Abstract
Two experiments compared Spontaneously Hypertensive Rats (SHRs; a rodent model of attention-deficit/hyperactivity disorder) and Wistars (a normoactive control strain), on the acquisition of a set-shifting strategy. In Experiment 1, SHRs and Wistars were equivalent in trials to criterion to learn a brightness or a texture discrimination but SHRs were faster than Wistars in shifting to the opposite discrimination when there was one or two days between the initial discrimination and the shift. In Experiment 2, SHRs and Wistars were equivalent in shifting when the shift between discriminations occurred immediately after a criterion had been met in the first discrimination. The results are discussed in terms of a failure of SHRs to store or retrieve an initial discrimination and/or latent inhibition over a delay, leading to faster acquisition of a set-shift. This failure in storage or retrieval may be the result of a hypoactive dopamine system in the prefrontal cortex and nucleus accumbens shell as well as abnormalities in entorhinal cortex in SHRs.
Keywords: SHR, spontaneously hypertensive rat, ADHD, set-shifting, extradimensional
Attention-deficit/hyperactivity disorder (ADHD) is a neurobiological disorder that typically manifests by the age of seven years and is more prevalent in boys than in girls. It affects approximately 2-12% of children and is defined by three central clinical symptoms: Inattention, hyperactivity, and impulsivity. These symptoms can manifest as distractibility, difficulty sustaining attention, and a failure to appropriately control motor responses. Some have suggested that all of these symptoms can be attributed to a primary deficit of behavioral inhibition (e.g. Nigg, 2001; Barkley, 1997).
Executive functions, such as working memory, inhibition, and set-shifting, rely on prefrontal cortex (PFC) and are generally thought to be impaired in children and adults with ADHD. Set-shifting is a cognitive function that requires attending to relevant stimuli while ignoring irrelevant stimuli (i.e., forming an attentional set) and subsequently shifting the allocation of attention, either within “dimensions” (e.g., discriminating first on the basis of one color and then shifting attention to discriminate on the basis of another color) or between “dimensions” (e.g., discriminating first on the basis of color and then shifting attention to discriminate on the basis of shape). This latter type of shift was utilized in the current studies, and is referred to as an extradimensional set-shift, where the subject learns that the previously relevant dimension (e.g., color) is now irrelevant and the previously irrelevant dimension (e.g. shape) is now rewarded (Slamecka, 1968; George et al., 2010).
Set-shifting in children and adults with ADHD has been assessed with a number of different neuropsychological tests, and whether set-shifting is impaired in participants with ADHD may depend upon which test is administered. For example, on the Wisconsin Card Sorting Test (WCST), the results of some studies suggest that children and adults with ADHD do not make more perseverative errors than controls (e.g., Biederman, Petty, Doyle, Spencer, Henderson, Marion, & Fried, 2008; Boonstra, Kooij, Oosterlaan, Sergeant, & Buitelaar, 2010; Pasini et al., 2007; Willcutt, Pennington, Boada, Ogline, Tunick, & Chhabildas, 2001) while the results of other studies suggest that they do (Lawrence, Houghton, Douglas, Durkin, Whiting, & Tannock, 2004; Mulas, Capilla, Fernandez, Etchepareborda, Campo, Maestu et al., 2006). Perseverative errors are defined as continuing to respond on the basis of the initial, relevant set, even after that set is no longer reinforced. Other tests of set-shifting have also yielded mixed results. For example, in the attentional set-shifting paradigm from the Attentional Battery of the Cambridge Neuropsychological Test Automated Battery (CANTAB), in which the requirement is to discriminate between two stimuli that differ in color and shape, children and adults with ADHD sometimes make more errors in a set-shift (e.g., Kempton, Vance, Maruff, Luk, Costin, & Pantelis, 1999; McLean, Dowson, Toone, Young, Bazanis, Robbins, & Sahakian, 2004) and sometimes do not (e.g., Goldberg, Mostofsky, Cutting, Mahone, Astor, Denckla, & Landa, 2005). On the other hand, the Trail Making Test (TMT), Part B from the Halstead-Reitan battery generally takes longer to complete (an indication of slower set-shifting) in children and adults with ADHD (e.g., Loo, Rich, Ishii, McGough, McCracken, Nelson, & Smalley, 2008; Muller, Gimbel, Keller-Pliesnig, Sartory, Gastpar, & Davids, 2007; Pasini, Paloscia, Allesandrelli, Profirio, & Curatolo, 2007; Toplak, Bucciarelli, Jain, & Tannock, 2009; but see Willcutt et al., 2001). As with all neuropsychological testing, the limitations of these studies include variability in diagnosis and sample demographics. In reviewing set-shifting and ADHD, Nigg (2006) has also pointed out that these different tests can require various cognitive and motor skills that are not necessarily intrinsic to set-shifting, including working memory (e.g., WCST) and fine motor control (e.g., TMT). In addition, set-shifting performance can be assessed with different dependent measures, including number of errors (e.g., WCST, CANTAB) and reaction time (e.g., TMT). Assessing set-shifting in an animal model of ADHD can help address some of these limitations and provide a basis for elucidating relatively precise neural mechanisms.
In the last decade, two types of set-shifting tasks, based on the human neuropsychological literature, have been used with rodents to explore the neural substrates of set-shifting (for a review, see Floresco, Zhang, & Enomoto, 2009). One type, introduced by Birrell and Brown (2000), is a rodent analog of the multi-stage set formation and set-shifting task in the CANTAB (Robbins, James, Owen, Sahakian, Lawrence, McInnes, & Rabbitt, 1998). This task involves training rats to discriminate between rewarded and non-rewarded bowls on the basis of odor and texture of the digging medium. Rats are then put through a reversal, an intradimensional set-shift, and an extradimensional set-shift. The other measure of set-shifting in rodents, more akin to the WCST (in which there is typically only a discrimination followed by an extradimensional set-shift) and the task that we used in the current experiments, involves a cross-maze (Ragozzino, Detrick, & Kesner 1999; Stefani, Groth, & Moghaddam, 2003). In this task, each trial is conducted with the cross maze in a ‘T’ configuration, the maze is rotated after each trial, and a new arm is blocked. The arms can be discriminated along two dimensions; in the current study, the arms could be discriminated on the basis of texture (rough or smooth) versus brightness (white or black).
Rats are initially trained to discriminate rewarded arms from non-rewarded arms on the basis of one dimension (e.g., rewarded arms are white). Subsequently, rats can be required to make a reversal (e.g., rewarded arms are black) or an extradimensional set-shift (e.g., rewarded arms are smooth). Set-shifting is critically dependent upon the medial PFC (mPFC; Birrell & Brown, 2000; Floresco, Block, & Tse, 2008; Ragozzino et al., 1999; Rich & Shapiro, 2007; Stefani et al., 2003; Stefani & Moghaddam, 2005) while reversal is critically dependent upon the orbitofrontal cortex (OFC; Ghods-Sharifi, Haluk, & Floresco, 2008; McAlonan & Brown, 2003). The nucleus accumbens (NAc) core (Floresco, Ghods-Sharifi, Vexelman, & Magyar, 2006) and the caudate-putamen (CPu; Ragozzino, Ragozzino, Mizumori, & Kesner, 2002) appear to be necessary for the maintenance of the new strategy after the set-shift but not for the initial shift in strategies, while the mediodorsal (MD) thalamic nuclei appear to be necessary for the initial shift in strategies but not the maintenance of the new strategy (Block, Dhanji, Thompson-Tardif, & Floresco, 2007).
The Spontaneously Hypertensive Rat (SHR) has been investigated extensively for its utility as an animal model of attention-deficit/hyperactivity disorder (ADHD) (see Sagvolden et al., 2005 for a review). These studies have examined a wide variety of neurochemical, genetic, and behavioral characteristics of the SHR to evaluate the extent to which the SHR models genotypic and phenotypic features observed in ADHD. In terms of executive functions, only one study to date has specifically addressed the nature of set-shifting ability in SHRs and it was reported that SHRs exhibited poorer set-shifting compared to inbred Wistar-Kyoto (WKY) rats (Kantak, Singh, Kerstetter, Dembro, Mutebi, Harvey et al., 2008). However, that study also reported that SHRs were poorer in the initial discrimination (Set 1) compared to WKY rats, making interpretation of poorer performance in Set 2 difficult. For example, rather than difficulty with set-shifting, it may be that sensory, motor, or motivational variables explain the poorer performance of SHRs in discrimination in general. Given the importance of set-shifting for understanding ADHD, we believed that further examination of this issue was warranted.
Experiment 1
The purpose of Experiment 1 was to compare set-shifting in a rat model of ADHD, the SHR, to set-shifting in an outbred control strain, the Wistar rat. In the current experiment, we observed better, not worse, set-shifting in SHRs after equivalent performance in an initial discrimination, in contrast to Kantak et al. (2008).
Method
Subjects
Subjects were 9 male Wistar rats and 9 male SHRs (SHR/NHsd) from Harlan (Indianapolis, IN). Rats were between 59 and 63 days old when they arrived in the colony. All rats were given at least 5 days of acclimation in the colony following their arrival, during which time they were provided unlimited access to food and water. Rats were housed individually. Prior to training on the set-shifting task, baseline weights were obtained and rats were then food-deprived to 85% of their initial free-feeding weight. The University of Vermont Institutional Animal Care and Use Committee approved all procedures.
Apparatus
The set-shift apparatus and procedures are modifications of those previously described by Stefani et al., 2003. The cross-maze was on a table located in a quiet, nondescript room with minimal overhead lighting (illumination at the top of the center square of the maze = 48 lux; illumination at the floor of the center square = 26 lux). During all procedures, a white noise generator underneath the table that the maze was on was used to produce a constant background masking noise (53 db measured at the floor of the center square of the maze). The maze was constructed of painted polycarbonate, and consisted of a square central platform (each side measured 14.0 cm), to which four arms were attached. Each arm was immediately adjacent to another arm, so that there was no space in between the arms. Each arm was 14.0 cm wide, 40.6 cm long, and 20.3 cm high. A food well was located 2.5 cm from the end of each arm, and measured 1.9 cm in diameter and 0.63 cm deep. The food pellets could not be detected visually from the arm entrance. The four arms varied along two dimensions: brightness and texture. Two of the arms were painted black, while two of the arms were painted white. Of the four arms, two had a smooth texture, while two of the arms had a rough texture that was achieved by mixing a small amount of sand into the paint. Thus, the arms were black/smooth, black/rough, white/smooth, and white/rough. The central platform was painted with a grey primer. A polycarbonate insert (also painted with a grey primer) could be positioned between the central platform and any one of the arm entrances to create a T configuration with the remaining open arms and the central platform (see Stefani et al., 2003 for diagram). The rats were held in a gray polycarbonate holding chamber (35.6 cm × 35.6 cm × 35.6 cm) containing animal bedding during the intertrial intervals (ITIs).
Habituation
Approximately 5-7 days following arrival in the colony, a daily handling regimen commenced. During the first three days of this handling regimen, each rat was handled for 3-5 minutes, and then weighed. On the third day of handling and weighing, food was taken away. On subsequent days, rats were handled, weighed, and then fed an amount of food that would maintain the rats at 85% of their baseline weight. Once the baseline weight was achieved, habituation training began. The first phase of habituation involved “open-arm” habituation. This phase was designed to acquaint the rats with the maze and shape the rat so that they reliably ate from all of the food wells relatively quickly. On the first day of open-arm habituation, 45-mg pellets (TestDiet) were scattered throughout all arms of the maze, the central platform, and in each food well. Each rat was allotted 10 minutes to freely explore the entire maze and eat the pellets. The rat was first placed in the distal end of the “start arm,” which was randomly selected. A timer was started, and the rat was allowed to explore and eat for 10 minutes. At the end of the 10 minutes, rats were removed and placed in the holding chamber for two minutes. The rats were then placed back into their home cages where they received ~1 gram of the 45-mg pellets used in the maze, in addition to their daily food rationing. This helped acquaint the rats with the taste of the new pellets and encourage them to eat during subsequent days in the maze. On the second day of “open-arm” habituation, each food well was baited with several pellets, and pellets were scattered only in the half of each arm closest to the food well. Rats were allowed to explore the maze for five minutes or until all pellets were consumed. Rats were again placed in the holding chamber for two minutes at the conclusion of their session. On the third day of habituation, four pellets were placed in each of the four food wells (for a total of 16 pellets). Rats were again allowed to explore the maze until five minutes had elapsed or all pellets had been consumed. On the fourth day of open-maze habituation, a single pellet was placed in each food well. Rats were given two minutes to consume all of the food pellets, and were placed in the holding chamber for two minutes at the conclusion of the session. If rats did not meet the criterion of consuming all four food pellets within two minutes, they were given an additional session of open-arm habituation.
The next phase of habituation was “blocked arm habituation” and consisted of eight trials per day over two consecutive days. The purpose of this habituation phase was to acquaint the animal with the T configuration of the maze, as well as being repeatedly moved from the maze to the holding chamber in quick succession. The polycarbonate insert was placed between the central platform and one of the arms. The start arm (the stem of the T) was randomly selected and differed for each rat. The other two arms that remained accessible from the central platform were baited with a single pellet each. Rats were placed in the distal end of the start arm, allowed to choose one of the arms and consume the food reward located in the food well of the chosen arm. Rats were then removed from the maze and placed in the holding chamber for a 15-s ITI. During the ITI, the maze was rotated so that the rat would begin the next trial from a different start arm. The start arm was altered on each trial to discourage the development of habit- or place-based strategies. On the second trial, the arm opposite of the chosen arm on the previous trial was baited. The rat was again placed in the start arm, allowed to choose one of the arms, obtain a reward if the correct arm was chosen, and was removed to the holding chamber for the 15-s ITI. This procedure was repeated for 6 more trials. The arms were baited randomly, such that the probability of being rewarded for any particular arm choice (e.g. black arms, smooth arms, left arms) was equal. The goal was to have the rat make an arm choice in order to receive a food pellet. At this stage, it was not desirable for the rats to make associations between particular responses and reinforcement. If rats obtained a reinforcer on three consecutive trials, reward was completely omitted from the subsequent trial (e.g. neither of the arms were baited). A second day of blocked-arm habituation was conducted.
Set-Shift Procedure
The set-shifting procedure comprised two daily sessions, Set 1 and Set 2. For 6 SHRs and 6 Wistars, the sets were one day apart. For 3 SHRs and 3 Wistars, the sets were two days apart. Prior to the set-shifting portion of the experiment, rats were randomly assigned to a stimulus-reward contingency (e.g. stimulus dimension: Texture; rewarded stimulus attribute: Smooth). During Set 1, the rat was first placed in the holding chamber for two minutes. Next, the rat was placed in the start arm (which was changed for each trial), and was allowed to make an arm choice. If the rat chose the rewarded stimulus attribute (correct arm), the rat would find a pellet at the end of the arm. If the rat chose a non-rewarded stimulus attribute (incorrect arm), the rat would not find a pellet. The rat was then removed to the holding chamber for the 15-s ITI, the maze was rotated and re-baited, and the rat would receive further trials until a criterion of eight consecutive correct choices was reached. An arm entry was only considered if all four paws were in contact with the arm. If a rat put two paws into an arm and then turned around and chose the alternate arm, the investigation of the first arm was not considered a committed arm entry because the rat needed to experience the tactile stimuli in order to make an informed arm entry choice.
For Set 2, the rewarded stimulus dimension was shifted. For example, a rat that was previously rewarded for choosing smooth arms might now be rewarded for choosing white arms. The shift in stimulus-reward contingency always occurred across stimulus dimensions. In other words, if texture (smooth or rough) was rewarded during Set 1, brightness (black or white) would be rewarded during Set 2. Unlike during Set 1, all rats were trained for at least 80 trials, regardless of how many trials were required to reach the criterion of eight consecutive correct arm choices. Time to reach criterion was recorded for both sets.
Data Analysis
Trials to criterion for Set 1 was analyzed by one-way analysis of variance (ANOVA). If a rat did not reach the criterion of 8 correct choices in a row within 120 trials of Set 2, it was assigned a criterion of 120 trials and a time to criterion of 120 minutes. Because of this imposed ceiling, we analyzed trials to criterion in Set 2 with the Kruskal-Wallis H test (“ANOVA by Ranks”) for nonparametric data as well as ANOVA. We also computed time per trial for Set 2 to assess motor abilities and motivation. Time per trial was computed by dividing the total time to completion of Set 2 by the total number of trials in Set 2.
For the analysis of overall performance across trial blocks for Set 2, percent correct scores were calculated for 10 consecutive blocks of 8 trials each. Perseverative responding in Set 2 was defined as choosing an arm that had been reinforced in Set 1 but was not reinforced in Set 2. Some researchers refer to these as “incongruent trials” (e.g., Floresco et al., 2009). Perseverative responding was evaluated across blocks by computing the percentage of correct choices from the two start arms, or perseveration arms, where choosing according to the Set 1 rule was no longer correct. Some researchers differentiate between errors of this type that occur early during the shift session and those errors that occur later during the shift session as “perseverative” and “regressive,” respectively (e.g., Floresco et al., 2009). Non-perseverative responding in Set 2 was defined as choosing an arm that continued to be reinforced in Set 2 after being reinforced in Set 1. Some researchers refer to these trial types as “congruent trials” (e.g., Floresco et al., 2009). Non-perseverative responses were evaluated across blocks by computing the percentage of correct choices from the other two start arms, or reinforcement arms, where choosing according to the Set 1 rule was still correct. Some researchers refer to errors on these trials as “never-reinforced errors” (e.g., Floresco et al., 2009). Performance across trial blocks for the two types of trials was analyzed with 2 (Strain) X 10 (Block) repeated-measures ANOVAs.
Data were analyzed with SPSS 17.0.2. Alpha was 0.05 for all tests.
Results
Of the 18 rats, two (1 SHR, 1 Wistar) were excluded from the analyses. The Wistar was excluded because he was overly aggressive and could not be tested in the maze. Because we trained and tested rats in contemporaneous strain pairs, we also excluded the SHR that was partnered with the excluded Wistar. The remaining rats included 8 SHRs and 8 Wistars.
Set 1: Trials to Criterion
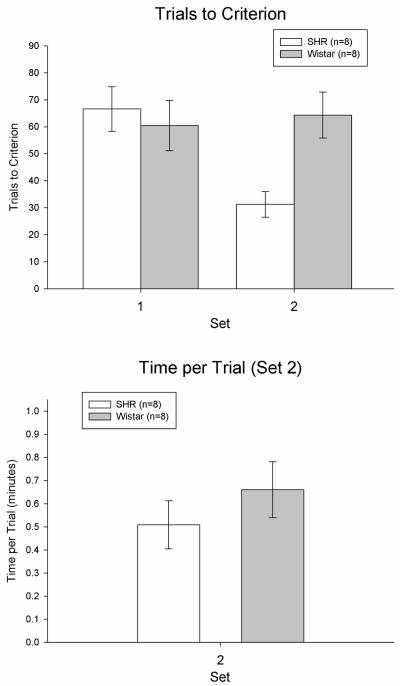
SHRs and Wistars learned the initial discrimination in Set 1 equivalently, based on comparable trials to criterion (Figure 1). This observation was confirmed with a one-way ANOVA on trials to criterion in Set 1, F(1,15) = 0.24, p > 0.63. The same results were obtained when data were analyzed with the Kruskal-Wallis H test for non-parametric data (p = 0.60). The same results were also obtained when discrimination in Set 1 (brightness or texture) was entered as a covariate into the ANOVA (p > 0.29).
Figure 1.
Trials to a criterion of 8 consecutive correct arm choices as a function of set in SHRs and Wistars (top panel) and time per trial as a function of set in SHRs and Wistars (bottom panel) in Experiment 1. Error bars are SEM.
Set 2: Trials to Criterion and Time per Trial
SHRs outperformed Wistars in the set-shift in Set 2 (Figure 1). This observation was confirmed with the Kruskal-Wallis H test for non-parametric data on trials to criterion in Set 2, H = 7.47, p < 0.01. The same results were obtained when data were analyzed with a one-way ANOVA (p < 0.01). In addition, the same results were also obtained when interval between Sets 1 and 2 (one day for 5 SHRs and 5 Wistars, two days for 3 SHRs and 3 Wistars) was entered as a covariate into the ANOVA (p < 0.01). Finally, the same results were obtained when discrimination in Set 2 (brightness or texture) was entered as a covariate into the ANOVA (p < 0.01).
Time per trial was used as a measure of motivation and motor abilities. SHRs and Wistars showed an equivalent time per trial although SHRs were generally faster (Figure 1). This observation was confirmed with a Kruskal-Wallis H test on time per trial in Set 2, H = 3.21, p = 0.07. The same results were obtained when data were analyzed with a one-way ANOVA (p = 0.35). In addition, the same results were also obtained when interval between Sets 1 and 2 (one day for 5 SHRs and 5 Wistars, two days for 3 SHRs and 3 Wistars) was entered as a covariate into the ANOVA (p = 0.31). Finally, the same results were obtained when discrimination in Set 2 (brightness or texture) was entered as a covariate into the ANOVA (p > 0.37).
Set 2: Performance Across Trial Blocks
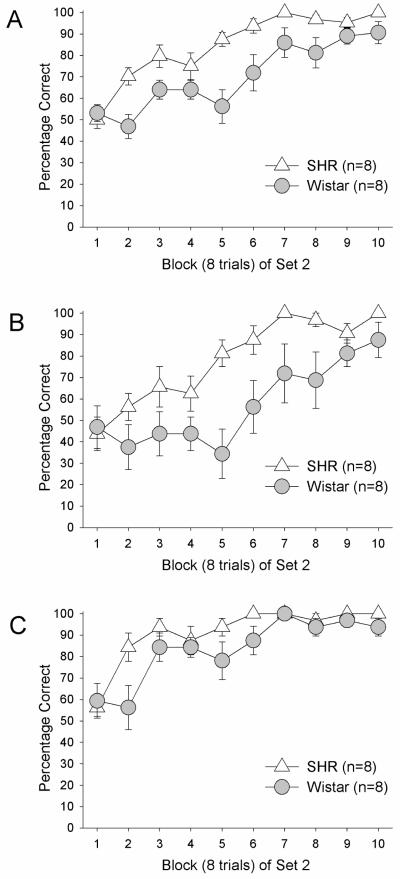
SHRs and Wistars both began at chance levels (~50%) of overall correct choices in block 1 of Set 2 and learned the new set across trial blocks. However, SHRs outperformed Wistars in the initial blocks (Figure 2A). This was confirmed by a 2 (Strain) X 10 (8-trial Block) repeated-measures ANOVA on percentage of correct choices which revealed significant Block, F(9,126) = 26.91, p < 0.01 and Strain, F(1,14) = 11.97, p < 0.01 main effects that were qualified by a significant Block X Strain interaction effect, F(9,126) = 2.69, p < 0.01. Follow-up t-tests comparing strains within each block revealed significant differences in Block 2 (p < 0.01), Block 3 (p < 0.04), Block 5 (p < 0.01), and Block 6 (p=0.03). The Strain main effect was still significant when interval between Sets 1 and 2 was entered as a covariate, p < 0.01. Finally, the Strain main effect was still significant when discrimination in Set 2 was entered as a covariate, p < 0.01.
Figure 2.
(A) Percentage of correct arm choices in Set 2 as a function of block of 8 trials in SHRs and Wistars in Experiment 1; (B) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is an incorrect arm choice in Set 2 in Experiment 1; (C) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is a correct arm choice in Set 2 in Experiment 1. Error bars are SEM.
Block data were also analyzed based on start arm (perseverative or reinforcement). Both SHRs and Wistars performed at chance levels (50%) from perseverative arm starts in Block 1 of Set 2. One sample t-tests comparing percentage of correct choices from perseverative arm starts in Block 1 of Set 2 to a 50% test value (chance performance) indicated that both SHRs (p > 0.45) and Wistars (p > 0.76) chose arms at levels not difference from chance in Block 1 of Set 2. Rats of both strains made fewer perseverative choices across trial blocks and SHRs outperformed Wistars (Figure 2B). This was confirmed by a 2 (Strain) X 10 (8-trial Block) repeated-measures ANOVA on percentage of correct choices from perseverative arm starts which revealed significant Block, F(9,126) = 12.94, p < 0.01 and Strain, F(1,14) = 7.92, p < 0.02 main effects. The Block X Strain interaction effect was not significant, F < 1.9. The Strain main effect was still significant when interval between Sets 1 and 2 was entered as a covariate, p < 0.02 and when discrimination in Set 2 was entered as a covariate, p < 0.01.
SHRs and Wistars both continued to choose arms that had been reinforced in Set 1 and were still reinforced in Set 2 and SHRs outperformed Wistars (Figure 2C). This was confirmed by a 2 (Strain) X 10 (8-trial Block) repeated-measures ANOVA on percentage of correct choices from reinforcement arm starts which revealed significant Block, F(9,126) = 15.82, p < 0.01 and Strain, F(1,14) = 4.64, p = 0.049 main effects. The Block X Strain interaction effect was not significant, F’s < 1.8. The Strain main effect approached but did not quite attain statistical significance when interval between Sets 1 and 2 was entered as a covariate, p = 0.058 and when discrimination in Set 2 was entered as a covariate, p = 0.068. One sample t-tests comparing percentage of correct choices from reinforcement arm starts in Block 1 of Set 2 to a 50% test value (chance performance) indicated that both SHRs (p = 0.17) and Wistars (p > 0.28) chose arms at levels not difference from chance in Block 1 of Set 2.
Discussion
The results of Experiment 1 suggest that SHRs are faster at set-shifting than Wistars. This finding appears contradictory to the only other published study to examine set-shifting in SHRs, which found that they performed more poorly than WKY/NCrl rats (Kantak et al., 2008; Experiment 3). There are some important differences between the current experiment and that of Kantak et al. (2008) that may explain the discrepant results. First, the dimensions of the initial discrimination and the set-shift were different between experiments. Our dimensions were somatosensory (rough vs. smooth texture) and visual (black vs. white color) while those of Kantak et al. (2008) were motor (left vs. right turn) and visual (presence of a black and white striped panel vs. absence of the panel). Second, in our experiment, SHRs and Wistars performed equivalently in the initial discrimination (Set 1), suggesting that there were no baseline sensory, motor, or motivational differences between strains that would explain the faster set-shifting in SHRs. In contrast, Kantak et al. (2008) reported that SHRs were significantly poorer in the initial discrimination (Set 1) than WKY/NCrl rats, which makes interpretation of SHRs’ poorer performance in Set 2 more difficult in their experiment. For example, it is difficult to know whether SHRs were simply poorer at discrimination in their experiment. Third, the rats in Kantak et al. (2008) were not experimentally naïve when they were tested in the initial discrimination and set-shifting. Indeed, they had been through extensive testing in the same apparatus using a win-shift task followed by a win-stay task. Although their rats were given a 4-week rest between win-stay testing and set-shifting, carry-over effects are a possibility, which was not the case in our experiment. Fourth, we used the Wistar as a control strain while Kantak et al. (2008) used WKY/NCrl. Sagvolden and colleagues have recently argued that WKYs from Charles River, such as those used by Kantak et al. (2008), may be inattentive compared to other strains (Sagvolden, Johansen, Woien, Walaas, Storm-Mathisen, Bergersen et al., 2009). Finally, the rats in Kantak et al. came from a different supplier (Charles River) than the rats used in the current study (Harlan). However, Sagvolden and colleagues suggest that there is no evidence for behavioral or genetic differences between SHRs from Charles River USA and those from Harlan USA (Sagvolden et al., 2009).
Experiment 2
The faster set-shifting in SHRs compared to Wistars in Experiment 1 was surprising. One possibility is that, counterintuitively, the faster set-shifting in SHRs might represent a memory deficit. That is, it is possible that SHRs remember the mechanics or basic procedure of the task in general but forget the specific rewarded dimension in Set 1 across time. If this hypothesis is correct, reducing the time between Sets 1 and 2 should reduce the advantage of SHRs over Wistars in Set 2. This hypothesis was investigated in Experiment 2 by conducting Sets 1 and 2 with no temporal delay between them. Note that conducting Sets 1 and 2 without a delay is more akin to how set-shifting is typically investigated in humans. For example, the shift in correct dimensions in the Wisconsin Card Sort Test usually occurs within a test session without warning (for a recent review, see Nyhus & Barcelo, 2009).
Methods
Subjects
Subjects were 8 male Wistar rats and 8 male SHRs from Harlan (Indianapolis, IN). Rats were between 59 and 63 days old when they arrived in the colony. All other details were identical to Experiment 1 above.
Apparatus, Habituation, Set-Shift Procedure, and Data Analysis
The apparatus, habituation, set-shift procedure, and data analysis for Experiment 2 were identical to those described for Experiment 1, except that there was no delay between these two sets (i.e., they were conducted on the same day, back-to-back).
Results
Set 1: Trials to Criterion
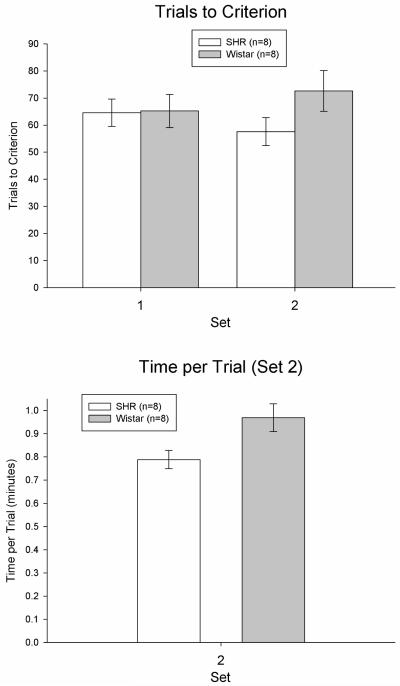
SHRs and Wistars learned the initial discrimination in Set 1 equivalently, based on comparable trials to criterion and time to criterion scores (Figure 3). This observation was confirmed with a one-way ANOVA on trials to criterion in Set 1, F(1,15) = 0.00, p > 0.93. The same results were obtained when data were analyzed with the Kruskal-Wallis H test for non-parametric data (p’s > 0.22). The same results were also obtained when discrimination in Set 1 (brightness or texture) was entered as a covariate into the ANOVA (p > 0.93).
Figure 3.
Trials to a criterion of 8 consecutive correct arm choices as a function of set in SHRs and Wistars (top panel) and time per trial as a function of set in SHRs and Wistars (bottom panel) in Experiment 2. Error bars are SEM.
Set 2: Trials to Criterion and Time per Trial
SHRs performed similarly to Wistars in the set-shift in Set 2 as measured by trials to criterion (Figure 3). This observation was confirmed with the Kruskal-Wallis H test for non-parametric data on trials to criterion in Set 2, H = 2.49, p = 0.11. The same result was obtained when data were analyzed with one-way ANOVAs (p = 0.12). Finally, the same results were obtained when discrimination in Set 2 (brightness or texture) was entered as a covariate into the ANOVA (p > 0.10).
Time per trial was used as a measure of motivation and motor abilities. Unlike Experiment 1 and despite the fact that SHRs and Wistars did not differ in trials to criterion, SHRs were significantly faster than Wistars in Set 2 (Figure 1). This observation was confirmed with a Kruskal-Wallis H test on time per trial in Set 2, H = 5.59, p < 0.02. The same results were obtained when data were analyzed with a one-way ANOVA (p < 0.03). Finally, the same results were obtained when discrimination in Set 2 (brightness or texture) was entered as a covariate into the ANOVA (p < 0.03).
Set 2: Performance Across Trial Blocks
SHRs and Wistars both began at chance levels (~50%) of correct choices in Block 1 of Set 2 and learned the new set across trial blocks (Figure 4A). Unlike Experiment 1, both strains learned Set 2 equivalently across trial blocks. This was confirmed by a 2 (Strain) X 10 (8-trial Block) repeated-measures ANOVA on percentage of correct choices which revealed a significant Block main effect, F(9,126) = 36.41, p < 0.01. Neither the Strain main effect, F(1,14) = 2.78, p = 0.12 nor the Block X Strain interaction effect, F(9,126) = 0.61, p > 0.78 were significant. Finally, the Strain main effect was still not significant when discrimination in Set 2 was entered as a covariate, p > 0.11.
Figure 4.
(A) Percentage of correct arm choices in Set 2 as a function of block of 8 trials in SHRs and Wistars in Experiment 2; (B) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is an incorrect arm choice in Set 2 in Experiment 2; (C) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is a correct arm choice in Set 2 in Experiment 2. Error bars are SEM.
Both SHRs and Wistars made a very high percentage of perseverative choices in block 1 of Set 2 (i.e., fewer than 10% correct choices from perseverative arm starts) (Figure 4B). This suggests a much greater level of proactive interference in Experiment 2 (when Set 2 followed Set 1 immediately) compared to Experiment 1 (when there was at least a day between Sets 1 and 2; Figure 2B). One sample t-tests comparing percentage of correct choices from perseverative arm starts in Block 1 of Set 2 to a 50% test value (chance performance) indicated that both SHRs (p < 0.01) and Wistars (p < 0.01) chose arms at levels significantly below chance in Block 1 of Set 2. Rats of both strains made fewer perseverative choices across trial blocks and, unlike Experiment 1, this improvement was equivalent between strains (Figure 4B). These observations were confirmed by a 2 (Strain) X 10 (8-trial Block) repeated-measures ANOVA on percentage of correct choices from perseverative arm starts which revealed a significant Block main effect, F(9,126) = 34.21, p < 0.01. Neither the Strain main effect, F(1,14) = 1.81, p = 0.20 nor the Block X Strain interaction effect, F(9,126) = 0.57, p > 0.81 were significant. The Strain main effect was still not significant when discrimination in Set 2 was entered as a covariate, p > 0.18.
SHRs and Wistars both continued to choose arms that had been reinforced in Set 1 and were still reinforced in Set 2 and they did so equivalently (Figure 4C). This was confirmed by a 2 (Strain) X 10 (8-trial Block) repeated-measures ANOVA on percentage of correct choices from reinforcement arm starts which revealed a significant Block main effect, F(9,126) = 2.21, p < 0.03. Neither the Strain main effect nor the Block X Strain interaction effect were significant, F’s < 2.0. The Strain main effect was still not significant when discrimination in Set 2 was entered as a covariate, p = 0.20. One sample t-tests comparing percentage of correct choices from reinforcement arm starts in Block 1 of Set 2 to a 50% test value (chance performance) indicated that both SHRs (p < 0.01) and Wistars (p < 0.01) chose arms at levels significantly above chance in Block 1 of Set 2.
Discussion
The results of Experiment 2 confirmed our hypothesis that the faster set-shifting by SHRs in Experiment 1 was due to a reduction in proactive interference in Set 2 from Set 1 across the delay between Sets 1 and 2. That is, in Experiment 1, SHRs showed greater forgetting than Wistars of the rewarded arms and non-rewarded arms from Set 1 by the time of Set 2, allowing them to learn the new discrimination faster than Wistars, who were subject to proactive interference in Set 2 from the Set 1 discrimination. In Experiment 2, where we conducted Sets 1 and 2 back-to-back, the advantage of SHRs over Wistars in Set 2 was almost entirely gone. Further confirmation that conducting Sets 1 and 2 back-to-back in Experiment 2 produced more proactive interference compared to conducting Sets 1 and 2 on separate days in Experiment 1 came in the form of perseverative arm choices. Specifically, rats in Experiment 2 began Set 2 making arm choices almost entirely based on Set 1 (i.e., fewer than 10% correct choices from perseverative arm starts; see Figure 4B). In contrast, rats in Experiment 1 began Set 2 making arm choices not significantly different from 50% chance levels (i.e., approximately 45% correct choices from perseverative arm starts; see Figure 2B). This suggests that, in Experiment 1, both strains forgot the Set 1 discrimination by the time of Set 2 to some extent. However, Wistars displayed more retention of the Set 1 discrimination (i.e., fewer correct choices from perseverative arms) across trial blocks than SHRs.
A caveat to the above interpretation is that, in Experiment 1, SHRs also outperformed Wistars from reinforcement arm starts in Set 2. Since these are arm starts in which choosing based on Set 1 is correct, this would suggest better, not worse, retention of Set 1 in SHRs. However, it appeared that the difference between strains in Set 2 was much more apparent in performance from perseverative arm starts, since both strains attained high levels of performance from reinforcement arm starts early in Set 2. If this was the case, then most of the performance differences between strains in Set 2 of Experiment 1 were caused by Wistars perseverating on the Set 1 rule, rather than SHRs remembering the Set 1 rule better.
To address this possibility, we performed a post-hoc analysis to determine on which block of 8 trials, during Set 2 of Experiment 1, each strain achieved a performance level significantly above chance (50%) from perseverative arm starts and from reinforcement arm starts. One sample t-tests comparing performance from perseverative arm starts to a 50% test value in each block of Set 2 revealed that SHRs were performing significantly above chance by Block 5 while Wistars weren’t performing significantly above chance until Block 9. In contrast, one sample t-tests comparing performance from reinforcement arm starts to a 50% test value in each block of Set 2 revealed that SHRs were performing significantly above chance by Block 2 while Wistars were performing significantly above chance by Block 3. These analyses suggest that the difference between SHRs and Wistars in Set 2 performance in Experiment 1 was driven primarily by performance from perseverative arm starts, as good performance from reinforcement arm starts was rapidly attained by both strains.
General Discussion
In Experiment 1, we showed that SHRs, a rat model of ADHD, were faster than a Wistar control strain in set-shifting conducted at least 24 hours after learning an initial discrimination. In Experiment 2, we showed that SHRs were not different from Wistars in set-shifting conducted immediately after learning an initial discrimination.
The pattern of results is consistent with the hypothesis that SHRs are faster at set-shifting when time elapses between sets because they forget more easily or do not retrieve the initial discrimination as well as a control strain. SHRs may generally have difficulty storing/retrieving information across/after a 24 hour delay. SHRs are poor at remembering the location of a hidden platform in the Morris water maze compared to Wistars or Wistar-Kyoto rats across testing sessions separated by a day (Gattu, Pauly, Boss, Summers, & Buccafusco, 1997; Gattu, Terry, Pauly, & Buccafusco, 1997). They are also showed poorer inhibitory avoidance compared to Wistars and WKYs, when tested for retention after a 48 hour delay (Gattu, Pauly et al., 1997; Gattu, Terry et al., 1997). Similarly, SHRs do not exhibit contextual freezing to a context in which they received a footshock the day before (Calzavara, Medrano, Levin, Kameda, Andersen, Tufik et al., 2009). In contrast, SHRs can perform quite well when there is no delay between training and testing. For example, in the radial-arm maze, where rats have to remember within a session which arms they’ve visited and which arms they still need to visit, 3 month old SHRs made fewer errors than 3 month old Sprague-Dawley rats (Wyss, Fisk, & van Groen, 1992).
Performance from reinforcement arm starts in Set 2 constitutes a relatively direct test of memory for Set 1, since rats just need to continue choosing the arm that was reinforced in Set 1. There was some indication that SHRs showed better, not worse, performance than Wistars from reinforcement arm starts in Set 2. However, both strains quickly attained high performance levels, making it somewhat difficult to say for certain that SHRs showed better memory. Furthermore, the data from perseverative arm starts in Experiments 1 and 2, discussed in more detail below, suggest that the level of proactive interference from Set 1 to Set 2 plays a strong role in Set 2 performance and that the level of proactive interference was greater in Wistars than in SHRs after a 24 hour delay. This is consistent with a memory deficit in SHRs.
The abolishment of the SHR advantage in set-shifting when the delay between sets is removed suggests that SHRs suffer less proactive interference in Set 2 from Set 1 when there is a delay between sets. Better set-shifting has been discussed in terms of a reduction in one or both of two forms of proactive interference from the initial discrimination: acquired equivalence from set formation and latent inhibition. Acquired equivalence is the process whereby stimuli that lead to the same outcome become more similar to each other (George et al., 2010). Formation of an attentional set may involve acquired equivalence between arms that lead to the same outcome that could then proactively interfere with a set-shift. If, for example, black arms are rewarded in the initial discrimination and white arms are not rewarded, the two black arms may undergo acquired equivalence and the two white arms may undergo acquired equivalence. During the set-shift, the acquired equivalence must be “tuned out” (cf. Oswald, Yee, Rawlins, Bannerman, Good, & Honey, 2001), since now, for example, rough arms are rewarded and one of the rough arms is white while the other is black. In other words, neither white arms nor black arms are now “equivalent” in terms of outcome. If acquired equivalence fades abnormally quickly in SHRs, they may not have had to “tune out” one dimension (e.g., brightness) and “tune in” another (e.g., texture) to the same extent as Wistars.
Acquired equivalence, at least between contexts, requires the mPFC (Iordanova, Killcross, & Honey, 2007). There is evidence that 6-OHDA lesions that deplete DA and NE from prefrontal cortex by destruction of DA and NE terminals improve set-shifting in marmoset monkeys (Roberts, De Salvia, Wilkinson, Collins, Muir, Everitt, & Robbins, 1994) and this may be due to an inability to form an attentional set in the initial compound discrimination (Crofts, Dalley, Collins, Van Denderen, Everitt, Robbins, & Roberts, 2001; but see George et al., 2010). Infusion of a D1 (Ragozzino, 2002) or a D2 (Floresco, Magyar, Ghods-Sharifi, Vexelman, & Tse, 2006) antagonist into mPFC impair set-shifting in rats, but infusions of a D4 antagonist into mPFC improve set-shifting (Floresco, Magyar et al., 2006). A number of studies have shown that SHRs have a hypoactive DA system, in terms of vesicular storage of DA, DA release and presynaptic D2 autoreceptors, in PFC (Russell, de Villiers, Sagvolden, Lamm, & Taljaard, 1995, 1998), NAc (Kirouac & Ganguly, 1993; Kujirai, Przedborski, Kostic, Jackson-Lewis, Fahn, & Cadet, 1990; Russell et al., 1995; Russell, 2003; Vaughn, van den Buuse, & Roland, 1999), and CPu (de Jong, Linthorst, & Versteeg, 1995; Linthorst, van Giersbergen, Gras, Versteeg, & de Jong, 1994; Linthorst, van den Buuse, de Jong, & Versteeg, 1990; Russell et al., 1995, 1998; Russell, de Villiers, Sagvolden, Lamm, & Taljaard, 2000). Thus, a hypoactive DA system in the PFC of SHRs might impair their ability to store an attentional set formed during the initial discrimination, which might, counterintuitively, make them faster at set-shifting because of a reduction in proactive interference in Set 2 from acquired equivalence in Set 1. Note that we are not arguing that the mPFC is completely dysfunctional, which would produce poorer, not faster, set-shifting. Rather, we are arguing that SHRs might treat each set relatively independently.
A second form of proactive interference in Set 2 is latent inhibition, a process whereby learning is slowed to a stimulus that has been previously experienced as non-reinforced. Our set-shifting procedure requires a discrimination between reinforced and non-reinforced arms, and the non-reinforced arms may undergo latent inhibition much like a Pavlovian conditioned stimulus (CS) that is presented repeatedly prior to being paired with an unconditioned stimulus (US). There is evidence that in a Pavlovian discrimination (CSA+, CSB−), the non-reinforced CSB undergoes latent inhibition that reveals itself as slower learning in a subsequent reversal phase when CSB is now reinforced (e.g., Bouton & Brooks, 1993). There is some evidence that SHRs are impaired in latent inhibition (Calzavara et al., 2009). Given the fact that the advantage of SHRs over Wistars in set-shifting was diminished when the interval between Sets 1 and 2 was decreased in Experiment 2, SHRs may develop latent inhibition to non-reinforced arms in Set 1 relatively normally but this latent inhibition may then fade abnormally quickly. Latent inhibition can be attenuated by treatments consistent with promotion of “forgetting” or “retrieval failure” (cf. Bouton & Brooks, 1993), including long retention intervals between stimulus preexposure and conditioning (e.g., De La Casa & Timberlake, 2006; Kraemer & Roberts, 1984).
It may be that an impairment in SHRs’ retention of latent inhibition is due to abnormalities in a neural circuit involving DA release in the NAc shell and regulation of this release by entorhinal cortex. Both the NAc shell (Gal, Schiller, & Weiner, 2005; Jongen-Relo, Kaufmann, & Feldon, 2002; Pedroza-Llinas, Ramirez-Lugo, Guzman-Ramos, Zavala-Vega, & Bermudez-Rattoni, 2009; Pothuizen, Jongen-Relo, Feldon, & Yee, 2006; Weiner, Gal, Rawlins, & Feldon, 1996) and the entorhinal cortex (Coutureau, Lena, Dauge, & DiScala, 2002; Coutureau, Galani, Gosselin, Majchrzak, & DiScala, 1999; Lewis & Gould, 2007; Oswald, Yee, Rawlins, Bannerman, Good, & Honey, 2002; Seiller, Dieu, Herbeaux, DiScala, Will, & Majchrzak, 2007; Shohamy, Allen, & Gluck, 2000) are necessary for latent inhibition (but see Pothuizen et al., 2006). Dopamine is released in the NAc shell during stimulus preexposure (Jeanblanc, Hoeltzel, & Louilot, 2002; Murphy, Pezze, Feldon, & Heidbreder, 2000). Inactivation of left entorhinal cortex during stimulus preexposure abolishes latent inhibition and latent inhibition-associated DA release in the NAc medial shell (Jeanblanc, Peterschmitt, Hoeltzel, & Louilot, 2004). Lesions of the NAc shell can improve set-shifting in a cross-maze (Floresco et al., 2006). Given the fact that glutamate-stimulated release of DA from the NAc shell is lower in SHRs than WKYs (Russell, 2003) and entorhinal cortex may also be abnormal in SHRs (cf. Terry, Hernandez, Buccafusco, & Gattu, 2000; Wyss & van Groen, 1992) it may be that SHRs show faster set-shifting because of abnormalities in a neural circuit involving DA release in the NAc shell regulated by entorhinal cortex, leading to an impairment in retention of latent inhibition.
What are the implications of these experiments for set-shifting in children and adolescents with ADHD? It seems likely that the amount of proactive interference at the time of the shift makes a contribution to observations of deficient set-shifting in persons with ADHD. What our experiments suggest is that impairments in set-shifting associated with ADHD may be more likely to be revealed with a very short time interval between the initial discrimination and shifting, which would increase proactive interference. Related to this is that filling the interval between the initial discrimination and the extradimensional set-shift with other discriminations, as in the CANTAB, may further increase proactive interference in the set-shift and reveal deficits in persons with ADHD. On the other hand, our experiments suggest that persons with ADHD might be less affected by proactive interference when a certain amount of time elapses between discriminations. This might be related to the finding of deficient strategic (effortful and organized) retrieval processes associated with ADHD (e.g., Pollak, Kavana-Vax, & Hoofien, 2008). If strategic retrieval becomes particularly important when time elapses between encoding and retrieval, persons with ADHD and SHRs may be less affected by proactive interference because they do not engage in the active memory search for the original stimulus dimensions.
In conclusion, we showed that SHRs and Wistars discriminate rewarded from non-rewarded arms equally quickly but SHRs outperform Wistars during a set-shift when an interval of at least a day is imposed between the initial discrimination and the set-shift. The superior set-shifting in SHRs is abolished when the shift occurs immediately after the initial discrimination. A reduction in proactive interference from acquired equivalence and/or latent inhibition over a delay in SHRs is a possible explanation for the pattern of results.
Acknowledgements
Support for this research came from NIMH (RO1 MH082893).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Barkley R. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Doyle AE, Spencer T, Henderson CS, Marion B, Fried R. Stability of executive function deficits in girls with ADHD: A prospective longitudinal followup study into adolescence. Developmental Neuropsychology. 2008;33:44–61. doi: 10.1080/87565640701729755. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cerebral Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Kooij JJS, Oosterlaan J, Sergeant JA, Buitelaar JK. To act or not to act, that’s the problem: Primarily inhibition difficulties in adult ADHD. Neuropsychology. 2010;24:209–221. doi: 10.1037/a0017670. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Brooks DC. Time and context effects on performance in a Pavlovian discrimination reversal. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:165–179. [Google Scholar]
- Calzavara MB, Medrano WA, Levin R, Kameda SR, Andersen ML, Tufik S, Silva RH, Frussa-Filho R, Abilio VC. Neuroleptic drugs revert the contextual fear conditioning deficit presented by spontaneously hypertensive rats: A potential animal model of emotional context processing in schizophrenia? Schizophrenia Bulletin. 2009;35:748–759. doi: 10.1093/schbul/sbn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutureau E, Galani R, Gosselin O, Majchrzak M, Scala GD. Entorhinal but not hippocampal or subicular lesions disrupt latent inhibition in rats. Neurobiology of Learning and Memory. 1999;72:143–157. doi: 10.1006/nlme.1998.3895. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Lena I, Dauge V, Scala GD. The entorhinal cortex-nucleus accumbens pathway and latent inhibition: A behavioral and neurochemical study in rats. Behavioral Neuroscience. 2002;116:95–104. doi: 10.1037//0735-7044.116.1.95. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Denderen JCMV, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cerebral Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- de Jong W, Linthorst AC, Versteeg HG. The nigrostriatal dopamine system and the development of hypertension in the spontaneously hypertensive rat. Archives des Maladies du Coeur et des Vaisseaux. 1995;88:1193–1196. [PubMed] [Google Scholar]
- De La Casa LG, Timberlake W. Effects of preexposure and retention interval placement on latent inhibition and perceptual learning in a choice-maze discrimination task. Learning & Behavior. 2006;34:193–201. doi: 10.3758/bf03193194. [DOI] [PubMed] [Google Scholar]
- Floresco S, Block AE, Tse MTL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. Journal of Neuroscience. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MTL. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behavioural Brain Research. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Gal G, Schiller D, Weiner I. Latent inhibition is disrupted by nucleus accumbens shell lesion but is abnormally persistent following entire nucleus accumbens lesion: The neural site controlling the expression and disruption of the stimulus preexposure effect. Behavioural Brain Research. 2005;162:246–255. doi: 10.1016/j.bbr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Gattu M, Pauly JR, Boss KL, Summers JB, Buccafusco JJ. Cognitive impairment in spontaneously hypertensive rats: Role of central nicotinic receptors. I. Brain Research. 1997;771:89–103. doi: 10.1016/s0006-8993(97)00793-2. [DOI] [PubMed] [Google Scholar]
- Gattu M, Terry AV, Pauly JR, Buccafusco JJ. Cognitive impairment in spontaneously hypertensive rats: Role of central nicotinic receptors. Part II. Brain Research. 1997;771:104–114. doi: 10.1016/s0006-8993(97)00794-4. [DOI] [PubMed] [Google Scholar]
- George DN, Duffaud A, Killcross S. Neural correlates of attentional set. In: Mitchell CJ, Le Pelley ME, editors. Attention and Associative Learning: From Brain to Behaviour. Oxford University Press; New York: 2010. pp. 351–383. [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiology of Learning and Memory. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Mostofsky SH, Cutting LE, Mahone EM, Astor BC, Denckla MB, Landa RJ. Subtle executive impairment in children with autism and children with ADHD. Journal of Autism and Developmental Disorders. 2005;35:279–293. doi: 10.1007/s10803-005-3291-4. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Killcross AS, Honey RC. Role of the medial prefrontal cortex in acquired distinctiveness and equivalence of cues. Behavioral Neuroscience. 2007;121:1431–1436. doi: 10.1037/0735-7044.121.6.1431. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Hoeltzel A, Louilot A. Dissociation in the involvement of dopaminergic neurons innervating the core and shell subregions of the nucleus accumbens in latent inhibition and affective perception. Neuroscience. 2002;111:315–323. doi: 10.1016/s0306-4522(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Peterschmitt Y, Hoeltzel A, Louilot A. Influence of the entorhinal cortex on accumbal and striatal dopaminergic responses in a latent inhibition paradigm. Neuroscience. 2004;128:187–200. doi: 10.1016/j.neuroscience.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Jongen-Relo AL, Kaufmann S, Feldon J. A differential involvement of the shell and core subterritories of the nucleus accumbens of rats in attentional processes. Neuroscience. 2002;111:95–109. doi: 10.1016/s0306-4522(01)00521-8. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, Deschepper CF, Dwoskin LP. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behavioral Neuroscience. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C. Executive function and attention deficit hyperactivity disorder: Stimulant medication and better executive function performance in children. Psychological Medicine. 1999;29:527–538. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Ganguly PK. Up-regulation of dopamine receptors in the brain of the spontaneously hypertensive rat: An autoradiographic analysis. Neuroscience. 1993;52:135–141. doi: 10.1016/0306-4522(93)90188-l. [DOI] [PubMed] [Google Scholar]
- Kraemer PJ, Roberts WA. The influence of flavor preexposure and test interval on conditioned taste aversions in the rat. Learning & Motivation. 1984;15:259–278. [Google Scholar]
- Kujirai K, Przedborski S, Kostic V, Jackson-Lewis V, Fahn S, Cadet JL. Autoradiography of dopamine receptors and dopamine uptake sites in the spontaneously hypertensive rat. Brain Research Bulletin. 1990;25:703–709. doi: 10.1016/0361-9230(90)90046-3. [DOI] [PubMed] [Google Scholar]
- Lawrence V, Houghton S, Douglas G, Durkin K, Whiting K, Tannock R. Executive function and ADHD: A comparison of children’s performance during neuropsychological testing and real-world activities. Journal of Attention Disorders. 2004;7:137–149. doi: 10.1177/108705470400700302. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Gould TJ. Reversible inactivation of the entorhinal cortex disrupts the establishment and expression of latent inhibition of cued fear conditioning in C57BL/6 mice. Hippocampus. 2007;17:462–470. doi: 10.1002/hipo.20284. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, van den Buuse M, de Jong W, Versteeg DH. Electrically stimulated [3H]dopamine and [14C]acetylcholine release from nucleus caudatus slices: Differences between spontaneously hypertensive rats and Wistar-Kyoto rats. Brain Research. 1990;509:266–272. doi: 10.1016/0006-8993(90)90551-l. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, van Giersbergen PL, Gras M, Versteeg DH, de Jong W. The nigrostriatal dopamine system: Role in the development of hypertension in spontaneously hypertensive rats. Brain Research. 1994;639:261–268. doi: 10.1016/0006-8993(94)91739-6. [DOI] [PubMed] [Google Scholar]
- Loo SK, Rich EC, Ishii J, McGough J, McCracken J, Nelson S, Smalley SL. Cognitive functioning in affected sibling pairs with ADHD: Familial clustering and dopamine genes. Journal of CHild Psychology and Psychiatry. 2008;49:950–957. doi: 10.1111/j.1469-7610.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McLean A, Dowson J, Toone B, Young S, Bazanis E, Robbins TW, Sahakian BJ. Characteristic neurocognitive profile associated with adult attention-deficit/hyperactivity disorder. Psychological Medicine. 2004;34:681–692. doi: 10.1017/S0033291703001296. [DOI] [PubMed] [Google Scholar]
- Mulas F, Capilla A, Fernandez S, Etchepareborda MC, Campo P, Maestu F, Fernandez A, Castellanos FX, Ortiz T. Shifting-related brain magnetic activity in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2006;59:373–379. doi: 10.1016/j.biopsych.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Muller BW, Gimbel K, Keller-Pliesnig A, Sartory G, Gastpar M, Davids E. Neuropsychological assessment of adult patients with attention-deficit/hyperactivity disorder. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:112–119. doi: 10.1007/s00406-006-0688-9. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Pezze M, Feldon J, Heidbreder C. Differential involvement of dopamine in the shell and core of the nucleus accumbens in the expression of latent inhibition to an aversively conditioned stimulus. Neuroscience. 2000;97:469–477. doi: 10.1016/s0306-4522(00)00043-9. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychological Bulletin. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT. What causes ADHD? Guilford; New York: 2006. [Google Scholar]
- Nyhus E, Barcelo F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: A critical update. Brain and Cognition. 2009;71:437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Oswald CJP, Yee BK, Rawlins JNP, Bannerman DB, Good M, Honey RC. Involvement of the entorhinal cortex in a process of attentional modulation: Evidence from a novel variant of an IDS/EDS procedure. Behavioral Neuroscience. 2001;115:841–849. doi: 10.1037//0735-7044.115.4.841. [DOI] [PubMed] [Google Scholar]
- Oswald CJP, Yee BK, Rawlins JNP, Bannerman DB, Good M, Honey RC. The influence of selective lesions to components of the hippocampal system on the orienting response, habituation and latent inhibition. European Journal of Neuroscience. 2002;15:1983–1990. doi: 10.1046/j.1460-9568.2002.02028.x. [DOI] [PubMed] [Google Scholar]
- Pasini A, Paloscia C, Alessandrelli R, Profirio MC, Curatolo P. Attention and executive functions profile in drug naive ADHD subtypes. Brain & Development. 2007;29:400–408. doi: 10.1016/j.braindev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Pedroza-Llinas R, Ramirez-Lugo L, Guzman-Ramos K, Zavala-Vega S, Bermudez-Rattoni F. Safe taste memory consolidation is disrupted by a protein synthesis inhibitor in the nucleus accumbens shell. Neurobiology of Learning and Memory. 2009;92:45–52. doi: 10.1016/j.nlm.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Pollack Y, Kahana-Vax G, Hoofien D. Retrieval processes in adults with ADHD: A RAVLT study. Developmental Neuropsychology. 33:62–73. doi: 10.1080/87565640701729789. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Jongen-Relo AL, Feldon J, Yee BK. Latent inhibition of conditioned taste aversion is not disrupted, but can be enhanced, by selective nucleus accumbens shell lesions in rats. Neuroscience. 2006;137:1119–1130. doi: 10.1016/j.neuroscience.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The effects of dopamine D1 receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learning & Memory. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJY, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral Neuroscience. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. Journal of Neuroscience. 2007;27:4747–4755. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Salvia MAD, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. Journal of Neuroscience. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philosophical Transactions of the Royal Society. B Biolological Sciences. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PMA. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Journal of the International Neuropsychological Society. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of Attention-Deficit Hyperactivity Disorder - the spontaneously hypertensive rat. Brain Research. 1995;676:343–351. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Differences between electrically-, ritalin-, and D-amphetamine-stimulated release of [3H]dopamine from brain slices suggest impaired vesicular storage of dopamine in an animal model of Attention-Deficit Hyperactivity Disorder. Behavioural Brain Research. 1998;94:163–171. doi: 10.1016/s0166-4328(97)00177-0. [DOI] [PubMed] [Google Scholar]
- Russell VA. In vitro glutamate-stimulated release of dopamine from nucleus accumbens core and shell of spontaneously hypertensive rats. Metabolic Brain Disease. 2003;18:161–168. doi: 10.1023/a:1023819220840. [DOI] [PubMed] [Google Scholar]
- Russell VA, de Villiers AS, Sagvolden T, Lamm MC, Taljaard JJ. Methylphenidate affects striatal dopamine differently in an animal model for attention-deficit/hyperactivity disorder -- the spontaneously hypertensive rat. Brain Research Bulletin. 2000;53:187–192. doi: 10.1016/s0361-9230(00)00324-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Woien G, Walaas SI, Storm-Mathisen J, Bergersen LH, Hvalby O, Jensen V, Aase H, Russell VA, Killeen PR, DasBanerjee T, Middleton FA, Faraone SV. The spontaneously hypertensive rat model of ADHD – The importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57:619–626. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Seillier A, Dieu Y, Herbeaux K, Scala GD, Will B, Majchrzak M. Evidence for a critical role of entorhinal cortex at pre-exposure for latent inhibition disruption in rats. Hippocampus. 2007;17:220–226. doi: 10.1002/hipo.20260. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Allen MT, Gluck MA. Dissociating entorhinal and hippocampal involvement in latent inhibition. Behavioral Neuroscience. 2000;114:867–874. [PubMed] [Google Scholar]
- Slamecka NJ. A methodological analysis of shift paradigms in human discrimination learning. Psychological Bulletin. 1968;69:423–438. doi: 10.1037/h0025762. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behavioral Neuroscience. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behavioral Neuroscience. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Terry AV, Hernandez CM, Buccafusco JJ, Gattu M. Deficits in spatial learning and nicotinic-acetylcholine receptors in older, spontaneously hypertensive rats. Neuroscience. 2000;101:357–368. doi: 10.1016/s0306-4522(00)00377-8. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Bucciarelli SM, Jain U, Tannock R. Executive functions: Performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychology. 2009;15:53–72. doi: 10.1080/09297040802070929. [DOI] [PubMed] [Google Scholar]
- Vaughn CE, van den Buuse M, Roland BL. Brain dopamine D2 receptor mRNA levels are elevated in young spontaneously hypertensive rats. Neuroscience Research. 1999;34:199–205. doi: 10.1016/s0168-0102(99)00037-1. [DOI] [PubMed] [Google Scholar]
- Weiner I, Gal G, Rawlins JN, Feldon J. Differential involvement of the shell and core subterritories of the nucleus accumbens in latent inhibition and amphetamine-induced activity. Behavioural Brain Research. 1996;81:123–133. doi: 10.1016/s0166-4328(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA. A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 2001;110:157–172. doi: 10.1037//0021-843x.110.1.157. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Fisk G, van Groen T. Impaired learning and memory in mature spontaneously hypertensive rats. Brain Research. 1992;592:135–140. doi: 10.1016/0006-8993(92)91668-5. [DOI] [PubMed] [Google Scholar]
- Wyss JM, van Groen T. Early breakdown of dendritic bundles in the retrosplenial granular cortex of hypertensive rats: Prevention by antihypertensive therapy. Cerebral Cortex. 1992;2:468–476. doi: 10.1093/cercor/2.6.468. [DOI] [PubMed] [Google Scholar]