Abstract
Non-invasive measurement of human islet cell mass in pancreas or following islet transplantation by nuclear imaging has yet to be achieved. It has been shown using mouse tumor models that pretargeting imaging strategies are sensitive and can greatly increase target to non-target signal ratios. The objective now is to demonstrate the specific pretargeting of human islet cells in mice. Our pretargeting strategy uses an anti-human islet cell antibody HPi1, conjugated to a phosphorodiamidate morpholino oligomer (MORF) that binds specifically to a 99mTc labeled complimentary MORF (cMORF). Sensitivity and specificity of the pretargeting were first validated in culture using a human beta cell line (betalox5) and a negative control human cell line (HEK293). Pretargeting was then used to target and visualize these two cell lines and human islets transplanted subcutaneously in NOD-scid IL2rγnull mice. In culture, 99mTc accumulation on the betalox5 cells pretargeted by MORF-HPi1 was 100-fold higher than on untreated betalox5 cells or following treatment with native HPi1 and much higher than on the MORF-HPi1 pretargeted control HEK293 cells. Small animal imaging readily localized the transplanted betalox5 cells and human islets, but not the HEK293 cells. Ex vivo counting demonstrated threefold higher 99mTc accumulation in the transplanted betalox5 cells and human islets than in the control HEK293 cells. The target accumulation was also shown to increase linearly with increased numbers of the implanted betalox5 cells. These results demonstrate specific binding of radioactivity and successful imaging of human betalox5 cells and human islets transplanted in mice. Thus MORF/cMORF pretargeting may be useful to measure noninvasively human islet cell mass within the pancreas or following islet transplantation.
Keywords: Pretargeting, Islet imaging, Antibody, Morpholino oligomer
Introduction
Development of a non-invasive approach to image human islet cell mass within the pancreas or following islet transplantation is of considerable interest for the diagnosis and prognosis of pre-diabetes or ongoing rejection of transplanted islets and for determining the efficacy of therapeutic interventions 1–8. However, two major difficulties must first be overcome. First, islets of Langerhans comprise only 1–2% of the normal human pancreas and each islet contains 40–70% beta cells. Thus the beta cell mass within a healthy pancreas constitutes only a small percentage of the total pancreas mass, and in diabetic patients this percentage is even lower. In islet transplant recipients, the preferred engraftment approach is via the intraportal venous route 9, which leads to islets scattered through the hepatic vascular tissue. Fortunately, the current non-invasive imaging modalities that include magnetic resonance imaging (MRI) 10, 11, nuclear imaging by either positron emission tomography (PET) or single photon emission computed tomography (SPECT) all exhibit extreme detection sensitivity favorable to the detection of deeply seated organs such as the pancreas or liver. Secondly, the pancreas is surrounded anatomically by the liver, spleen, and intestines that often accumulate high levels of imaging agents following intravenous administration. The accumulation of the radioactive tracer in these and other surrounding non-target tissues can reduce target/non-target ratios and therefore interfere with pancreas imaging.
Previous attempts using nuclear imaging for detection of the pancreatic islets have mainly focused on radiolabeled small molecules directly targeting the vesicular monoamine transporter-type 2 receptor (VMAT2R) and radiolabeled peptides directly targeting the glucagon-like peptide-1 receptor (GLP-1R) 12–17. In addition to the common challenges of imaging islet cell mass, each of these approaches has its own caveats. Apart from beta cells, VMAT2R is also expressed in cells of the central nervous system, in chromaffin cells of the adrenal medulla, and in enterochromaffin-like cells of the stomach 18. Nonspecific accumulation in the pancreas is also a concern 18, 19. In the case of the GLP-1R targeting peptides, the target level fluctuates with the concentration of blood glucose and therefore may be an unreliable indicator of beta cell mass. A third approach using directly labeled antibodies specific for beta cells is under consideration 20, 21, but like all labeled large proteins, intact antibodies accumulate slowly in their target and clear slowly from surrounding tissues, often leading to unfavorable target/non-target ratios. Smaller single chain antibodies displaying faster pharmacokinetics are now under development to address the slow target accumulation and tissue clearance 22.
Pretargeting is an attractive alternative that addresses the slow pharmacokinetics and unfavorable target/non-target ratios observed in the direct targeting approach using labeled antibodies. Pretargeting increases target to non-target ratios and has been successful for imaging tumors 23–27 and infection 28, 29. We have validated a novel pretargeting strategy using a complementary pair of phosphorodiamidate morpholino oligomers (MORF/cMORF) in mouse tumor models 30–35, showing both high sensitivity and specificity and favorable target to non-target ratios.
In the present report, we designed an imaging approach to extend the benefits of MORF/cMORF pretargeting experienced in tumor imaging to a non-cancerous tissue, in this case, islets. We used the human islet cell-specific antibody HPi1 36 to target human islets and a human insulinoma cell line (betalox5) 37, 38 following their transplantation into NOD-scid IL2rγnull mice. We now report that the pretargeting MORF conjugated antibody specifically binds to human islet cells and the labeled cMORF specifically binds to the pretargeting MORF-antibody both in vitro and in vivo. We observed that our pretargeting strategy readily allows non-invasive imaging of human islets and betalox5 cells transplanted into immunodeficient mice.
Materials and Methods
Animals, cells, and materials
Mice
NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD-scid IL2rγnull, abbreviated as NSG) mice were obtained from colonies developed by LDS at The Jackson Laboratory, Bar Harbor, ME 39. All mice were housed in a pathogen free facility in microisolator cages, given autoclaved food and maintained on acidified autoclaved water and sulfamethoxazole-trimethoprim medicated water (Goldline Laboratories, Ft. Lauderdale, FL) on alternate weeks. All animal use was in accordance with the guidelines of the Animal Care and Use Committee of the University of Massachusetts Medical School and The Jackson Laboratory and conformed to the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 1996).
Cell Lines and human islets
The betalox5 cell line was a gift from Dr. Pamela Itkin-Ansari (San Diego, CA). This cell line was derived from adult beta cells and has been described previously 37. The human embryonic kidney (HEK) 293 cell line was obtained from American Tissue Culture Collection. Both cell lines were grown in our laboratory as well as in the tissue culture core facility of our institute. The primary human islets were obtained from the Integrated Islet Distribution Program (IIDP) supported by NIDDK and JDRF.
HPi1 Antibody
The mouse anti-human-islet IgG1 antibody HPi1 was developed at Oregon Health & Science University, Portland, OR 36. This antibody was developed following immunization of BALB/c mice with human islet cells. Immunohistochemistry and flow cytometry both revealed islet cell selectivity and cell surface reactivity.
MORFs and Other Materials
The 3’equivilent terminus amine-derivatized MORF and cMORF were obtained from Gene-Tools (Philomath, OR) with the following base sequences: 5’-TCTTCTACTTCACAACTA and 5’-TAGTTGTGAAGTAGAAGA respectively. The Hydralink kit used for the antibody conjugation with MORF was obtained from Solulink (San Diego, California). The commercial PD-10 column was obtained from NeoRex Corp (Seattle, WA); The Sephadex G100 gel was obtained from Pharmacia Biotech (Uppsala, Sweden). The succinimidyl ester of S-acetylmercaptoacetyltriglycine (NHS-MAG3) was prepared in house 40. The 99Mo-99mTc generator was obtained from Perkin Elmer Life Science Inc (Boston, MA). All other chemicals were reagent grade and were used without further purification.
Synthesis and quality assurance of the MORF-HPi1 pretargeting antibody
Using the commercial Hydralink method, MORF-HPi1 was prepared in a similar manner to that of other MORF-antibodies 33, 35, 41. Briefly, the HPi1 was conjugated with (CH3)2C=NNH-Py-CO2-NHS and, at the same time, the amine derivatized MORF was conjugated with HCO-Ph-CO2-NHS. After purification, the modified antibody and the modified MORF were combined to form a hydrazone link.
The antibody, MORF, and cMORF concentrations were measured by UV spectrophotometry and analyzed for quality assurance by size exclusion HPLC using a Superose-12 column (Amersham Pharmacia Biotech, Piscataway, NJ). The HPLC was equipped with in-line UV and radioactivity detectors. The eluant was 0.10 M pH 7.2 phosphate buffer at a flow rate of 0.60 mL/min. Recovery of radioactivity was routinely measured in the HPLC analysis of all radiolabeled samples and was always over 90%.
The average number of MORFs per antibody (gpm, group per molecule) was determined by adding a known excess of 99mTc-labeled cMORF to a known quantity of MORF-HPi1 as described previously 31. The ability of MORF to hybridize with its cMORF complement was confirmed during the gpm measurement as the radioactive cMORF HPLC peak shifted to a shorter retention time after binding to the MORF-HPi1. The labeling of the cMORF effector after conjugation with NHS-MAG3 was as described previously 42.
The preserved specificity of the HPi1 after conjugation was confirmed in a competitive assay against native HPi1 on betalox5 cells in culture using MORF-HPi1 radiolabeled by adding 99mTc-cMORF at a MORF/cMORF molar ratio of 3. The betalox5 cells were seeded at 0.4×106 cells into each well of 12-well tissue culture plates. After culturing in 1 mL culture medium (DMEM#11885 buffer containing 10% FBS, 5.5 mM glucose 10 mM non-essential amino acids) for 2 days, the medium was removed. To each well was then added 0.2 mL of fresh 37oC culture medium containing 0.3 ng of 99mTc radiolabeled MORF-HPi1 and varying amounts of native HPi1 (0–15 μg, N= 3 for each dosage). After incubation at 37oC for 1 hour, the medium was removed, the cells were rinsed, and both medium and cells were counted for radioactivity.
Specific bindings of cMORF to MORF-HPi1 and MORF-HPi1 to cells in culture
The culture medium for betalox5 cells was the same as above, while that for HEK293 cells was DMEM#11965 buffer containing 10% FBS, 10 mM non-essential amino acids, P/S, and G418. Nine wells were seeded with betalox5 cells and another nine with HEK293 cells as a negative control, each at 0.4×106/well. Two days later the wells for each cell line were divided into 3 groups (A, B, and C; N=3). Medium in each well was replaced with 0.2 ml of culture media containing 30 ng of MORF-HPi1 antibody (group A), 30 ng of native HPi1 (group B) or cell culture medium only (group C). All cells were incubated for 1 h at 37oC. Then, the medium was removed and the cells were washed three times with medium. Each well subsequently received 0.2 mL of culture medium containing 0.6 ng of 99mTc-cMORF. Five minutes later, the medium was removed, the cells were rinsed, and the radioactivity of both the medium and cells was counted.
Animal Studies
Three separate animal studies were performed. The first evaluated the specific binding of the labeled cMORF to the MORF-HPi1 in the betalox5 cell transplanted mice. While we had validated specific binding in vivo of the identical labeled cMORF to several MORF conjugated antitumor antibodies in tumor xenografts 31, 33, 35, it was necessary to confirm that the binding is also specific in connection with islet cell imaging. The second study then measured the specific accumulation of the MORF-HPi1 in the targets of betalox5 cells and human islets by pretargeting, using negative HEK293 cells as control. To further confirm the specific accumulation by pretargeting, the number of the implanted belaox5 cells was varied in the third study so that the cell number could be correlated with the signal strength.
Specific Binding of labeled cMORF to MORF-HPi1 in vivo
Three groups of four NSG mice each received 20×106 betalox5 cells subcutaneously in the right flank. Five days later, mice in group 1 each received via the tail vein 15 μg of MORF-HPi1 (gpm=1.21) in 0.1 mL, mice in group 2 each received 15 μg of native HPi1 and mice in group 3 were left untreated. Two days later, all mice each received 2.2 μg (60 μCi) of radiolabeled cMORF in 0.1 mL via a tail vein. These dosages were previously determined to be optimal for tumor targeting but may not be optimal for targeting islet cells in this model. However, absolute optimization is not required to confirm the target specific accumulation of cMORF. At 3 h post-injection of radioactivity, all mice were euthanized for biodistribution analysis. Organs were harvested and counted along with standards for decay correction and conversion of the counts to percent of injected dosage (%ID). Organ weights were also measured to calculate the %ID/g values. At necropsy, the transplantation sites were visible in the flank but were too small to be reliably separated and weighted. As such, the target flank and the contralateral flank were both excised (including the skin and the muscle) and the accumulation in the target was calculated by the following formula: %ID in the target = (%ID/g of the target flank - %ID/g of the contralateral flank) × the weight of the target flank.
Specific binding of MORF-HPi1 to islet cells in vivo
To confirm that the accumulation of the MORF-antibody in the target was due to specific antibody binding to islet cells but not due to non-specific factors related to any possible local inflammation after transplantation, the study betalox5 cells were transplanted in NSG mice as before. Transplantation of the negative HEK293 cells served as a control. As a more clinically relevant model, another study group was added, in which primary human islets were transplanted in the same manner. Mice in the three groups were randomly selected for imaging before necropsy.
Specifically, 4 mice each received 1) 4000 IEQ human islets, 2) 20×106 betalox5 cells, or 3) 20×106 HEK293 cells in the right flank. Five days later, each animal received 30 μg of the MORF-HPi1 via the tail vein followed 2 days later by intravenous injection of 4.3 μg of radiolabeled cMORF. Both the antibody dosage and the cMORF dosage were increased 2-fold from the dosages used in our first animal study, so that sufficient radioactivity (1.5 mCi) could be administered to each animal for convenient imaging. Mice from each group were imaged on a small animal NanoSPECT/CT camera (Bioscan, Washington DC) 3 h to 7 h post radioactivity injection. Each CT acquisition required about 1–2 min while each SPECT acquisition required about 50 min. The acquisitions were reconstructed and analyzed using InVivoScope 1.37 software (Bioscan). All animals were euthanized at 7 h for biodistribution analysis. Confirmation that the radioactivity signal in the image at the transplant site was localized to the grafts and not to off-target sites such as in the intestine was made by imaging the same animal after all internal organs had been removed.
Correlation of the beta cell number implanted with the signal strength
As a further validation of specific accumulation to target cells by pretargeting, 16 mice in 4 groups (N = 4) were implanted with varying numbers of betalox5 cells from 5 to 20 ×106 in 0.1 mL cell medium. Five days later, each animal received 15 μg of MORF-HPi1 (gpm=1.21). Another three days later, each mouse received 2.2 μg (60 μCi) of radiolabeled cMORF via a tail vein. Three hours later, they were euthanized and the biodistribution data were collected as described above.
Statistics
Statistical analyses used the unpaired Student’s T test. P values less than 0.05 were considered significant.
Results
Synthesis and quality assurance of the MORF-HPi1 pretargeting antibody
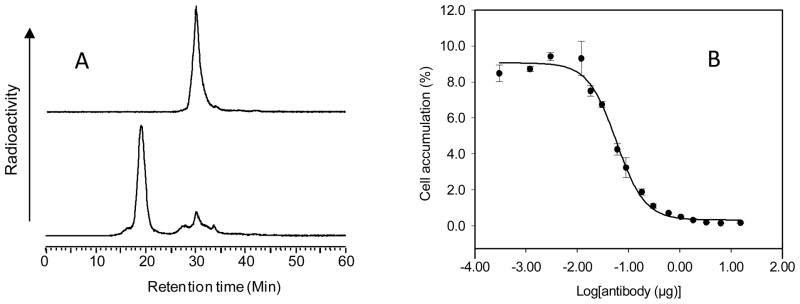
The conjugation of the HPi1 antibody with MORF provided an average gpm of 1.21 and the conjugated antibody retained the ability to hybridize with cMORF. Fig 1A presents an HPLC radiochromatogram of the MORF-HPi1 antibody after adding labeled cMORF at a MORF/cMORF molar ratio of 3, showing a shift in the profile to higher molecular weight resulting from hybridization. Fig 1B shows that the cell binding curve of 99mTc-cMORF/MORF-HPi1 when in competition with increasing concentrations of native HPi1 has a shape typical of specific binding inhibition by unlabeled antibody.
Fig 1.
(A) HPLC radiochromatograms of 99mTc-cMORF before (top) and after (bottom) adding an excess of MORF-HPi1, showing a shift to higher molecular weight resulting from hybridization and (B) results of adding 99mTc-cMORF/MORF-HPi1 along with increasing dosage of unlabeled native HPi1 to betalox5 cells that show a relationship characteristic of preserved binding affinity.
Specific binding of cMORF to MORF-HPi1 and MORF-HPi1 to cells in culture
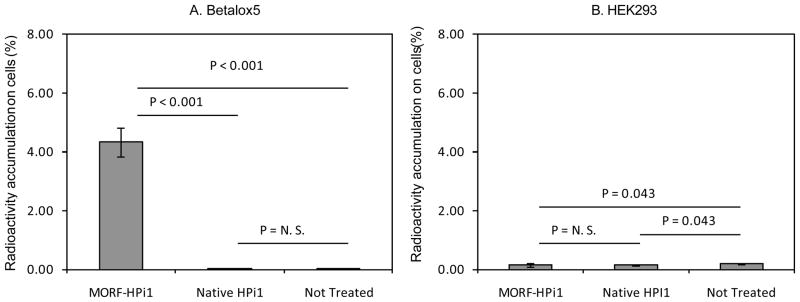
After 1 h incubation, the accumulation of the 99mTc-cMORF effector in the betalox5 cells pretreated with MORF-HPi1 was about100-fold higher compared to accumulations in the betalox5 cells either pretreated or untreated by native HPi1 (both p values <0.05), indicating MORF-HPi1 mediated specific cell binding of labeled cMORF (Fig 2A). Accumulations of the radiolabeled cMORF in the HEK293 control cells treated with MORF-HPi1 in the same manner were, as expected, 25-fold lower (Fig 2B) (p<0.05) and comparable to the accumulations in HEK293 cells pretreated by native HPi1 (0.17% v.s. 0.17%, p=0.46), and slightly lower than the accumulations in HEK293 cells without pretreatment (0.17% v.s. 0.23%, p<0.05). These results confirm that MORF-HPi1 binds specifically to betalox5 cells.
Fig 2.
Histograms presenting the cell accumulations of 99mTc-cMORF in betalox5 cells (panel A) and HEK293 cells (panel B) after treatment with MORF-HPi1, native HPi1, or untreated. Error bars signify one SD (N=3). No significant differences were observed among values in panel B.
Specific binding of labeled cMORF to MORF-HPi1 in vivo
The accumulation of 99mTc-cMORF in the betalox5 transplant site of mice pretargeted with MORF-HPi1 was more than 8-fold higher than the accumulations in the transplant sites of the control mice pretargeted with the unmodified native HPi1 and the transplant sites of control mice not pretargeted (Fig 3). These data demonstrate the specific accumulation of cMORF to the MORF-HPi1 in the target site in vivo.
Fig 3.
Histograms presenting the accumulations of 99mTc-cMORF in the transplantation site 3 h post administration in mice transplanted with betalox5 cells and pretargeted with MORF-HPi1, native HPi1 or nothing. Error bars signify one SD (N=4).
Specific binding of pretargeting antibody to islet cells in vivo
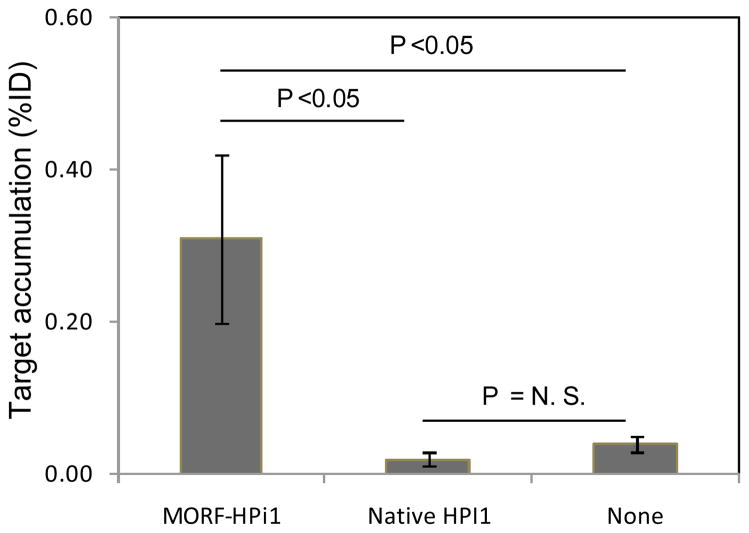
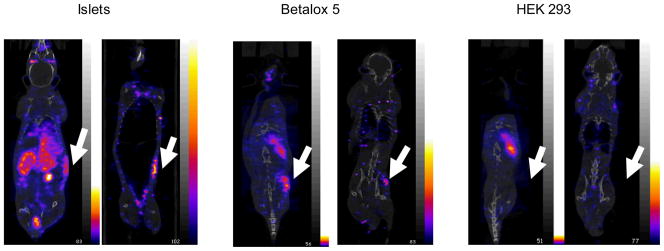
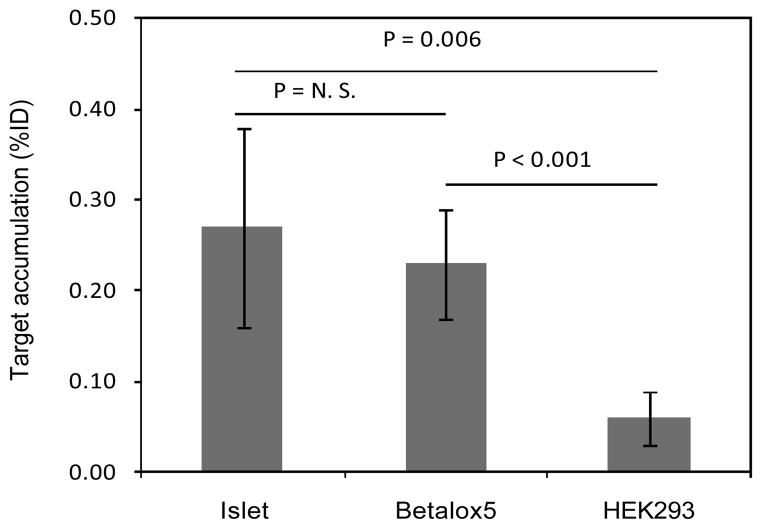
The study was performed in three transplant models, NSG mice transplanted with betalox5 cells, HEK293 cells, or human islets. For each transplant model, Fig 4 shows one coronal slice of one animal on the left in each panel. On the right of each panel is a coronal slice at the same level from the same animal but after removal of internal organs. A focal radioactivity accumulation is evident in the betalox5 and human islet transplanted mice but not in the HEK293 transplanted animals. That the accumulation remained after the removal of internal organs confirms that the accumulation is at the site of transplantation. Fig 5 presents target accumulations at 7 h postinjection of the radioactive cMORF by necropsy, confirming the results of live animal imaging. However, the non-specific accumulation in the HEK293 control (due to non-specific MORF-antibody still in circulation) remains considerable and may suggest that further optimization of the pretargeting parameters (dosage and timing) will be needed to improve further the target/non-target ratios. Nevertheless, the 4-fold higher accumulations in study groups verify the accumulation is predominately the result of antibody/antigen specific binding of the MORF-HPi1 antibody to islet cells.
Fig 4.
Coronal slices at the same level before (left panels) and after (right panels) removal of internal organs. One representative animal for each of the three groups (left to right): transplanted with islets, betalox5 cells, and HEK293 cells, all pretargeted with MORF-HPi1 and receiving 99mTc-cMORF 48 h later. The presence of a focal radioactivity accumulation in the human islet and betalox5 transplanted mice but not in the HEK293 transplanted mice and the continuing presence of signal after the removal of internal organs confirms that the accumulations are at the site of transplantation and are the result of specific binding of the antibody to the target.
Fig 5.
Histograms presenting target accumulations of 99mTc-cMORF at 7 h in MORF-HPi1 pretargeted mice transplanted with human islets, betalox5 cells, or HEK293 cells. Error bars represent one standard deviation (N = 4).
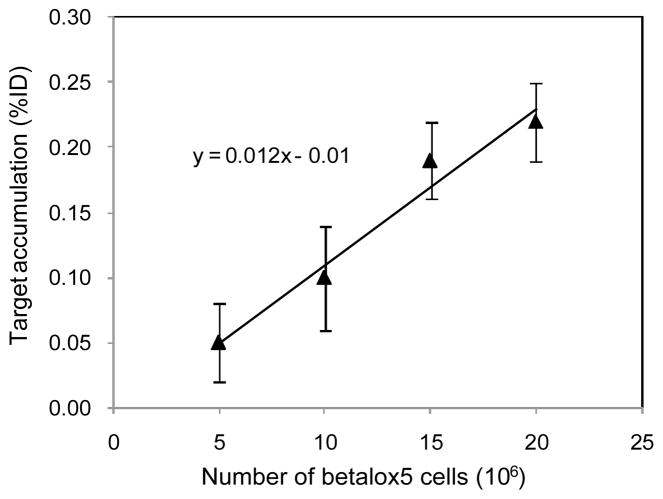
Correlation of the beta cell number implanted with the signal strength
Fig 6 shows that the signal accumulation by pretargeting (%ID) increases linearly with the number of implanted beta cells. These data provide additional evidence that the signal accumulation is due to the beta cell binding of the radioactivity. Extrapolation of the fitted line intersects the Y axis very close to the origin at 0.01 %ID.
Fig 6.
Results of a linear regression fit between the number of betalox5 cells transplanted in the flank of mice and the target accumulation (%ID). Error bars represent one standard deviation (N = 4).
Discussion
In this manuscript, we have presented evidence both in vitro and in vivo confirming that the MORF/cMORF pretargeting strategy can be used to specifically target human islet cells using primary human islets and human islet cell line transplants in mice. We have also demonstrated that islets and islet cell transplants can be readily imaged. While non-specific accumulation at low levels remains evident due to incomplete clearance of MORF-conjugated antibodies from the circulation, we expect that further optimization of the dosage and timing will reduce the non-specific accumulation even further. However, even in the presence of this low level non-specific antibody accumulation, the target accumulations clearly show evidence of specific binding of labeled cMORF to MORF-HPi1 and MORF-HPi1 to the human islet cells in vivo.
Pretargeting with antibodies directed at epitopes expressed by human islet cells has not previously been reported. While MORF/cMORF pretargeting has provided high target to non-target ratios and sensitive tumor detection in mouse models 23–27, this investigation represents our first attempt to apply MORF/cMORF pretargeting to targeting non cancer cells, in this case, islet cells. Apart from the need for improved methods of islet imaging, selecting diabetes as a non-malignant disease offers a particularly difficult test of the pretargeting strategy.
Two recent advances made these experiments possible. First, the studies described in this manuscript represent a new approach for human islet cell imaging using human islet cell-specific antibodies. These studies were possible due to the recent development and characterization of a number of antibodies against human pancreatic islet cells, including HPi1 36. Second, novel immunodeficient mice as recipients for transplantation of human islets for in vivo islet cell imaging are now available 39, 43–45. NOD-scid IL1rγnull mice have recently been used to document the function of transplanted human islets 46, 47, and in the current study, to permit in vivo investigations of human islet cell pretargeting.
The ultimate goal of this research is to develop an islet cell imaging approach that is noninvasive and capable of measuring islet (or beta) cell mass by imaging. The results of this investigation are encouraging in that specific accumulations were detectable by imaging in animals transplanted with a limited number of human betalox5 cells or primary human islets. Our data indicate that a linear relationship exists between the number of transplanted beta cells and signal intensity. The utility of this approach will need further validation in additional studies with varying target type (beta cells versus islets) and transplant location. In addition, intraportal islet transplantation may be more clinically relevant than the subcutaneous islet or islet cell transplant locations used in this investigation, but islets located in the hepatic vascular structure may be even more accessible or easier for targeting. Optimization of MORF/cMORF pretargeting or other pretargeting approaches may ultimately permit this strategy to be useful for imaging islets within an intact pancreas.
Acknowledgments
This work was supported by the Juvenile Diabetes Research Foundation International (JDRF 37-2009-7) and grants DK082894, CA94994, DK72473, AI46629, AI050864, and Diabetes Endocrinology Research Center grant DK32520 from the National Institutes of Health. Some data in this report were orally presented in the symposium on beta-cell imaging on the occasion of the 46th Annual Meeting of the European Association for the Study of Diabetes (EASD) in Stockholm, Sweden, and at the 57th Society of Nuclear Medicine annual meeting in Salt Lake City, Utah, USA. We thank Dr Pamela Itkin-Ansari from the University of California San Diego and Sanford-Burnham Medical Research Institute, San Diego, CA for her generous gift of the betalox5 cell line and Louise Ohrn of Tissue Culture Core Facility of UMass Medical School for cell maintenance. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1.Paty BW, Bonner-Weir S, Laughlin MR, McEwan AJ, Shapiro AM. Toward development of imaging modalities for islets after transplantation: insights from the National Institutes of Health Workshop on Beta Cell Imaging. Transplantation. 2004;77:1133–1137. doi: 10.1097/01.tp.0000113231.90613.0e. [DOI] [PubMed] [Google Scholar]
- 2.Souza F, Freeby M, Hultman K, Simpson N, Herron A, Witkowsky P, Liu E, Maffei A, Harris PE. Current progress in non-invasive imaging of beta cell mass of the endocrine pancreas. Curr Med Chem. 2006;13:2761–2773. doi: 10.2174/092986706778521940. [DOI] [PubMed] [Google Scholar]
- 3.Moore A. Advances in beta-cell imaging. Eur J Radiol. 2009;70:254–257. doi: 10.1016/j.ejrad.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malaisse WJ, Louchami K, Sener A. Noninvasive imaging of pancreatic beta cells. Nat Rev Endocrinol. 2009;5:394–400. doi: 10.1038/nrendo.2009.103. [DOI] [PubMed] [Google Scholar]
- 5.Brom M, Andrałojč K, Oyen WJ, Boerman OC, Gotthardt M. Development of radiotracers for the determination of the beta-cell mass in vivo. Curr Pharm Des. 2010;16:1561–1567. doi: 10.2174/138161210791164126. [DOI] [PubMed] [Google Scholar]
- 6.Martinic MM, von Herrath MG. Real-time imaging of the pancreas during development of diabetes. Immunol Rev. 2008;221:200–213. doi: 10.1111/j.1600-065X.2008.00581.x. [DOI] [PubMed] [Google Scholar]
- 7.Villiger M, Goulley J, Martin-Williams EJ, Grapin-Botton A, Lasser T. Towards high resolution optical imaging of beta cells in vivo. Curr Pharm Des. 2010;16:1595–1608. doi: 10.2174/138161210791164153. [DOI] [PubMed] [Google Scholar]
- 8.Medarova Z, Moore A. MRI as a tool to monitor islet transplantation. Nat Rev Endocrinol. 2009;5:444–452. doi: 10.1038/nrendo.2009.130. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 10.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006;12:44–48. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 11.Antkowiak PF, Tersey SA, Carter JD, Vandsburger MH, Nadler JL, Epstein FH, Mirmira RG. Noninvasive assessment of pancreatic beta-cell function in vivo with manganese-enhanced magnetic resonance imaging. Am J Physiol Endocrinol Metab. 2009;296:E573–578. doi: 10.1152/ajpendo.90336.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goland R, Freeby M, Parsey R, Saisho Y, Kumar D, Simpson N, Hirsch J, Prince M, Maffei A, Mann JJ, Butler PC, Van Heertum R, Leibel RL, Ichise M, Harris PE. 11C-dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J Nucl Med. 2009;50:382–389. doi: 10.2967/jnumed.108.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kung MP, Hou C, Lieberman BP, Oya S, Ponde DE, Blankemeyer E, Skovronsky D, Kilbourn MR, Kung HF. In vivo imaging of beta-cell mass in rats using 18F-FP-(+)-DTBZ: a potential PET ligand for studying diabetes mellitus. J Nucl Med. 2008;49:1171–1176. doi: 10.2967/jnumed.108.051680. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Dang H, Middleton B, Zhang Z, Washburn L, Stout DB, Campbell-Thompson M, Atkinson MA, Phelps M, Gambhir SS, Tian J, Kaufman DL. Noninvasive imaging of islet grafts using positron-emission tomography. Proc Natl Acad Sci USA. 2006;103:11294–11299. doi: 10.1073/pnas.0603909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48:736–743. doi: 10.2967/jnumed.106.038679. [DOI] [PubMed] [Google Scholar]
- 16.Wild D, Béhé M, Wicki A, Storch D, Waser B, Gotthardt M, Keil B, Christofori G, Reubi JC, Mäcke HR. [Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J Nucl Med. 2006;47:2025–2033. [PubMed] [Google Scholar]
- 17.Kwan EP, Gaisano HY. Glucagon-like peptide 1 regulates sequential and compound exocytosis in pancreatic islet beta-cells. Diabetes. 2005;54:2734–2743. doi: 10.2337/diabetes.54.9.2734. [DOI] [PubMed] [Google Scholar]
- 18.Saisho Y, Harris PE, Butler AE, Galasso R, Gurlo T, Rizza RA, Butler PC. Relationship between pancreatic vesicular monoamine transporter 2 (VMAT2) and insulin expression in human pancreas. J Mol Histol. 2008;39:543–551. doi: 10.1007/s10735-008-9195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson O, Jahan M, Johnström P, Korsgren O, Sundin A, Halldin C, Johansson L. In vivo and in vitro characterization of [18F]-FE-(+)-DTBZ as a tracer for beta-cell mass. Nucl Med Biol. 2010;37:357–363. doi: 10.1016/j.nucmedbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Moore A, Bonner-Weir S, Weissleder R. Noninvasive in vivo measurement of beta-cell mass in mouse model of diabetes. Diabetes. 2001;50:2231–2236. doi: 10.2337/diabetes.50.10.2231. [DOI] [PubMed] [Google Scholar]
- 21.Saudek F, Brogren CH, Manohar S. Imaging the Beta-cell mass: why and how. Rev Diabet Stud. 2008;5:6–12. doi: 10.1900/RDS.2008.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueberberg S, Meier JJ, Waengler C, Schechinger W, Dietrich JW, Tannapfel A, Schmitz I, Schirrmacher R, Köller M, Klein HH, Schneider S. Generation of novel single-chain antibodies by phage-display technology to direct imaging agents highly selective to pancreatic beta- or alpha-cells in vivo. Diabetes. 2009;58:2324–2334. doi: 10.2337/db09-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karacay H, Brard PY, Sharkey RM, Chang CH, Rossi EA, McBride WJ, Ragland DR, Horak ID, Goldenberg DM. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11:7879–7885. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 24.Sharkey RM, Cardillo TM, Rossi EA, Chang CH, Karacay H, McBride WJ, Hansen HJ, Horak ID, Goldenberg DM. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med. 2005;11:1250–1255. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 25.Pagel JM, Hedin N, Subbiah K, Meyer D, Mallet R, Axworthy D, Theodore LJ, Wilbur DS, Matthews DC, Press OW. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101:2340–2348. doi: 10.1182/blood-2002-03-0874. [DOI] [PubMed] [Google Scholar]
- 26.Subbiah K, Hamlin DK, Pagel JM, Wilbur DS, Meyer DL, Axworthy DB, Mallett RW, Theodore LJ, Stayton PS, Press OW. Comparison of immunoscintigraphy, efficacy, and toxicity of conventional and pretargeted radioimmunotherapy in CD20-expressing human lymphoma xenografts. J Nucl Med. 2003;44:437–445. [PubMed] [Google Scholar]
- 27.Magnani P, Paganelli G, Modorati G, Zito F, Songini C, Sudati F, Koch P, Maecke HR, Brancato R, Siccardi AG, Fazio F. Quantitative comparison of direct antibody labeling and tumor pretargeting in uveal melanoma. J Nucl Med. 1996;37:967–971. [PubMed] [Google Scholar]
- 28.Boerman OC, van Eerd J, Oyen WJG, Corstens FHM. 3-step pretargeting strategy to image infection. J Nucl Med. 2001;42:1405–1411. [PubMed] [Google Scholar]
- 29.Rusckowski M, Fritz B, Hnatowich DJ. Localization of infection using streptavidin and biotin: an alternative to nonspecific polyclonal IgG. J Nucl Med. 1992;33:1810–1815. [PubMed] [Google Scholar]
- 30.Liu G, Mang’era K, Liu N, Gupta S, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using 99mTc-labeled morpholino, a DNA analog. J Nucl Med. 2002;43:384–391. [PubMed] [Google Scholar]
- 31.Liu G, He J, Dou S, Gupta S, Vanderheyden JL, Rusckowski M, Hnatowich DJ. Pretargeting in tumored mice with radiolabeled morpholino oligomer showing low kidney uptake. Eur J Nucl Med Mol Imaging. 2004;31:417–424. doi: 10.1007/s00259-003-1393-9. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Dou S, Mardirossian G, He J, Zhang S, Liu X, Rusckowski M, Hnatowich DJ. Successful radiotherapy of tumor in pretargeted mice by 188Re radiolabeled phosphorodiamidate morpholino oligomer, a synthetic DNA analog. Clin Cancer Res. 2006;12:4958–4964. doi: 10.1158/1078-0432.CCR-06-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Dou S, Pretorius PH, Liu X, Rusckowski M, Hnatowich DJ. Pretargeting CWR22 prostate tumor in mice with MORF-B72.3 antibody and radiolabeled cMORF. Eur J Nucl Med Mol Imaging. 2008;35:272–280. doi: 10.1007/s00259-007-0606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu G, Cheng D, Dou S, Chen X, Liang M, Pretorius H, Rusckowski M, Hnatowich DJ. Replacing 99mTc with 111In improves MORF/cMORF pretargeting by reducing intestinal accumulation. Mol Imag Biol. 2009;11:303–307. doi: 10.1007/s11307-009-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Dou S, Pretorius PH, Liu X, Chen L, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using phosphorodiamidate morpholino oligomer (MORF) conjugated CC49 antibody and radiolabeled complimentary MORF effector. Q J Nucl Med Mol Imaging. 2010;54:333–340. [PMC free article] [PubMed] [Google Scholar]
- 36.Dorrell C, Abraham SL, Lanxon-Cookson KM, Canaday PS, Streeter PR, Grompe M. Isolation of major pancreatic cell types and long-term culture-initiating cells using novel human surface markers. Stem Cell Res. 2008;1:183–194. doi: 10.1016/j.scr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 37.de la Tour D, Halvorsen T, Demeterco C, Tyrberg B, Itkin-Ansari P, Loy M, Yoo SJ, Hao E, Bossie S, Levine F. Beta-cell differentiation from a human pancreatic cell line in vitro and in vivo. Mol Endocrinol. 2001;15:476–483. doi: 10.1210/mend.15.3.0604. [DOI] [PubMed] [Google Scholar]
- 38.Itkin-Ansari P, Geron I, Hao E, Demeterco C, Tyrberg B, Levine F. Cell-based therapies for diabetes: progress towards a transplantable human beta cell line. Ann N Y Acad Sci. 2003;1005:138–147. doi: 10.1196/annals.1288.015. [DOI] [PubMed] [Google Scholar]
- 39.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2rgnull mice engrafted with mobilized human hematopoietic stem cell. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 40.Winnard P, Jr, Chang F, Rusckowski M, Mardirossian G, Hnatowich DJ. Preparation and use of NHS-MAG3 for technetium-99m labeling of DNA. Nucl Med Biol. 1997;24:425–432. doi: 10.1016/s0969-8051(97)00027-9. [DOI] [PubMed] [Google Scholar]
- 41.He J, Liu G, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. An improved method for covalently conjugating morpholino oligomers to antitumor antibodies. Bioconjug Chem. 2007;18:983–988. doi: 10.1021/bc060208v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu G, Dou S, He J, Yin D, Gupta S, Zhang S, Wang Y, Rusckowski M, Hnatowich DJ. Radiolabeling of MAG3-morpholino oligomers with 188Re at high labeling efficiency and specific radioactivity for tumor pretargeting. Appl Radiat Isot. 2006;64:971–978. doi: 10.1016/j.apradiso.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 44.Shultz LD, Pearson T, King M, Giassi L, Carney L, Gott B, Lyons B, Rossini AA, Greiner DL. Humanized NOD/LtSz-scid IL2 receptor common gamma chain knockout mice in diabetes research. Ann N Y Acad Sci. 2007;1103:77–89. doi: 10.1196/annals.1394.002. [DOI] [PubMed] [Google Scholar]
- 45.Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Curr Protoc Immunol. 2008;Chapter 15(Unit 15.21) doi: 10.1002/0471142735.im1521s81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, Atkinson MA, Wasserfall C, Herold KC, Woodland RT, Schmidt MR, Woda BA, Thompson MJ, Rossini AA, Greiner DL. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Pearson T, Shultz LD, Lief J, Burzenski L, Gott B, Chase T, Foreman O, Rossini AA, Bottino R, Trucco M, Greiner DL. A new immunodeficient hyperglycaemic mouse model based on the Ins2Akita mutation for analyses of human islet and beta stem and progenitor cell function. Diabetologia. 2008;51:1449–1456. doi: 10.1007/s00125-008-1057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]