Abstract
Traumatic brain injury often negatively impacts episodic memory; however studies of the neural substrates of this impairment have been limited. In this study, both encoding and recognition of visually presented stimuli were examined with functional magnetic resonance imaging. Twelve adults with chronic complicated mild, moderate, and severe injuries were compared with a matched group of twelve controls. Behavioral task performance did not differentiate the groups. During neuroimaging, however, the group of individuals with traumatic brain injury exhibited increased activation, as well as increased bilaterality and dispersion as compared to controls. Findings are discussed in terms of increased resource recruitment.
Traumatic brain injury (TBI) is defined as an injury to the brain incurred through externally inflicted trauma. Such injuries may result in significant impairment of an individual’s physical, cognitive and psychosocial functioning. One specific type of cognitive ability known to be compromised after TBI is episodic memory (Himanen, Porthin, Isoniemi, Helenius, & Kurki, 2006; Levin et al., 1990; Millis & Ricker, 1994; Ricker, et al., 2001; Wiegner & Donders, 1999; Wright, Schmitter-Edgecombe & Woo, 2010). As classically defined (e.g., by Baddeley, Harris, Sunderland, Watts & Wilson, 1987), the term episodic memory describes the ability to recall information regarding previously experienced stimuli, or events linked to a specific point in time. While the term “episodic memory” encompasses several types of cognitive tasks and operations, in the clinical setting, episodic memory is most frequently assessed by having individuals learn a stimulus set for recall and/or recognition at a later point in time.
While it may be argued that other persisting cognitive deficits after TBI may cause even greater functional disablement than episodic memory (i.e., executive control deficits), individuals with TBI have been shown to have significant awareness of memory difficulties (Anderson & Schmitter-Edgecombe, 2009). In addition, assistance with memory issues has been one of the most frequently cited needs of individuals at one year post injury (e.g., Corrigan, Whiteneck, & Mellick, 2004). This awareness of need may be due to the fact that episodic memory difficulties can have a significant impact on daily living: in day to day functioning, impairment in episodic memory may contribute to difficulties with learning and retaining new information, as well as recall of functional tasks, appointments, assignments and obligations. While such difficulties may range from mild to severe, it is important to note that the diffuse and complex nature of TBI may result in multiple areas of cognitive deficit, with additive effects. As a result, even mild episodic memory impairment, when combined with executive control and other cognitive difficulties, may potentially have a significant impact on the ability of an individual to complete household tasks or function in some employment settings.
There have been numerous behavioral studies of episodic memory impairment following TBI (e.g., Blanchet, Paradis-Giroux, Pepin, & McKerral, 2009; Deshpande, Millis, Reeder, Fuerst, & Ricker, 1996; Millis & Ricker, 1994; Tsirka et al., 2010; Wiegner & Donders, 1999). Taken together, these have clarified the nature of such deficits after injury (emphasizing the multifactorial nature of impairment in the acquisition of novel information, rather than solely a failure in retrieval). Although these contributions to the literature have been significant, there has been surprisingly minimal exploration of the cerebral substrates of this type of cognitive difficulty.
Our capacity to characterize the cerebral correlates of cognitive function in both healthy and clinical populations has been greatly expanded with the development of functional neuroimaging technologies. In studying TBI, such methods provide unique opportunities for identifying the pathophysiology of cognitive dysfunction related to injury in areas such as episodic memory. In the first study to apply functional imaging technology to the examination of episodic memory in persons with TBI, Ricker and colleagues (2001) utilized oxygen-15 positron emission tomography (O-15 PET) to study a group of persons with severe TBI at 1 to 3 years post injury. In comparison to matched controls, persons with TBI displayed increased overall activation, as well as increased bilaterality in activation patterns during an episodic memory task, even when task performance was not significantly different. In addition, activation patterns were noted to be more diffuse and more posterior as compared to healthy individuals. The authors asserted that the increased blood flow, more widespread dispersion and altered lateralization found in the TBI groups may indicate that increased cognitive effort is required to complete the same cognitive task as healthy controls due to decreased cognitive efficiency associated with brain injury. In addition, they suggested that the more posterior activation may represent the employment of a different strategy for remembering, or differential processing of the stimuli. In a subsequent O-15 PET study of memory following TBI, Levine and colleagues (2002) demonstrated that although individuals with TBI did not perform significantly differently on particular behavioral memory tasks and did engage the frontal, temporal and parietal areas normally associated with memory retrieval in healthy individuals, they also displayed relative increases in activation of the frontal, anterior cingulate and occipital regions and decreased activation in the right dorsomedial thalamus. Overall, the authors concluded that the TBI participants displayed alterations in the functional neuroanatomical networks used by healthy controls for memory. They further concluded that this may represent neural reorganization due to injury-related disconnection.
Despite the differences in technologies, fMRI studies of memory have demonstrated similar findings to those reported above. It should be noted, however, that the majority of fMRI studies in persons with TBI have examined working memory, while the study of episodic memory in this population remains quite novel. In fact, in addition to the study presented here, our search of the literature revealed only two other fMRI studies of TBI which employed episodic memory paradigms. The first of these studies (Strangman et al., 2008) involved the teaching of a verbal learning strategy to a group of 54 individuals with documented memory impairment after TBI. FMRI scanning was then conducted during the encoding phase only of a verbal learning paradigm. After scanning, free recall and recognition for the word lists was tested. The aim of the study was to assess the efficacy of fMRI results at the baseline session to predict rehabilitation outcome after a subsequent 12 session rehabilitation program focused on teaching of internal strategies for memory improvement. The rehabilitation program was followed by 2 post-test sessions of neuropsychological testing, with no repeat fMRI. For primary and secondary fMRI analysis, 6 regions of interest (ROI) were selected for prediction analysis based on areas activated using the same learning paradigm with a matched group of healthy controls. The findings of this study supported the assertion that the left prefrontal areas, are related to strategic verbal learning, as these areas of activation were observed in the TBI group, as well as the control group. They also observed that BOTH under and overactivation in the TBI group was associated with reduced performance on memory tasks after rehabilitation, creating an inverted-U quadratic relationship between performance and activation, which was therefore predictive of outcome. The authors asserted that underactivation may represent structural injury to the gray or white matter within the region of interest or possibly injury to areas projecting to that region, while overactivation may indicate intact and engaged cortical areas, which, despite, “effortful utilization”, failed to produce improvement in functional memory as tested.
Strangman and colleagues (2009) conducted an additional study of 20 participants with TBI and 20 healthy controls using the same behavioral and fMRI paradigm as the 2008 study. The aims of this study were to evaluate whether individuals with TBI and controls activated the same networks during verbal learning, and whether TBI affected neural activity during encoding when participants were instructed to use semantic clustering. Despite baseline testing indicating significant differences in behavioral performance between groups, direct comparison of group fMRI activation across all tasks, indicated no significant differences, suggesting that individuals in the TBI group activated the same general networks as healthy controls. Additional analysis and modeling focused on regions of interest (ROIs) with known anatomical connectivity which had been identified as being differentially activated by both groups during semantic (directed) strategy encoding. These comparisons indicated decreased activity for individuals with TBI in these key ROI’s, as compared to controls. The authors postulated that these decreases may be reflective of the decreased behavioral performance by the TBI group, or may indicate the use of different networks or strategies by the TBI group during this specific type of encoding. Further functional connectivity analysis which suggested a functional (but not anatomical) breakdown in connectivity between the left DLPFC and other areas normally associated with strategic control.
Together, these studies indicate significant alterations in functional cerebral processing of memory following TBI, as has been shown in previous PET studies. It is relevant to note that fMRI working memory studies have also shown that, relative to healthy controls, persons with TBI demonstrate alterations in hemodynamic response in terms of both blood flow and dispersion of cortical activation associated with working memory tasks conducted during fMRI. This has been found with individuals with moderate to severe injury (Christodoulou, et al., 2001; Perlstein, et al., 2004) as well as with individuals with mild TBI (Chen, et al., 2004; McAllister et al., 1999, 2001). In addition, other studies conducted using different working memory load conditions have shown altered patterns of activation between groups, and have suggested differential recruitment of cortical areas for individuals with TBI as compared to controls (Newsome et al., 2007, Turner & Levine 2008).
Given the relative novelty of neuroimaging studies of episodic memory following TBI, the goal of the present study was to add significantly to the literature in this critical area through further characterization of the cerebral activation during episodic memory task performance with fMRI. As alterations in cerebral activation have been noted for individuals with various levels of injury, even when behavioral performance was within functional limits, we included individuals with complicated mild, moderate, and severe levels of TBI, along with a healthy control group. For group comparison, each participant completed neuropsychological testing of cognition, as well as both encoding and recognition phases of an episodic memory task within an fMRI scanner. Given previous findings using O-15PET and fMRI during memory tasks, it was anticipated that alterations in activation, dispersion, and laterality would be observed in the current study for the group with TBI as compared to the control group. Because of our expectations that differences in activation patterns for participants with TBI may occur outside of those activated by healthy controls, we chose not to follow an ROI approach as used by Strangman et al. (2008, 2009). Our assessment was that a voxel-based approach would allow an opportunity to capture and evaluate activation which may occur outside of regions prescribed by previous studies of healthy controls. In doing so, our aim was to gain additional information which may help to characterize the changes in neural functioning following TBI, as change may involve altered recruitment of networks and functional brain areas. As so few neuroimaging studies of episodic memory after TBI have been conducted, we felt that exploring these characteristics in a more holistic fashion was critical to providing a base for further neuroimaging studies of episodic memory after TBI.
In addition, it should be noted that prior memory studies have generally conducted fMRI during encoding or retrieval individually. In the present study we chose to examine activation patterns of both encoding and recognition within the same fMRI session. Based on the limited neuroimaging studies of individuals with TBI, and what is known from studies of healthy individuals, we hypothesized that individuals with TBI would demonstrate greater cerebral activation as well as differential dispersion of activation during both encoding and recognition.
In addition we felt that it was important to document whether or not these alterations would in fact be observed, and also to begin to describe differences based on memory process. No other study has made this comparison in individuals with TBI.
Method
Participants
Participating in this study were 15 persons with TBI and 14 uninjured controls. Because of technical difficulties and/or subject movement, the data from some fMRI sessions were not usable. Thus, included in the current paper are 12 controls (2 F) and the 12 persons with TBI (3 F) with usable fMRI data. All participants were right hand dominant and were free of any history of neurological disease or insult (excepting the traumatic brain injury for the injury group), psychiatric illness, and alcohol or drug abuse. No participant met any of the standard exclusionary criteria for MRI, including metal in the body or pregnancy. All participants gave consent approved by the Institutional Review Board of the University of Pittsburgh.
We included TBI participants with moderate and severe, as well as complicated mild initial traumatic brain injury based on history confirmed by medical record data. Classification of moderate to severe injury was made based on the lowest Glasgow Coma Scale (GCS; Teasdale & Jennett, 1974) score in the first 24 hours after injury (moderate defined as GCS of 9 – 12 and severe defined as GCS of 3-8), as well as objective medical documentation indicating TBI. Those with mild initial injuries (i.e., GCS ≥ 13) were only included if there was sufficient documentation of significant medical complications following injury, such as positive neuroradiologic findings. Those persons with “complicated mild” injuries were included, as research has shown that such persons have outcomes more similar to those of persons with moderate TBI (Kashluba, Hanks, Casey, & Millis, 2008; Williams, Levin, & Eisenberg, 1990). Initial GCS scores in our study group ran the full range from 3–15 (M = 8.9, SD = 5.5), with best GCS scores in the first 24 hours ranging from 7–15 (M = 12.2, SD = 3.4). These scores suggest that, on average, persons in the TBI group sustained relatively severe injuries. Due to hemodynamic alterations that are associated with acuity rather than cognitive activation in the first several months after injury (Yamaki, et al., 1996), TBI participants were at least one year post-injury, but not greater than 3 years post-injury (M = 1.7 years, SD = 0.6 years, range = 1.06–2.61 years). Though inclusion for the TBI participants was originally determined through medical records and prior scans, structural scans collected for this study were examined. The majority of the residual findings seen on the T2 structural scans were diffuse. Residual focal lesions in the TBI group were few, and there was no consistent trend towards any contusion location (1 bifrontal, 1 right temporal, 1 basal ganglia). Control scans all appeared to be age-appropriate and within normal limits.
Controls were matched to the TBI group for age, gender, and number of years of education. Age matching was achieved through age stratification with five-year strata. Within each age stratum, participants were matched for educational level within one year. The groups included in this paper were not significantly different in age (controls: M = 26.5, SD = 8.7, range = 19–50; TBI: M = 33.1, SD = 12.9, range = 18–54; t(22) = -1.46, p = 0.16), or number of years of education (controls: M = 16.2, SD =2.8, range = 12–22; TBI: M = 14.8, SD = 1.9, range = 12–18; t(22) = 1.37, p = 0.19). Complete participant demographics for the TBI group are presented in Table 1.
Table 1.
Participant Demographics for the TBI Group
| Gender | Age | Yrs. Educ. | Initial GCS | Best GCS/24 hrs. | Yrs. Since Injury | Injury Cause |
|---|---|---|---|---|---|---|
| M | 35 | 12 | 3(T) | 7 | 1.9 | MVA |
| M | 18 | 12 | 15 | 15 | 1.1 | Sports |
| M | 31 | 15 | 3(T) | 7 | 2.2 | MCA |
| F | 21 | 12 | 15 | 15 | 1.9 | MVA |
| F | 20 | 15 | 12 | 15 | 2.4 | Fall |
| M | 23 | 14 | 3(TP) | 11 | 2.2 | MVA |
| M | 54 | 16 | 3(TP) | 7 | 1.1 | MCA |
| M | 41 | 18 | 7 | 11 | 1.1 | Falling object |
| M | 30 | 16 | 13 | 15 | 2.0 | MCA |
| M | 47 | 16 | 15 | 15 | 1.1 | Bicycle |
| F | 24 | 16 | 4 | 14 | 1.1 | MVA |
| M | 53 | 16 | 14 | 14 | 2.6 | Fall |
GCS = Glasgow Coma Score, T = intubated, P = given paralytic medication, MVA = motor vehicle accident, MCA = motorcycle accident
Materials and Design
During the imaging session, four pairs of stimulus blocks were presented. For each pair, the first was an encoding block and the second was a recognition block. The four types of stimuli used were black line drawings (pictures), black outline shape arrays (shapes), black capitalized words (words), and black capitalized non-pronounceable letter strings (letters). All were displayed on a white background. The pictures were from Snodgrass & Vanderwart (1980), the words were developed from their original set (as used by Nolde, Johnson, & D’Esposito, 1998; Raye, Johnson, Mitchell, Nolde, & D’Esposito, 2000), and both words and pictures were high-frequency, easily named and visualized items. Shape stimuli were randomly generated arrays composed of primary shapes, and letter stimuli were unpronounceable 4–8 letter strings. During encoding blocks, participants were shown sets of items of one of the four stimulus types, and for each item made a yes/no pleasantness judgment (e.g., McDermott, et al., 1999) for the purpose of enhancing attention and encoding. During each block, only one type of stimulus was presented, and each block comprised an approximately 6-minute run for the fMRI session. During recognition blocks, stimulus sets consisted of half previously seen items and half new items. Participants were asked to identify if a presented item had been previously seen or not by pressing one of two buttons on a response pad. While the recognition block for a particular stimulus type was presented immediately following the corresponding encoding block, the ordering of the four pairs of blocks was counterbalanced among participants. Items within each block were randomly ordered. When considering the eight functional scans together with the structural scans, each scanning session totaled around 1 hour and 15 minutes.
E-prime software (www.pstnet.com) was used for stimulus presentation. Trials were set up so that following a 500 msec fixation cross, an item was presented for 2500 msec. The stimulus screen was followed by a blank screen presented for 1000, 3000, or 5000 msec.
Procedure
After informed consent was obtained, the questionnaires and neuropsychological assessments were completed. The Token Test (DeRenzi & Vignolo, 1962; Spreen & Benton, 1969) was given in order to ensure participants could understand and follow verbal instructions. An estimate of overall intelligence was obtained using the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001). Although TBI and control group members were matched, as previously described, by age and education level, the average WTAR standard score for the TBI group fell in the average range (mean = 101, SD = 9.3), while the average WTAR standard score for the Control Group fell in the high average range (mean = 114, SD = 10.2). The Brief Symptom Inventory (BSI; Derogatis, 1993) was administered to screen for psychiatric disorders. Individuals with significant and acute psychiatric symptoms were not included as study participants. The Michigan Alcoholism Screening Test (MAST; Selzer, 1971) was administered as an assessment of recent alcohol use in order to screen out individuals with suspected current alcohol abuse or dependence. No significant current alcohol abuse or dependence was suggested by individual MAST scores for participants in either the TBI or Control groups, nor did average MAST scores for each group suggest significant use (Average MAST score for control group = 0.5, SD = 1, Average MAST score for the TBI group = 0.8, SD = 1.2)
The cognitive domain of interest in this study was assessed using the California Verbal Learning Test - II (CVLT-II; Delis, Kramer, Kaplan & Ober, 2000). In order to ensure that TBI participants had a deficit in episodic memory, persons with TBI were only included in the study if they performed at least 1 standard deviation (SD) below normative standards on any index of the CVLT-II. This test is a reliable and valid measure used to examine episodic memory, and has been well studied in the TBI population (e.g., DeJong & Donders, 2009; Jacobs & Donders, 2007, 2008; Wolfe, et al., 2009). To be eligible for participation in this study, healthy controls were required to perform no lower than 1 SD below normative standards in all categories. All participants included in the study met the criteria set for their group. Of a total of 21 consented persons with TBI, seven were excused from further participation for having CVLT-II scores in the normal range. Four of 17 consented controls also had to be excluded for scoring outside the normal range. Three other consented participants (2 TBI and 1 control) also had to be excluded for MRI contraindications not disclosed at original screening. Following the neuropsychological measures, a short practice task was run outside the scanner to familiarize participants with the episodic memory task. MRI safety screening was then completed by the technologist and participants then completed the imaging session. Scans were conducted on a 3-Tesla Siemens Allegra head-dedicated scanner.
fMRI Parameters
Structural scans acquired during these sessions included axial T2 weighted images (39 contiguous 3mm slices, TR = 6440 ms, TE = 73 ms, 256 × 256 matrix, FOV = 200mm, flip angle = 150°) and a sagittal 3-D MPRAGE sequence (224 contiguous 0.78 mm slices, TR = 1680ms, TE = 2.48ms, 256×256 matrix, FOV = 200mm, flip angle = 8°). Functional images consisted of sets of 39 contiguous 3mm axial slices (TR = 2000ms, TE = 25ms, 64×64 matrix, FOV = 200mm, flip angle = 79°).
fMRI Analysis
All pre-processing steps and analyses were conducted with SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/). For pre-processing, images were first corrected for subject motion using a 5th degree B-spline algorithm with a 6 parameter rigid body spatial transformation, and then registered to a mean image. Coregistration of the structural to functional images was accomplished with a normalized mutual information function. Images were then segmented, and normalized into a standard space. The final preprocessing step was smoothing with a Gaussian kernel of 10 mm FWHM.
Data analysis was conducted using a voxel-based approach on each individual participant’s data, with motion regressed out. An explicit mask (created using the ART program— http://www.nitrc.org/projects/artifact_detect) was used. Individual first-level analyses were entered into a two-sample t-test. The MNI coordinates (Mazziotta, Toga, Evans, Fox, & Lancaster, 1995) of activated areas were converted to Talairach (Talairach & Torneau, 1998) coordinates with the use of the icbm2tal algorithm described by Lancaster, et al. (2007). These coordinates were then localized using the Talairach Client (www.talairach.org/client.html Lancaster, et al., 2000; Lancaster, et al., 1997).
Results
Behavioral Data
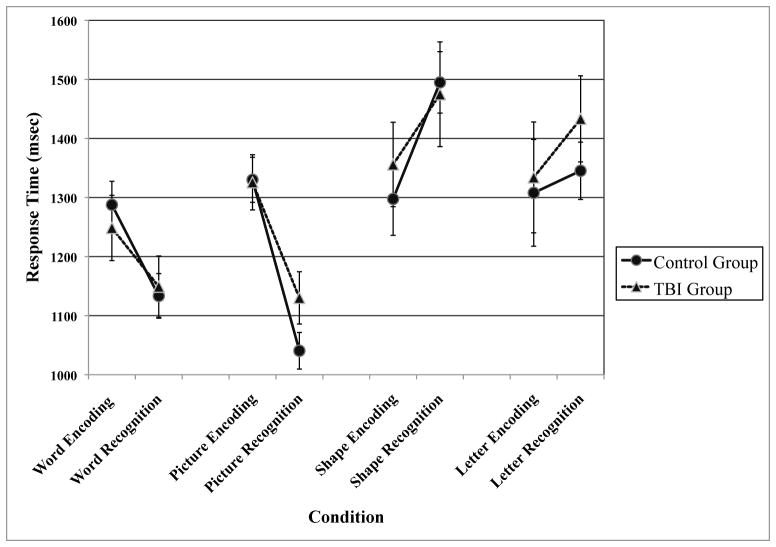
A 2 × 8 analysis of variance (ANOVA) was run to see the effects of Group (Control, TBI) and Condition (encoding and recognition conditions for each of the four stimulus types) on response times. As indicated by Figure 1, the conditions with picture and word stimuli resulted in the respective recognition conditions being faster than the encoding conditions. Letters and shapes both exhibited the opposite pattern, with the recognition conditions being slower than the encoding conditions. Shape conditions were especially affected, with shape recognition contributing the slowest response times in the experiment. In the 2×8 ANOVA, there was a significant main effect of condition (F (7, 154) = 14.71, p < 0.001) but no effect of group (F (1, 22) = .28, p = 0.60), and no interaction (F (7, 154) = 0.50, p = 0.83). T-tests were run to see if the TBI group performed slower on any individual conditions than controls, but none of these comparisons proved to be significant (all ps > 0.11, two-tailed).
Figure 1.
Response times by group and condition.
A similar analysis was completed on the accuracy data. Because “pleasantness” is subjective, accuracy values are only available for the recognition conditions. A 2 (Group) × 4 (Condition) repeated measures ANOVA was conducted on these data, and a resulting significant main effect of condition was reported (F (3, 66) = 130.36, p < 0.001), again with no main effect of group (F (1, 22) = 0.60, p = 0.45) or group by condition interaction (F (3, 66) = 1.11, p = 0.35). Overall, the patterns observed in the accuracy data and the response time data were similar in that increased accuracy on a task corresponded with faster response times for that task, with pictures being responded to most accurately (88%, by all participants), followed by words (82%), letters (60%), and shapes (45%). T-tests were conducted to see if the control group was more accurate for any given condition, but no significant differences between the groups were found (all ps were greater than 0.20, two-tailed). It should be noted that performance on the shape condition was quite poor, with accuracy scores falling to chance level in both groups, and increased response times associated with these items. While the finding that the shape condition was too difficult is problematic, it could not have been predicted due to the fact that this kind of condition has not previously been examined in the TBI population. In light of these issues, it was thought best that further analysis not be conducted on the shape data, as any results would be uninterpretable.
Imaging Data
Given that some previous imaging studies have shown examples of equivalent behavioral performance between TBI and control groups on episodic memory tasks (Ricker, et al., 2001, recognition; Levine, et al., 2002, cued recall), it was not surprising that our data for the recognition condition also did not differentiate between the two groups. This finding suggests that the TBI and control groups were equally able to perform the tasks at hand, and it now falls to the imaging data to show whether there are differences in the activation record associated with yielding that equivalence of behavioral performance. As discussed in the Introduction, previous research in persons with TBI has provided evidence that they may recruit additional brain areas to accomplish the same task (Ricker, et al., 2001), and in some cases do this despite achieving the same level of behavioral performance as control participants (see discussion of subgroup in Levine, et al., 2002).
To examine differences between controls and persons with TBI on these encoding and recognition tasks, three models were run for each individual, and the individual contrast maps were then entered into group analyses to compare the activation patterns of the two groups. The three contrasts that were examined included one focused on process (Word Encoding vs. Word Recognition), as well as two directly comparing the processing of the different stimulus types (Word Encoding vs. Picture Encoding and Word Recognition vs. Picture Recognition). In our initial analysis, considerable individual variation was noted even within the two groups (see, e.g., Miller et al. 2009, for a discussion of individual variation in fMRI). As a result, a threshold of p = 0.01 uncorrected along with a cluster size of 10 voxels were used for initial description of the data from the three contrasts. We then implemented more restrictive thresholding (p < 0.001, uncorrected, cluster size > 10 voxels) and further evaluated the areas which remained.
Word Encoding vs. Word Recognition
As compared to word recognition, word encoding did not elicit much extra activation in either group. For the recognition portion, however, both groups had some additional areas of activation. The activation seen in the TBI group was much more diverse, with three times as many clusters as in the control group. Most of these clusters were located posteriorly, and there were roughly equal numbers of clusters located in the right and left hemispheres. Controls, on the other hand, had fewer clusters, with nearly all located in the right hemisphere, though this pattern of findings is likely due to the direct comparison between both processes. While these data are preliminary, they are suggestive that persons with TBI recruited more areas in service of the same task. As noted in the description of the behavioral data, above, this pattern of activation was associated with equal task performance. When this contrast was subjected to the more conservative threshold, the only activation which remained was for the TBI group in the word recognition condition. Clusters found included bilateral areas in the temporal lobe (BA 21 and 22, extending to 39 on the right), right inferior frontal gyrus (BA 45), and the inferior parietal lobule on the left (BA 40). These areas suggest that the language and memory systems may have been working harder in the TBI group during word recognition.
Word Encoding vs. Picture Encoding
Again for this contrast, there were no voxels for word encoding which survived thresholding for either group. The controls also did not have any extra voxels associated with the picture encoding condition. The TBI group, however, experienced a great deal of activation associated with the picture encoding condition (see Figure 2). These areas of activation were more commonly seen towards the posterior regions of the brain, but were split fairly evenly between left and right hemisphere areas. Again there was evidence for an increase in the areas of activation associated with performing a task (and achieving control-equivalent behavioral performance), as well as for bilateral activation of areas. Many of these areas survived more stringent thresholding (p uncorrected < 0.001, cluster size > 10 voxels), including bilateral areas in the supramarginal gyri (BA 40), the superior frontal gyri (BA 6 and 8 on the left and 8 on the right), and the middle occipital gyri (BA 19). There was also activation in BA 37 bilaterally, though the cluster on the right was in the fusiform gyrus, while the cluster on the left was in the middle temporal gyrus. Additional lateralized areas included right superior temporal gyrus (BA 22), and left cerebellum. These areas could be more involved in the processing of pictures as opposed to words, as well as possible subvocal rehearsal. In any case, there is again clear evidence of bilateral activations as well as more areas of activity for the TBI group.
Figure 2.
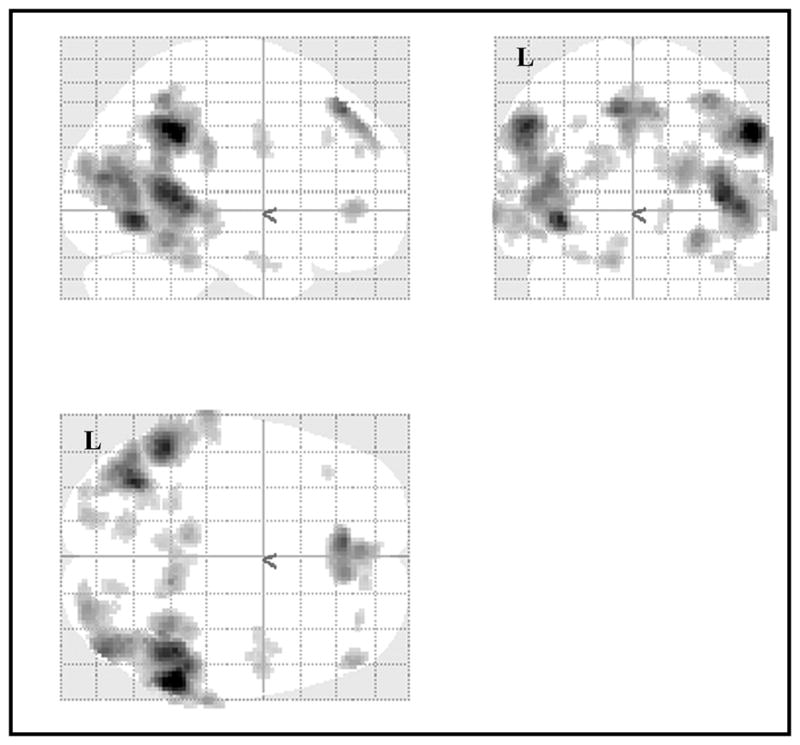
Activation for Picture Encoding as compared to Word Encoding for the TBI group at p < 0.005, uncorrected, excluding clusters with fewer than 10 voxels.
Word Recognition vs. Picture Recognition
Overall, this comparison generated less activation for each group, but there were some locations that warrant discussion. The controls, for word recognition, activated some additional areas associated with memory and language processing (i.e., anterior cingulate cortex, inferior frontal gyrus, superior temporal gyrus, and cingulate gyrus, all on the left). While the areas activated by the control group were cortical, those activated by the TBI group were subcortical (i.e., the right pulvinar and caudate). This distinction will be considered in more detail below. No additional areas survived thresholding for the controls in the picture recognition side of the contrast. In comparison, the TBI group had several additional active areas for picture recognition, including areas which may be involved in the demands of processing more visually demanding stimuli (i.e., right fusiform gyrus, right precuneus, middle occipital gyrus, left posterior cingulate, left medial frontal gyrus, right inferior parietal lobule, and right middle temporal gyrus). The only area that remained at the more rigorous threshold (p uncorrected < 0.001, cluster size > 10 voxels) was the right precuneus (BA 7) in the TBI group during picture recognition. As compared to the controls, these results again represent a more distributed pattern of activation, as well as one slightly more skewed towards the posterior of the brain. The previously-discussed pattern of nearly equal right-left hemispheric distribution does not hold for this contrast.
Together these results suggest that, regardless of process or modality, there is a general tendency for the TBI group to activate more areas than controls, and that the active areas tend to be distributed between the two hemispheres and include more posterior clusters.
Discussion
In this study, we obtained several results which are consistent with previous imaging and behavioral findings in TBI, as well as one which may be novel. First of all, our study results indicated equivalent behavioral performance between the healthy control and TBI groups, in terms of reaction times, accuracy, and performance pattern on all task types. Although these have been carefully considered and analyzed, as our sample size was small it is possible that these negative results reflect an issue of inadequate power. Other studies with similar numbers have, however, seen differences between TBI groups and controls. In any case, despite seemingly equivalent performance, differences were found in the patterns of activation seen in the control and TBI groups. This finding is consistent with previous studies reviewed above. In a sense, it is actually ideal to design a memory task in which individuals with TBI and known memory issues are able to perform equivalently to healthy controls. This is preferable because performance differences are now removed as a factor, and we are able to interpret the differences in brain activation as related to injury, and not simply related to divergent level of performance. Secondly, as seen in previous imaging studies of cognition after TBI (e.g., Ricker et al., 2001), results of the current study found conditions under which a more bilateral pattern was seen for the TBI group. Also consistent, in nearly every contrast there was evidence for more areas of activation and a more distributed pattern of activation surviving threshold for the persons with TBI, as compared to the control group.
In the final, and potentially novel, comparison, results of this study indicated a difference between the groups such that the TBI group showed more subcortical involvement while the activation for the control group was more cortical in nature. It is not known whether this finding represents something which will be confirmed by future studies of episodic memory after TBI, or whether it is unique to this experiment and the condition of word recognition. It should be noted, however, that there is evidence that the structures shown to be active in this portion of the experiment are important in language processing (see Crosson & Haaland, 2003, for a review) as well as other cognitive functions. Even if these subcortical structures are not specifically damaged after TBI (as suggested by Primus, et al., 1997), they are highly interconnected through white matter tracts known to be especially susceptible to injury via TBI (e.g., Whyte, Hart, Laborde, & Rosenthal, 2005), and other researchers (e.g., Little, et al., 2010), have found differences in cognitive functioning associated with damage to thalamic projection fibers. In either case, given the small number of imaging studies of episodic memory after TBI, a resolution of this issue is not yet possible, but it has the potential to be of interest for future study.
There are, however, some factors which likely impacted our findings. First of all, the study design was originally meant to have high-level controls in the Letter and Shape conditions. Unfortunately, behavioral results seem to indicate that participants did not respond to them as control conditions. As a result, Shape and Letter conditions were not useful for comparison in the manner initially intended. The resulting comparisons had to then be made without benefit of a control condition, creating increased likelihood of washout of observable brain activation between the compared conditions. It should be noted, however, that the study finding of little differential group activation during word encoding, (as found in the word encoding vs. word recognition comparison and the word encoding vs. picture encoding comparison) was consistent with the findings of Strangman (2009), despite the fact that methods and analyses were different between studies. Further study will be required to further assess whether this consistency is a true pattern in neural activation during memory encoding.
There was also a great deal of individual variation, both within groups as well as between members of the two groups. Part of this is likely due to factors unique to the individuals who participated. Miller, et al., (2009) investigated individual differences on three tasks employing different kinds of memory (episodic, semantic, and working) and found that activation was more similar within a single participant across the three tasks than it was across participants doing the same task. Hester, Fassbender, & Garavan (2004) have also addressed this problem to a lesser extent but did find individual differences in activation as well. Another issue which may be affecting our results is that our participants were quite diverse in age, ranging from 18 to 54 across a group of only 12 participants. At the lower end of the age range, participants’ brains are likely to not be fully myelinated (Sowell, et al., 2003), while at the higher end of the spectrum normal aging processes may already be underway (see, e.g., Rajah & D’Esposito, 2005, for an example of how aging can lead to differences in fMRI activation). The TBI sample was also necessarily rather heterogeneous. Participants in the TBI group were in the same early chronic phase of injury, but there were differences in the time since injury. Variation in severity and pathology could have also contributed to the heterogeneity of the TBI sample. While necessary to ensure adequate recruitment, including participants with any such differences could lead to lower data quality in a group analysis situation. In sum, it is highly likely that both individual differences and a wide age distribution played a role in our limited ability to appreciate changes in brain activation due to our experimental conditions.
An additional issue which has seen more discussion in literatures dealing with populations other than TBI is that of the reasons behind recruitment of “extra” brain regions during task performance. This is seen quite often in the literature on aging. When older participants are found to have more activation for a given task, it is sometimes attributed to greater recruitment due to neural inefficiency (e.g., Duverne, Habibi, & Rugg, 2008). Another possibility is that increased or divergent activation is due to some form of compensation for brain systems that are not working as they did before being injured. While the current study is not designed specifically to address this question, the results may suggest an explanation nearer to functional compensation, given that the “extra” areas of activation seen in the TBI group were outside the typical memory areas. In fact, they seemed to be drawing more heavily on other cognitive systems which support memory. This finding is not surprising, and the influence of cognitive functions like attention or executive control on episodic memory is beginning to come to light (e.g., Cabeza, Ciaramelli, Olson, & Moscovitch, 2008). These functions are known to be commonly affected by TBI, and disconnection of white matter tracts contributing to these cognitive functions is also of concern in the TBI population. Future studies from our lab are planning to address these issues by using an episodic memory paradigm that directly manipulates cognitive load during encoding and by considering data from DTI and functional connectivity analyses as well as fMRI.
Finally, given the novelty of this area of research, this study represents a step forward in the efforts to characterize the neural substrates of episodic memory after TBI. While Strangman et al. (2008, 2009) chose an ROI approach to the analysis of memory circuits based on those activated by healthy controls, it should be kept in mind that their purpose was to evaluate whether currently available cognitive rehabilitation strategies could be evaluated for usefulness depending on fMRI findings, even though the more general correlates of fMRI activation associated with episodic memory performance after TBI had yet to be established. Our goal in this study was broader, as we felt that, given the paucity of published studies in this area, it was critical to establish information regarding changes to those memory systems after TBI, and to learn more about changes which might occur outside of normal circuitry. As a result, we felt that a voxel-based approach was more readily applicable for this purpose. It is our hope that, as we continue to learn more about episodic memory functioning after TBI, our increased ability to create new and innovative approaches to assessment and treatment which utilize this knowledge will allow for improvements in quality of life for those who have sustained TBI.
Acknowledgments
This study was supported in part by a grant (NIH-NINDS R01NS048178-01) awarded to Dr. Ricker.
References
- Anderson JW, Schmitter-Edgecombe M. Predictions of episodic memory following moderate to severe traumatic brain injury during inpatient rehabilitation. Journal of Clinical and Experimental Neuropsychology. 2009;31:425–438. doi: 10.1080/13803390802232667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Harris J, Sunderland A, Watts KP, Wilson BA. Closed head injury and memory. In: Levin HS, Grafman J, Eisenberg HM, editors. Neurobehavioral Recovery from Head Injury. New York: Oxford University Press; 1987. pp. 296–317. [Google Scholar]
- Blanchet S, Paradis-Giroux AA, Pepin M, McKerral M. Impact of divided attention during verbal learning in young adults following mild traumatic brain injury. Brain Injury. 2009;23:111–122. doi: 10.1080/02699050802649688. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, DeLuca J, Ricker JH, Madigan NK, Bly BM, Lange G, et al. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. Journal of Neurology, Neurosurgery, & Psychiatry. 2001;71:161–168. doi: 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, Whiteneck G, Mellick D. Perceived needs following traumatic brain injury. Journal of Head Trauma Rehabilitation. 2004;19:205–216. doi: 10.1097/00001199-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Crosson B, Haaland KY. Subcortical functions in cognition: Toward a consensus. Journal of the International Neuropsychological Society. 2003;9:1027–1030. [Google Scholar]
- DeJong J, Donders J. A confirmatory factor analysis of the California Verbal Learning Test Second Edition (CVLT-II) in a traumatic brain injury sample. Assessment. 2009;16:328–336. doi: 10.1177/1073191109336989. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- DeRenzi E, Vignolo LA. The Token Test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. BSI Brief Symptom Inventory: Administration, Scoring, and Procedure Manual. 4. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Deshpande SA, Millis SR, Reeder KP, Fuerst D, Ricker JH. Verbal learning subtypes in traumatic brain injury: A replication. Journal of Clinical and Experimental Neuropsychology. 1996;18:836–42. doi: 10.1080/01688639608408306. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiology of Aging. 2008;29:1902–16. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H. Individual differences in error processing: A review and reanalysis of three event-related fMRI studies using the go/nogo task. Cerebral Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- Himanen L, Porthin R, Isoniemi H, Helenius H, Kurki T. Longitudinal cognitive changes in traumatic brain injury: A 30-year follow-up study. Neurology. 2006;66:187–192. doi: 10.1212/01.wnl.0000194264.60150.d3. [DOI] [PubMed] [Google Scholar]
- Jacobs ML, Donders J. Performance discrepancies on the California Verbal Learning Test Second Edition (CVLT-II) after traumatic brain injury. Archives of Clinical Neuropsychology. 2008;23:113–118. doi: 10.1016/j.acn.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Jacobs ML, Donders J. Criterion validity of the California Verbal Learning Test Second Edition (CVLT-II) after traumatic brain injury. Archives of Clinical Neuropsychology. 2007;22:143–149. doi: 10.1016/j.acn.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Kashluba S, Hanks RA, Casey JE, Millis SR. Neuropsychologic and functional outcome after complicated mild traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2008;89:904–911. doi: 10.1016/j.apmr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Gary HE, Jr, Eisenberg HM, Ruff RM, Barth JT, Kreutzer J, et al. Neurobehavioral outcome 1 year after severe head injury. Journal of Neurosurgery. 1990;73:699–709. doi: 10.3171/jns.1990.73.5.0699. [DOI] [PubMed] [Google Scholar]
- Levine B, Cabeza R, McIntosh AR, Black SE, Grady CL, Stuss DT. Functional reorganisation of memory after traumatic brain injury: A study with H2150 positron emission tomography. Journal of Neurology, Neurosurgery, & Psychiatry. 2002;73:173–181. doi: 10.1136/jnnp.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little DM, Kraus MF, Joseph J, Geary EK, Susmaras T, Zhou XJ, et al. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–64. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probablistic atlas of the human brain: Theory and rationale for its development. NeuroImage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, et al. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Ojemann JG, Petersen SE, Ollinger JM, Snyder AZ, Akbudak E, et al. Direct comparison of episodic encoding and retrieval of words: An event-related fMRI study. Memory. 1999;7:661–678. doi: 10.1080/096582199387797. [DOI] [PubMed] [Google Scholar]
- Miller MB, Donovan CL, Van Horn JD, German E, Sokol-Hessner P, Wolford GL. Unique and persistent individual patterns of brain activity across different memory retrieval tasks. Neuroimage. 2009;48:625–635. doi: 10.1016/j.neuroimage.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis SR, Ricker JH. Verbal learning patterns in moderate and severe traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 1994;16:498–507. doi: 10.1080/01688639408402661. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Steinberg JL, Troyanskaya M, Sharma RG, Rauch RA, et al. Working memory brain activation following severe traumatic brain injury. Cortex. 2007;43:95–111. doi: 10.1016/s0010-9452(08)70448-9. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D’Esposito M. Left prefrontal activation during episodic remembering: An event-related FMRI study. NeuroReport. 1998;9:3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Cole MA, Demery JA, Seignourel PJ, Dixit NK, Larson M, et al. Parametric manipulation of working memory load in traumatic brain injury: Behavioral and neural correlates. Journal of the International Neuropsychological Society. 2004;10:724–741. doi: 10.1017/S1355617704105110. [DOI] [PubMed] [Google Scholar]
- Primus EA, Bigler ED, Anderson CV, Johnson SC, Mueller RM, Blatter D. Corpus striatum and traumatic brain injury. Brain Injury. 1997;11:577–86. doi: 10.1080/026990597123278. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Nolde SF, D’Esposito M. FMRI investigations of left and right PFC to episodic remembering. Psychobiology. 2000;28:197–206. [Google Scholar]
- Ricker JH, Müller RA, Zafonte RD, Black KM, Millis SR, Chugani H. Verbal recall and recognition following traumatic brain injury: A [0–15] water positron emission tomography study. Journal of Clinical & Experimental Neuropsychology. 2001;23:196–206. doi: 10.1076/jcen.23.2.196.1204. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test (MAST): The quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning & Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton A. Neurosensory Center Comprehensive Examination for Aphasia. Victoria, BC: University of Victoria, Department of Psychology, Neuropsychology Laboratory; 1969. [Google Scholar]
- Strangman GE, Goldstein R, O’Neil-Pirozzi TM, Kelkar K, Supelana C, Burke D, et al. Neurophysiological alterations during strategy-based verbal learning in traumatic brain injury. Neurorehabilitation and Neural Repair. 2009;23:226–236. doi: 10.1177/1545968308324225. [DOI] [PubMed] [Google Scholar]
- Strangman GE, O’Neil-Pirozzi TM, Goldstein R, Kelkar K, Katz DI, Burke D, et al. Prediction of memory rehabilitation outcomes in traumatic brain injury by using functional magnetic resonance imaging. Archives of Physical Medicine and Rehabilitation. 2008;89:974–981. doi: 10.1016/j.apmr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: a 3-dimensional proportional system, an approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Teasdale G, Jennet B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:480–487. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tsirka V, Simos P, Vakis A, Vourkas M, Arzoglou V, Syrmos N, et al. Material-specific difficulties in episodic memory tasks in mild traumatic brain injury. International Journal of Neuroscience. 2010;120:184–91. doi: 10.3109/00207450903585308. [DOI] [PubMed] [Google Scholar]
- Turner GR, Levine B. Augmented neural activity during executive control processing following diffuse axonal injury. Neurology. 2008;71:812–818. doi: 10.1212/01.wnl.0000325640.18235.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. London: Psychological Corporation; 2001. [Google Scholar]
- Whyte J, Hart T, Laborde A, Rosenthal M. Rehabilitation issues in traumatic brain injury. In: DeLisa JA, et al., editors. Physical Medicine & Rehabiliation: Principles and Practice. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 1677–1713. [Google Scholar]
- Wiegner S, Donders J. Performance on the California Verbal Learning Test after traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 1999;21:159–170. doi: 10.1076/jcen.21.2.159.925. [DOI] [PubMed] [Google Scholar]
- Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Wolfe PL, Millis SR, Hanks R, Fichtenberg N, Larrabee GJ, Sweet JJ. Effort indicators within the California Verbal Learning Test-II (CVLT-II) The Clinical Neuropsychologist. 2010;24:153–168. doi: 10.1080/13854040903107791. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Schmitter-Edgecombe M, Woo E. Verbal memory impairment in severe closed head injury: The role of encoding and consolidation. Journal of Clinical and Experimental Neuropsychology. 2010;19:1–9. doi: 10.1080/13803390903512652. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaki T, Yoshino E, Fujimoto M, Ohmori Y, Imahori Y, Ueda S. Chronological positron emission tomographic study of severe diffuse brain injury in the chronic stage. Journal of Trauma—Injury, Infection, and Critical Care. 1996;40:50–56. doi: 10.1097/00005373-199601000-00010. [DOI] [PubMed] [Google Scholar]