Jr, Weihrauch D, Olson LE, Kress TT, Hettrick DA, Pagel PS, Warltier DC, Kersten JR. Reactive oxygen species modulate coronary wall shear stress and endothelial function during hyperglycemia. Am J Physiol Heart Circ Physiol. 2003;284:H1552–1559. doi: 10.1152/ajpheart.01013.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 17.Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–186. doi: 10.1042/EB0410173. [DOI] [PubMed] [Google Scholar]
- 18.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 19.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 20.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 21.Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am. 2009;93:569–582. doi: 10.1016/j.mcna.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell ST, Eley H, Tisdale MJ. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal. 2007;19:1797–1806. doi: 10.1016/j.cellsig.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 24.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 25.Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE. Immunohistochemical localization of rat angiotensin II AT1 receptor. Am J Physiol. 1993;264:F989–995. doi: 10.1152/ajprenal.1993.264.6.F989. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20:604–612. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 30.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 31.Li WG, Miller FJ, Jr, Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H(2)O(2)-induced O(2) production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang DX, Chen YF, Campbell WB, Zou AP, Gross GJ, Li PL. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ Res. 2001;89:1177–1183. doi: 10.1161/hh2401.101752. [DOI] [PubMed] [Google Scholar]
- 33.Brandes RP. Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension. 2005;45:847–848. doi: 10.1161/01.HYP.0000165019.32059.b2. [DOI] [PubMed] [Google Scholar]