Abstract
Functional and structural neuroimaging have identified abnormalities of the brain that are likely to contribute to the neuropathophysiology of attention-deficit/hyperactivity disorder (ADHD). In particular, hypofunction of the brain regions comprising the cingulo-frontal-parietal (CFP) cognitive-attention network have been consistently observed across studies. These are major components of neural systems that are relevant to ADHD, including cognitive/attention networks, motor systems and reward/feedback-based processing systems. Moreover, these areas interact with other brain circuits that have been implicated in ADHD, such as the “default mode” resting state network. ADHD imaging data related to CFP network dysfunction will be selectively highlighted here to help facilitate its integration with the other information presented in this special issue. Together, these reviews will help shed light on the neurobiology of ADHD.
INTRODUCTION
Dysfunction of cingulate, frontal and parietal cortical regions has been implicated in the pathophysiology of attention-deficit/hyperactivity disorder (ADHD) by convergent data from a variety of sources, including neuroimaging, neuropsychological neurochemical and genetics studies (1–7). Earlier in this special issue, the groundwork has been laid which shows how cingulate, frontal and parietal cortical regions interact with striatal, cerebellar and other brain regions in healthy humans and animals during cognitive processes relevant to ADHD. This review will highlight studies that have found functional and structural abnormalities of the cingulo-frontal-parietal (CFP) cognitive-attention network in ADHD. However, at no time should the narrow focus of this review be taken to suggest that CFP network abnormalities are the only factors responsible for ADHD. Clearly, they are only part of the pathophysiology of ADHD. To fully characterize the disorder, the findings herein will need to be integrated with the wider literature on neurocircuitry models of ADHD—such as data on possible dysfunction of a proposed “default mode” network of the brain and/or reward/motivation networks—as reviewed in this issue and elsewhere (8).
Cingulo-Frontal-Parietal Attention Network
Imaging studies have attempted to identify the pathophysiology of ADHD by looking for abnormalities of brain regions that are normally involved in attention, cognition, executive function, motor control, response inhibition and working memory. This typically led to investigations centered on dorsal anterior midcingulate cortex (daMCC), dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), and to a lesser extent, parietal cortex. Together, these regions comprise the main components of the cingulo-frontal-parietal [CFP] cognitive-attention network (see Figure 1). These areas, along with striatum, premotor areas, thalamus and cerebellum have been identified as nodes within parallel networks of attention and cognition (9–21).
Figure 1. The Cingulo-Frontal-Parietal Cognitive/Attention Network.
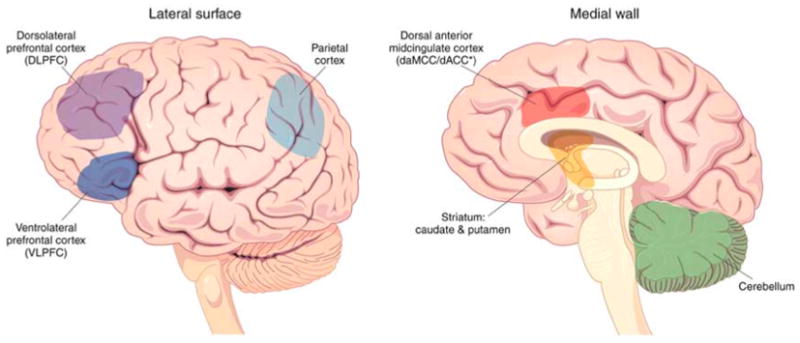
The dorsal anterior midcingulate cortex [daMCC], dorsolateral prefrontal cortex [DLPFC], ventrolateral prefrontal cortex [VLPFC] and parietal cortex comprise the CFP network. These regions work in concert with each other and other regions such as striatum and cerebellum to support normal cognition, attention and motor control processes. All of these brain regions have been found to display functional and structural abnormalities in ADHD.
Dorsal Anterior Midcingulate Cortex: Cognition, Attention and Motivation/Reward
The most consistent cross-study and cross-modality data identifying a region as dysfunctional in ADHD has been provided for the dorsal anterior midcingulate cortex (daMCC) (22). The daMCC, located on the medial surfaces of the frontal lobes, refers to areas 24c′/32′ in humans. The nomenclature of cingulate subdivisions has evolved over the past few decades (22–24). To clarify, the more recent term, daMCC, is equivalent to dorsal anterior cingulate cortex (dACC). As monkey connection studies have shown, it maintains strong reciprocal connections with other cognitive/attention and motor regions including DLPFC, parietal cortex, premotor cortex and striatum, and these differential connections may be correlated with different cognitive, motor and reward functions (25, 26).
The daMCC plays critical roles in attention, cognitive processing, target detection, novelty detection, response selection, response inhibition, error detection and motivation. Particularly relevant to reward/motivation and cognitive theories of ADHD, the daMCC is a key modulator of moment-to-moment adjustments in behavior via its primary role in feedback-based decision-making. As detailed elsewhere (22, 27, 28), this feedback-based decision-making conceptualization of daMCC is based on evidence from single unit studies in monkeys and humans as well as on human neuroimaging studies. The daMCC encompasses a local intracortical network comprised of functionally heterogeneous cells that variously anticipate and signal motivationally relevant targets, indicate novelty, encode reward values, signal errors and influence motor responses. The daMCC integrates goal and feedback related information from various sources and uses this information to modulate activity in executive brain regions that direct attention and produce motor responses. The daMCC thus acts within cognitive-reward-motor networks to increase the efficiency of decision-making and execution, and its proper function is therefore germane to ADHD.
Numerous functional, structural, connectionist, neurochemical and pharmacological imaging studies have identified abnormalities of daMCC in ADHD. Specifically, many fMRI, PET and event-related potential (ERP) studies have reported daMCC hypofunction in ADHD using a variety of tasks and techniques. Zametkin and colleagues (29) reported that even after normalization for an observed 8.1% global reduction of cerebral glucose metabolism in 25 treatment-naïve ADHD adults (compared to 50 adult controls), regional metabolism was specifically lower in ADHD in daMCC, premotor and somatosensory areas during a continuous performance task. As shown in Figure 2, the first fMRI study to specifically interrogate daMCC integrity in ADHD found that daMCC was hypoactive in ADHD adults during cognitive/attention task performance (30). Subsequent fMRI studies using a variety of tasks have similarly found relative daMCC hypofunction in ADHD compared to controls. These have included an fMRI study of adolescent boys with ADHD using a Go/NoGo task (31) (see Figure 3), as well as others using response inhibition and timing tasks (32–39).
Figure 2. The daMCC Shows Hypofunction in ADHD during Counting Stroop.
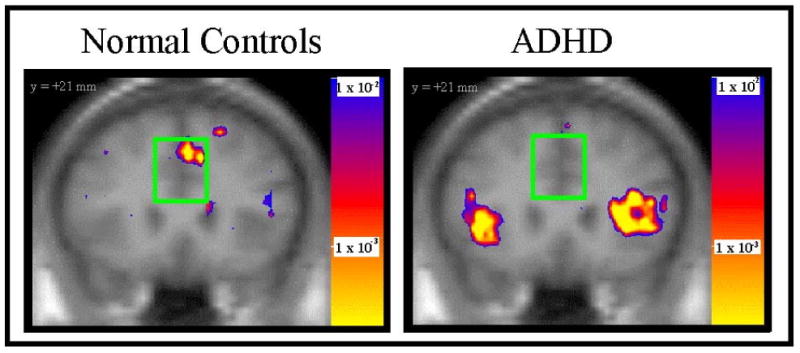
Dorsal anterior midcingulate cortex (daMCC) activated in healthy controls, but not in subjects with ADHD, during the Counting Stroop (30).
Figure 3. The daMCC Shows Hypofunction in ADHD during Response Inhibition.
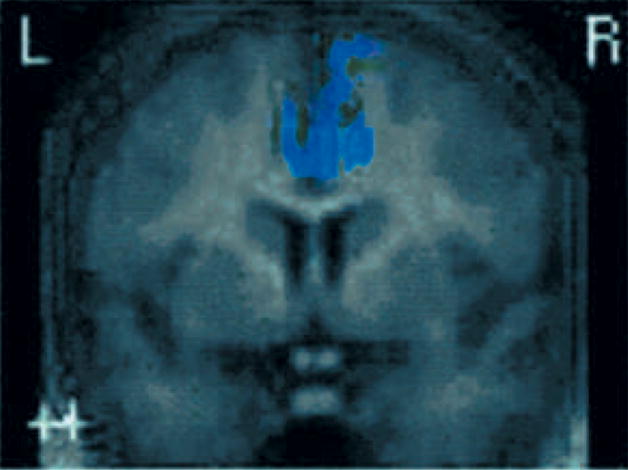
Tamm and colleagues (31) showed that daMCC was hypoactive in an ADHD group relative to control group during a response inhibition Go/NoGo task.
Importantly, a meta-analysis of neuroimaging studies by Dickstein and colleagues (40), shown in Figure 4, found daMCC to be among a limited number of brain regions that were hypoactive in ADHD relative to healthy controls. Using an activation likelihood estimate meta-analytic method (41), this review provided a relatively unbiased overview of ADHD imaging findings. ADHD was additionally found to be associated with significant hypoactivity of DLPFC, VLPFC, superior parietal cortex, caudate, and thalamus.
Figure 4. Meta-Analysis Shows daMCC and CFP Dysfunction in ADHD.
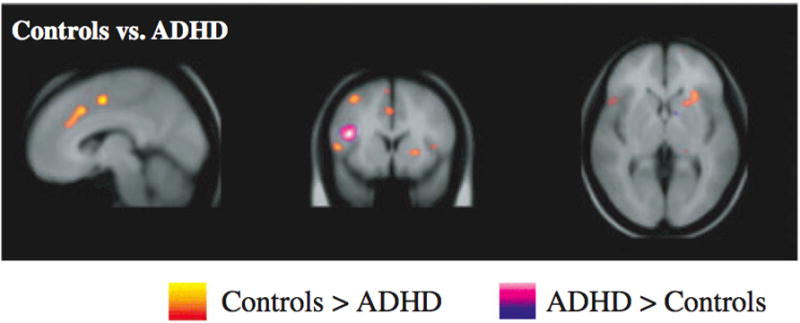
Dickstein and colleagues (40), via an activation likelihood meta-analysis, found daMCC to be among a limited number of brain regions that were hypoactive in ADHD relative to healthy controls. CFP network abnormalities were also reported.
Structural studies have also reported abnormalities within defined regions of cingulate cortex and lateral prefrontal cortex in ADHD. Smaller cingulate cortical volumes have been reported in adults (42) and children (43) with ADHD. More recently, Makris and colleagues (44) reported pilot study results showing that both treatment-naïve as well as treated adults with ADHD displayed significantly reduced ACC volumes. An earlier study of ADHD children, relevant to default mode network studies, showed a reduction in posterior cingulate volume in ADHD (45). Cortical thickness quantification via high-resolution MRI structural scans has been recently applied to the study of ADHD. Children with ADHD had significant global thinning of the cortex, most prominently in the cingulate and superior prefrontal regions (46). These data in children were generally consistent with the findings Makris et al (47) that showed selective cortical thinning of the daMCC and CFP attention networks in adults with ADHD. Connection studies using diffusion tensor imaging (DTI) by Makris and colleagues (48) have also identified abnormalities of cingulum bundle and superior longitudinal fascicle II in adults with ADHD—connection pathways that subserve attention and executive functions, and are thus highly relevant to ADHD.
Functional pharmacoimaging has begun to identify alterations in the CFP neural circuitry that may underlie ADHD and to characterize the mechanisms-of-action of medications used to treat it. Pharmacoimaging studies using fMRI complement dopaminergic imaging studies (discussed elsewhere in this issue) by highlighting the cingulate and frontal effects. Such reports now suggest that stimulant medications may work in part by increasing and therefore by possibly “normalizing” the generally observed hypoactivation of the CFP cognitive/attention network and striatum in ADHD.
Specifically, a recent study by Bush and colleagues (24) used fMRI in conjunction with a specialized cognitive task, the Multi-Source Interference Task (MSIT) (49), to determine if methylphenidate would increase activation in the daMCC and other fronto-parietal regions that subserve attention. This randomized, placebo-controlled, 6-week, pre/post study found a group x scan interaction and t-test confirmation of higher activation in the daMCC at 6 weeks in the MPH group, as compared to the placebo group. Moreover, the MSIT enabled single subject daMCC volume-of-interest analyses which confirmed the group-averaged findings and suggested that daMCC activity might be related to clinical response (see Figure 5). Beyond daMCC, 6 weeks of MPH also increased activation of many structures implicated in ADHD pathophysiology, including DLPFC, VLPFC, parietal cortex, caudate, thalamus and temporal lobe. These findings indicated that MPH may act, in part, by helping to normalize daMCC and wider CFP hypofunction in ADHD.
Figure 5. Methylphenidate increases daMCC and CFP activity in ADHD.
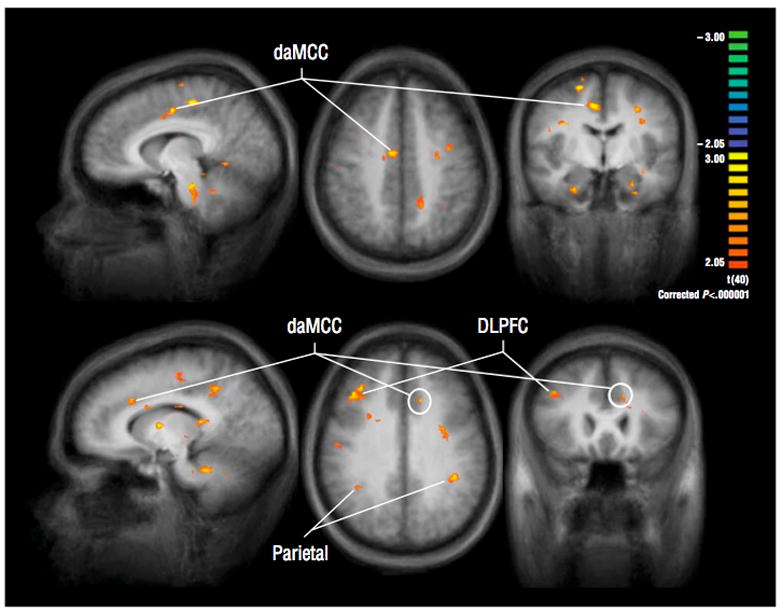
Using the MSIT and fMRI in 21 adults with ADHD, Bush and colleagues (24) showed that at 6 weeks, daMCC activation was higher in the group that received methylphenidate (N = 11) than in the group that received placebo (N = 10). Similar results were observed in dorsolateral prefrontal cortex (DLPFC), parietal cortex and networked regions.
Biochemical and electrophysiology studies mesh well with these pharmacoimaging findings. A magnetic resonance spectroscopy study found decreased choline and increased N-acetyl-aspartate (NAA) levels in daMCC following treatment of ADHD with 5–6 weeks of MPH, indicating biochemical changes occur in daMCC with MPH treatment (50). Pliszka and colleagues (51) found stimulant treatment increases ACC activity in ADHD using event-related potentials. Data on subsets of children from a 1 year follow-up fMRI study of ADHD suggested long-term effects of MPH on cingulate cortex, insula and putamen (52).
Recently, Brown and colleagues (53) provided new evidence on how genetic variations of the dopamine system dopamine transporter gene [SLC6A3] may be linked to alterations of daMCC function. In 42 adults with ADHD performing the Multi-Source Interference Task (MSIT) during fMRI, ADHD subjects homozygous for the 10R allele showed significant hypoactivation of left daMCC compared to 9R-carriers. Clearly, the accumulated data from these fMRI, PET and ERP studies, along with the biochemical, genetic, cortical thickness and volumetric data reviewed above, provide compelling evidence that daMCC dysfunction likely contributes to the pathophysiology of ADHD.
Lateral Prefrontal Cortex
While daMCC dysfunction likely contributes to the pathophysiology of ADHD, many brain regions have also been implicated, including other areas within the CFP cognitive/attention network. Research has focused mainly on DLPFC and VLPFC, as these regions are thought to support vigilance, selective and divided attention, attention shifting, planning, executive control, and working memory functions (10, 21). Also, VLPFC in particular has been associated with behavioral inhibition (32, 54). Though speculative, it may be that some of the inconsistency surrounding lateral prefrontal findings may be due in large part to the relatively increased spatial variability in the anatomic locations of these structures between subjects (i.e., centromedial brain structures, such as daMCC, show relatively less morphologic variability in probabilistic atlases than do lateral/peripheral regions such as DLPFC or VLPFC). Despite this though, structural and functional data support the conclusion that lateral prefrontal cortex abnormalities contribute to ADHD.
Structural imaging studies of ADHD have identified both 3–4% smaller global cerebral volumes in ADHD, as well as specifically smaller prefrontal volumes in ADHD (55, 56). More recently, Monuteaux et al (57) found that among adults with ADHD, subjects with the 7-repeat allele of the dopamine D4 receptor (DRD4) gene had a significantly smaller mean volume of superior frontal cortex and cerebellum compared to subjects without this allele. Cortical thickness maturation delays have been found in ADHD, with delays most prominent in lateral prefrontal cortex, especially the superior and DLPFC regions (58). In a separate study combining cortical thickness and genetics by the same group, cortical thinning of multiple regions, including orbitofrontal cortex, inferior prefrontal cortex and posterior parietal cortex, was associated with possession of the DRD4 7-repeat allele in both healthy children and those with ADHD (59). These brain regions were generally thinner in ADHD than controls, though a complicating factor was that ADHD patients with the DRD4 7-repeat allele did better clinically. Cortical thickness results have not, however, always been consistent. Though Wolosin et al (60) reported overall decreases of total cerebral and cortical volumes, and a significant decrease in cortical folding bilaterally in ADHD children, they did not find significant differences in cortical thickness between ADHD and healthy children.
Diffusion tensor imaging and fMRI have been combined to help identify abnormalities of connections of prefrontal cortical areas in ADHD. Casey and colleagues (61) used fMRI maps from a go/nogo task to identify portions of VLPFC and striatum involved in suppressing an inappropriate action in parent-child dyads with and without ADHD. They reported fractional anisotropy in right prefrontal fiber tracts was correlated with both functional activity in inferior frontal gyrus and caudate nucleus, and with performance of a go/nogo task in parent-child dyads with ADHD. Prefrontal fiber tract measures were also associated between ADHD parents and their children, suggesting disruption of fronto-striatal connections may play a role in ADHD. The work of Silk and colleagues (62) further indicates fronto-striatal and fronto-parietal circuitry abnormalities exist in children with ADHD.
A number of functional imaging studies have reported prefrontal cortical abnormalities in ADHD. In particular, dysfunction of DLPFC and VLPFC have been identified (3–5, 7, 38, 39, 54, 63). Fronto-temporal abnormalities were found in ADHD via a working memory task and PET (64). Ernst et al (65), employing a gambling task, provided data implicating VLPFC and daMCC in ADHD and highlighting the need to further examine cognitive, emotional and motivational interactions in its pathophysiology. Also, beyond the global and daMCC hypometabolism discussed above, the PET study by Zametkin and colleagues (29) also showed regional hypoactivity of superior prefrontal cortex and premotor cortex.
Importantly, the aforementioned meta-analysis by Dickstein and colleagues (40) provided confirmatory evidence of wider CFP neurocircuitry dysfunction in ADHD (cf. Figure 4). Also, by limiting their focus to response inhibition task studies, as suggested by the work of Durston et al (33) and Aron & Poldrack (66), they identified a more limited set of regions including VLPFC, daMCC, parietal cortex, caudate and precentral gyrus. Notably DLPFC was not included on this list. These data helped clarify that DLPFC and VLPFC abnormalities may contribute to ADHD in different ways.
Pharmacoimaging studies have further supported the conclusion that CFP hypofunction in occurs in ADHD. In the previously mentioned study by Bush and colleagues (24) it was also reported that beyond the daMCC findings, 6 weeks of methylphenidate also increased activation of DLPFC and VLPFC (and also parietal cortex, caudate, thalamus and temporal lobe). Non-stimulant medications used for ADHD have also been studied in healthy male adults with fMRI. Atomoxetine, a selective noradrenaline reuptake inhibitor, was found to increase both inhibitory control on a stop-signal task and right VLPFC activation (67).
Parietal Cortex
Parietal cortex, a third component of the CFP cognitive-attention network, has long been known to play important roles in attention and spatial processing. Specifically, parietal cortex plays key roles in attention allocation, and encompasses polymodal sensory convergence areas (68–71). Although parietal cortex has been the a priori focus of only a few ADHD functional imaging studies, it has been identified as displaying altered function in ADHD.
Tamm and colleagues (72) have shown that ADHD subjects performing a visual oddball task had significantly lower activation of parietal cortex, including superior parietal gyrus and multiple areas of inferior parietal lobe. Vance et al (73) reported that ADHD subjects performing a spatial working memory mental rotation task displayed significantly less inferior parietal lobe activation, in addition to lower parieto-occipital and caudate activation. In another study, ADHD children showed less activation than controls in multiple areas of parietal cortex, DLPFC and putamen. A lack of a difference in daMCC in this study may have been attributable to higher error rates in the ADHD group, since errors activate daMCC (74). Parietal hypofunction has also been observed in ADHD in tasks of mental rotation/spatial processing (75), task switching (76) and sequential finger tapping (77).
It has been suggested that such findings of parietal hypofunction might reflect secondary problems rather than primary neuroanatomical abnormalities. For example, hypoactivation during fMRI might occur due to abnormal input from regions that are connected to what would be otherwise normally functional parietal cortex. Although structural (cortical thickness) abnormalities in the parietal cortices of those with ADHD (47) further support the conclusion that parietal cortex functional abnormalities do play a role in ADHD pathophysiology, they do not resolve whether the observed parietal cortex differences are primary or secondary. Thus, while it is clear that hypofunctioning parietal cortical subdivisions likely play roles in ADHD pathophysiology, the challenges ahead will be in specifically parsing how different areas contribute to create the observed symptoms.
CFP Network Interactions and Conclusions
Advances have been made in identifying hypofunction within the CFP in ADHD. Specifically, it should be noted that while the data reviewed here strongly support the premises that (1) the CFP neural circuitry supports attention, cognition, motor control and motivation/reward processes in healthy humans, and (2) dysfunction of components of the CFP neural circuitry likely contributes to the pathophysiology of ADHD, the exact mechanisms by which such dysfunction leads to the symptoms of ADHD has yet to be determined.
In broad terms, the multiple functions of daMCC, DLPFC, VLPFC and parietal cortical regions alone provide a great many possibilities. Simplistically, it could be the case that for healthy humans, DLPFC is more responsible for overall planning and goal-setting, VLPFC and daMCC are responsible for inhibiting excessive or inappropriate motor behavior, heteromodal parietal cortex assists with target detection and attention shifts, and daMCC integrates information from these inputs and helps to execute such plans by modifying behavior on a trial-by-trial basis. Dysfunction within components of the CFP network in ADHD could therefore lead to inattention by failing to detect targets or inadequately filtering noise within the system. Such dysfunction could also lead to hyperactivity by failing to adequately inhibit motor activity that is not in line with motivated goals, or by failing to use reward and error feedback to modify behavior. Similarly, impulsivity could be produced by insufficient encoding of motivational goals and/or the impaired ability to preferentially pursue long-term goals over short-term goals.
Of course, the reality is much more complex. Beyond just the CFP intranetwork communications, it has been shown how the CFP network interacts with striatum, premotor cortex, cerebellum, superior temporal sulcus, thalamus, and the brain stem reticular activating system to support cognitive-motor processing. Also, reward/motivational information (encoded by striatum, daMCC, nucleus accumbens and orbitofrontal cortex) is integrated with information from default mode network regions (perigenual ACC, medial PFC, portions of VLPFC, amygdala and posterior cingulate cortex).
Interactions within such networks and the specific roles of each region are starting to be parsed out experimentally. For example, Corbetta and colleagues have postulated that a “reorienting response” relies on the coordinated action of a dorsal frontoparietal network that links stimuli and responses and helps select actions, along with a predominantly right hemispheric ventral frontoparietal network which serves to interrupt and reset ongoing activity. Further, they hypothesize that when attention for a specific task is required, the ventral network is suppressed to prevent reorienting to distracting events (78). Distinct and separable roles for DLPFC, daMCC and parietal cortex in cognitive processing have also been suggested by Liston et al. (79). Dosenbach and colleagues have suggested that parallel “hybrid” control systems are possible in which transient activity of a fronto-parietal network reflects trial-by-trial adjustments, while sustained activity of cingulo-opercular regions throughout trials may indicate that it is more responsible for set maintenance (80, 81). Recent work has utilized event-related fMRI and functional connectivity analyses to identify how different elements of proposed interacting networks are responsible for the maintenance of attention on a target, cued shifts of attention, and reorienting to an unexpected target (82).
Translations of such network models into testable predictions about ADHD network circuitry have commenced. For example, it has been hypothesized (83, 84) that in ADHD, abnormal activity in “default mode” brain systems that normally subserve resting state and vigilance functions (85, 86) may interfere with CFP-modulated attention systems. Castellanos and colleagues (87, 88) have reported abnormal connectivity within default network structures (VMPFC, precuneus, and posterior cingulate cortex) and furthermore altered functional connectivity between the daMCC and default network areas (precuneus and posterior cingulate cortex). Finally, Liston and colleagues recently reported that psychosocial stress reversibly and selectively impairs attention control and disrupts functional connectivity within a frontoparietal network that mediates attention shifts (89). While admittedly speculative, it would be interesting to extend beyond these findings to determine if the chronic stress within those with ADHD could (1) parametrically contribute to the disruption of functional attention network integrity in ADHD and (2) if stress-reduction techniques such as relaxation response training, meditation or yoga could be used to alleviate some portion of ADHD morbidity by strengthening CFP network connections. For the interested reader, fuller explanations for how such observed CFP cognitive-attention network abnormalities described here might lead to specific ADHD symptoms appear elsewhere within this special issue and also in other sources (8, 22, 80, 81).
Lastly, although this narrow review focuses on CFP network abnormalities, it is important to recall that many other systems have been implicated, Most prominently, studies of subcortical dysfunction and dopaminergic modulatory functions have been reported and must be integrated with CFP neurocircuitry models. The interested reader can find reviews of dopaminergic imaging relevant to ADHD (90, 91) as well as the roles various neurotransmitters may play in the pathophysiology of ADHD (92-94). Dopamine plays roles on attention, cognition and reward processes (92, 95–97) and can increase the neuronal signal-to-noise ratio both by boosting signal and dampening background noise (98). Dopamine also displays an inverted-U influence such that it optimizes neural transmission within a range but may adversely affect performance at lower or higher levels (92). Volkow and colleagues showed specific activity of methylphenidate in basal ganglia (99), that it blocks the dopamine transporter (DAT) (100), and that methylphenidate increases extracellular dopamine in striatum (101). Spencer et al (102) confirmed how striatal effects of methylphenidate match behavioral effects using immediate and extended release formulations. Studies of the dopamine transporter (DAT), which is primarily responsible for presynaptic reuptake of dopamine, have shown that methylphenidate blocks striatal DAT and increases extracellular dopamine (90, 91, 98, 103, 104). These studies dovetail nicely with imaging studies that illustrate striatum dysfunction in ADHD by Durston, Casey, Vaidya, Epstein and colleagues (7, 105–111).
In conclusion, functional, structural, biochemical and connectionist imaging data have identified abnormalities of brain regions within CFP networked functional systems, and pharmacoimaging has helped to identify ways that medications used to treat ADHD exert their effects. It remains to be determined how the CFP network functions during cognitive and reward processing, and more specifically how dysfunction of the component regions contribute to the pathophysiology of ADHD.
Acknowledgments
The author wishes to thank Jennifer Holmes, Scott Rauch, Michael Jenike, Michael Posner, Brent Vogt, Joseph Biederman, Thomas Spencer and the MGH Pediatric Psychopharmacology Clinic staff for invaluable assistance, support, mentoring and collaboration related to the topics discussed herein; as well as the anonymous reviewers for their many helpful comments.
Footnotes
Disclosure/Conflict of Interest
This review was produced without direct support or compensation. Indirect support has been provided to the author for cingulate and ADHD-related work over the past decade in the form of grant or general support by the Centers for Disease Control (CDC), the National Institutes of Mental Health (NIMH), the National Science Foundation (NSF), the Mental Illness and Neuroscience Discovery (MIND) Institute, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Benson-Henry Institute for Mind-Body Medicine at Massachusetts General Hospital, the David Judah Fund, the McIngvale Fund, the Johnson and Johnson Center for the Study of Psychopathology, the Center for Functional Neuroimaging Technologies (P41RR14075), McNeil Pharmaceuticals, Pfizer Pharmaceuticals and Eli Lilly & Co. The author has, or has had in the past, a relationship with one or more organizations listed below as follows: former advisory board member and speaker’s honoraria from Eli Lilly and Company and Novartis Pharmaceuticals; and has received speaker’s honoraria from Shire U.S. Inc., Janssen Pharmaceuticals, Johnson & Johnson and McNeil Pharmaceuticals. The author does not now and has not at any time had a financial interest in any of these entities.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zametkin AJ, Liotta W. The neurobiology of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 1998;59(Suppl 7):17–23. [PubMed] [Google Scholar]
- 2.Giedd JN, Blumenthal J, Molloy E, Castellanos FX. Brain imaging of attention deficit/hyperactivity disorder. Ann N Y Acad Sci. 2001;931:33–49. doi: 10.1111/j.1749-6632.2001.tb05772.x. [DOI] [PubMed] [Google Scholar]
- 3.Durston S. A review of the biological bases of ADHD: what have we learned from imaging studies? Ment Retard Dev Disabil Res Rev. 2003;9:184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- 4.Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biological Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Schneider M, Retz W, Coogan A, Thome J, Rosler M. Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD)--a neurological view. Eur Arch Psychiatry Clin Neurosci. 2006;256(Suppl 1):i32–41. doi: 10.1007/s00406-006-1005-3. [DOI] [PubMed] [Google Scholar]
- 6.Kelly AM, Margulies DS, Castellanos FX. Recent advances in structural and functional brain imaging studies of attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2007;9:401–407. doi: 10.1007/s11920-007-0052-4. [DOI] [PubMed] [Google Scholar]
- 7.Vaidya CJ, Stollstorff M. Cognitive neuroscience of Attention Deficit Hyperactivity Disorder: current status and working hypotheses. Dev Disabil Res Rev. 2008;14:261–267. doi: 10.1002/ddrr.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 10.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 11.Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 13.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- 14.Colby CL. The neuroanatomy and neurophysiology of attention. J Child Neurol. 1991;6(Suppl):S90–118. doi: 10.1177/0883073891006001s11. [DOI] [PubMed] [Google Scholar]
- 15.Berman R, Colby C. Attention and active vision. Vision Res. 2008 doi: 10.1016/j.visres.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 17.Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 18.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morecraft RJ, Geula C, Mesulam MM. Architecture of connectivity within a cingulo-fronto-parietal neurocognitive network for directed attention. Arch Neurol. 1993;50:279–284. doi: 10.1001/archneur.1993.00540030045013. [DOI] [PubMed] [Google Scholar]
- 20.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 21.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 22.Bush G. Dorsal anterior midcingulate cortex: Roles in normal cognition and disruption in attention-deficit/hyperactivity disorder. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford University Press; 2009. pp. 245–274. [Google Scholar]
- 23.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bush G, Spencer TJ, Holmes J, Shin LM, Valera E, Seidman LJ, et al. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the Multi-Source Interference Task. Archives of General Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- 25.Morecraft RJ, Tanji J. Cingulofrontal interactions and the cingulate motor areas. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford University Press; 2009. pp. 113–144. [Google Scholar]
- 26.Rolls ET. Cingulate Neurobiology and Disease. Oxford University Press; 2009. The anterior and midcingulate cortices and reward; pp. 191–206. [Google Scholar]
- 27.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- 29.Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med. 1990;323:1361–1366. doi: 10.1056/NEJM199011153232001. [DOI] [PubMed] [Google Scholar]
- 30.Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 31.Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 32.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 33.Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage. 2003;20:2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Zang YF, Jin Z, Weng XC, Zhang L, Zeng YW, Yang L, et al. Functional MRI in attention-deficit hyperactivity disorder: evidence for hypofrontality. Brain Dev. 2005;27:544–550. doi: 10.1016/j.braindev.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:643–651. doi: 10.1016/j.biopsych.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- 37.Durston S, Davidson MC, Mulder MJ, Spicer JA, Galvan A, Tottenham N, et al. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 38.Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, 3rd, Xiong J, et al. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry. 2006;163:1052–1060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- 39.Smith AB, Taylor E, Brammer M, Halari R, Rubia K. Reduced activation in right lateral prefrontal cortex and anterior cingulate gyrus in medication-naive adolescents with attention deficit hyperactivity disorder during time discrimination. J Child Psychol Psychiatry. 2008;49:977–985. doi: 10.1111/j.1469-7610.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 40.Dickstein SG, Bannon K, Xavier Castellanos F, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 41.Lancaster JL, Laird AR, Fox PM, Glahn DE, Fox PT. Automated analysis of meta-analysis networks. Hum Brain Mapp. 2005;25:174–184. doi: 10.1002/hbm.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, et al. Dorsolateral Prefrontal and Anterior Cingulate Cortex Volumetric Abnormalities in Adults with Attention-Deficit/Hyperactivity Disorder Identified by Magnetic Resonance Imaging. Biological Psychiatry. 2006:60. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 43.Semrud-Clikeman M, Pliszka SR, Lancaster J, Liotti M. Volumetric MRI differences in treatment-naive vs chronically treated children with ADHD. Neurology. 2006;67:1023–1027. doi: 10.1212/01.wnl.0000237385.84037.3c. [DOI] [PubMed] [Google Scholar]
- 44.Makris N, Seidman LJ, Valera EM, Biederman J, Monuteaux MC, Kennedy DN, et al. Anterior cingulate volumetric alterations in treatment-naive adults with ADHD: a pilot study. J Atten Disord. 2010;13:407–413. doi: 10.1177/1087054709351671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overmeyer S, Bullmore ET, Suckling J, Simmons A, Williams SC, Santosh PJ, et al. Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychol Med. 2001;31:1425–1435. doi: 10.1017/s0033291701004706. [DOI] [PubMed] [Google Scholar]
- 46.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 47.Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 48.Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cereb Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- 49.Bush G, Shin LM. The Multi-Source Interference Task: An fMRI Task that Reliably Activates the Cingulo-Frontal-Parietal Cognitive/Attention Network in Individual Subjects. Nature Protocols. 2006;1:308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- 50.Kronenberg G, Ende G, Alm B, Deuschle M, Heuser I, Colla M. Increased NAA and reduced choline levels in the anterior cingulum following chronic methylphenidate. A spectroscopic test-retest study in adult ADHD. Eur Arch Psychiatry Clin Neurosci. 2008;258:446–450. doi: 10.1007/s00406-008-0810-2. [DOI] [PubMed] [Google Scholar]
- 51.Pliszka SR, Liotti M, Bailey BY, Perez R, 3rd, Glahn D, Semrud-Clikeman M. Electrophysiological effects of stimulant treatment on inhibitory control in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:356–366. doi: 10.1089/cap.2006.0081. [DOI] [PubMed] [Google Scholar]
- 52.Konrad K, Neufang S, Fink GR, Herpertz-Dahlmann B. Long-term effects of methylphenidate on neural networks associated with executive attention in children with ADHD: results from a longitudinal functional MRI study. J Am Acad Child Adolesc Psychiatry. 2007;46:1633–1641. doi: 10.1097/chi.0b013e318157cb3b. [DOI] [PubMed] [Google Scholar]
- 53.Brown AB, Biederman J, Valera EM, Doyle AE, Bush G, Spencer T, et al. Effect of dopamine transporter gene (SLC6A3) variation on dorsal anterior cingulate function in attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:365–375. doi: 10.1002/ajmg.b.31022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 55.Seidman LJ, Valera EM, Bush G. Brain function and structure in adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:323–347. doi: 10.1016/j.psc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Monuteaux MC, Seidman LJ, Faraone SV, Makris N, Spencer T, Valera E, et al. A preliminary study of dopamine D4 receptor genotype and structural brain alterations in adults with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1436–1441. doi: 10.1002/ajmg.b.30870. [DOI] [PubMed] [Google Scholar]
- 58.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw P, Gornick M, Lerch J, Addington A, Seal J, Greenstein D, et al. Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- 60.Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Hum Brain Mapp. 2009;30:175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 62.Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: A diffusion tensor imaging study. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobel M, Bechtel N, Weber P, Specht K, Klarhofer M, Scheffler K, et al. Effects of methylphenidate on working memory functioning in children with attention deficit/hyperactivity disorder. Eur J Paediatr Neurol. 2008 doi: 10.1016/j.ejpn.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Schweitzer JB, Faber TL, Grafton ST, Tune LE, Hoffman JM, Kilts CD. Alterations in the functional anatomy of working memory in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2000;157:278–280. doi: 10.1176/appi.ajp.157.2.278. [DOI] [PubMed] [Google Scholar]
- 65.Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, et al. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160:1061–1070. doi: 10.1176/appi.ajp.160.6.1061. [DOI] [PubMed] [Google Scholar]
- 66.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 67.Chamberlain SR, Hampshire A, Muller U, Rubia K, Del Campo N, Craig K, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 68.Culham J. Dissociations within association cortex. Neuron. 2002;33:318–320. doi: 10.1016/s0896-6273(02)00584-6. [DOI] [PubMed] [Google Scholar]
- 69.Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 70.Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci U S A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 72.Tamm L, Menon V, Reiss AL. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am J Psychiatry. 2006;163:1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- 73.Vance A, Silk TJ, Casey M, Rinehart NJ, Bradshaw JL, Bellgrove MA, et al. Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Mol Psychiatry. 2007;12:826–832. 793. doi: 10.1038/sj.mp.4001999. [DOI] [PubMed] [Google Scholar]
- 74.Cao Q, Zang Y, Zhu C, Cao X, Sun L, Zhou X, et al. Alerting deficits in children with attention deficit/hyperactivity disorder: event-related fMRI evidence. Brain Res. 2008;1219:159–168. doi: 10.1016/j.brainres.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 75.Silk T, Vance A, Rinehart N, Egan G, O'Boyle M, Bradshaw JL, et al. Fronto-parietal activation in attention-deficit hyperactivity disorder, combined type: functional magnetic resonance imaging study. Br J Psychiatry. 2005;187:282–283. doi: 10.1192/bjp.187.3.282. [DOI] [PubMed] [Google Scholar]
- 76.Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- 77.Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, et al. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 78.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, et al. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and Basal Ganglia-cortical networks. J Neurosci. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 84.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007 doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 85.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 87.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 89.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spencer TJ, Biederman J, Madras BK, Faraone SV, Dougherty DD, Bonab AA, et al. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biol Psychiatry. 2005;57:1293–1300. doi: 10.1016/j.biopsych.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 91.Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- 92.Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arnsten AF. Modulation of prefrontal cortical-striatal circuits: relevance to therapeutic treatments for Tourette syndrome and attention-deficit hyperactivity disorder. Adv Neurol. 2001;85:333–341. [PubMed] [Google Scholar]
- 94.Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- 95.Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- 96.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 97.Schultz W. The phasic reward signal of primate dopamine neurons. Adv Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- 98.Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Ding YS, Fowler JS, Volkow ND, Dewey SL, Wang GJ, Logan J, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacology (Berl) 1997;131:71–78. doi: 10.1007/s002130050267. [DOI] [PubMed] [Google Scholar]
- 100.Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 101.Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spencer T, Biederman J, Ciccone P, Madras B, Dougherty D, Bonab A, et al. A PET Study Examining Pharmacokinetics, Detection and Likeability, and Dopamine Transporter Receptor Occupancy Of Short and Long-Acting Orally Administered Formulations of Methylphenidate in Adults. American Journal of Psychiatry. 2006;163:387–395. doi: 10.1176/appi.ajp.163.3.387. [DOI] [PubMed] [Google Scholar]
- 103.Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6(Suppl 1):S31–43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- 104.Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 105.Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 106.Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 107.Casey BJ, Nigg JT, Durston S. New potential leads in the biology and treatment of attention deficit-hyperactivity disorder. Curr Opin Neurol. 2007;20:119–124. doi: 10.1097/WCO.0b013e3280a02f78. [DOI] [PubMed] [Google Scholar]
- 108.Durston S. Converging methods in studying attention-deficit/hyperactivity disorder: what can we learn from neuroimaging and genetics? Dev Psychopathol. 2008;20:1133–1143. doi: 10.1017/S0954579408000539. [DOI] [PubMed] [Google Scholar]
- 109.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2005;162:1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A, et al. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J Child Psychol Psychiatry. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]


