Abstract
Inflammation plays a major role in the destruction of cartilage in osteoarthritis (OA), with the interaction of multiple mediators, immune cells, fibroblasts and chondrocytes. Current 2D studies in vitro with cell lines, as well as animal models, are limited in terms of providing insight into pathogenic mechanisms related to the human system. Hence, an in vitro human 3D cartilage tissue system was established to study the impact of inflammatory mediators on chondrocytes and matrices as an initial approach to emulating early stages of OA. An in vitro 3D humancartilage tissue system was established by culturing primary chondrocytes in silk protein porous scaffolds up to 21 days in static culture, with and without cytokine (IL-1β and TNF-α) exposure or with the use of macrophage conditioned medium (MCM). To assess chondrocyte responses, transcript levels, histology and immunohistochemistry were used to assess changes in cell viability and in cartilage matrix composition, including collagen type II and aggrecan. Chondrocyte hypertrophy and apoptosis were assessed via collagen type X and caspase-3. RT-PCR revealed that the cytokines and the MCM regulated matrix-related gene expression of chondrocytes, but with different outcomes. For anabolic-encoding genes, MCM suppressed collagen type II and up-regulated aggrecan. In contrast, the cytokines suppressed aggrecan formation and had no effect on collagen type II. For catabolic-encoded genes, both cytokines and MCM upregulated MMP1, MMP3, MMP13 and ADAMTS4, with cytokines preferentially upregulating MMP13 and MCM upregulating ADMTS4. MCM down-regulated ADAMTS5. In addition, MCM stimulation led to hypertrophy and apoptosis of chondrocytes, outcomes not found with the cytokine treatment group. A decrease in aggrecan content with cytokines and MCM stimulation was found, while MCM resulted in greater reduction than the cytokine treatment. The results demonstrated that OA-like features, such as changes in matrix synthesis gene expression, increase of collagense gene expression and loss of aggrecan, were initiated within this 3D chrondrocyte human tissue system upon stimulation of the cultures with cytokines and MCM. MCM was a better inducer of immune-related features of OA, because besides the features found with cytokine stimulation, the MCM treatment also initiated collagen X expression and deposition and apoptosis of chondrocytes, important features of human OA. The results obtained with this new in vitro tissue model provide an initial step towards the development of an early stage OA system to allow for more systematic study and insight into the origins and outcomes with this disease.
1. Introduction
Osteoarthritis (OA) is a major cause of disability during aging. By the age of 60, close to 100% of the population will have histological changes indicating degeneration of knee cartilage, and over 80% will have radiographic evidence of OA in at least one joint [1]. The mechanisms involved in osteoarthritis remain elusive. Animal models and monolayer cultured cells are most often used to study OA-related features. For animals, there are OA models such as the STR/ort mouse model [2]; genetic models such as knock-outs of matrix proteins [3, 4], knock-outs of signaling molecules [5, 6], and surgical instability induced OA models, such as joint damage by transsection of the collateral and anterior cruciate ligaments (ACL) [7, 8]. Animal models are limited in terms of utility due to differences with human systems, and primary tissues are limited by source and individual differences. Chondrocytes isolated from animal joints and cartilage tissue from surgery are also used for the study of OA in monolayer cultures [9]. 3D cell-cell and cell–extracellular matrix interactions [10], together with the development of central hypoxia [11], are important for the study of cartilage, and these conditions are poorly reflected by conventional two-dimensional (2D) cell culture systems. The inherent limitations of conventional methods, both in vitro and in vivo, prompt the need for improved options to study OA using 3D human tissue systems.
Biomaterials have been used to construct supporting scaffolds for cartilage, and each of the materials has advantages and limitations. For example, poly lactic acid (PLA), poly glycolic acid (PGA) and poly lactide-co-glycolide (PLGA), induce unfavorable inflammatory responses in vivo [12], and natural polymers like collagen and alginate are limited in utility due to rapid degradation or insufficient mechanical properties, and agarose is limited by poor biodegradability [13]. Silk protein scaffold systems provide mechanically robust, 3D matrices suitable for long term sustained culture due to the slow degradation of the protein matrix. Further, these scaffold systems demonstrate biocompatibility, utility in vitro, ease of reproduction, convenience, and can be sterilized by autoclaving [14]. We previously reported the formation of human cartilage tissue by combining either human mesenchymal stem cells (hMSCs) or primary chondrocytes with highly porous silk scaffolds [15, 16]. The silk scaffolds supported cells for at least 3 weeks in vitro, and also supported chondrognesis from hMSCs and the redifferentiation of primary chondrocytes. Cartilage-related transcripts were upregulated and cartilage-specific extracellular matrix (ECM0 deposition was characterized. Thus, tissue engineered human cartilage, as demonstrated previously using 3D porous silk scaffolds, was selected as a suitable starting point for the implementation of in vitro disease systems related to OA.
OA is associated with pain, the main clinical manifestation, as well as the structural deterioration of cartilage [17]. Thus a change of the main matrix components, collagen and aggrecan, in both quantity and quality are associated with the disease. Chondrocytes, as the main cell type in cartilage, are fully differentiated cells with low metabolic activity. During the development of OA, the equilibrium between metabolism and phenotype of chondrocytes changes. In early OA, there is evidence of increased matrix synthesis and degenerative activity of chondrocytes, including the upregulation of aggrecanase (ADAMTS) and collagenase (MMP), promoting the synthesis of matrix components such as collagen type II and IX [18]. At the same time, collagens which are not normally found in cartilage appear, including the hypertrophy marker collagen X, which reflects phenotypic modulation of chondrocytes. Apoptotic death of chondrocytes is involved in normal physiological processes for these cells, however, aberrant apoptosis contributes to cartilage degradation and has been implicated in the pathogenesis of OA [19].
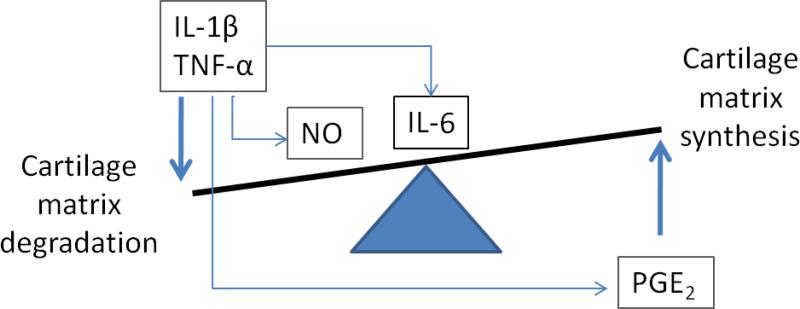
OA is frequently associated with inflammation [20]. In clinical studies, the presence of mononuclear cell infiltrates consisting of T cells and macrophages in the synovial membrane of patients with OA has been reported [21, 22]. Macrophages generate a broad spectrum of cytokines and immune factors including interleukin-1(IL-1), tumor necrosis factor (TNF), interleukin-6 (IL-6), prostaglandin-E2 (PGE2), nitric oxide (NO), interferon-alfa (IFN-α) and transforming growth factor-beta (TGF-β), with roles in OA development and progression [23-25]. Together with cytokines and chemokines produced by chondrocytes and synoviocytes, these inflammatory mediators participate in cartilage metabolism [26, 27] (Error! Reference source not found. Briefly, IL-1β, TNF-α alone or together improve collagenase and aggrecanase production, and also suppress condrocyte synthesis of aggrecan and type II collagen required to restore the ECM [27-30], resulting in the degradation of cartilage matrix. IL-6, a major target of IL-1, was upregulated slightly in OA cartilage [31], and was not capable of stimulating cartilage degradation directly. Modulation of both catabolic and anabolic activities of IL-6 has been shown, indicating that IL-6 may have dual roles in OA pathogenesis [27]. Nitric oxide, in response to pro-inflammatory cytokines, is a catabolic factor produced in OA, and promotes cartilage degradation by inhibiting collagen and aggrecan synthesis, activating MMPs, increasing susceptibility of chondrocytes to injury by other oxidants, and causing apoptosis [32]. NO may also play a protective role at certain levels. Like NO, PGE2 is also increased in OA, but unlike NO, it can reverse aggrecan degradation induced by IL-1β and stimulate collagen type II gene expression. Thus PGE2 is considered a positive regulator [33, 34]. In contrast, anti-anabolic effects of PGE2 on articular cartilage have been demonstrated [35].
Among mediators, IL-1β and TNF-α play important roles in the development and progression of OA [27, 36, 37]. IL-1β and TNF-α have been used as OA inducers in studies with chondrocytes in vitro or with cartilage tissue from joint replacement surgery [28, 38]. However, the development and progression of OA occurs in an environment where multiple mediators are active, and IL-1β and TNF-α alone can not fully represent the complex signaling generated by immune cells, activated chondrocytes and other cells found in the joint environment. Further, the impact of these mediators related to OA are likely subtle and long acting, thus sustained or long term tissue systems are essential to begin to elucidate cause and effect.
The goal in the present study was to begin to develop a more relevant 3D tissue system for OA, using tissue engineering and the combined effects of inflammatory mediators. Our hypothesis was that sustained 3D cartilage culture with human cells and interactions with cytokines as well as macrophage conditioned medium would simulate an inflammatory environment for OA formation. This system mimicked the inflammatory environment of OA cartilage with more than one cytokine, vs. traditional investigations. This in vitro OA tissue system may find utility in the study of OA formation and progression to aid in the investigation of the disease as well as for drug screening.
2. Materials and Methods
2.1. Silk purification and scaffold fabrication
Silk fibroin was purified and fabricated into 3-D scaffolds following our prior studies [39]. Briefly, to remove sericin, Bombyx mori cocoons were boiled in a solution of 0.02 M Na2CO3 for 30 min and then washed with distilled water. The extracted fibroin protein was then dissolved in 9.3M LiBr for 4 hours to yield a 15 w/v% silk solution. The LiBr was removed by dialysis against distilled water using Slide-a-Lyzer dialysis cassettes. The silk scaffolds were prepared by adding 4 g of granular NaCl (500-600 um) into 2 ml 6-8% silk solution in Teflon containers and then left at room temperature for 24 hrs. Subsequently, the NaCl particles were leached out by dissolving in distilled water. The scaffolds were punched into discs 5 mm in diameter and 3 mm thick. Scaffolds were autoclaved and conditioned with Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS) overnight prior to cell culture.
2.2. Chondrocyte culture and tissue engineered cartilage construction
Adult human knee chondrocytes (NHAC-kn) were obtained from Lonza (Walkersville, MD, USA). All cells were cultured in normal human chondrocyte cell growth medium (BulletKit® , Lonza) and maintained in 5% CO2 in a 37°C incubator. Before seeding into silk scaffolds, cells were sub-cultured 2-3 times to less than 85% confluency and the medium was changed twice a week. The cells were trypsinized with 0.25% trypsin/EDTA and resuspended. The cells, 1.5 million, were suspended in 30 ul medium and seeded into each scaffold evenly dropwise. To allow the cells to diffuse and attach, the scaffolds were incubated for 2.5 hours before 3 ml fresh medium was added. After 2 days, all cell-seeded scaffolds were transferred to chondrogenesis medium with chondrocyte differentiation basal medium, supplements and growth factors (R3-IGF-1, TGF-β1, transferrin, insulin, FBS and gentamicin/ amphotericin-B) (Chondrocyte Differentiation Medium BulletKit®, Lonza) and cultured for 4 weeks to generate tissue engineered cartilage. Medium was replaced twice a week.
2.3. THP-1 cell culturing and differentiation
Human acute monocytic leukemia cell line (THP-1) cells were obtained from ATCC (Manassas, VA) and cultured in medium with 90% RPMI 1640, 10% fetal bovine serum (FBS), 2.5 mM HEPEs, 1% Antibiotic-Antimycotic (Invitrogen, Carlsbad, CA) and 0.05 mM 2-mercaptoethanol (Sigma, St. Louis, MO). THP-1 cells were differentiated following routine protocols [40], and the dose of inducer was optimized by cell morphology and cytokine secretion (data not shown). Specifically, monocytes was induced in 6-well plate with 200 ng/ml PMA (phorbol 12-myristate-13-acetate) for 6 days; each well contained 0.3 million cells. Cells were cultured fresh when a 6-day conditioned medium sample was needed for chondrocyte treatment during a period of 3 weeks.
2.4. Cytokines and macrophage conditioned medium
After 6 days of PMA induction, 0.3 million THP-1 cells secrete TNF-α and IL-1β in concentrations of about 500 pg/ml, thus we added a 0.5 ml aliquot of monocyte conditioned medium to 4.5 ml of TE cartilage culture medium for each scaffold, to yield a final concentration of 50 pg/ml in TNF-α and 50 pg/ml in IL-1β. Correspondingly, the same dose of recombinant cytokines TNF-α (cat # 210-TA/CF, lot # AA3308062, R & D system, Minneapolis, MN) and IL-1β (Prod# RIL1BI, lot # JG125416, Pierce Biotech, Rockford, IL) were added to the culture medium directly. All supplements were refreshed when medium was replaced twice a week.
2.5. Total RNA extraction, reverse transcription and RT-PCR analysis
Whole scaffolds were put into 0.5 ml Trizol (Invitrogen, Carlsbad, CA) and after homogenization, the RNA was extracted using phase separation with chloroform (Sigma, St. Louis, MO) in a volume ratio of 5:1 with the Trizol. Total RNA was purified using an RNeasy Mini kit (Qiagen, Valencia, CA, USA) following the protocol supplied. cDNA was reverse transcribed from RNA using High-Capacity cDNA archive kits (Applied Biosystems, Carlsbad, CA) according to standard protocol from the vendor. TaqMan Gene Expression assay kits (Applied Biosystems, Carlsbad, CA) were used to analyze transcription levels of matrix-related and remodeling genes including: collagen type II (NM_001844), ACAN (NM_013227.2), Col type X (NM_000493.3), MMP1 (NM_002421.2), MMP3 (NM_002422.3), MMP13 (NM_002427.2), ADAMTS4 (NM_005099.4), and ADAMTS5 (NM_007038.3). RT-PCR processing and final data analysis were conducted using Mx3000 and Mxpro™ QPCR software, version 4.01 (Stratagene, La Jolla, CA). Gene expression was normalized to GAPDH (NM_002046.3).
2.6. Immunohistochemistry
After 1 week and 3 weeks of treatment, the TE cartilage were collected in 4% formalin, and processed and stained following standard procedures. Briefly, samples were dehydrated through a series of gradient alcohols, embedded in paraffin and sectioned at 5 um thickness and then rehydrated through a series of gradient alcohols. A primary antibody of caspase-3 (Abcam, Cambridge MA USA) and collagen II (Santa Cruz, CA USA) were incubated on sections separately, followed by mouse HRP-labeled secondary antibody. Sections were counterstained with hematoxylin.
2.7. sGAG and DNA quantification
sGAG was analyzed by using a Sulfated Glycosaminoglycan Assay (Blyscan™ Kit, Biocolor UK) following the protocol provided. Briefly, 125 μg/mL of papain solution was added to samples and incubated in 60°C for 24 hrs. Subsequently, dye was added into the samples and mixed for 30 minutes to form a glycosaminoglycan-dye complex. After centrifugation and removing unbound dye, the insoluble pellet was re-dissolved and absorbance was read at 656 nm by a microplate reader (VERSAmax microplate reader, Molecular Devices). Correspondingly, DNA from the same sample was quantified with Picogreen kit (Invitrogen, Carlsbad, CA) following the protocol provided. Samples were read at 480 nm/528 nm in the micxroplate reader (VERSAmax), and DNA concentration was determined by standard curve.
2.8. ELISA
To confirm IL-1β TNF-α and PGE2 concentrations in culture, ELISA kits (B&D system, Sparks, MD) were used following the protocols provided. Briefly, supernatant taken from tissue culture media were added into antibody pre-coated microplates and incubated for 1 hour, then enzyme-linked polyclonal antibody specific for target molecules was added to the well, followed by a substrate solution for color development. At each step, unbound material and antibody-enzyme were washed away. Color intensity was measured at 450 nm by a microplate reader (VERSAmax), with a wavelength correction set to 540 nm.
2.9. Measurement of Nitric Oxide
Supernatants from THP-1 cultures were collected after 6 days of inducing in 200 ng/ml PMA, and nitrite was assayed with the Griess reagent kit (Molecular Probes, Eugene, OR). Briefly, a 150ul aliquot of culture supernatant was mixed with 20 ul of Griess reagent (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride and 1% sulfanilic acid in 5% phosphoric acid) and diluted with 130ul of deionized water. Absorbance was measured at 548 nm and the amount of nitrite in the sample was calculated from a sodium nitrite standard curve.
2.10. Statistical analysis
Results are presented as means +/-SD with N≥3. Unless otherwise noted, one-way ANOVA was used for comparisons assuming equal variances. P<0.05 was considered statistically significant.
3. Results
3.1. Proinflammatory factor release
THP-1 cells were differentiated with 200 ng/ml PMA for 6 days. After secretion of proinflammatory factors, including TNF-α, IL-1β, PGE2 and NO, each factor was determined separately. In 5 ml of differentiation medium there were 504.1±42.24 pg/ml TNF-α, 491.28±31.79 pg/ml IL-1 β, 68.54±10.11pg/ml PGE2 and 0.39±0.06 uM/ml NO, respectively (Error! Reference source not found.)
3.2. Type II collagen expression
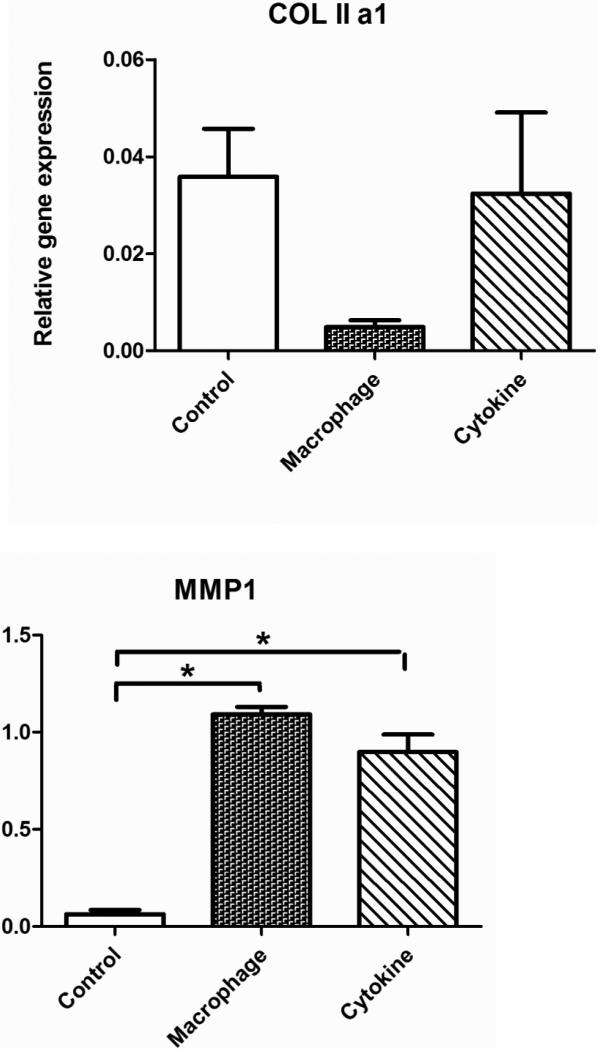
Collagen expression is regulated by an overbalance of cytokines, and we found that collagen level was regulated by cytokine culture and MCM differently in the TE cartilage (Error! Reference source not found.). Specifically, at the transcriptional level, thecollagen type II gene was down-regulated in the MCM treatment group but not in the cytokine treatment group; the cytokine treatment resulted in a dramatic up-regulation of collagenase encoding genes, including MMP1, MMP3, MMP13. There was no statistical difference in MMP1 and MMP13 regulation between the cytokine and MCM treatment groups, but the cytokine treatment had a more significant effect on MMP3 up-regulation. With immunohistochemistry, no obvious qualitative differences in collagen type II levels were observed between the tissue engineered cartilage study groups with cytokines or MCM stimulation, or in the control without treatment (Error! Reference source not found.Error! Reference source not found.).
3.3. sGAG expression
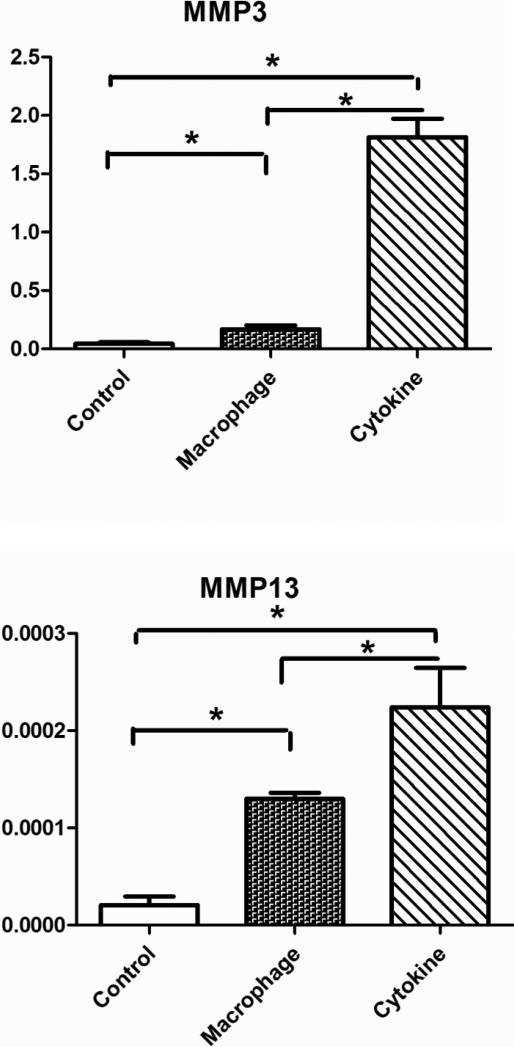
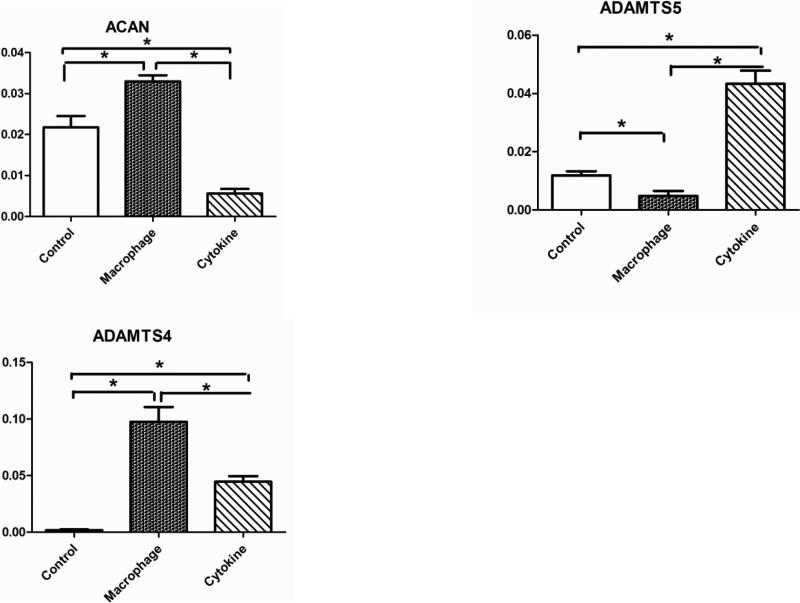
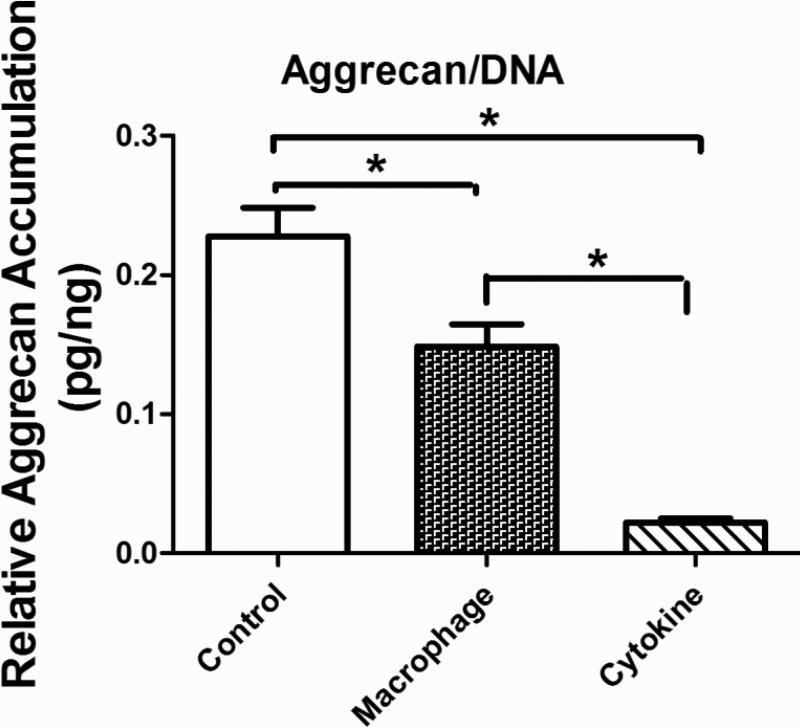
At the transcrip level, aggrecan (ACAN) was down-regulated in the cytokine treatment group but up-regulated in the MCM treatment group (Error! Reference source not found.). The cytokine treatment resulted in a dramatic up-regulation of aggrecanase encoding genes ADAMTS4, and ADAMTS5 and the MCM treatment had a greater effect on ADAMTS4. When normalized by cell number both the MCM and cytokine treatment groups showed a reduction in aggrecan content after 3 weeks of stimulation (Error! Reference source not found.Error! Reference source not found.).
3.4. Chondrocyte hypertrophy
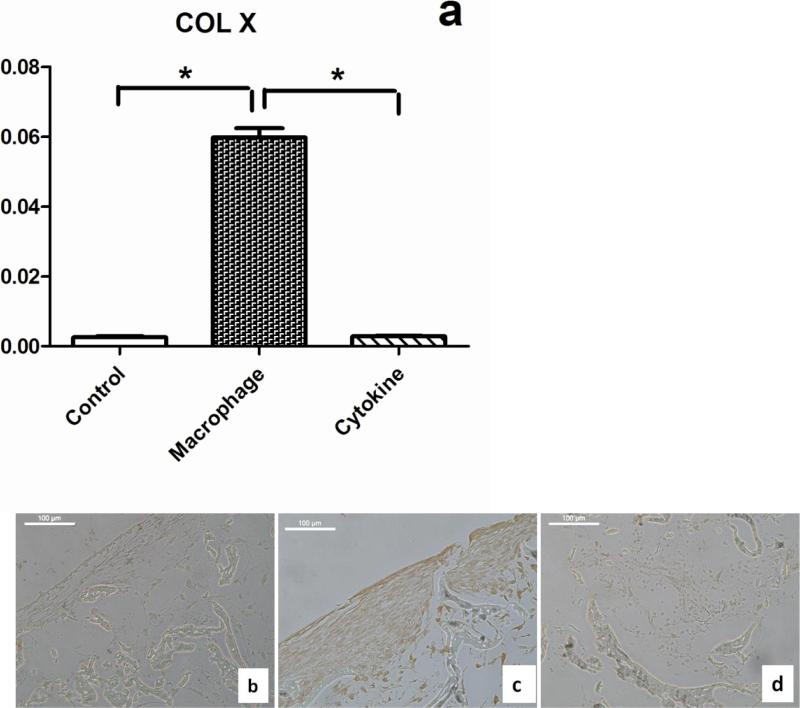
Hypertrophy is found in the lower zone of osteoarthritis cartilage [41] and is the main force driving endochondral ossification. Therefore, this marker is used histopathology of cartilage from OA patients to grade disease severity. Thus we examined collagen types X expression in both MCM and cytokine treatment groups in the present study. The cytokine treatment did not cause a significant change in type X collagen expression at the transcript level compared to the control group, but the MCM treatment up-regulated expression in chondrocytes significantly (21±3 fold) (Error! Reference source not found.a). Furthermore, collagen type X expression was confirmed at the protein level by immunohistology staining (Error! Reference source not found.b,c,d).
3.5. Apoptosis
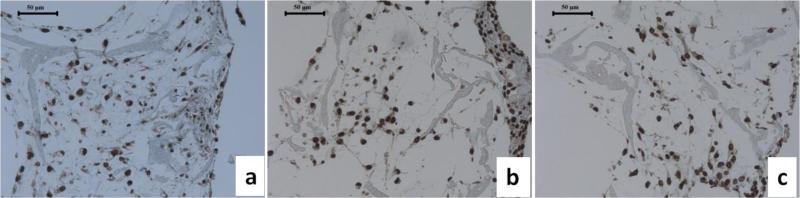
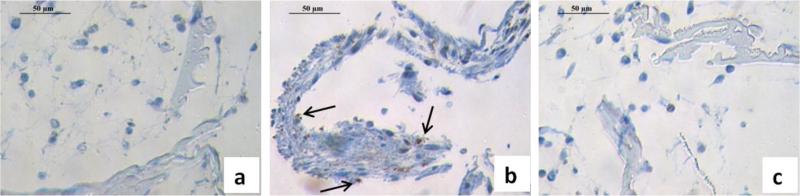
Chondrocyte death has been reported in OA joints and most studies have reported increased apoptotic cell death. This outcome is recognized as a central feature associated with the failure of cartilgae structure and function in OA [42-45]. After 3 weeks of countinuous stimulation, apoposis, as measured by caspase-3 positive staining, was found in the MCM treatment group (Error! Reference source not found.b), but not in the control and cytokine treatment groups (Error! Reference source not found.a,c).
4. Discussion
Synovial inflammation likely contributes to the dysregulation of chondrocyte function, favoring changes between catabolic and anabolic activities during cartilage metabolism and remodeling [18]. The transcriptional levels of matrix-remodeling genes were differently regulated by the MCM and the cytokine treatments in the cartilage constructs. IL-1β and TNF-α affect the activity of chondrocytes by suppressing the synthesis of aggrecan and type II collagen, as reported for articular chondrocytes [28, 29]. In the present study, a decrease of aggrecan in the cytokine treatment group was observed, but suppression of collagen type II did not occur. A possible explanation could be that IL-1β can induce PGE2 expression from chondrocytes, and PGE2 opposes the inhibitory effects of IL-1 on matrix synthesis by stimulating type II collagen expression [20] (Figure 1). For the suppression of collagen type II in the MCM group, NO could play a key role. NO is present at high levels in OA cartilage [46], and has been shown to inhibit the synthesis of collagen type II and aggrecan [47], independent of PGE2. A slight increase in aggrecan expression in the MCM treatment group could be due to the effect from PGE2, which was reported to improve aggrecan synthesis in vitro [48].
Figure 1.
Scheme for role of inflammatory mediators in cartilage matrix metabolism
It has been documented that the production of MMPs is stimulated by TNF-α and IL-1β [49], which explains the up-regulation of all MMPs in the cytokine treatment group. However, factors like PGE2 play a compensating role on cytokine effects, especially IL-1β. PGE2 caused the down-regulation of IL-1-induced MMP-3 production in fibroblasts [50]. Thus differential regulation of MMP3 in the MCM treatment group could be a result of combined mediator activity. ADAMTS4 and ADAMTS5 enzymes were identified as highly selective aggrecanases induced by proinflammatory cytokines [51]. In the present cartilage system the up-regulation of ADAMTS4 and ADAMTS5 with cytokine treatment is consistent with previous in vitro studies with human explants [52]. Compared with the cytokine treatment, the MCM study group induced a down-regulation of ADAMTS5 in the cartilage (Error! Reference source not found.). Although both ADAMTS4 and ADAMTS5 are known to constitute early events in osteoarthritic cartilage damage [53], research has shown that their regulation and function are different [54-56]. Previous human data has shown that there is predominant expression of ADAMTS4 in osteoarthritic cartilage, while ADAMTS5 is constitutively expressed in osteoarthritic and normal cartilage [57], which is more closely akin to the MCM treatment results shown here.
The RT-PCR results demonstrated that cytokine stimulation induced catabolic transcripts related to collagen II by upregulating collagenase MMP1, 3 and 13, with no effect on anabolic transcript for collagen type II (Error! Reference source not found. COL IIa1, Error! Reference source not found. MMP1,3,13). In contrast, MCM stimulation suppressed gene collagen II transcript levels but with a reduction in MMP3 to a lesser extent compared with the cytokine stimulation study group (Error! Reference source not found. COL IIa1, Error! Reference source not found. MMP1,3,13). Therefore, the cytokine and MCM treatments could result in a similar level of collagen type II turnover, but through different mechanisms. In addition, the accumulation of collagen type II in TE cartilage was lower than in native cartilage tissue due to the limited secretion function of primary chondrocytes in vitro, an issue that should be addressed in future studies. Thus, a significant change in type II collagen protein levels was difficult to detect.
The finding of a reduction in aggrecan content after 3 weeks of stimulation is consistent with previous reports from OA patients and from in vitro studies [34, 58, 59]. More specifically, the cytokine treatment showed a further decrease in aggrecan content when compared with the MCM group. In RT-PCR results, ACAN expression was down-regulated and ADAMTS4,5 upregulated in the cytokine treatment group, thus the net effect of cytokine treatment was a statistically significant decrease in aggrecan, consistent with the aggrecan quantification results. For the MCM treatment group, the decrease in aggrecan was moderate compared with the cytokine group (Error! Reference source not found.), which is due to the competing effects from both aggrecan synthesis and degradation. The RT-PCR results showed that expression of ACAN was upregulated by treatment with MCM and ADAMTS5 was dramatically down-regulated, both leading to the anabolism of aggrecan. ADAMTS4 was upregulated, which suppressed the anabolism activity. Thus interacting mechanisms result in remodeling of this cartilage component by degradation of aggreacan and replacement with newly synthesized aggrecan. At a certain stage in the progression of OA, the chondrocytes are unable to compensate for aggrecan loss even in the presence of increased synthesis, resulting in a net loss of matrix [60, 61]. In this study, the transcript results from the MCM treatment supported this mechanism.
Phenotypic modulation of chondrocytes in early stages of OA is reflected by the presence of collagen not normally found in cartilage, including the hypertrophic chondrocyte marker, type X collagen [18]. There is no literature showing that IL-1β or TNF-α induce collagen X expression from chondrocytes, consistent with the cytokine treatment group in the present study. Other inflammation mediators secreted by macrophages can affect collagen X expression and hypertrophy in cartilage, including NO, PGE2, and TGF-β. In previous studies, the expression and localization of iNOS showed an association with NO and hypertrophy in human chondrocytes [62], which suggested NO as an inducer of hypertrophy in chondrocytes. In contrast, PGE2 inhibited chondrocyte collagen X expression and suppressed hypertrophy [63, 64]. Our results confirmed that IL-1β or TNF-α are not initial factors responsible for collagen X expression in OA-related tissue systems, extrapolating from the current tissue system as a test model. Since NO and PGE2 play opposite roles, together with other existing mediators in MCM, the synergy and competition between these factors results in an acceleration of collagen X expression in the cartilage studied here.
The effects of IL-1β and TNF-α on chondrocyte apoptosis are debatable. In some studies, chondrocytes are resistant to induction of apoptosis by IL-1 treatment alone [65, 66], however, conflicting data have shown IL-1β-induced apoptosis in human articular chondrocytes [67]. There is increasing evidence that excess NO is important in the pathology of OA; regulating chondrocyte apoptosis as one function [65, 68]. Both chondrocytes and synoviocytes can produce NO, and previous research has shown that exogenous NO produced by synoviocytes will induce chondrocyte apoptosis [19,64,69], with possible mechanisms of reduing mitochondrial potential, ATP generation, and increasing caspase activity [70, 71]. Thus the role of NO on tissue engineered chondrocytes is as an inducer of apoptosis [34]. The function of PGE2 on chondrocytes apoptosis is unclear. Some research has indicated that PGE2 had no effect on apoptosis by itself, but enhanced NO-induced apoptosis in chondrocytes [72]. Other data indicated that PGE2 induced chondrocyte apoptosis in vitro via a cAMP-dependent pathway [73]. The data in the present study demonstrated that the addition of IL-1β and TNF-α, without other inflammatory factors, did not lead to apoptosis of chondrocytes in the TE cartilage, thus the presence of NO, PGE2 and other mediators in MCM must play a crucial role in the apoptosis induction process. In addition, since chondrocytes can also produce NO and PGE2 in response to some inflammatory factors, in future studies these mediators should be monitored in the culture medium to better elucidate their roles on chondrocyte apoptosis and the initiation of other OA-like features.
The data indicate that chondrocytes responded to both cytokine and MCM induced effects in the TE cartilage systems studied here, and matrix turnover related to the disease condition is at least mimicked in part in these early stages of study. Chondrocytes respond differently to the cytokines and MCM treatments due to the complexity of impacts of inflammation mediators in the MCM. Table 3 shows a systematic comparison of matrix-remodeling gene expression and cell responses comparing in vivo, 2D culture systems and 3D systems. The data show that traditional 2D cultures are sufficient for studying cell response to cytokines, but limited in the study of ECM and the dynamic process involved. Moreover, the comparisons suggest that the tissue engineered cartilage stimulated with MCM was a more appropriate tissue model to use to study certain aspects of OA progression, such as the impacts due to NO and PGE2.
Table 3.
Changes of OA markers in 2D cell culture studies, in vivo studies, and in the present in vitro systems
| OA markers | In vivo cartilage study | 2D culture w/ | Current study - in | ||
|---|---|---|---|---|---|
| Early stage | Late stage | TNF-α/IL-1 | MCM | Cytokines | |
| MMP1 | ↑[74] | ↓[75] | ↑[76] | ↑ | ↑ |
| MMP3 | ↑[74, 77] | ↓[74, 76, 77] | ↑[76, 78] | ↑ | ↑ |
| MMP13 | X [76] | ↑[75-77] | ↑[76, 78] | ↑ | ↑ |
| ACAN | ↑,↓[79, 80] | ↓[79] | ↓ [81, 82] | ↑ | ↓ |
| COL II | ↑ [83] | ↓↑[77, 79] | ↓ [28, 81, 82] | ↓ | ↑ |
| ADAMTS4 | ↑[57] | ↑ [75] | ↑[76] | ↑ | ↑ |
| ADAMTS5 | →[57] | →↑[57, 75] | →[56] | ↓ | ↑ |
| Loss of COL II | √ [18] | √[18] | Not applicable | X | X |
| Loss of sGAG | √ [18] | √[58] | Not applicable | √ | √ |
| hypertrophy | √ [18, 41] | X [84] | no ref found | √ | X |
| Chondrocyte apoptosis | √ Progressive | X no ref found | √ [85], X [66] | √ | X |
(↑ increase, ↓decrease, →consistent in gene level, √ detectable, X not detectable, -- no change).
5. Conclusions
An in vitro human cartilage 3D tissue system was developed to allow the study of the combined effects of inflammatory factors and chondrocyte functions in a controlled and systematic fashion. Silk porous scaffolds provided a compatible environment for three-dimensional tissue engineered cartilage formation, and in the presence of cytokines the tissue engineered cartilage showed some OA-like phenotype. The tissue engineered cartilage was induced with a proinflammatory environment, a macrophage conditioned medium containing the full spectrum of cytokines, growth factors, small molecules and other mediators, to mimic the in vivo situation. The regulation and remodeling of chondrocyte extracellular matrix was influenced by multiple immune mediators and their balance in the MCM treatment resulted in different outcomes than the cytokine-specific simulation, which is a closer approximation to early stage symptoms of OA. The results reported here set the stage for future studies where mechanical forces, dose dependent effect of inflammatory mediators, longer term cultivation and hypoxia can be explored related to OA. In the long term, a 3D tissue system such as that initiated here would offer a useful platform to study disease formation, progression and modes of intervention
Figure 2.
Transcriptional levels of human collagen type II and collagenase MMP1, MMP3, MMP13 in TE cartilage without stimulation (control), with stimulation by macrophage conditioned medium (macrophage) or with cytokine additions to the medium (cytokines) for 1 week. Significant differences between groups at p<0.05.
Figure 3.
Histology of cross-sections of tissue engineered cartilage with different study groups on collagen type II expression at 3 weeks stimulation: (a) control, (b) MCM stimulation (c) cytokine stimulation. Scale bars, 50 um.
Figure 4.
Transcriptional levels of human aggrecan and aggrecanases ADAMTS4, ADAMTS5 in tissue engineered cartilage without stimulation (control), with stimulation by macrophage conditioned medium (macrophage) or with cytokine additions to the medium (cytokines) for 1 week. Significant differences between groups at p<0.05.
Figure 5.
Accumulation of sGAG quantified by sulfated glycosaminoglycan assay in the group without treatment (Control), macrophage conditioned medium stimulation group (macrophage) and cytokine stimulation group (cytokines), normalized by DNA content at 3 weeks stimulation. Significant differences between groups at p<0.05
Figure 6.
Hypertrophy markers in chondrocytes. (a) Transcript level of collagen X in no stimulation group (control) macrophage conditioned medium treated group (Macrophage) and cytokines stimulation groups (Cytokines) after 1 week treatment. *Significant differences between groups at p<0.05. (b) Collagen X immunohistology staining of cross-sections of the cartilage with the different stimulation conditions, (b) Control group without stimulation, (c) with MCM stimulation, and (d) with cytokines stimulation. Scale bars, 100 um
Figure 7.
Histological analysis of apoptosis (brown dots are positive hypoxyprobe staining) in tissue engineered cartilage without stimulation (a) after stimulation with macrophage conditioned medium, and (b) cytokines (c) for 3 weeks. Scale bars, 50 um.
Table 1.
THP-1 derived macrophage secretion of inflammatory factors
| Proinflammatory | Concentration in macrophage |
|---|---|
| TNF-α | 504.1±42.24 pg/ml |
| IL-1β | 491.28±31.79 pg/ml |
| PGE2 | 68.54±10.11 pg/ml |
| NO | 0.39±0.06 uM/ml |
Table 2.
Study groups and abbreviations
| No proinflammatory factor added | Control |
| Macrophage conditioned medium stimulation, added 0.5 ml MCM to 4.5 ml culture medium | Macrophage |
| Cytokine stimulation, added 50 pg/ml TNF-α, on d 5 0 pg/m l IL-1 β to culture medium | Cytokine |
Acknowledgements
The authors thank Annette Shepard-Barry in tufts medical center for her help on histology and immunohistochemistry. We thank the National Institutes of Health (EB002520) Tissue Engineering Resource Center for support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loeser RF., Jr Aging and the etiopathogenesis and treatment of osteoarthritis. Rheum Dis Clin North Am. 2000;26:547–67. doi: 10.1016/s0889-857x(05)70156-3. [DOI] [PubMed] [Google Scholar]
- 2.Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;65:1414–21. doi: 10.1136/ard.2005.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–16R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 4.Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, et al. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum. 2006;54:2891–900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- 5.de Hooge AS, van de Loo FA, Bennink MB, Arntz OJ, de Hooge P, van den Berg WB. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage. 2005;13:66–73. doi: 10.1016/j.joca.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, et al. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2:e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appleton CT, McErlain DD, Pitelka V, Schwartz N, Bernier SM, Henry JL, et al. Forced mobilization accelerates pathogenesis: characterization of a preclinical surgical model of osteoarthritis. Arthritis Res Ther. 2007;9:R13. doi: 10.1186/ar2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts MJ, Adams SB, Jr., Patel NA, Stamper DL, Westmore MS, Martin SD, et al. A new approach for assessing early osteoarthritis in the rat. Anal Bioanal Chem. 2003;377:1003–6. doi: 10.1007/s00216-003-2225-2. [DOI] [PubMed] [Google Scholar]
- 9.Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of MMP-13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–71. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirsch T. Cell-cell and cell-matrix interactions during development and pathogenesis. Current Opinion in Orthopedics. 2006;17:387–9. [Google Scholar]
- 11.Pfander D, Gelse K. Hypoxia and osteoarthritis: how chondrocytes survive hypoxic environments. Curr Opin Rheumatol. 2007;19:457–62. doi: 10.1097/BOR.0b013e3282ba5693. [DOI] [PubMed] [Google Scholar]
- 12.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 13.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–82. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Kim UJ, Blasioli DJ, Kim HJ, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082–94. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Blasioli DJ, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006;27:4434–42. doi: 10.1016/j.biomaterials.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 17.Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332:639–42. doi: 10.1136/bmj.332.7542.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 19.Goggs R, Carter SD, Schulze-Tanzil G, Shakibaei M, Mobasheri A. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. Vet J. 2003;166:140–58. doi: 10.1016/s1090-0233(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 20.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–7. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haywood L, McWilliams DF, Pearson CI, Gill SE, Ganesan A, Wilson D, et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–7. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- 22.Sakkas LI, Platsoucas CD. The role of T cells in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:409–24. doi: 10.1002/art.22369. [DOI] [PubMed] [Google Scholar]
- 23.Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004:S37–46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- 24.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pujol JP, Chadjichristos C, Legendre F, Bauge C, Beauchef G, Andriamanalijaona R, et al. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res. 2008;49:293–7. doi: 10.1080/03008200802148355. [DOI] [PubMed] [Google Scholar]
- 26.Borzi RM, Mazzetti I, Marcu KB, Facchini A. Chemokines in cartilage degradation. Clin Orthop Relat Res. 2004:S53–61. doi: 10.1097/01.blo.0000143805.64755.4f. [DOI] [PubMed] [Google Scholar]
- 27.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–65. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 28.Goldring MB, Birkhead J, Sandell LJ, Kimura T, Krane SM. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988;82:2026–37. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson DW, Dodge GR. Effects of interleukin-1beta and tumor necrosis factor-alpha on expression of matrix-related genes by cultured equine articular chondrocytes. Am J Vet Res. 2000;61:624–30. doi: 10.2460/ajvr.2000.61.624. [DOI] [PubMed] [Google Scholar]
- 30.Aigner T, Soeder S, Haag J. IL-1beta and BMPs--interactive players of cartilage matrix degradation and regeneration. Eur Cell Mater. 2006;12:49–56. doi: 10.22203/ecm.v012a06. discussion. [DOI] [PubMed] [Google Scholar]
- 31.Fan Z, Soder S, Oehler S, Fundel K, Aigner T. Activation of Interleukin-1 Signaling Cascades in Normal and Osteoarthritic Articular Cartilage. Am J Pathol. 2007;171:938–46. doi: 10.2353/ajpath.2007.061083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scher JU, Pillinger MH, Abramson SB. Nitric oxide synthases and osteoarthritis. Curr Rheumatol Rep. 2007;9:9–15. doi: 10.1007/s11926-007-0016-z. [DOI] [PubMed] [Google Scholar]
- 33.Nedelec E, Abid A, Cipolletta C, Presle N, Terlain B, Netter P, et al. Stimulation of cyclooxygenase-2-activity by nitric oxide-derived species in rat chondrocyte: lack of contribution to loss of cartilage anabolism. Biochem Pharmacol. 2001;61:965–78. doi: 10.1016/s0006-2952(01)00559-7. [DOI] [PubMed] [Google Scholar]
- 34.Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–9. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60:513–23. doi: 10.1002/art.24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Towle CA, Hung HH, Bonassar LJ, Treadwell BV, Mangham DC. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage. 1997;5:293–300. doi: 10.1016/s1063-4584(97)80008-8. [DOI] [PubMed] [Google Scholar]
- 37.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004:S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 38.Dodge GR, Poole AR. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989;83:647–61. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–85. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 40.Park EK, Jung HS, Yang HI, Yoo MC, Kim C, Kim KS. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res. 2007;56:45–50. doi: 10.1007/s00011-007-6115-5. [DOI] [PubMed] [Google Scholar]
- 41.Girkontaite I, Frischholz S, Lammi P, Wagner K, Swoboda B, Aigner T, et al. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol. 1996;15:231–8. doi: 10.1016/s0945-053x(96)90114-6. [DOI] [PubMed] [Google Scholar]
- 42.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–9. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 43.Johnson EO, Charchandi A, Babis GC, Soucacos PN. Apoptosis in osteoarthritis: morphology, mechanisms, and potential means for therapeutic intervention. J Surg Orthop Adv. 2008;17:147–52. [PubMed] [Google Scholar]
- 44.Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8:333–45. doi: 10.2174/138945007779940025. [DOI] [PubMed] [Google Scholar]
- 45.Aigner T, Kim HA, Roach HI. Apoptosis in osteoarthritis. Rheum Dis Clin North Am. 2004;30:639–53. xi. doi: 10.1016/j.rdc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Karan A, Karan MA, Vural P, Erten N, Tascioglu C, Aksoy C, et al. Synovial fluid nitric oxide levels in patients with knee osteoarthritis. Clin Rheumatol. 2003;22:397–9. doi: 10.1007/s10067-003-0761-y. [DOI] [PubMed] [Google Scholar]
- 47.Mastbergen SC, Bijlsma JW, Lafeber FP. Synthesis and release of human cartilage matrix proteoglycans are differently regulated by nitric oxide and prostaglandin-E2. Ann Rheum Dis. 2008;67:52–8. doi: 10.1136/ard.2006.065946. [DOI] [PubMed] [Google Scholar]
- 48.El Hajjaji H, Marcelis A, Devogelaer JP, Manicourt DH. Celecoxib has a positive effect on the overall metabolism of hyaluronan and proteoglycans in human osteoarthritic cartilage. J Rheumatol. 2003;30:2444–51. [PubMed] [Google Scholar]
- 49.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 50.Ruwanpura SM, Noguchi K, Ishikawa I. Prostaglandin E2 regulates interleukin-1beta-induced matrix metalloproteinase-3 production in human gingival fibroblasts. J Dent Res. 2004;83:260–5. doi: 10.1177/154405910408300315. [DOI] [PubMed] [Google Scholar]
- 51.Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–52. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 52.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–85. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 53.Bondeson J, Wainwright S, Hughes C, Caterson B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin Exp Rheumatol. 2008;26:139–45. [PubMed] [Google Scholar]
- 54.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–6. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 56.Fosang AJ, Rogerson FM, East CJ, Stanton H. ADAMTS-5: the story so far. Eur Cell Mater. 2008;15:11–26. doi: 10.22203/ecm.v015a02. [DOI] [PubMed] [Google Scholar]
- 57.Naito S, Shiomi T, Okada A, Kimura T, Chijiiwa M, Fujita Y, et al. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathol Int. 2007;57:703–11. doi: 10.1111/j.1440-1827.2007.02167.x. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–35. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 59.Block E, Stuhlsatz H, Leur E, Greiling H. 016 Influence of cytokines on the biosynthesis of proteoglycans in human chondrocytes. Fresenius’ Journal of Analytical Chemistry. 1992;343:67–8. [Google Scholar]
- 60.Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22:351–84. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Abramson SB. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res Ther. 2008;10(Suppl 2):S2. doi: 10.1186/ar2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishida K, Doi T, Matsuo M, Ishiwari Y, Tsujigiwa H, Yoshida A, et al. Involvement of nitric oxide in chondrocyte cell death in chondro-osteophyte formation. Osteoarthritis Cartilage. 2001;9:232–7. doi: 10.1053/joca.2000.0380. [DOI] [PubMed] [Google Scholar]
- 63.Li TF, Zuscik MJ, Ionescu AM, Zhang X, Rosier RN, Schwarz EM, et al. PGE2 inhibits chondrocyte differentiation through PKA and PKC signaling. Exp Cell Res. 2004;300:159–69. doi: 10.1016/j.yexcr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 64.Tchetina EV, Di Battista JA, Zukor DJ, Antoniou J, Poole AR. Prostaglandin PGE2 at very low concentrations suppresses collagen cleavage in cultured human osteoarthritic articular cartilage: this involves a decrease in expression of proinflammatory genes, collagenases and COL10A1, a gene linked to chondrocyte hypertrophy. Arthritis Res Ther. 2007;9:R75. doi: 10.1186/ar2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146:75–85. [PMC free article] [PubMed] [Google Scholar]
- 66.Kuhn K, Hashimoto S, Lotz M. IL-1 beta protects human chondrocytes from CD95-induced apoptosis. J Immunol. 2000;164:2233–9. doi: 10.4049/jimmunol.164.4.2233. [DOI] [PubMed] [Google Scholar]
- 67.Fischer BA, Mundle S, Cole AA. Tumor necrosis factor-alpha induced DNA cleavage in human articular chondrocytes may involve multiple endonucleolytic activities during apoptosis. Microsc Res Tech. 2000;50:236–42. doi: 10.1002/1097-0029(20000801)50:3<236::AID-JEMT7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 68.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 69.Wu GJ, Chen TG, Chang HC, Chiu WT, Chang CC, Chen RM. Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J Cell Biochem. 2007;101:1520–31. doi: 10.1002/jcb.21268. [DOI] [PubMed] [Google Scholar]
- 70.Svetlana Krasnokutsky JS, Steven B. Abramson. Osteoarthritis in 2007 Bulletin of the NYU Hospital for Joint Diseases. 2007;65:222–8. [PubMed] [Google Scholar]
- 71.Maneiro E, Lopez-Armada MJ, de Andres MC, Carames B, Martin MA, Bonilla A, et al. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann Rheum Dis. 2005;64:388–95. doi: 10.1136/ard.2004.022152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martel-Pelletier J, Pelletier JP, Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003;33:155–67. doi: 10.1016/s0049-0172(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 73.Miwa M, Saura R, Hirata S, Hayashi Y, Mizuno K, Itoh H. Induction of apoptosis in bovine articular chondrocyte by prostaglandin E(2) through cAMP-dependent pathway. Osteoarthritis Cartilage. 2000;8:17–24. doi: 10.1053/joca.1999.0266. [DOI] [PubMed] [Google Scholar]
- 74.Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56:542–9. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davidson RK, Waters JG, Kevorkian L, Darrah C, Cooper A, Donell ST, et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther. 2006;8:R124. doi: 10.1186/ar2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–57. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 77.Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44:2777–89. doi: 10.1002/1529-0131(200112)44:12<2777::aid-art465>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 78.Flannery CR, Little CB, Caterson B, Hughes CE. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 1999;18:225–37. doi: 10.1016/s0945-053x(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 79.Eid K, Thornhill TS, Glowacki J. Chondrocyte gene expression in osteoarthritis: Correlation with disease severity. J Orthop Res. 2006;24:1062–8. doi: 10.1002/jor.20137. [DOI] [PubMed] [Google Scholar]
- 80.Aurich M, Squires GR, Reiner A, Mollenhauer JA, Kuettner KE, Poole AR, et al. Differential matrix degradation and turnover in early cartilage lesions of human knee and ankle joints. Arthritis Rheum. 2005;52:112–9. doi: 10.1002/art.20740. [DOI] [PubMed] [Google Scholar]
- 81.Reginato AM, Sanz-Rodriguez C, Diaz A, Dharmavaram RM, Jimenez SA. Transcriptional modulation of cartilage-specific collagen gene expression by interferon gamma and tumour necrosis factor alpha in cultured human chondrocytes. Biochem J. 1993;294(Pt 3):761–9. doi: 10.1042/bj2940761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldring MB, Fukuo K, Birkhead JR, Dudek E, Sandell LJ. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem. 1994;54:85–99. doi: 10.1002/jcb.240540110. [DOI] [PubMed] [Google Scholar]
- 83.Lorenz H, Wenz W, Ivancic M, Steck E, Richter W. Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Res Ther. 2005;7:R156–65. doi: 10.1186/ar1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brew CJ, Clegg PD, Boot-Handford RP, Andrew JG, Hardingham T. Gene expression in human chondrocytes in late osteoarthritis is changed in both fibrillated and intact cartilage without evidence of generalised chondrocyte hypertrophy. Ann Rheum Dis. 2008;69:234–40. doi: 10.1136/ard.2008.097139. [DOI] [PubMed] [Google Scholar]
- 85.Carames B, Lopez-Armada MJ, Cillero-Pastor B, Lires-Dean M, Vaamonde C, Galdo F, et al. Differential effects of tumor necrosis factor-alpha and interleukin-1beta on cell death in human articular chondrocytes. Osteoarthritis Cartilage. 2008;16:715–22. doi: 10.1016/j.joca.2007.10.006. [DOI] [PubMed] [Google Scholar]