Abstract
Early-life exposure to organophosphate pesticides leads to subsequent hyperresponsiveness of β-adrenergic receptor-mediated cell signaling that regulates hepatic gluconeogenesis, culminating in metabolic abnormalities resembling prediabetes. In the current study, we evaluated the effects of chlorpyrifos or parathion on presynaptic sympathetic innervation to determine whether the postsynaptic signaling effects are accompanied by defects in neuronal input. We administered either chlorpyrifos or parathion to newborn rats using exposure paradigms known to elicit the later metabolic changes but found no alterations in either hepatic or cardiac norepinephrine levels in adolescence or adulthood. However, shifting chlorpyrifos exposure to the prenatal period did evoke changes: exposure early in gestation produced subsequent elevations in norepinephrine, whereas later gestational exposure produced significant deficits. We also distinguished the organophosphate effects from those of the glucocorticoid, dexamethasone, a known endocrine disruptor that leads to later-life metabolic and cardiovascular disruption. Postnatal exposure to dexamethasone elicited deficits in peripheral norepinephrine levels but prenatal exposure did not. Our results indicate that early-life exposure to organophosphates leads to subsequent abnormalities of peripheral sympathetic innervation through mechanisms entirely distinct from those of glucocorticoids, ruling out the possibility that the organophosphate effects are secondary to stress or disruption of the HPA axis. Further, the effects on innervation were separable from those on postsynaptic signaling, differing in critical period as well as tissue- and sex-selectivity. Organophosphate targeting of both presynaptic and postsynaptic β-adrenergic sites, each with different critical periods of vulnerability, thus sets the stage for compounding of hepatic and cardiac functional abnormalities.
Keywords: β-Adrenergic receptor, Dexamethasone, Glucocorticoids, Heart, Liver, Norepinephrine, Organophosphate insecticides, Parathion, Sympathetic nervous system
INTRODUCTION
There is increasing evidence that early-life exposures to environmental chemicals contribute to the explosive, worldwide rise in obesity and diabetes [18,29,46]. These include some of the most commonly-used pesticides [18,27,38] such as the organophosphates, which account for nearly 50% of worldwide insecticide use [9]. Human exposure to organophosphates is virtually ubiquitous [28] and populations with the greatest organophosphate exposures also have the highest rates of obesity [10,12,13,31,32,37,48].
Originally, organophosphates were considered primarily to be neurotoxicants but these agents also evoke increases in body weight and diabetes-like changes in hepatic energy metabolism upon exposure in adulthood [1,24]. Developing animals appear to be even more sensitive, showing persistent and late-emerging metabolic abnormalities after otherwise nonsymptomatic, early-life exposures; these culminate in deficits in carbohydrate and lipid metabolism resembling prediabetes, and in sensitization to the adverse effects of elevated dietary fat intake [19,20,36,40,42]. We were able to show that one of the triggers for the metabolic effects was the emergence of abnormal hepatic cell signaling, reflecting a potentiation of gluconeogenic signals mediated by β-adrenergic receptors (βARs) and glucagon receptors [2,4,25,40]. Further, we found that the magnitude of the organophosphate effects on hepatic function were as large as those seen after comparable developmental exposures to the glucocorticoid, dexamethasone [3]; excess glucocorticoids are considered to be among the most definitive contributors to the cardiovascular and metabolic components comprising the “fetal origins of adult disease” [6,11]. At the same time, though, we were able to distinguish the cell signaling effects of the organophosphates from those of dexamethasone in terms of different critical periods, tissue selectivity (liver vs. heart) and sex-selectivity of the outcomes [3,4,26]. In turn, this suggested that, although the organophosphates converged on similar metabolic targets as dexamethasone, the pesticides were not eliciting their effects through secondary mechanisms such as stress or other actions on the hypothalamus-pituitary-adrenal (HPA) axis.
Nevertheless, it is not known whether the hyperreactivity of hepatic cell signaling evoked by early-life organophosphate exposure represents a primary target leading to metabolic defects, or instead whether this is an adaptation to alterations in neurohumoral input. For example, increased postsynaptic reactivity to βAR input could be a compensation for deficits in sympathetic noradrenergic innervation. Accordingly, in the current study, we evaluated the effects of chlorpyrifos or parathion exposure during different fetal and neonatal stages, on norepinephrine (NE) levels in the liver measured in adolescence and adulthood, and contrasted this with the effects of dexamethasone; to evaluate tissue selectivity, we made comparisons with NE levels in the heart. The treatments and exposure periods were chosen to match the critical periods for effects of each of the test agents on cell signaling as shown in our previous studies [3,4,26]. For the organophosphates, we evaluated doses straddling the threshold for barely-detectable inhibition of cholinesterase, exposures that are otherwise nonsymptomatic [35,44,45]. For dexamethasone, we explored the effects of doses well below (0.05 mg/kg) or within the recommended therapeutic range (0.2 or 0.8 mg/kg) for its use in the management of preterm labor [15].
METHODS
Animal treatments
All procedures utilized tissues that were archived from earlier studies and maintained frozen at −45° C, so that no additional animals were actually used for this study. Details of animal husbandry, institutional approvals, maternal and litter characteristics, and growth curves, have all been presented in earlier work from the original animal cohorts: dexamethasone [3,17], chlorpyrifos [5,26] and parathion [4,43]. Timed-pregnant Sprague-Dawley rats (Charles River, Raleigh, NC) were housed individually and given free access to food and water. For studies of gestational dexamethasone exposure, dams received daily subcutaneous injections of 0.05, 0.2 or 0.8 mg/kg dexamethasone phosphate (Sigma Chemical Co., St. Louis, MO) on GD17-19, whereas controls received equivalent volumes (1 ml/kg) of isotonic saline vehicle. On the day after birth, all pups were randomized within their respective treatment groups and redistributed to the nursing dams, maintaining a litter size of 10 to ensure standard nutrition. Randomization was repeated every 3–4 days and in addition, dams were rotated among litters to obviate any differences in maternal caretaking. Cross-fostering of dexamethasone-exposed pups to control dams does not alter its developmental effects, nor does fostering of normal pups by dexamethasone-treated dams elicit developmental abnormalities [30]. For studies of the effects of postnatal dexamethasone treatment, pups were given the same doses on PN1-3 or PN7-9 and the same randomization procedures were followed.
For studies of organophosphate exposure, chlorpyrifos or parathion (both from Chem Service, West Chester, PA) were dissolved in dimethylsulfoxide to consistent absorption [47] and were injected subcutaneously in a volume of 1 ml/kg body weight; control animals received vehicle injections on the same schedules. For exposure on GD9-12 or GD17-20, dams were injected daily with chlorpyrifos at 1 or 5 mg/kg of body weight. These doses span the threshold for inhibition of fetal brain cholinesterase activity, fetal growth impairment and reduced maternal weight gain, all of which become evident at or above 5 mg/kg [14,35]. For studies of chlorpyrifos or parathion effects in the first few days after birth, animals were given 1 mg/kg s.c. chlorpyrifos, or 0.1 or 0.2 mg/kg parathion, daily on PN1-4; for studies in older animals, which tolerate higher organophosphate exposures [8,33,34,47], we administered 5 mg/kg chlorpyrifos on PN11-14. The doses of chlorpyrifos and parathion used on PN1-4 were chosen to be toxicodynamically equivalent, as assessed through measurements of brain cholinesterase inhibition [35,44,45]. For the organophosphate treatment regimens, the same randomization procedures were carried out as described for dexamethasone.
Animals were weaned on PN21 and samples were obtained at ages ranging from adolescence through adulthood. Determinations were carried out using 12 animals per treatment group at each age for the dexamethasone and parathion studies, and 12–24 for the chlorpyrifos studies. Each group had equal numbers of males and females, with no more than one male and one female used from any single litter (defined from the final litter assignment in the randomization procedure).
Assays and data analysis
Tissues were thawed on ice and deproteinized by homogenization in 0.1 N perchloric acid containing 3,4-dihydroxybenzylamine (Sigma) as an internal standard. Homogenates were sedimented at 26,000 × g for 10 minutes, the supernatant solutions were decanted, and NE was then trace-enriched by alumina adsorption, separated by reverse-phase high performance liquid chromatography and quantitated by electrochemical detection [39]; values were corrected for recovery of the internal standard.
Data are presented as means and standard errors, with treatment differences established by ANOVA utilizing the factors of treatment, sex and age. Post-hoc tests for individual treatment effects were established with Fisher’s Protected Least Significant Difference Test. Significance was assumed at p < 0.05. Because each treatment paradigm involved a separate cohort of animals, treatment comparisons were made only to the matched control group from the same cohort; similarly, the specific age points differed among the various study groups but always ranged within the period from the onset of adolescence (PN30) to full adulthood (PN100).
RESULTS
Dexamethasone
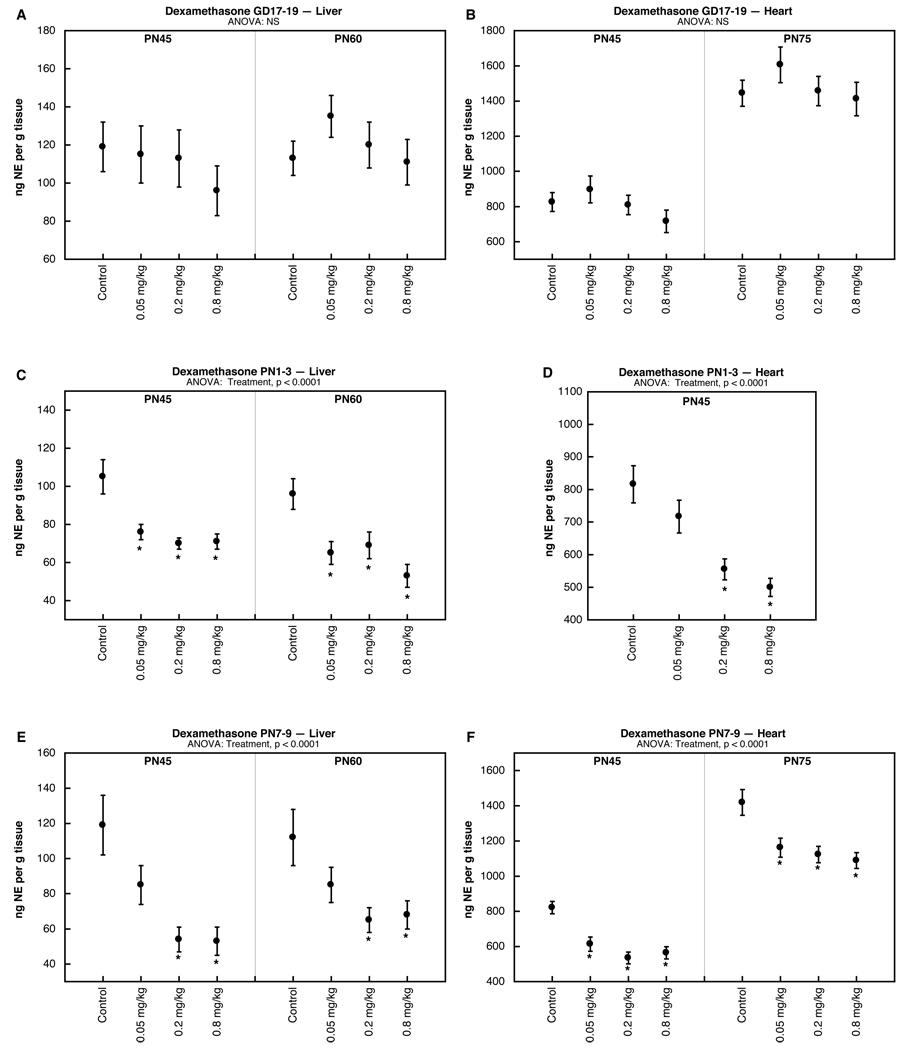
The results of multivariate ANOVA (treatment, age, sex) did not identify any interactions of treatment × sex, so the results are shown combined for males and females. Exposure to dexamethasone in late gestation (GD17-19) had no effect on either liver (Fig. 1A) or heart (Fig. 1B) NE in adolescence or adulthood. In contrast, the same dexamethasone doses given to pups on PN1-3 produced significant deficits. In the liver, even the lowest dose elicited a robust decline in NE that was present in adolescence and persisted into adulthood (Fig. 1C); deficits were also seen in the heart at the two higher dexamethasone doses (Fig. 1D). Shifting the dexamethasone exposure to a later neonatal period (PN7-9) elicited a similar pattern, with deficits in both liver (Fig. 1E) and heart (1F) present in adolescence and extending into adulthood.
Figure 1.
Effects of dexamethasone administered on GD 17–19 (A,B), PN1-3 (C,D) or PN7-9 (E,F), assessed in liver (A,C,E) and heart (B,D,F). Data represent means and standard errors obtained from 12 animals in each treatment group at each age; results from males and females were combined because of the absence of treatment × sex interactions. ANOVA appears at the top of each panel and asterisks denote individual treatment groups that differ significantly from the corresponding control group.
Chlorpyrifos and parathion
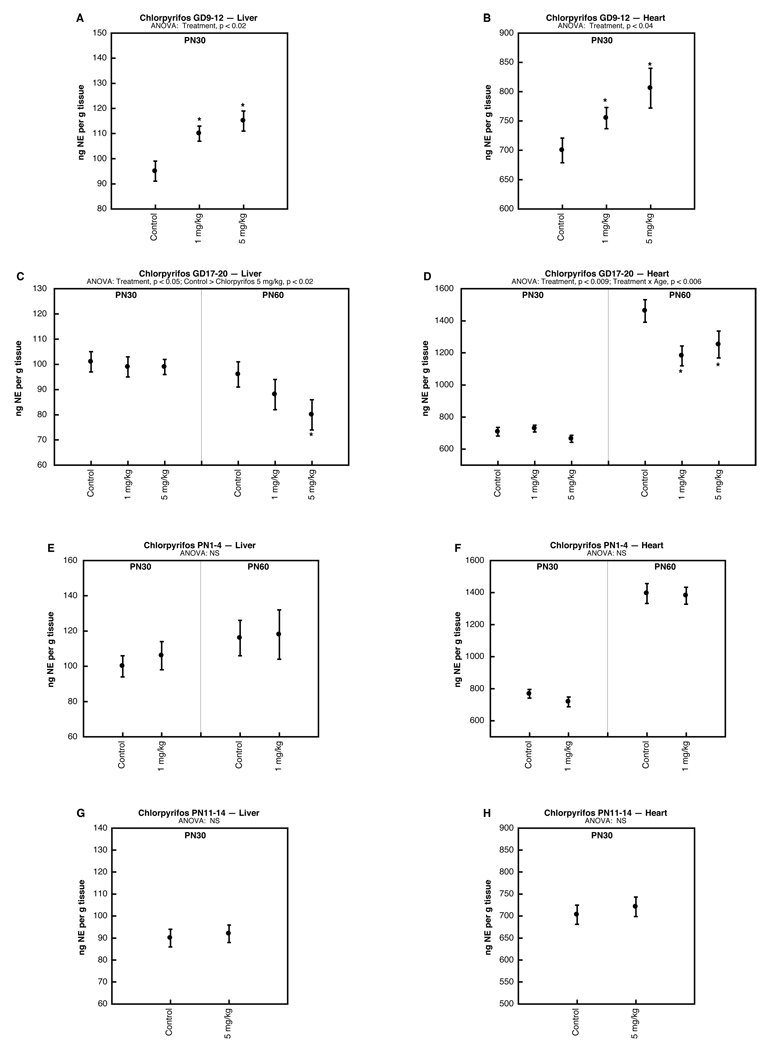
Just as for dexamethasone, multivariate ANOVAs for the effects of organophosphates did not reveal any sex-selective effects (i.e. no treatment × sex interaction), so results were combined for males and females. Chlorpyrifos exposure early in gestation (GD9-12) produced significant increases in NE concentrations in both the liver (Fig. 2A) and heart (Fig. 2B). In contrast, exposure later in gestation (GD17-20) failed to evoke changes initially but then an opposite effect, i.e. deficits in NE, emerged by adulthood in both the liver (Fig. 2C) and heart (Fig. 2D).
Figure 2.
Effects of chlorpyrifos administered on GD9-12 (A,B), GD17-20 (C,D), PN1-4 (E,F) or PN11-14 (G,H), assessed in liver (A,C,E,G) and heart (B,D,F,H). Data represent means and standard errors obtained from 12–24 animals in each treatment group at each age; results from males and females were combined because of the absence of treatment × sex interactions. ANOVA appears at the top of each panel and asterisks denote individual treatment groups that differ significantly from the corresponding control group.
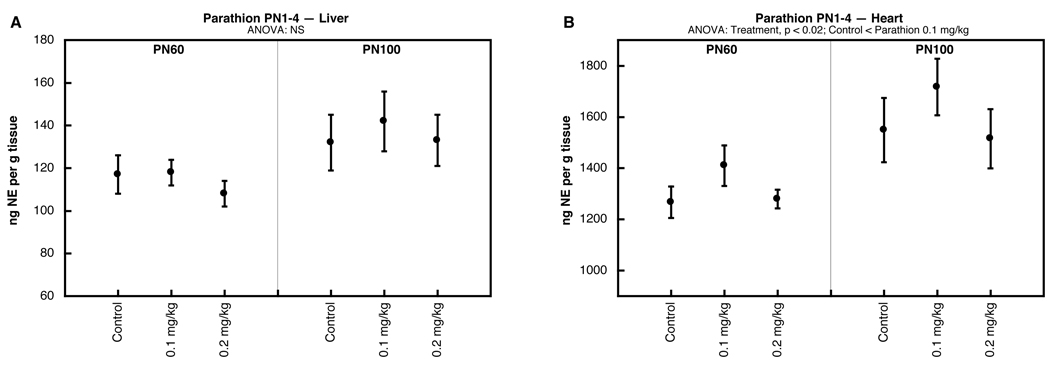
Postnatal chlorpyrifos exposure failed to alter NE levels, whether given on PN1-4 (Fig. 2E,F) or PN11-14 (Fig. 2G,H). Notably, the same treatments elicit major alterations in postsynaptic βAR signaling in adolescence and adulthood [26]. We also evaluated parathion given on PN1-4 and similarly found little or no change in NE concentrations in the liver (Fig. 3A); the heart showed a slight elevation at the low dose of parathion that was significant when measured across both age points (main treatment effect), but not for either age point separately (Fig. 3B).
Figure 3.
Effects of parathion administered on PN1-4, assessed in liver (A) and heart (B). Data represent means and standard errors obtained from 12 animals in each treatment group at each age; results from males and females were combined because of the absence of treatment × sex interactions. ANOVA appears at the top of each panel and asterisks denote individual treatment groups that differ significantly from the corresponding control group.
DISCUSSION
The results obtained here indicate that prenatal exposure to organophosphates leads to subsequent abnormalities of peripheral sympathetic innervation that could contribute to adverse metabolic and cardiovascular responses. Importantly, these effects are distinct from those of glucocorticoids, ruling out the possibility that they represent actions secondary to stress or disruption of the HPA axis. Further, the critical period, tissue selectivity and sex-specificity of the organophosphate effects on noradrenergic innervation seen here differ from those reported earlier for postsynaptic cell signaling [2,4,25,40]. Accordingly, hyperresponsiveness to hepatic gluconeogenic signals is not simply a compensation for presynaptic defects, nor are the effects on norepinephrine an adaptation to abnormalities in postsynaptic function. The combination of separate presynaptic and postsynaptic defects, each with different critical periods of vulnerability, thus sets the stage for compounding of both hepatic and cardiac functional abnormalities.
Before discussing the effects of the organophosphates, it is useful to examine those elicited by dexamethasone to provide a comparative basis against a known endocrine disruptor that leads to metabolic and cardiovascular dysregulation. Previously, we showed that early-life exposure to glucocorticoids produces immediate suppression of the development of peripheral sympathetic projections, accompanied by corresponding functional deficits [7,21,41]. Later, in adolescence and adulthood, hyperresponsiveness emerges in postsynaptic cell signaling mediated by βARs but with a distinct preferential effect in males [3]. Here, we found that postnatal exposure to dexamethasone, either on PN1-3 or PN7-9, resulted in deficits in hepatic and cardiac norepinephrine in adolescence and adulthood; however, unlike the effects on postsynaptic responses, there was no sex preference, indicating that the presynaptic defects represent an entirely separate target. Notably, we did not see any corresponding effect with prenatal dexamethasone exposure, indicating that the critical period for the glucocorticoid effect is in the postnatal period. Because the rat is an altricial species, the neonatal stage corresponds to human fetal development within the window (24–34 weeks’ gestation) in which glucocorticoids are the consensus treatment in preterm labor [15]; our results were obtained using dexasmethasone at doses spanning those recommended for preterm use and thus could provide mechanisms for later-life metabolic and cardiovascular abnormalities.
For the organophosphates, our earlier results likewise showed βAR hyperresponsiveness in adolescence and adulthood after early-life exposures to either chlorpyrifos or parathion, with a corresponding emergence of metabolic abnormalities resembling prediabetes [4,19,20,26,36,40,42]. Notably, the tissue and sex selectivity for these postsynaptic effects did not match those seen for dexamethasone [3], indicating that the organophosphates were not producing their long-term defects by eliciting stress or through HPA axis disruption. Here, when we evaluated corresponding effects on presynaptic noradrenergic innervation, we did not find any effects of postnatal chlorpyrifos or parathion exposure, an outcome totally distinct from the profound effects seen with postnatal dexamethasone treatment. However, when we shifted the exposure period to prenatal stages, chlorpyrifos exposure did produce later-life alterations in both hepatic and cardiac norepinephrine levels; again, this is entirely distinct from prenatal dexamethasone, which had no such effects. Indeed, we were able to define two distinct critical periods for the effects of chlorpyrifos: exposure early in gestation (GD9-12) led to subsequent elevations in norepinephrine, whereas exposure late in gestation (GD17-20) produced decrements. Thus, not only are the effects of this organophosphate on presynaptic noradrenergic innervation entirely separable from those of dexamethasone, but they are also distinct from those on postsynaptic cell signaling, which is prominently targeted by the same postnatal exposure paradigms that, as shown here, failed to affect norepinephrine [4,26]. Again, then, our results point to discrete effects on presynaptic innervation and postsynaptic signaling, rather than a coordinated adaptation of one to the other. Notably, the disparities for the peripheral effects among the different critical exposure windows parallels those reported earlier for behavioral outcomes for the same four exposure paradigms [16,22,23]. This reinforces the principle that the outcomes after developmental exposure to environmental toxicants are highly dependent on the stage at which exposure occurs.
To our knowledge, this is the first report to explore the developmental neurotoxicity of organophosphates directed toward peripheral sympathetic pathways, rather than the more typical focus on the central nervous system. Given the explosive worldwide increase in the incidence of obesity and diabetes, our results point to the likelihood that early-life exposures to common environmental chemical contaminants could contribute to these outcomes through perturbation of the neurohumoral pathways that are critical to metabolic homeostasis.
Acknowledgments
Acknowledgments/disclaimers: Research was supported by NIH ES10356. The study sponsors had no role in the study design; collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication. TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Weltchek Mallahan & Weltchek (Lutherville MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Carter Law (Peoria IL), Gutglass Erickson Bonville & Larson (Madison WI), The Killino Firm (Philadelphia PA), Alexander Hawes (San Jose, CA) and the Shanahan Law Group (Raleigh NC).
Abbreviations
- ANOVA
analysis of variance
- βAR
β-adrenergic receptor
- GD
gestational day
- HPA
hypothalamus-pituitary-adrenal axis
- NE
norepinephrine
- PN
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abdollahi M, Donyavi M, Pournourmohammadi S, Saadat M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to malathion. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2004;137:343–347. doi: 10.1016/j.cca.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Adigun AA, Wrench N, Levin ED, Seidler FJ, Slotkin TA. Neonatal parathion exposure and interactions with a high-fat diet in adulthood: adenylyl cyclase-mediated cell signaling in heart, liver and cerebellum. Brain Res. Bull. 2010;81:605–612. doi: 10.1016/j.brainresbull.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adigun AA, Wrench N, Seidler FJ, Slotkin TA. Neonatal dexamethasone treatment leads to alterations in cell signaling cascades controlling hepatic and cardiac function in adulthood. Neurotoxicol. Teratol. 2010;32:193–199. doi: 10.1016/j.ntt.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adigun AA, Wrench N, Seidler FJ, Slotkin TA. Neonatal organophosphorus pesticide exposure alters the developmental trajectory of cell signaling cascades controlling metabolism: differential effects of diazinon and parathion. Environ. Health Perspect. 2010;118:210–215. doi: 10.1289/ehp.0901237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum M, Ortiz L, Quan A. Fetal origins of cardiovascular disease. Curr. Opin. Pediatr. 2003;15:166–170. doi: 10.1097/00008480-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Bian X-P, Seidler FJ, Bartolome J, Kavlock RJ, Bartolome M, Slotkin TA. Dose-dependent effect of prenatal dexamethasone treatment on β-adrenergic receptor coupling to ornithine decarboxylase and cyclic AMP. J. Dev. Physiol. 1990;14:125–130. [PubMed] [Google Scholar]
- 8.Campbell CG, Seidler FJ, Slotkin TA. Chlorpyrifos interferes with cell development in rat brain regions. Brain Res. Bull. 1997;43:179–189. doi: 10.1016/s0361-9230(96)00436-4. [DOI] [PubMed] [Google Scholar]
- 9.Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem. Res. Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- 10.Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W, Coronado G, Thompson B. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ. Health Perspect. 2002;110:A787–A792. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin. Sci. 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- 12.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenske RA, Kissel JC, Lu C, Kalman DA, Simcox NJ, Allen EH, Keifer MC. Biological based pesticide dose estimates for children in an agricultural community. Environ. Health Perspect. 2000;108:515–520. doi: 10.1289/ehp.00108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: effects on neurospecific proteins indicate changing vulnerabilities. Environ. Health Perspect. 2003;111:297–303. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilstrap LC, Christensen R, Clewell WH, D'Alton ME, Davidson EC, Escobedo MB, Gjerdingen DK, Goddard-Finegold J, Goldenberg RL, Grimes DA, Hansen TN, Kauffman RE, Keeler EB, Oh W, Susman EJ, Vogel MG, Avery ME, Ballard RA, Crowley P, Garite T, Hankins GDV, Jobe AH, Koppe JG, Maher JE, Merkatz IR, Shankaran S, Simpson KN, Sinclair JC, Slotkin TA, Taeusch HW, Wright LL. Effect of corticosteroids for fetal maturation on perinatal outcomes. J. Am. Med. Assoc. 1995;273:413–418. [Google Scholar]
- 16.Icenogle LM, Christopher C, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, Slotkin TA, Levin ED. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol. Teratol. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- 18.La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt. Sinai J. Med. 2011;78:22–48. doi: 10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassiter TL, Ryde IT, Levin ED, Seidler FJ, Slotkin TA. Neonatal exposure to parathion alters lipid metabolism in adulthood: interactions with dietary fat intake and implications for neurodevelopmental deficits. Brain Res. Bull. 2010;81:85–91. doi: 10.1016/j.brainresbull.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassiter TL, Ryde IT, MacKillop EA, Brown KK, Levin ED, Seidler FJ, Slotkin TA. Exposure of neonatal rats to parathion elicits sex-selective reprogramming of metabolism and alters the response to a high-fat diet in adulthood. Environ. Health Perspect. 2008;116:1456–1462. doi: 10.1289/ehp.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau C, Slotkin TA. Maturation of sympathetic neurotransmission in the rat heart. VII. Supression of sympathetic responses by dexamethasone. J. Pharmacol. Exp. Ther. 1981;216:6–11. [PubMed] [Google Scholar]
- 22.Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol. Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- 23.Levin ED, Addy N, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev. Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 24.Meggs WJ, Brewer KL. Weight gain associated with chronic exposure to chlorpyrifos in rats. J. Med. Toxicol. 2007;3:89–93. doi: 10.1007/BF03160916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer A, Seidler FJ, Aldridge JE, Slotkin TA. Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide, chlorpyrifos. Toxicol. Appl. Pharmacol. 2005;203:154–166. doi: 10.1016/j.taap.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Meyer A, Seidler FJ, Slotkin TA. Developmental effects of chlorpyrifos extend beyond neurotoxicity: critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ. Health Perspect. 2004;112:170–178. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan DP, Lin LI, Saikaly HH. Morbidity and mortality in workers occupationally exposed to pesticides. Arch. Environ. Contam. Toxicol. 1980;9:349–382. doi: 10.1007/BF01057414. [DOI] [PubMed] [Google Scholar]
- 28.Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, Wilson NK, Lyu CW. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J. Exposure Anal. Environ. Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- 29.Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Intl. J. Androl. 2008;31:201–208. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 30.Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids: fetal effect or maternal influence? J. Endocrinol. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- 31.Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, Diaz D, Dietrich J, Reyes A, Kinney PL. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Petchuay C, Visuthismajarn P, Vitayavirasak B, Hore P, Robson MG. Biological monitoring of organophosphate pesticides in preschool children in an agricultural community in Thailand. Intl. J. Occup. Environ. Health. 2006;12:134–141. doi: 10.1179/oeh.2006.12.2.134. [DOI] [PubMed] [Google Scholar]
- 33.Pope CN, Chakraborti TK. Dose-related inhibition of brain and plasma cholinesterase in neonatal and adult rats following sublethal organophosphate exposures. Toxicology. 1992;73:35–43. doi: 10.1016/0300-483x(92)90168-e. [DOI] [PubMed] [Google Scholar]
- 34.Pope CN, Chakraborti TK, Chapman ML, Farrar JD, Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68:51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- 35.Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: What is the vulnerable period? Environ. Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res. Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothlein J, Rohlman D, Lasarev M, Phillips J, Muniz J, McCauley L. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and nonagricultural Hispanic workers. Environ. Health Perspect. 2006;114:691–696. doi: 10.1289/ehp.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saldana TM, Basso O, Hoppin JA, Baird DD, Knott C, Blair A, Alavanja MCR, Sandler DP. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care. 2007;30:529–534. doi: 10.2337/dc06-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seidler FJ, Slotkin TA. Development of central control of norepinephrine turnover and release in the rat heart: responses to tyramine, 2-deoxyglucose and hydralazine. Neuroscience. 1981;6:2081–2086. doi: 10.1016/0306-4522(81)90047-6. [DOI] [PubMed] [Google Scholar]
- 40.Slotkin TA. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reprod. Toxicol. 2010 doi: 10.1016/j.reprotox.2010.07.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slotkin TA, Barnes G, Lau C, Seidler FJ, Trepanier P, Weigel SJ, Whitmore WL. Development of polyamine and biogenic amine systems in brains and hearts of neonatal rats given dexamethasone: role of biochemical alterations in cellular maturation for producing deficits in ontogeny of neurotransmitter levels, uptake, storage and turnover. J. Pharmacol. Exp. Ther. 1982;221:686–693. [PubMed] [Google Scholar]
- 42.Slotkin TA, Brown KK, Seidler FJ. Developmental exposure of rats to chlorpyrifos elicits sex-selective hyperlipidemia and hyperinsulinemia in adulthood. Environ. Health Perspect. 2005;113:1291–1294. doi: 10.1289/ehp.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slotkin TA, Levin ED, Seidler FJ. Developmental neurotoxicity of parathion: progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol. Teratol. 2009;31:11–17. doi: 10.1016/j.ntt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ. Health Perspect. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol. Appl. Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- 46.Trasande L, Cronk C, Durkin M, Weiss M, Schoeller DA, Gall EA, Hewitt JB, Carrel AL, Landrigan PJ, Gillman MW. Environment and obesity in the National Children's Study. Environ. Health Perspect. 2009;117:159–166. doi: 10.1289/ehp.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol. Appl. Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- 48.Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, Garfinkel R, Andrews H, Hoepner L, Barr DB. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol. Appl. Pharmacol. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]