Abstract
Pei′ai64S, an indica sterile variety with photoperiod and thermo-sensitive genic male sterile (PTGMS) genes, has been widely exploited for commercial seed production for “two-line” hybrid rice in China. One PTGMS gene from Pei′ai64S, pms1(t), was mapped by a strategy of bulked-extreme and recessive-class approach with simple sequence repeat (SSR) and insert and deletion (In-Del) markers. Using linkage analysis for the F2 mapping population consisting of 320 completely male sterile individuals derived from a cross between Pei′ai64S and 93-11 (indica restorer) lines, the pms1(t) gene was delimited to the region between the RM21242 (0.2 cM) and YF11 (0.2 cM) markers on the short arm of chromosome 7. The interval containing the pms1(t) locus, which was co-segregated with RM6776, is a 101.1 kb region based on the Nipponbare rice genome. Fourteen predicted loci were found in this region by the Institute for Genomic Research (TIGR) Genomic Annotation. Based on the function of the locus LOC_Os07g12130 by bioinformatics analysis, it is predicted to encode a protein containing a Myb-like DNA-binding domain, and may process the transcript with thermosensory response. The reverse transcription-polymerase chain reaction (RT-PCR) results revealed that the mRNA levels of LOC_Os07g12130 were altered in different photoperiod and temperature treatments. Thus, the LOC_Os07g12130 locus is the most likely candidate gene for pms1(t). These results may facilitate not only using the molecular marker assisted selection of PTGMS genes, but also cloning of the pms1(t) gene itself.
Keywords: Super hybrid rice, pms1(t), Photoperiod and thermo-sensitive male sterile gene, Genetic mapping
1. Introduction
Discovery and successful application of male sterility in hybrid rice breeding have made a tremendous contribution to food security in the world. A photoperiod and thermo-sensitive genic male sterile (PTGMS) rice, named Nongken 58S, was first found as a spontaneous mutant in the japonica variety Nongken 58 in China (Shi, 1985). PTGMS rice has a unique trait distinguishing it from the other varieties, in which its pollen fertility is altered by day-length and temperature (Zhang and Yuan, 1987). Pollens of the PTGMS line show sterility under long day and high temperature (LD/HT), whereas they become fertile during short day and low temperature (SD/LT). Consequently, as a desirable germplasm in developing ‘‘two-line’’ hybrid rice, the PTGMS strain serves as a sterile line under LD/HT conditions to produce hybrid seeds, whereas various degrees of male fertility under SD/LT conditions allow it to be used as a maintainer to propagate itself. Therefore, large-scale hybrid rice seeds can be developed by this “two-line” seed production system less expensively and more efficiently. In addition, regardless of the relationship of restoration and maintenance, PTGMS rice has a broad spectrum of restoration compared with the “three-line” system. A further advantage is that the performance of PTGMS hybrids avoids the negative effects of male sterile cytoplasm (Virmani et al., 2003).
The fertility trait shows a typical single-locus segregation in the F2 population from a cross between Nongken 58S and Nongken 58. This locus was designated as pms3 (Mei et al., 1999a; 1999b), and was delimited into a 28.4-kb interval on chromosome 12 (Lu et al., 2005). In contrast, the fertility trait of 32001S, an indica PGMS line also derived from Nongken 58S, displays a two-locus segregation pattern in the progeny resulting from a cross with the indica cultivar Minghui 63 (Zhang et al., 1994), indicating that the fertility trait segregation is dependent on the genetic backgrounds of the materials used in the crosses. The two loci were designated as pms1 and pms2, and were localized on chromosomes 7 and 3, respectively. The effect of pms1 was found to be two to three times larger than that of pms2 (Zhang et al., 1994). Liu et al. (2001) constructed a physical map of the pms1 region and further localized the pms1 locus to an 85-kb region on chromosome 7. Compared with the TGMS, the genetic mechanism of PTGMS is far more complex. The sterile lines derived from Nongken 58S show diversity of photoperiod and thermo-sensitive characteristics, and the thermo-sensitive genes are not always allelic under different genetic backgrounds (Chen and Lu, 1996; Xiang et al., 2002; Zhou et al., 2005). Recently cloned PGMS genes OSMYOXIB (Jiang et al., 2007) and Ugp1 (Chen et al., 2007) cosuppressing plants show TGMS phenotype, but are different from the PTGMS genes of Nongken 58S.
Pei′ai64S as a widely-applied PTGMS line, which shows significant thermo-sensitive characteristics, was also derived from the japonica variety Nongken 58S. It contains complex germplasm backgrounds such as indica and javanica (Luo et al., 2000). A new elite “two-line” hybrid rice variety named Liangyoupeijiu (Pei′ai64S/93-11) with Pei′ai64S as a male sterile line has been widely extended as a super hybrid rice variety in the southern provinces of China. However, the genetic factor(s) responsible for the PTGMS trait in the above line is still unclear. Thus, it is necessary to further investigate and map the sterile genes responsible for PTGMS in Pei′ai64S. In this study, we constructed a larger F2 population with a total of 320 sterile plants, and attempted to make fine genetic mapping of PTGMS genes in Pei′ai64S. Here, we show that the pms1(t) gene is located in a 101.1-kb region between RM21242 (0.2 cM) and YF11 (0.2 cM) markers, and was predicted to encode a protein containing a Myb-like DNA-binding domain. The results of this study will greatly facilitate the final isolation of the pms1(t) gene by a map-based cloning approach and would be useful for molecular marker-assisted selection for new PTGMS lines in hybrid rice breeding.
2. Materials and methods
2.1. Plant materials and mapping population
The maternal parent Pei′ai64S, as an indica type of the PTGMS variety with a low critical temperature for fertility transfer, is widely applied in China (Luo et al., 2000). The paternal parent 93-11 is a typical indica variety, and its whole genome has been sequenced (Yu et al., 2002). The F1 (Pei′ai64S/93-11) and F2 populations were planted in the field under LD/HT conditions on the experimental farm at Zhejiang University, Hangzhou, China in 2005. The heading stage was arranged from late July to mid-August, during which the day length and average daily temperature were favorable for the identification of sterile plants in the F2 population. The sterile control (Pei′ai64S) was sowed five times at 10 d intervals from the first 10 d interval of May to the second 10 d interval of June.
2.2. Pollen fertility examination
Plants were classified as sterile based on the anther’s shape (not dehiscing) and without dispersed pollens during the heading stage. For pollen fertility, three panicles per sterile plant were sampled after the heading stage, but before flowering, and were fixed in 37% (v/v) formaldehyde/glacial acetic acid (FAA) solution. Pollen grains from broken anthers were stained with a 0.01 g/ml iodine and iodine-potassium solution (I2-KI). More than 600 pollen grains from each individual were examined under a microscope to estimate the percentage of fertile stainable pollen. Plants with 100% sterile pollens were considered to be completely male sterile. Pollen fertility of each sterile plant was tested at least three times during the heading stage. All sterile individuals were planted in the field during the summer of 2006 to reconfirm their sterility.
2.3. Molecular marker development and assay
Whole genomic sequences of Oryza sativa subspecies indica (93-11) and japonica (Nipponbare) were downloaded from ftp://ftp.genomics.org.cn/pub/ricedb/SynVs9311/9311/Sequence/Chromosome/ and http://rgp.dna.affrc.go.jp/IRGSP/Build4/build4.html websites, respectively. Information about 18 828 simple sequence repeats (SSRs) was obtained from the supplementary Table 18 of Matsumoto et al. (2005). Other SSR loci in the target genomic region were identified by the online software Simple Sequence Repeat Identification Tool (SSRIT; http://www.gramene.org/gramene/searches/ssrtool) (Temnykh et al., 2001). Polymerase chain reaction (PCR) based insert and deletion (In-Del) markers were developed for the regions in defect of SSR markers. Sequence differences between the corresponding genomic regions of 93-11 and Nipponbare were identified by bl2seq (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi).
Primers for the candidate SSR or In-Del markers were designed based on the flanking sequence of SSR loci or In-Dels using the Primer3 program (http://frodo.wi.mit.edu/primer3/) (Rozen and Skaletsky, 2000). In order to predict the polymorphism of markers between 93-11 and Nipponbare, all primers were analyzed by electronic PCR (e-PCR) (ftp://ftp.ncbi.nlm.nih.gov/pub/schuler/e-PCR/) before synthesizing.
Plant DNA used for constructing the DNA pool of fertility or sterility was extracted from 5 g of fresh leaf tissues using the hexadecyltrimethy ammonium bromide (CTAB) method described by Murray and Thompson (1980). DNA extraction from other sterile plants was carried out following a rapid one-tube genomic DNA extraction method described by Steiner et al. (1995).
PCR was performed in a 15-μl reaction volume containing 25 ng of the template DNA, 1.5 μl 10× PCR buffer, 0.2 mmol/L deoxy-ribonucleoside triphosphate (dNTP), 0.2 μmol/L primer pairs, and 0.5 U Taq DNA polymerase. The amplification protocol included an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 30 s denaturation at 94 °C, 30 s annealing at 55 °C, and extension of 1 min at 72 °C, and a final extension step of 5 min at 72 °C in a DNA Engine Thermal Cycler (MJ Research, USA). Taq DNA polymerase and dNTP were purchased from Dingguo Biotech Co., Ltd. (Beijing, China). Primers were synthesized by Sangon Co., Ltd. (Shanghai, China). PCR products were separated on a 0.06 g/ml polyacrylamide gel, and the amplified DNA fragments were silver-stained for visualization.
2.4. Genetic mapping and linkage analysis
Linkage between molecular markers and the PTGMS gene was determined following bulked-extreme and recessive-class approaches (Zhang et al., 1994). Fertile (BF pool) and sterile (BS pool) DNA bulks were prepared by mixing equal amounts of DNA from 10 highly fertile (>95% fertile pollen) plants and 10 completely sterile (100% sterile pollen) plants, respectively. Bulked segregation analysis was used to identify all of the markers linked to the PTGMS gene (Michelmore et al., 1991). Markers that could detect polymorphism between the two bulks were further used to analyze the completely sterile population.
2.5. Expression analysis
The plants tested for sterile traits were grown under two natural conditions: LD/HT and SD/LT. TRIZOL™ kit (Invitrogen, Shanghai, China) was used for RNA isolation with the manufacturer’s recommended protocol. The total RNA was extracted from the developing young panicles with length of 2, 10, and 20 cm at the boot stage of the plants. Semi-quantitative reverse transcription (RT)-PCR experiments were performed with PrimeScript™ 1st strand cDNA synthesis kit (TaKaRa, Dalian, China). Total RNA was reverse transcribed into single-stranded cDNA. The cDNA transcribed from the total RNA (20 μl reaction volume) was used as the template according to the manufacturer’s instructions. A 1-µl cDNA solution of each sample was used for RT-PCR. Base on information from the Institute for Genomic Research (TIGR) rice genome (http://rice.plantbiology.msu.edu/cgi-bin/gbrowse/rice/), sequences of five putative genes located in the target region were used for primer design to study their expressions. Primers were designed using the Primer3 program (http://cbr-rbc.nrc-cnrc.gc.ca/cgi-bin/primer3_www.cgi) (Rozen and Skaletsky, 2000). To exclude contamination of DNA, all primer pairs were designed to span at least one intron. Details of primers specific for these genes are shown in Table 1. RT-PCR amplification with all the primers was carried out at an annealing temperature of 55 °C. The ubiquitin gene was used as a control. The PCR reactions consisted of an initial denaturation for 5 min at 95 °C followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 40 s. The PCR products were electrophoresed on 0.01 g/ml agarose gels. The experiments were repeated in triplicate.
Table 1.
Primers for the RT-PCR analysis
| Gene name | Primer name | Primer sequence (5′→3′) |
| LOC_Os07g12130 | G2130F | AGTGGGCAGAGATTGCAAAG |
| G2130R | CTTGCACCTATCACCCCACT | |
| LOC_Os07g12140 | G2140F | GGTCCCAGTTTCCTGCAATA |
| G2140R | TCCGTGCAATCAAATCAAGA | |
| LOC_Os07g12160 | G2160F | CCTGAAGATGCAAGGAAAGC |
| G2160R | GCCTCCAAATACCCCTTGAT | |
| LOC_Os07g12170 | G2170F | CTGTTCGCGAAGAAGGAGAT |
| G2170R | TCATTCAGCATCCTGTGGAG | |
| LOC_Os07g12240 | G2240F | TGTTGGTACTGGGACCATTG |
| G2240R | TGATCCTCCCATCTCTGTCC | |
| Ubiquitin | UbiqF | AGAAGGAGTCCACCCTCCAC |
| UbiqR | CACGGTTCAACAACATCCAG |
2.6. Sequencing and data analysis
To calculate the linkage distance between molecular markers and pms1(t) locus, the data were analyzed with MAPMAKER/EXP 3.0 (Lincoln et al., 1992) at a limit of detection (LOD) threshold of 3.0. A linkage map was constructed using MapDraw V2.01 (Liu and Meng, 2003). The candidate gene sequencing was made by Invitrogen Corporation. The obtained gene sequences were used for gene prediction through Genscan (http://genes.mit.edu/GENSCAN.html) and FGENESH (http://mendel.cs.rhul.ac.uk/mendel.php?topic=fgen), and subjected to multiple sequence alignment with CLUSTALW (http://clustalw.genome.jp/).
3. Results
3.1. Construction of a completely sterile population and genetic analysis of male fertility
The day length and temperature are suitable for PTGMS gene expression during the heading stage from late July to mid August in Hangzhou. By pollen grain fertility test, a total of 320 completely sterile plants were obtained from a large F2 population consisting of 6 384 individuals. These plants were used to construct a completely sterile population for PTGMS gene mapping.
A total of 1 008 plants were randomly selected from the F2 population for genetic analysis. Pollen fertility of each plant was investigated under the LD/HT conditions. In this population, 59 plants classified as completely sterile showed no dehiscing anther (A3, B3 in Fig. 1) with 100% sterile pollen as the maternal parent Pei′ai64S (Table 2; C3, C4 in Fig. 1). Another 193 plants classified as partially sterile had a thin anther with some dehiscing occurring at the top (A2, B2 in Fig. 1) and contained about 6.20% of dark stained fertile pollens (Table 2; C2 in Fig. 1). The anther shape and pollen fertility of the rest of the 756 fertile plants were similar to those of the paternal parent 93-11 (A1, B1, C1 in Fig. 1). The numbers of fertile plus partially sterile and completely sterile individuals fit the expected 15:1 ratio (χ 2=0.21, P>0.9), indicating that the PTGMS trait of Pei′ai64S was controlled by two recessive genes. The expected segregation ratio was also confirmed by distribution of seed setting rate (data not shown).
Fig. 1.
Anthers observed by naked eyes and pollen grains observed under light microscope
A1–A4 showed the shapes of anthers from two parents and their F2 individuals. A1: 93-11 as the fertile control with dispersed normal fertility pollens; A2: F2 individual with partially dehiscent anther; A3: F2 individual with no dehiscent anther; A4: Pei′ai64S as the sterile control under the LD/HT condition. B1–B4 and C1–C4 showed anthers and pollen grains stained with I2-KI solution, corresponding to column specimens of A1–A4. The black-stained pollen grains are considered viable, while shrink and unstained sterile pollen grains are abortive
Table 2.
Pollen fertilities of F2 sterile individuals and control by the observation under light microscope
| F2 sterile individuals and control | n | Pollen fertility (%) |
|||
| TS | RS | SS | NF | ||
| Extreme sterility | 59 | 99.23 | 0.01 | 0.77 | 0.00 |
| Partial sterility | 193 | 77.56 | 0.30 | 15.94 | 6.20 |
| Pei′ai64S (CK) | 150 | 98.14 | 0.76 | 1.10 | 0.00 |
On the basis of their shapes and staining patterns, pollen grains could be classified into four categories, viz. typical sterile or unstained withered sterile (TS), round sterile or unstained spherical sterile (RS), stained sterile or stained round sterile (SS), and normal fertile or stained round fertile (NF), respectively
3.2. Analyses of linkage molecular markers using bulked segregant analysis (BSA) method
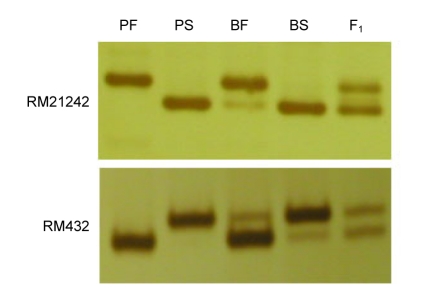
The sterile genes of Pei′ai64S were linked with the SSR marker on chromosome 7 based on previous study (data not shown). After analysis of the predicted amplification by e-PCR, 58 pairs of primers based on specific sequences of chromosome 7 were designed for screening the polymorphism between two parents (Pei′ai64S and 93-11), and between two bulks (fertile pool and sterile pool). Twenty-two pairs of primers were identified as polymorphic markers, which showed a linkage relationship with the PTGMS gene (Table 3; Fig. 2).
Table 3.
Primer sequences and sizes of polymorphic bands for the SSR and In-Del markers in the pms1(t) region
| Marker name | Synonym | Motif | No. of Repeat | Chr. | Primer sequence (5′→3′) | PCR product size (bp) |
|
| Nipponbare | 93-11 | ||||||
| RM21136 | AAG | 13 | 7 | Forward: GAAGCCAAACGCAACCAAGG; Reverse: TCGGTGAATTGTCCTGTATCAGC | 108 | 108 | |
| RM21216 | AT | 41 | 7 | Forward: GTGGCCACCTGTGGATACAA; Reverse: TATCTCATGCGAGCCAGATG | 385 | ||
| YF48 | AT | 8 | 7 | Forward: GGCTCTTAGCCATCAAGATACAA; Reverse: CAGTATGGAACAAAGAGCCAAG | 152 | ||
| RM21238 | CCG | 10 | 7 | Forward: GAGCTTCTCCTCACCCATCACC; Reverse: CTTCTGCAGAGGGTGTTCAACG | 189 | 273 | |
| RM21242 | GTC | 7 | 7 | Forward: GAGAGGAATGGAATGGAATGAGG; Reverse: GAACAGGCATGGTGAAGAGTGC | 131 | 134 | |
| RM6776 | RM21244 | TCC | 9 | 7 | Forward: AGCCCGGACATGCAAAAC; Reverse: GAAGCAGGCGAAATCTCCTC | 169 | |
| YF11 | A | 17 | 7 | Forward: ATACCTCACTTGGCCTGTGC; Reverse: TACCCTATACCGAACCGTGC | 190 | 194 | |
| YF15 | In-Del | 7 | Forward: TGGAGGCAAACCTAAAAAGG; Reverse: TAATCCAACCGCACGTGAT | 351 | 343 | ||
| YF22 | In-Del | 7 | Forward: TCGAGTCCGCTTATCTCCAG; Reverse: AGGGAGAGGGGAGAGGAGTA | 210 | 205 | ||
| YF25 | In-Del | 7 | Forward: TGGCGACATAGGAGTGTTTG; Reverse: GAGAGATATCCGCCAGTTGC | 386 | 377 | ||
| RM21247 | AG | 10 | 7 | Forward: GACGTCTCATCCTCCATGGTTCC; Reverse: TTGCGATGCCGAGGAGATAAGG | 236 | ||
| YF6 | In-Del | 7 | Forward: ACTTCAGATGAGAGTTCCCAACA; Reverse: GTCAGACCAGCGGCTATACTGT | 307 | 224 | ||
| YF10 | TC, TA | 8, 22 | 7 | Forward: ACGCCAGAGACCACTCACTC; Reverse: TGGGAGTAACGAACACCACA | 205 | 239 | |
| RM1253 | RM21249 | AG | 16 | 7 | Forward: CTGAACTTGCCTGAGAACTC; Reverse: GACGACCTCTCCATGCTCG | 175 | |
| RM7153 | RM21310 | ACAT | 8 | 7 | Forward: ATCCTAAAGCAGTTGCACGG; Reverse: GATCAGCAACCATCCAAAGG | 154 | |
| RM542 | RM21421 | AG | 22 | 7 | Forward: TGAATCAAGCCCCTCACTAC; Reverse: CTGCAACGAGTAAGGCAGAG | 114 | 92 |
| RM214 | RM21423 | AG | 32 | 7 | Forward: CTGATGATAGAAACCTCTTCTC; Reverse: AAGAACAGCTGACTTCACAA | 149 | 113 |
| RM7338 | RM21502 | ATCC | 9 | 7 | Forward: CTTATCTCTCGGCAAGCAGC; Reverse: CTCACACGCATGGATCAATC | 164 | 160 |
| RM445 | RM21582 | AG | 12 | 7 | Forward: CGTAACATGCATATCACGCC; Reverse: ATATGCCGATATGCGTAGCC | 251 | 259 |
| RM432 | RM21652 | ATCC | 9 | 7 | Forward: TTCTGTCTCACGCTGGATTG; Reverse: AGCTGCGTACGTGATGAATG | 187 | 179 |
| RM3691 | RM21671 | AG | 15 | 7 | Forward: GCTGATGGTCAAAGATCAGG; Reverse: ATGTGTCTGCTGGCACAGAG | 117 | 145 |
| RM11 | RM21672 | AG | 15 | 7 | Forward: TCTCCTCTTCCCCCGATC; Reverse: ATAGCGGGCGAGGCTTAG | 126 | 146 |
Fig. 2.
BSA analysis for SSR markers
PF, PS, BF, BS, and F1 represent 93-11, Pei′ai64S, fertile bulk, sterile bulk, and F1 hybrid of a cross of Pei′ai64S/93-11, respectively. The PCR products were separated in non-denatured 0.06 g/ml polyacrylamide gel electrophoresis, and visualized by quick silver-staining. The bands were amplified by two SSR primers (RM21242 and RM432)
3.3. Fine mapping of the pms1(t) gene
The pms1(t) locus was mapped using several polymorphic SSR markers that distributed randomly on chromosome 7 and were used to assay a population consisting of 218 completely sterile individuals separately one by one. The genotype data of the sterile population were obtained by reading the band of PCR products from the polyacrylamide gel electrophoresis. The recombination events between the pms1(t) locus and closely linked markers indicated that a marker closer to the gene had a smaller number of recombinants in the panel (Table 4). There are 42 recombinants between the farthest marker RM11 and pms1(t) with the genetic distance of 11.5 cM. For the closer linked marker RM7153, 10 recombinants occurred between the pms1(t) locus and RM7153, so the distance from RM7153 to pms1(t) was only 2.6 cM. On the other hand, 37 recombination events occurred between RM21136 and the pms1(t) locus with the genetic distance of 9.8 cM (Fig. 3).
Table 4.
Genotypes of sterile individuals for recombinant between sterile genes and molecular markers
| Marker | Genotype |
|||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| RM21136 | B | B | B | B | B | B | B | B | B | B | A | H | H | H |
| YF48 | B | B | B | B | B | B | B | B | B | B | B | B | B | H |
| RM21238 | B | B | B | B | B | B | B | B | B | B | B | B | B | H |
| RM21242 | B | B | B | B | B | B | B | B | B | B | B | B | B | H |
| RM6776 | B | B | B | B | B | B | B | B | B | B | B | B | B | B |
| YF11 | H | B | B | B | B | B | B | B | B | B | B | B | B | B |
| YF22 | H | B | B | B | B | B | B | B | B | B | B | B | B | B |
| YF25 | H | H | B | B | B | B | B | B | B | B | B | B | B | B |
| YF6 | H | H | H | B | B | B | B | B | B | B | B | B | B | B |
| RM1253 | H | H | H | B | B | B | B | B | B | B | B | B | B | B |
| RM7153 | H | H | H | H | H | H | H | H | H | H | A | H | H | B |
A, B, and H represent genotypes of 93-11, Pei′ai64S and their heterozygote, respectively. 1–14 represent the selected individuals
Fig. 3.
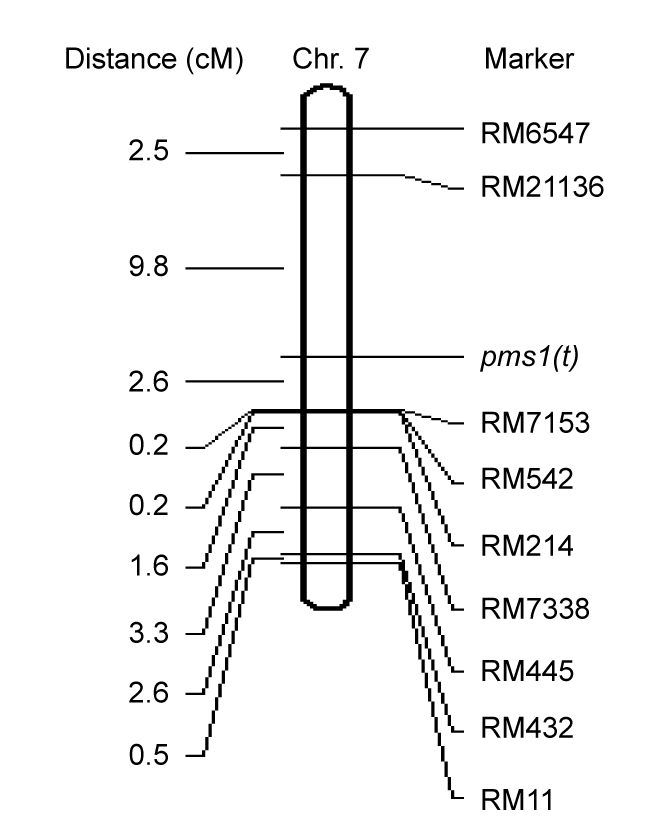
Location of the pms1(t) locus on the molecular linkage map of chromosome 7
In an additional 102 completely sterile individuals, 3 and 16 recombinants were detected at RM7153 and RM21136, respectively. Altogether, the data comprising 320 completely sterile individuals showed that the number of recombinant individuals was 13 at RM7153 and 53 at RM21136, respectively.
For fine physical mapping of pms1(t), a series of markers was further developed. As a result, we obtained 11 polymorphic molecular markers, including 7 SSR markers and 4 In-Del markers between RM21136 and RM7153 (Table 4). These markers were further used to screen the recombinants detected by RM7153 and RM21136. No recombination event was detected between the marker RM6776 and pms1(t), and only one recombination occurred between pms1(t) and its closest linked markers RM21242 and YF11 on both sides. Thus, the genetic distance decreased to 0.2 cM between pms1(t) and RM21242 (Table 5; Fig. 4).
Table 5.
Gene annotations and positions of start and end points of 14 gene loci within the 101.1-kb region from RM21242 to YF11 on chromosome 7
| No. | Gene locus | Gene annotation | Start | End |
| 1 | LOC_Os07g12120 | Hypothetical protein | 6775171 | 6776190 |
| 2 | LOC_Os07g12130 | Myb-like DNA-binding domain containing protein, expressed | 6779818 | 6777673 |
| 3 | LOC_Os07g12140 | DNA-binding protein, putative, expressed | 6790010 | 6794406 |
| 4 | LOC_Os07g12150 | Acyl carrier protein, mitochondrial precursor, putative, expressed | 6797368 | 6797839 |
| 5 | LOC_Os07g12160 | KHG/KDPG aldolase, putative, expressed | 6801745 | 6804236 |
| 6 | LOC_Os07g12170 | ADP-ribosylation factor 1, putative, expressed | 6807971 | 6810407 |
| 7 | LOC_Os07g12180 | Retrotransposon protein, putative, Ty1-copia subclass | 6816373 | 6813623 |
| 8 | LOC_Os07g12190 | Retrotransposon protein, putative, unclassified | 6818596 | 6817502 |
| 9 | LOC_Os07g12200 | ADP-ribosylation factor 1, putative, expressed | 6821343 | 6823745 |
| 10 | LOC_Os07g12210 | Conserved hypothetical protein | 6832221 | 6833291 |
| 11 | LOC_Os07g12220 | Conserved hypothetical protein | 6839803 | 6843124 |
| 12 | LOC_Os07g12230 | Hypothetical protein | 6846320 | 6851694 |
| 13 | LOC_Os07g12240 | Calmodulin TaCaM2-1, putative | 6854921 | 6855484 |
| 14 | LOC_Os07g12250 | 60S ribosomal protein L24, putative, expressed | 6863637 | 6864932 |
The above information is cited from the TIGR Rice Genome Annotation website http://rice.plantbiology.msu.edu/cgi-bin/gbrowse/rice/
Fig. 4.
High-resolution genetic and physical map of the pms1(t) locus
In the partial genetic map of the molecular markers, the numbers under the long horizontal line indicate the times of recombination events detected between the pms1(t) locus and its corresponding markers. The bold bars represent the BAC clones. The pms1(t) locus was covered by the thick horizontal line between the two vertical dashed lines from RM21242 to YF11, in which the genomic information was based on Nipponbare genome sequence (released 4.0), and some gene annotations of the gene loci represented with black bars were from TIGR database
3.4. Candidate genes of pms1(t)
Genomic sequence analysis indicated that RM21242 and YF11 were located on BAC clones AP005197 and AP003740, respectively. Based on the genome sequence of Nipponbare, the physical distance between these two markers is approximately 101.1 kb. Based on the gene annotation information (TIGR Rice Genome Annotation Release 5, Jan. 24, 2007; http://www.tigr.org/tdb/e2k1/osa1/pseudomolecules/info.shtml), about 14 predicted loci were found to be present in the above region, which may be considered as the candidate genes for pms1(t) (Fig. 4; Table 5). Among these loci, LOC_Os07g12130 encodes a Myb-like protein containing a DNA-binding domain, which hits 16 gene ontology (GO) terms. One of the gene ontology (GO) terms is GO:0009628, which responds to abiotic stimulus. Interestingly, GO:0009266, a child term for GO:0009628, responds to thermal stimulus. Two other second child terms, GO:0009408 and GO:0040040, also respond to heat stimulus and have the characteristics of thermosensory behavior, which are very closely related to the thermo-sensitive genic male sterile characteristics. However, for the other 13 candidate genes, we did not find a direct relationship with the photoperiod or thermo-sensitive response. These results suggest that the locus of LOC_Os07g12130 is more likely a thermo-sensitive candidate gene for pms1(t).
3.5. Expression analysis
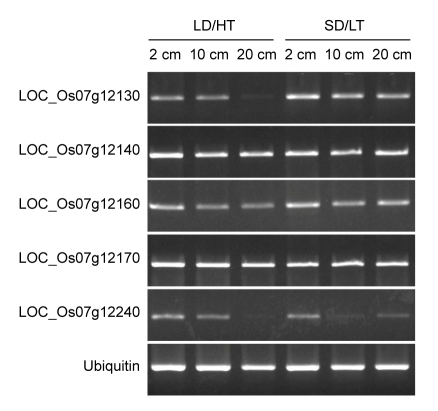
To investigate whether the expression levels of the candidate genes located in the target region change under different conditions, five putative genes annotated as expressed proteins were tested for their expressions in rice panicles at 2, 10, and 20 cm stages. These tissues were harvested from Pei′ai64S plants grown under two natural conditions, LD/HT and SD/LT. The results showed that expressions of LOC_Os07g12140, LOC_Os07g12160, and LOC_Os07g12170 were detected in all tested panicles, and their mRNA levels displayed similar expression patterns in different photoperiod and temperature treatments (Fig. 5). The band intensities of LOC_Os07g 12130 were higher at 2 and 10 cm panicles than that at 20 cm panicles under LD/HT conditions, but the band intensities from 2 and 10 cm panicles were lower under LD/HT conditions than those from same stage panicles under SD/LT conditions (Fig. 5), indicating that the expression of LOC_Os07g12130 is altered under different environmental conditions and/or different panicle growth stages. These expression patterns of LOC_Os07g12130 further support that the pms1(t) is a promising candidate gene. Similar diversities of the expression levels for LOC_Os07g 12240 were observed under different environments and/or panicle developmental stages. The band intensities of 20 cm panicles under LD/HT conditions and 10 cm panicles under SD/LT conditions were almost undetectable. Our results imply that LOC_Os07g12140 may also associate with photoperiod and thermo-sensitive male sterile traits, and its expression may be controlled by other factor(s).
Fig. 5.
Semi-quantitative RT-PCR results of five candidate genes for Pei′ai64S
The individuals were planted under LD/HT and SD/LT conditions, respectively. Also, samples were taken at three different stages of the young panicle sizes (2, 10, and 20 cm). LOC_Os07g12130, 12140, 12160, 12170, and 12240 were used as the candidate genes, and ubiquitin as a control in our experiment
4. Discussion
4.1. Genetic diversity of PTGMS rice
Nongken 58S, which was a photosensitive male sterile line discovered by the Shi (1985), has played a crucial role in “two-line” rice breeding in China; however, the studies on its photosensitive sterile gene and genetic mapping remain controversial. Early research suggested that the sterility trait for japonica PTGMS lines, involving Nongken 58S, is controlled by a single nuclear gene (Shi, 1985), which was named as pms3 (Mei et al., 1999a; 1999b), and mapped on chromosome 12 (Lu et al., 2005). However, subsequent studies showed that the sterile trait for PTGMS varieties derived from Nongken 58S was controlled by two recessive genes. The PGMS genes of the line 32001S consist of the two gene loci (pms1 and pms2), of which the pms1 gene was located on chromosome 7 (Zhang et al., 1994; Mei et al., 1999b; Liu et al., 2001; Lu et al., 2005), and another pms3 gene on chromosome 12 (Lu et al., 2005).
Localization studies showed that the TGMS genes from different sources may be located in different chromosomes. Examples include reports that tms1 (Wang et al., 1995), tms2 (Lopez et al., 2003), tms3 (Subudhi et al., 1997), tms4 (Dong et al., 2000), tms5 (Yang et al., 2007a), rtms1 (Jia et al., 2001), ms-h (Koh et al., 1999), and tms6 (Lee et al., 2005) were located on chromosomes 8, 7, 6, 2, 2, 10, 9, and 5, respectively. However, these TGMS materials are not to be applied in rice production because of the non-stability of their sterility. The Pei′ai64S used in this study has been widely used in the seed production of hybrid rice in China. This PTGMS line is developed through subspecies hybridization, and has a complex genetic background containing rice germplasms from indica, japonica, and javanica, although its sterility is also derived from the Nongken 58S. So it is of great significance in theory and practice to clarify the localization of the sterile gene.
4.2. Distribution of PTGMS genes on rice chromosome 7
In this study, we showed that the sterility trait for Pei′ai64S is controlled by two recessive genes. In our linkage analysis of SSR markers, we showed that the pms1(t) gene for the Pei′ai64S located on the short arm of chromosome 7 between two markers (RM7153 and RM21136) with a 12.4-cM genetic distance. Further we found that the pms1(t) gene was within the interval of the RM21242 and YF11 with a 0.4-cM distance, and was co-segregated with the RM6776. Based on sequence information from the Nipponbare genome, the gene range between RM21242 and YF11 covers close to 101.1 kb, in which there are 14 loci. By combining the above bioinformatics and gene expression analyses, we hypothesized that the LOC_Os07g12130 locus is most likely a candidate gene of pms1(t).
So far, two other TGMS genes, tms2 (Lopez et al., 2003) and tms5 (Zhou et al., 2006), were mapped on the long arm of rice chromosome 7 while the PTGMS gene pms1 was also mapped on the short arm of this chromosome (Liu et al., 2001). Therefore, we hypothesized that there may exist a “hotspot” of the TGMS genes on chromosome 7. Interestingly, the RG477 linked to pms1 gene with the interval of 0.25-cM (Liu et al., 2001), and was just located at the second intron position of the LOC_Os07g12130 locus. Further analysis showed that our positioning of the pms1(t) gene coincided with the location of the pms1 gene in the same BAC clone, but in the opposite direction (GenBank accession No. AP005197). We suggest that there are two following possible explanations:
The first one is the different genetic background between both tested PTGMS varieties (Pei′ai64S in this study and 32001S tested in Liu et al. (2001)). The former was developed from the complex crosses of indica/japonica, while the latter was derived from the cross of indica/indica, although both sterile varieties contained commonly the sterile genes from Nongken 58S. Therefore, we assume that a gene cluster (a set of two or more genes) may play the different effects on the male sterility, which was also found in the fertility restorer genes. There is a set of the Rf-1 homologous genes on chromosome 10 (Akagi et al., 2004; Komori et al., 2004).
The second one is that our experimental methods and identification standard of the sterile plants are different from those used by Liu et al. (2001), which will have an impact on the positioning results. Liu’s sterile standard was based on the seed setting rate under the natural conditions, in which the plants with seed setting rate lower than 10% were regarded as the sterile type. However, this estimated number of sterile plants was generally higher than that from pollen fertility (data not shown). Therefore, the adoption of identification standards with the pollen fertility was superior to that with the seed fertility, which can accurately calculate the crossing-over value of the sterile plants. In order to ensure the accuracy of the investigation, we also conducted two years of repeated testing and reproduced consistent results. Meanwhile, we also developed an optimal DNA silver staining method for SSR marker analysis on 0.06 g/ml polyacrylamide gel, which ensures gene mapping accuracy of pms1(t).
4.3. Correlation between Myb-like protein and PTGMS gene
Based on RT-PCR results, we sequenced the two candidate genes LOC_Os07g12130 and LOC_Os07g 12240 (supplementary Data 1–4), which displayed different expression levels under different environmental conditions. LOC_Os07g12130, as a pms1(t) candidate gene, encodes a Myb-like protein with a DNA binding domain. Myb proteins are the largest superfamily of transcription factors that play a regulating role in the process of development and defense reaction, such as regulation of plant cell shape, growth regulators, and responses to stress (Meissner et al., 1999). A new Myb-like gene (AtMYB103) showed a high level of mRNA expression in the early development of anther tapetum and middle-level performance in Arabidopsis thaliana (Li et al., 1999). The fertility of the mutant with double defects of anther development in myb33 and myb65 was enhanced in higher light and lower temperature conditions (Millar and Gubler, 2005). The over expression of AtMYB24, as a member of the R2R3 Myb gene family, usually causes abnormal pollen grains and anther indehiscence during the microspore development in Arabidopsis (Yang et al., 2007b). Zhu et al. (2004) identified an anther dehiscence 1 (aid1) gene, which encodes a new protein containing single Myb domains from a mutant of rice spikelet fertility. In addition, OsGAMYB gene knockout mutants produced abnormal flowers more frequently under high temperature conditions than under low temperature conditions (Kaneko et al., 2004). Thus, LOC_Os07g 12130 that may be regarded as a member of a signal transduction pathway affects anther development by regulating gene expression at different temperatures. In young panicles of Pei′ai64S, Myb transcription factors showed a significantly lower transcriptional abundance under the LD/HT than under the SD/LT conditions, which may lead to a significant gene inhibition in the normal process of transcription (Guo et al., 2006).
In this study, we hypothesized that there might be different expression patterns of pms1(t) gene under different photoperiod and temperature conditions. The RT-PCR results demonstrated that the mRNA level of pms1(t) (LOC_Os07g12130) was dramatically reduced under the LD/HT conditions, which suggests an association between male sterility and this gene. However, the detail on the pms1(t) expression pattern under different photoperiod and temperature conditions remains unclear. Further work is required to elucidate whether this gene product is similar to the UGP-like enzyme (Chen et al., 2007) in the rice spikelet.
The mutations that occurred in the promoter/untranslated regions (UTR) may lead to changes in gene expression profiles. Recently, by sequencing of the upstream of BnGID1 gene of a dwarf mutant from Brassica napus, a tripartite base mutant in the pyrimidine box of the BnGID1 promoter was found (Li et al., 2010), based on which we need to further confirm sequence mutations in the promoter/UTR of LOC_Os07g12130 in our further research.
Acknowledgments
We thank Dr. Jia-hua XIE and Dr. Farooqahmed KITTUR (North Carolina Central University, USA) for English language correction.
List of electronic supplementary materials
Supplementary Data 1 Multiple DNA sequence alignment for predicted protein of LOC_Os07g12130
Supplementary Data 2 Multiple DNA sequence alignment for LOC_Os07g12130 in Pei′ai64S S1, S2, and S3
Supplementary Data 3 Multiple DNA sequence alignment for LOC_Os07g12130 in Pei′ai64S, 93-11, and Nipponbare
Supplementary Data 4 Multiple DNA sequence alignment for LOC_Os07g12240 in Pei′ai64S, 93-11, and Nipponbare
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30571146), the Key Research Project of Zhejiang Province (No. 2003C22007), and the Rice Project of Zhejiang Province (No. 04-06), China
Electronic supplementary materials: The online version of this article (doi:10.1631/jzus.B1000306) contains supplementary materials, which are available to authorized users
References
- 1.Akagi H, Nakamura A, Yokozeki-Misono Y, Inagaki A, Takahashi H, Mori K, Fujimura T. Positional cloning of the rice Rf-1 gene, a restorer of BT-type cytoplasmic male sterility that encodes a mitochondria-targeting PPR protein. Theor Appl Genet. 2004;108(8):1449–1457. doi: 10.1007/s00122-004-1591-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen RZ, Zhao X, Shao Z, Wei Z, Wang YY, Zhu LL, Zhao J, Sun MX, He RF, He GC. Rice UDP-glucose pyrophosphorylase 1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell. 2007;19(3):847–861. doi: 10.1105/tpc.106.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Lu H. Allelism of thermo-sensitive genic male sterile genes of indica rice. Sci Agric Sin. 1996;29(1):27–33. (in Chinese) [Google Scholar]
- 4.Dong NV, Subudhi PK, Luong PN, Quang VD, Quy TD, Zheng HG, Wang B, Nguyen HT. Molecular mapping of a rice gene conditioning thermosensitive genic male sterility using AFLP, RFLP and SSR techniques. Theor Appl Genet. 2000;100(5):727–734. doi: 10.1007/s001220051345. [DOI] [Google Scholar]
- 5.Guo XQ, Dong HT, Zheng KL, Luo HM, Tan XL, Fang YQ, Wang YQ, Deng Y, Dai CG, Lou YC, et al. Gene expression profiling under different photoperiod/temperature conditions in a photoperiod-/thermo-sensitive genic male sterile line of rice (Oryza sativa L.) Chin Sci Bull. 2006;51(2):175–181. doi: 10.1007/s11434-005-1087-8. [DOI] [Google Scholar]
- 6.Jia JH, Zhang DS, Li CY, Qu XP, Wang SW, Chamarerk V, Nguyen HT, Wang B. Molecular mapping of the reverse thermo-sensitive genic male-sterile gene (rtms1) in rice. Theor Appl Genet. 2001;103(4):607–612. doi: 10.1007/PL00002916. [DOI] [Google Scholar]
- 7.Jiang SY, Cai M, Ramachandran S. ORYZA SATIVA MYOSIN XI B controls pollen development by photoperiod-sensitive protein localizations. Dev Biol. 2007;304(2):579–592. doi: 10.1016/j.ydbio.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko M, Inukai Y, Ueguchi-Tanaka M, Itoh H, Izawa T, Kobayashi Y, Hattori T, Miyao A, Hirochika H, Ashikari M, et al. Loss-of-function mutations of the rice GAMYB gene impair alpha-amylase expression in aleurone and flower development. Plant Cell. 2004;16(1):33–44. doi: 10.1105/tpc.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh HJ, Son YH, Heu MH, Lee HS, McCouch SR. Molecular mapping of a new genic male-sterility gene causing chalky endosperm in rice (Oryza sativa L.) Euphytica. 1999;106(1):57–62. doi: 10.1023/A:1003575016035. [DOI] [Google Scholar]
- 10.Komori T, Ohta S, Murai N, Takakura Y, Kuraya Y, Suzuki S, Hiei Y, Imaseki H, Nitta N. Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.) Plant J. 2004;37(3):315–325. doi: 10.1046/j.1365-313x.2003.01961.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee DS, Chen LJ, Suh HS. Genetic characterization and fine mapping of a novel thermo-sensitive genic male-sterile gene tms6 in rice (Oryza sativa L.) Theor Appl Genet. 2005;111(7):1271–1277. doi: 10.1007/s00122-005-0044-x. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Wang Y, Li X, Gao Y, Wang Z, Zhao Y, Wang M. A GA-insensitive dwarf mutant of Brassica napus L. correlated with mutation in pyrimidine box in the promoter of GID1 . Mol Biol Rep. 2010;38(1):191–197. doi: 10.1007/s11033-010-0094-2. [DOI] [PubMed] [Google Scholar]
- 13.Li SF, Higginson T, Parish RW. A novel myb-related gene from Arabidopsis thaliana expressed in developing anthers. Plant Cell Physiol. 1999;40(3):343–347. doi: 10.1093/oxfordjournals.pcp.a029548. [DOI] [PubMed] [Google Scholar]
- 14.Lincoln S, Daly M, Lander E. On Structing Genetic Maps with MAPMAKER/EXP 3.0. Whitehead Institute Technical Report. 3rd Ed. Cambridge: Whitehead Institute; 1992. [Google Scholar]
- 15.Liu N, Shan Y, Wang FP, Xu CG, Peng KM, Li XH, Zhang Q. Identification of an 85-kb DNA fragment containing pms1, a locus for photoperiod-sensitive genic male sterility in rice. Mol Genet Genomics. 2001;266(2):271–275. doi: 10.1007/s004380100553. [DOI] [PubMed] [Google Scholar]
- 16.Liu RH, Meng JL. MapDraw: a microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Hereditas. 2003;25(3):317–321. (in Chinese) [PubMed] [Google Scholar]
- 17.Lopez MT, Toojinda T, Vanavichit A, Tragoonrung S. Microsatellite markers flanking the tms2 gene facilitated tropical TGMS rice line development. Crop Sci. 2003;43(6):2267–2271. doi: 10.2135/cropsci2003.2267. [DOI] [Google Scholar]
- 18.Lu Q, Li XH, Guo D, Xu CG, Zhang Q. Localization of pms3, a gene for photoperiod-sensitive genic male sterility, to a 28.4-kb DNA fragment. Mol Genet Genomics. 2005;273(6):507–511. doi: 10.1007/s00438-005-1155-4. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Qiu Z, Li R. Pei-Ai64S-A dual-purpose sterile line whose sterility is induced by low critical temperature. Hybrid Rice. 2000;15(s2):4–5. (in Chinese) [Google Scholar]
- 20.Matsumoto T, Wu JZ, Kanamori H, Katayose Y, Fujisawa M, Namiki N, Mizuno H, Yamamoto K, Antonio BA, Baba T, et al. The map-based sequence of the rice genome. Nature. 2005;436(7052):793–800. doi: 10.1038/Nature03895. [DOI] [PubMed] [Google Scholar]
- 21.Mei MH, Chen L, Zhang ZH, Li ZY, Xu CG, Zhang Q. pms3 is the locus causing the original photoperiodsensitive male sterility mutation of ‘Nongken 58S’. Sci China (Ser C) 1999;42(3):316–322. doi: 10.1007/BF03183609. [DOI] [PubMed] [Google Scholar]
- 22.Mei MH, Dai XK, Xu CG, Zhang Q. Mapping and genetic analysis of the genes for photoperiod-sensitive genic male sterility in rice using the original mutant nongken 58S. Crop Sci. 1999;39(6):1711–1715. doi: 10.2135/cropsci1999.3961711x. [DOI] [Google Scholar]
- 23.Meissner RC, Jin HL, Cominelli E, Denekamp M, Fuertes A, Greco R, Kranz HD, Penfield S, Petroni K, Urzainqui A, et al. Function search in a large transcription factor gene family in Arabidopsis: assessing the potential of reverse genetics to identify insertional mutations in R2R3 MYB genes. Plant Cell. 1999;11(10):1827–1840. doi: 10.1105/tpc.11.10.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michelmore RW, Paran I, Kesseli RV. Identification of Markers Linked to Disease-Resistance Genes by Bulked Segregant Analysis—a Rapid Method to Detect Markers in Specific Genomic Regions by Using Segregating Populations. PNAS. 1991;88(21):9828–9832. doi: 10.1073/pnas.88.21.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millar AA, Gubler F. The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are MicroRNA-regulated genes that redundantly facilitate anther development. Plant Cell. 2005;17(3):705–721. doi: 10.1105/tpc.104.027920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray M, Thompson W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozen S, Skaletsky HJ. Primer3 on the WWW for General Users and for Biologist Programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 28.Shi M. The discovery and preliminary studies of the photoperiod-sensitive recessive male sterile rice (Oryza sativa L. subsp. japonica) Sci Agric Sin. 1985;(2):44–48. (in Chinese) [Google Scholar]
- 29.Steiner JJ, Poklemba CJ, Fjellstrom RG, Elliott LF. A rapid one-tube genomic DNA extraction process for PCR and RAPD analyses. Nucleic Acids Res. 1995;23(13):2569–2570. doi: 10.1093/nar/23.13.2569-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subudhi PK, Borkakati RP, Virmani SS, Huang N. Molecular mapping of a thermosensitive genetic male sterility gene in rice using bulked segregant analysis. Genome. 1997;40(2):188–194. doi: 10.1139/g97-027. [DOI] [PubMed] [Google Scholar]
- 31.Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S, McCouch S. Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 2001;11(8):1441–1452. doi: 10.1101/gr.184001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virmani S, Sun Z, Mou T, et al. Two-Line Hybrid Rice Breeding Manual. Los Baños, Laguna, Philippines: International Rice Research Institute; 2003. p. 88. [Google Scholar]
- 33.Wang B, Xu WW, Wang JZ, Wu W, Zheng HG, Yang ZY, Ray JD, Nguyen HT. Tagging and mapping the thermosensitive genic male-sterile gene in rice (Oryza sativa L.) with molecular markers. Theor Appl Genet. 1995;91(6-7):1111–1114. doi: 10.1007/BF00223928. [DOI] [PubMed] [Google Scholar]
- 34.Xiang Y, Li B, Wu H, Chen L. Studies on allelism of photosensitive and thermo-sensitive genic male-sterile rice genes. Seed. 2002;21(4):37–39. (in Chinese) [Google Scholar]
- 35.Yang QK, Liang CY, Zhuang W, Li J, Deng HB, Deng QY, Wang B. Characterization and identification of the candidate gene of rice thermo-sensitive genic male sterile gene tms5 by mapping. Planta. 2007;225(2):321–330. doi: 10.1007/s00425-006-0353-6. [DOI] [PubMed] [Google Scholar]
- 36.Yang XY, Li JG, Pei M, Gu H, Chen ZL, Qu LJ. Over-expression of a flower-specific transcription factor gene AtMYB24 causes aberrant anther development. Plant Cell Rep. 2007;26(2):219–228. doi: 10.1007/s00299-006-0229-z. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica) Science. 2002;296(5565):79–92. doi: 10.1126/science.1068037. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Shen BZ, Dai XK, Mei MH, Saghai Maroof MA, Li ZB. Using bulked extremes and recessive class to map genes for photoperiod-sensitive genic male sterility in rice. PNAS. 1994;91(18):8675–8679. doi: 10.1073/pnas.91.18.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang ZG, Yuan SC. The influence of photoperiod on pollen fertility change of Hubei photoperiod-sensitive genic male sterile rice. China J Rice Sci. 1987;1(3):137–143. (in Chinese) [Google Scholar]
- 40.Zhou CJ, Li J, Zou JC, Liang FS, Ye CJ, Jin DM, Weng ML, Wang B. Cloning and characterization of a second form of the rice adenine phosphoribosyl transferase gene (OsAPT2) and its association with TGMS. Plant Mol Biol. 2006;60(3):365–376. doi: 10.1007/s11103-005-4208-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Zhang Q, Xiang Y. Studies on the fertility variational rules of crosses F2 of Nongken 58S genetic source and its sterile lines derived from it which cross Nongken 58 and Xiang indica rice No. 13. Seed. 2005;24(4):16–20. (in Chinese) [Google Scholar]
- 42.Zhu QH, Ramm K, Shivakkumar R, Dennis ES, Upadhyaya NM. The ANTHER INDEHISCENCE1 gene encoding a single MYB domain protein is involved in anther development in rice. Plant Physiol. 2004;135(3):1514–1525. doi: 10.1104/pp.104.041459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data 1 Multiple DNA sequence alignment for predicted protein of LOC_Os07g12130
Supplementary Data 2 Multiple DNA sequence alignment for LOC_Os07g12130 in Pei′ai64S S1, S2, and S3
Supplementary Data 3 Multiple DNA sequence alignment for LOC_Os07g12130 in Pei′ai64S, 93-11, and Nipponbare
Supplementary Data 4 Multiple DNA sequence alignment for LOC_Os07g12240 in Pei′ai64S, 93-11, and Nipponbare