Abstract
Osmotic stress caused oxidative stress in rhesus macaque sperm, which was alleviated by antioxidant supplementation. The objective of the present study was to demonstrate that cryopreservation of rhesus macaque sperm also induces (reactive oxygen species) ROS production, and to determine whether ROS have an important role in cryopreservation-induced membrane. Additionally, we evaluated the antioxidant capacity of TEST buffer (with 20% egg yolk and 13% skim milk) and supplementation with antioxidants, superoxide dismutase (SOD), catalase (CAT), and α-tocopherol. There was a substantial level of ROS production in both the presence (15% increase in superoxide, P < 0.01; 14% increase in hydrogen peroxide, P < 0.01) and absence of egg yolk (EY) and skim milk (SM; 33% increase in superoxide, P < 0.001; 48% increase in hydrogen peroxide, P < 0.001). Superoxide dismutase provided little membrane protection against ROS, but increased post-thaw total and progressive motility by 10% (P < 0.01) and 15% (P < 0.05), respectively. Supplementation with CAT and α-tocopherol in the presence of EY and SM decreased H2O2 by 55% (P < 0.01) and 49% (P < 0.001), whereas supplementation with CAT and α-tocopherol in the absence of EY and SM reduced the level of lipid peroxidation by 61% (P < 0.05) and 28% (P < 0.01). In conclusion, this is apparently the first report that cryopreservation of rhesus macaque sperm induced a significant increase in ROS and that antioxidant supplementation can significantly decrease the extent of ROS-induced membrane damage.
Keywords: Cryopreservation, Sperm, Nonhuman primate, Oxidative stress, Lipid peroxidation
1. Introduction
The rhesus macaque provides an important model for human biomedical research; therefore, the ability to preserve and store the genetics from this non-human primate (NHP) is imperative. Maintaining NHP populations by conventional breeding in most cases is resource intensive, leaving sperm cryopreservation as a more viable option. Further, development of cryopreservation media without animal products is desirable for biosecurity of preserved and transported semen, for a variety of medically and economically important species. Despite the advances that have been made, cryopreservation still induces considerable damage to sperm as a result of the extreme temperature and osmolality changes to which cells are exposed [1–3]. With rapidly decreasing temperatures, extracellular solutes begin to freeze and the cell is exposed to an increasingly hyperosmolal environment, which turns extremely hypo-osmotic during thawing [4,5]. This fluctuation in osmolality causes substantial membrane stretching, which can disrupt the actin cytoskeleton and decrease the lateral packing of the lipids, leaving the cell membrane extremely vulnerable to oxidative damage [3,6,7].
There are several low-molecular weight antioxidants (enzymatic and non-enzymatic) present in seminal plasma to help protect sperm against oxidative damage by reactive oxygen species (ROS) [8–12] However, during preparation for cryopreservation, sperm are washed out of seminal plasma and re-suspended in cryopreservation extender, which leaves them vulnerable to oxidative stress due to loss of antioxidant protection [13]. The absence of seminal plasma, in combination with a membrane high in polyunsaturated fatty acids (PUFA), makes sperm particularly susceptible to lipid peroxidation and cell damage during cryopreservation [14]. Two significant types of ROS free radicals are generated under conditions of cellular stress are superoxide anion (O2−·) and hydrogen peroxide (H2O2), both of which have been associated with cryopreservation [15,16] and ensuing oxidative lipid and DNA damage [17–19].
Previous work in our laboratory demonstrated that osmotic stress of rhesus macaque sperm resulted in significant ROS production; therefore, we inferred that cryopreservation will also result in ROS production. Our primary goal was to demonstrate that cryopreservation of rhesus macaque sperm induces ROS production and to determine whether ROS have an important role in membrane damage sustained during cryopreservation. Additionally, we evaluated the antioxidant capacity of our cryopreservation buffer and whether supplementation with the antioxidants superoxide dismutase, catalase, or α–tocopherol, would provide additional protection against these ROS during cryopreservation.
2. Materials and methods
2.1. Chemicals
Superoxide dismutase (SOD), catalase (CAT), and α-tocopherol (Type IV, from vegetable oil) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Dihydroethidium (DHE), 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA), SYTOX® Green, 4,4-difluro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (BODIPY® 581/591), and the LIVE/DEAD sperm viability kit (Propidium Iodide and SYBR® 14) were obtained from Invitrogen (Eugene, OR, USA). In order to minimize premature oxidation, DHE and H2DCFDA were initially solubilized in DMSO, held under N2, and stored at −80°C until needed.
2.2. Experiment 1: cryopreservation of rhesus macaque sperm and the evaluation of ROS production
One ejaculate from each of five male rhesus macaques (Macaca mulatta) were obtained by electroejaculation, as previously described [20]. Animals were housed at California National Primate Research Center and maintained according to Institutional Animal Care and Use Committee protocols at the University of California. Semen samples were collected directly into 50 mL centrifuge tubes containing 5 mL of HEPES-Biggers, Whitten, and Whittingham (BWW) media [21]. Following semen collection, semen samples were diluted in an additional 5 mL HEPES-BWW containing 2 mg/mL polyvinylalcohol (PVA) for a final concentration of 1 mg/mL PVA. The samples were gently rocked for 5 min, after which the coagulum was removed and the sample was maintained at room temperature for 10 min to allow particulate matter to settle to the bottom. The upper 9.5 mL of semen was then transferred into a separate tube for determination of initial motility. Cells were then washed by centrifugation for 5 min at 300 × g and re-suspended to 100 × 106/mL in one of two diluents: 1) complete TEST buffer (4.325% TES, 1.027% Tris, 1.0% dextrose, pH.7.4) supplemented with 30% (v/v) fresh egg yolk (EY) and 20% (v/v) skim milk (SM) + 3% glycercol [22]; or 2) TEST buffer (no EY or SM) + 3% glycerol as a control. The complete TEST medium is routinely used for NHP sperm cryopreservation. It was unknown whether EY or SM would affect the level of ROS; therefore, we included a ‘no EY/no SM’ control TEST buffer.
Pre-freeze samples were diluted 1:1 with fresh cryopreservation media in the absence of glycerol, washed once by centrifugation for 5 min at 300 × g, and re-suspended in BWW + 1 mg/mL PVA to 25 × 106/mL. The following end points were then evaluated before and after cryopreservation: motility (CASA), viability (SYBR 14/PI), level of lipid peroxidation (BODIPY), and level of ROS production (DHE, and DCF).
Cells were taken for pre-freeze evaluation, as above, or loaded into 0.5 mL polyvinylchloride straws (IMV; Minneapolis, MN, USA) and sealed with polyvinyl chloride sealing powder (IMV). Straws were then transferred to a controlled rate programmable freezer (Planar Products, Ltd, Middlesex, UK) and frozen according to the following freezing curve: −0.5 °C/min from 22 °C to 8 °C, −17 °C/min from 8 °C to −110 °C, after which they were plunged into liquid nitrogen (−196 °C) for storage. Following cryopreservation for 24–48 h, one straw per treatment was thawed for 30 s in a 37 °C water bath. The samples were diluted 1:1 with fresh cryopreservation media in the absence of glycerol and washed once with BWW + 1 mg/mL PVA by centrifugation for 5 min at 300 × g. Cells were then re-suspended in BWW + 1 mg/mL PVA to 25 × 106/mL for post-thaw evaluation. After thawing, samples were equilibrated for 5 min prior to further evaluation.
2.3. Experiment 2: antioxidant supplementation of rhesus macaque sperm during cryopreservation
Five additional semen samples were collected from each of the previously used rhesus males and were processed as described above (in Experiment 1). All ejaculates were extended in complete TEST buffer + 3% glycerol or TEST buffer (no EY or SM) + 3% glycerol, with or without the following antioxidants: SOD (200 U/mL), CAT (200 U/mL), and α-tocopherol (100 μM) for 15 min prior to cryopreservation. Motility, viability, lipid peroxidation, and ROS production were evaluated both pre- and post-thaw.
2.4. Motility
Sperm motility was evaluated by means of computer-assisted sperm analysis (CASA) with HTM Ceros, version 12.2 g (Hamilton Thorne Biosciences, Inc, Beverly, MA, USA). At least 200 cells in a minimum of four fields were evaluated for each treatment. The following instrument settings were used for CASA analysis: frame rate, 60 Hz; frames acquired, 30; minimum contrast, 80; minimum cell size, 4 pixels; static VAP cutoff, 20 μ/s; static VSL cutoff, 10 μ/s; progressive VAP threshold, 25 μ/s; progressive STR threshold, 80%; static intensity limits, 0.6 – 1.4; static size limits, 0.6 – 2.31; and static elongation limits 0–80. Percent total motility (TM) and percent progressive (forward) motility (PM) were determined at all time points.
2.5. Viability
The membrane-permeant nucleic acid stain, SYBR-14, and the membrane-impermeant dye, propidium iodide (PI), were added to 500 μL of each sample, for final concentrations of 0.1 and 12 μM, respectively. Sperm were incubated for 10 min at room temperature, diluted to 1 × 106/mL, and analyzed by flow cytometry. The excitation wavelength used for both fluorochromes was 488 nm and the emission maxima of SYBR-14 and PI was 516 nm and 617 nm, respectively [6].
2.6. Measurement of ROS: the DHE and H2DCFDA assay
To measure the superoxide anion, dihydroethidium (DHE) (2 μM) and SYTOX green (0.05 μM) were added together for 15 min; thereafter, sperm were diluted to 1 × 106/mL and analyzed by flow cytometry [6,23]. Hydrogen peroxide was measured by evaluating the oxidation of the cell-permeant probe 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA). Both H2DCFDA (50 μM) and PI (5 μM) were added for 15 min after which sperm were diluted to 1 × 106/mL and analyzed by flow cytometry [6].
2.7. Measurement of lipid peroxidation: the BODIPY581/591C-11 assay
Lipid peroxidation was evaluated using the fluorescence lipid probe BODIPY581/591C-11 (4,4-difluro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic-acid). When this probe became incorporated into the sperm membrane, it fluoresced red until lipid radicals peroxidized the membrane, at which time the probe underwent an emission shift to green (red: non-peroxidized, green: peroxidized). Before cryopreservation, sperm were incubated in 1 μM BODIPY581/591C-11 for 30 min at room temperature to load the probe into the cell membrane. Sperm were then washed two times (300 × g, 5 min) to remove excess probe, and re-suspended in TEST buffer and processed for cryopreservation. Upon thawing, sperm were diluted to 25 × 106/mL in the presence of the lipid peroxidation promoters’ ferrous sulfate (1 μM) and sodium ascorbate (5 μM) for 30 min. During the last 5 min, PI (12 μM) was added, after which sperm were diluted to 1 × 106/mL and analyzed by flow cytometry.
2.8. Flow cytometric analysis
Flow cytometry was performed using a FACScan flow cytometer (Becton-Dickenson, Franklin Lakes, NJ, USA), equipped with a 488 nm excitation laser, and data was analyzed using CellQuest software (Becton-Dickenson, Franklin Lakes, NJ, USA). The SYBR 14, SYTOX green, and DCF were measured with a 530/30 bandpass filter, whereas PI was measured with a 585/42 bandpass filter and DHE was measured with a 650LP bandpass filter. Adjustments were made to address and eliminate fluorochrome spectral overlap, so that each cell population was seen as distinct. To limit the evaluation of DHE, DCF, and BODIPY fluorescence to viable sperm, only the subpopulation outside of SYTOX green or PI positive cells, respectively, were included in the evaluation. A total of 10,000 gated events were analyzed per sample.
2.9. Statistical analysis
Data were analyzed by a one-way analysis of variance (ANOVA) and individual means were compared using Tukey’s multiple range post-hoc test, by use of the Minitab statistical software (Minitab Inc, State College, PA, USA). In Experiment 2, the level of interaction between the cryopreservation media and supplemented antioxidant were determined using two-way ANOVA. All data were blocked by individual male and due to the absence of male differences, data for each treatment was pooled. Data are expressed as the mean ± SEM and the level of significance was set at P < 0.05.
3. Results
3.1. Experiment 1: cryopreservation of rhesus macaque sperm and the evaluation of ROS production
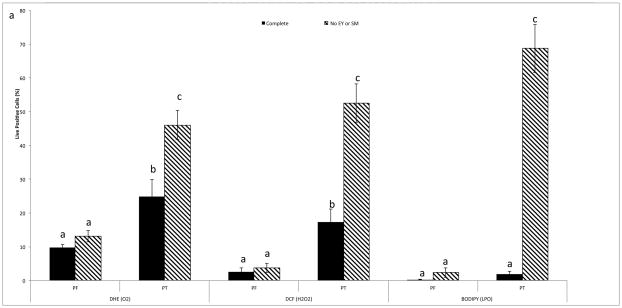
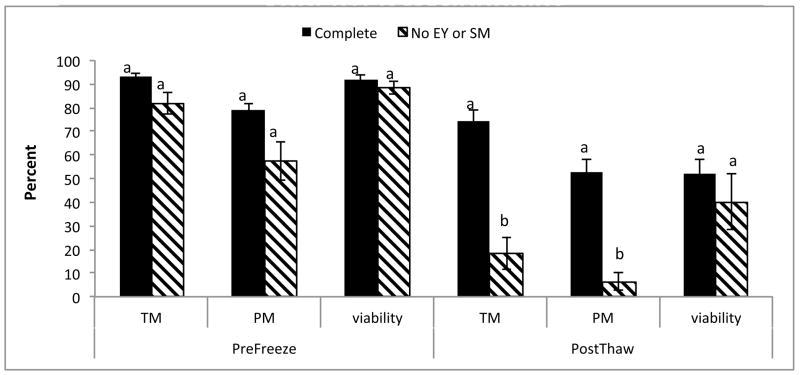
Cryopreservation of rhesus macaque sperm in complete TEST buffer + 3% glycerol increased both O2−·;. (P < 0.01) and H2O2 (P < 0.01) production; however, there was no increase in lipid peroxidation in this medium (Fig. 1). To determine whether EY and SM provided protection from ROS, rhesus macaque sperm were also cryopreserved in TEST buffer + 3% glycerol in the absence of those two components. Analysis of post-thaw samples revealed an increase in both O2−·. (P < 0.01) and H2O2 (P < 0.01), with a very high level of lipid peroxidation (P < 0.01). Comparison of post thaw ROS production between both mediums revealed a higher level of ROS production (P < 0.01), as well as higher levels of lipid peroxidation (P < 0.001; Fig. 1) in cells cryopreserved in the absence of EY and SM. There was also a noticeable decrease in both total (P < 0.01) and progressive (P < 0.01) motility when cells were cryopreserved in the absence of EY and SM, although there was no difference in viability (P = 0.49; Fig. 2).
Fig. 1.
Mean (± SEM) pre- and post-thaw ROS (superoxide - O2−· and hydrogen peroxide - H2O2)and lipid peroxidation (LPO) measured in rhesus macaque sperm cryopreserved in complete TEST buffer + 3% glycerol, or TEST buffer with 3% glycerol, but no egg yolk (EY) or skim milk (SM) + 3%.
a–cMeans without a common superscript between treatments differ (P < 0.05).
Fig. 2.
Mean (± SEM) pre- and post-thaw total (TM) and progressive (PM) motility of cryopreserved rhesus macaque sperms in TEST buffer + 3% glycerol in the absence of egg yolk (EY) or skimmed milk (SM).
a,bMeans without a common superscript differ (P < 0.05).
3.2. Experiment 2: antioxidant supplementation of rhesus macaque sperm during cryopreservation
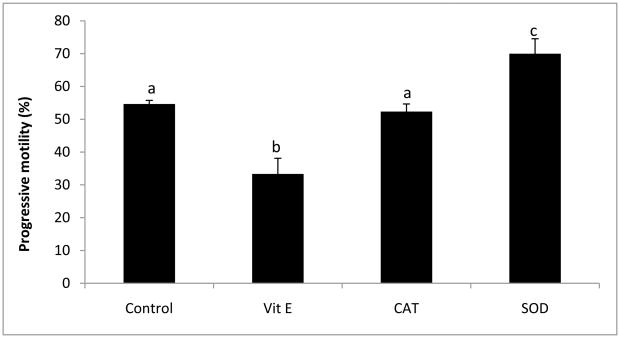
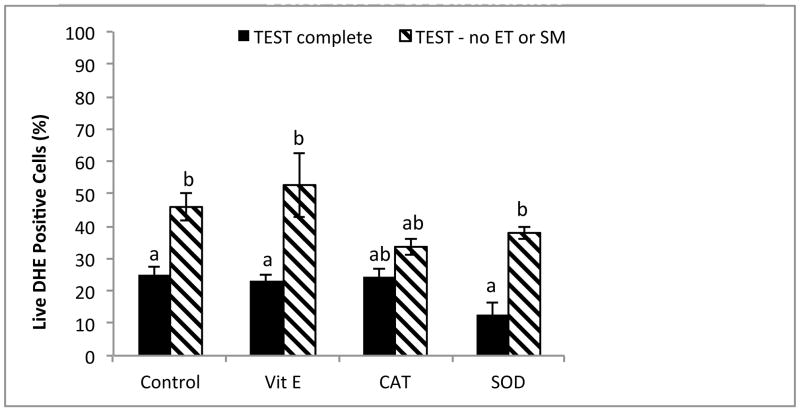
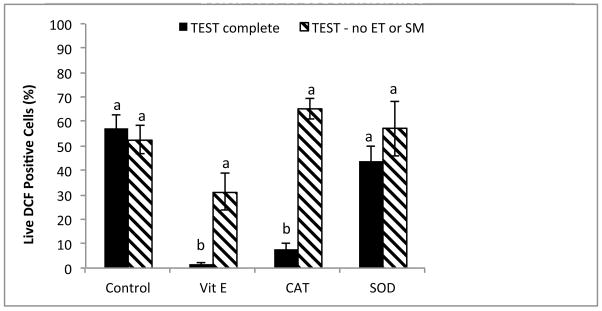
The addition of antioxidants to rhesus sperm cryopreserved in TEST buffer + 3% glycerol in the absence or presence of EY and SM affected both total and progressive motility. Whereas α-tocopherol decreased both total (P < 0.05) and progressive (P < 0.05) motility, the addition of SOD increased both total (P < 0.05, data not shown) and progressive (P < 0.05) motility to control levels (Fig. 3). No affect on viability was observed (P = 0.843, data not shown). Antioxidant protection against ROS was variable (Fig. 4 and 5). In the EY and SM free TEST buffer without antioxidants, O2−·. was increased (P < 0.05), and although SOD appeared to decrease the level of O2−·. production in both types of media, the effect was not significant (complete TEST, P = 0.03; TEST no EY or SM, P = 0.255). Interestingly, when compared to complete TEST buffer, rhesus sperm cryopreserved in the absence of EY and SM exhibited increased O2−·. production in all treatments (control, P < 0.05; α-tocopherol, P < 0.05; SOD, P < 0.01), except those with CAT added (P = 0.109; Fig. 4).
Fig. 3.
Mean (± SEM) progressive motility of cryopreserved rhesus macaque sperms in TEST buffer + 3% glycerol in the presence of the following antioxidants: α-tocopherol (Vit E), catalase (CAT), and superoxide dismutase (SOD).
a–cMeans without a common superscript among antioxidant treatments differ (P < 0.05).
Fig. 4.
Post-thaw superoxide production (O2−·: DHE positive) in rhesus macaque spermcryopreserved in the presence or absence of egg yolk (EY) or skimmed milk (SM), with the addition of antioxidants α-tocopherol (Vit E), catalase (CAT), and superoxide dismutase (SOD).
a,bMeans without a common superscript among antioxidant treatments differ (P < 0.05).
Fig. 5.
Post-thaw hydrogen peroxide (H2O2: DCF positive) production in rhesus macaque spermcryopreserved in the presence or absence of egg yolk (EY) or skimmed milk (SM), with the addition of the following antioxidants: α-tocopherol (Vit E), catalase (CAT), and superoxide dismutase (SOD).
a,bMeans without a common superscript among antioxidant treatments differ (P < 0.05).
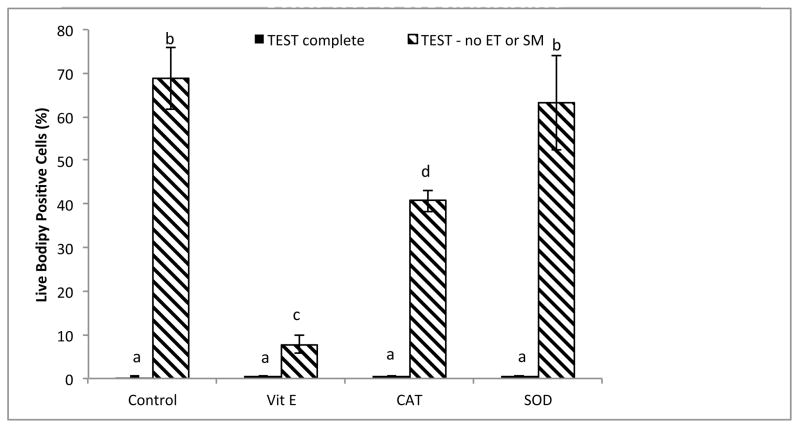
Addition of antioxidants in the absence of EY and SM did not significantly affect production of H2O2 (Fig. 5). However, the presence of EY and SM in combination with α-tocopherol or CAT had a synergistic effect; these antioxidants reduced H2O2 production well below control levels. The interaction between EY/SM and α-tocopherol (P < 0.05) decreased the levels of H2O2, as did the interaction between EY/SM and CAT (P < 0.01; Fig. 5). The most dramatic effect of antioxidants was observed at the level of lipid peroxidation (Fig. 6). In the presence of EY and SM, there was no increase in lipid peroxidation among any of the treatments. However, in the absence of EY and SM, the levels of lipid peroxidation increased dramatically for α–tocopherol and CAT (control, P < 0.01; α-tocopherol P < 0.05; CAT P < 0.01), but not SOD (P > 0.01). Addition of α-tocopherol to TEST buffer absent of EY and SM decreased the level of lipid peroxidation near levels of those observed in the complete TEST buffer. Addition of CAT (P < 0.01) also decreased lipid peroxidation, whereas SOD had little effect (P = 0.741; Fig. 6).
Fig. 6.
Post-thaw lipid peroxidation (% BODIPY581/591C-11 positive cells) levels in rhesus macaque sperm cryopreserved in the presence or absence of egg yolk (EY) or skimmed milk (SM), with the addition of antioxidants: α-tocopherol (Vit E), catalase (CAT), and superoxide dismutase (SOD).
a–dMeans without a common superscript among antioxidant treatments differ (P < 0.05).
4. Discussion
Sperm cryopreservation has become an extremely important tool for preserving the genetics of valuable animals, especially in the case of genetically unique, valuable, and endangered species. However, although commonly used, sperm cryopreservation still induces substantial cell damage as a result of cold stress, osmotic change, and intracellular ice crystal formation. Furthermore, current research has shown that cryopreservation-induced ROS may also be an important source of post-thaw cell damage, although this phenomenon has never been investigated for rhesus macaque sperm [16,24,25].
In the present study, we evaluated the effect cryopreservation on ROS production by rhesus macaque sperm and whether addition of antioxidants would reduce ROS production. To determine whether our cryopreservation extender (more specifically, the EY and SM) was acting as an ROS scavenger, comparisons were made between sperm frozen in a rhesus macaque sperm cryopreservation buffer [22] and sperm frozen in the same media without EY and SM (control TEST buffer + 3% glycerol). There was a significant increase in post thaw ROS production in both media; however, in the absence of EY and SM, the level of ROS production was much higher and a significant level of lipid peroxidation was also observed. Therefore, we inferred that EY, SM, or both, were playing an important antioxidant role in rhesus sperm cryopreservation.
The specific roles of EY and SM on sperm cell protection during cryopreservation were not clear. Although the casein component of milk was a strong inhibitor of lipid peroxidation [26], the majority of the protection was most likely the result of the EY. It is generally accepted that low-density lipoproteins (LDLs), more specifically phosphatidlycholine, present in EY provide protection against lipid peroxidation by associating with the membrane and stabilizing it [27]. This phenomenon appeared to be especially apparent during cryopreservation of rhesus macaque sperm. It is also notable that macaque sperm cryopreservation using TEST-yolk medium benefitted from a rather high concentration of EY (20%), which certainly was a higher concentration than that used for cryopreserving sperm of most species, except humans. Lower concentrations of EY in this medium have not been established to be beneficial regarding antioxidant and lipid peroxidation activity, although one study reported varying EY concentrations affected motility [28]. In our laboratory, osmotic stress alone, in the absence of EY and SM, caused significant increases in ROS and lipid peroxidation of rhesus macaque sperm [6]. However, cryopreservation-induced osmotic stress in the presence of TEST buffer did not produce the same levels of lipid peroxidation, even in the presence of substantial levels of ROS. This further emphasized the important role that EY and SM have in cell membrane protection. Additionally, Dong et al [28] recently demonstrated that in the absence of the permeable cryoprotectant glycerol, TEST buffer with EY provided sufficient protection during cryopreservation, effectively recovering 70–80% of the sperm samples compared to controls. Although post thaw motility was decreased by approximately 50% compared to samples frozen with glycerol and EY, these results clearly illustrated the high level of protection EY provided during cryopreservation of rhesus macaque sperm.
In an effort to decrease the level of cryopreservation induced ROS, antioxidant use has been investigated, with varying levels of success. Antioxidant supplementation during cryopreservation of equine [29], canine [25], bovine [30], and Iberian Red Deer sperm [31] increased post-thaw motility and viability. Although ROS were not specifically measured, we inferred that ROS were present and antioxidant supplementation was successful in protecting sperm against damage from these free radicals. Additionally, Li et al [24] reported that addition of ascorbate or CAT during cryopreservation of human sperm significantly decreased ROS, improved motility and viability, and concurrently decreased DNA damage and early apoptotic events.
In the presence of EY and SM, supplementation with SOD, CAT, or α-tocopherol had little effect on O2−·. production or lipid peroxidation, presumably due to the antioxidant effects exerted by EY and SM. Alternatively, in the absence of protein and lipids, CAT was an effective O2−· Scavenger, whereas both CAT and α-tocopherol were dramatic inhibitors of lipid peroxidation, even in the presence of high levels of ROS. It was noteworthy that alpha;-tocopherol was so effective in protecting cells against lipid peroxidation that the levels were brought down to near control levels. Addition of α-tocopherol during osmotic stress also elicited the same dramatic inhibition of lipid peroxidation [6]. In that study, the addition of 100 μM α-tocopherol to media without lipids and proteins significantly decreased the level of lipid peroxidation observed under both hypo- and hyperosmotic stress. These results were not surprising, since α-tocopherol is a powerful chain-breaking antioxidant that can scavenge O2−·, H2O2, and hydroxyl radicals that contribute to lipid peroxidation of the sperm membrane [32,33]. In the presence of O2−·, H2O2 can be broken down to form the hydroxyl radical (OH·), whereas the product of the interaction between O2−· and nitric oxide (NO.), peroxynitrite (ONOO−), can decompose to form OH· [34]. Although there are many intermediary ROS in the lipid peroxidation cascade, the dominant free radical initiator is OH· [34–35]. That neither CAT nor SOD were as effective as α-tocopherol in protecting against membrane damage, we inferred that OH· was the dominant ROS involved in the observed lipid peroxidation, and that α-tocopherol effectively scavenged this ROS.
Interestingly, the addition of α-tocopherol or CAT acted synergistically with EY and SM, maximizing antioxidant protection. This phenomenon could be the result of additional α-tocopherol naturally present in the EY [36], the antioxidant properties of EY phospholipids [37], or both. The lipid profile of EY can fluctuate, depending on the laying-hen diet [38–40]; however, a high level of the phospholipids, phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are always present [39]. Additionally, EY contains varying levels of lipid soluble carotenoids and vitamins [39,41], and α-tocopherol, which can range as high as 100 to 300 μg/g of EY [36]. Egg yolk clearly provides antioxidant protection during cryopreservation of rhesus macaque sperm; however, the chemical composition of EY can vary considerably. Based on the current studies, perhaps this variation could be minimized with the development of a non-biological cryopreservation medium using α-tocopherol in lieu of EY and SM.
The effect of α-tocopherol in the presence of EY and SM was less dramatic and even had a negative effect on total and progressive motility. Although there is a large body of evidence supporting the positive effect of α-tocopherol on post thaw sperm motility, Iberian Red Deer sperm, like rhesus macaque sperm, also exhibited decreased (albeit not significant) post-thaw motility in the presence of α-tocopherol [25,31,42–44]. Perhaps the effects of α-tocopherol on post thaw motility were species specific, to at least some extent. Since, the cryopreservation protocol for Iberian Red Deer sperm also included high EY content, perhaps supplemented α-tocopherol levels combined with levels present in the cryopreservation extender were just high enough to affect the motility of Iberian Red Deer and rhesus macaque sperm. Alternatively, the addition of SOD to complete TEST buffer enhanced motility. Sperm exposed to O2−· have increased levels of hyperactivation/capacitation, but the addition of SOD has been shown to prevent those effects [45]. By incubating and cryopreserving sperm with SOD, we may have prevented any premature hyperactivation resulting in improved post-thaw motility.
In conclusion, this is apparently the first report that cryopreservation of rhesus macaque sperm induced a significant increase in ROS. We also demonstrated that the addition of exogenous lipids and proteins had a significant protective effect against cryopreservation-induced cell damage. Both EY and SM exhibited a synergistic effect with α-tocopherol and CAT supplementation, resulting in a decrease in ROS production and a dramatic reduction in lipid peroxidation. These results clearly supported the protective effect of EY and SM when used in sperm cryopreservation; furthermore, in the absence of EY and SM, α-tocopherol or CAT were effective alternatives.
Acknowledgments
This work was supported by a grant from the National Center for Research Resources (NIH 2 R01 RR016581-05A1), National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rutllant J, Pommer AC, Meyers SA. Osmotic tolerance limits and properties of rhesus monkey (Macaca mulatta) spermatozoa. J Androl. 2003;24:534–41. doi: 10.1002/j.1939-4640.2003.tb02705.x. [DOI] [PubMed] [Google Scholar]
- 2.Pommer AC, Rutllant J, Meyers SA. The role of osmotic resistance on equine spermatozoal function. Theriogenology. 2002;58:1373–84. doi: 10.1016/s0093-691x(02)01039-7. [DOI] [PubMed] [Google Scholar]
- 3.Correa LM, Thomas A, Meyers SA. The macaque sperm actin cytoskeleton reorganizes in response to osmotic stress and contributes to morphological defects and decreased motility. Biol Reprod. 2007;77:942–53. doi: 10.1095/biolreprod.107.060533. [DOI] [PubMed] [Google Scholar]
- 4.Mazur P. Studies on rapidly frozen suspensions of yeast cells by differential thermal analysis and conductometry. Biophys J. 1963;3:323–53. doi: 10.1016/s0006-3495(63)86824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazur P. Principles of Cryobiology. Boca Raton, FL: CRC Press; 2004. [Google Scholar]
- 6.McCarthy MJ, Baumber J, Kass PH, Meyers SA. Osmotic stress induces oxidative cell damage to rhesus macaque spermatozoa. Biol Reprod. 2010;82:644–51. doi: 10.1095/biolreprod.109.080507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarty J, Banerjee D, Pal D, De J, Ghosh A, Majumder GC. Shedding off specific lipid constituents from sperm cell membrane during cryopreservation. Cryobiology. 2007;54:27–35. doi: 10.1016/j.cryobiol.2006.10.191. [DOI] [PubMed] [Google Scholar]
- 8.Moilanen J, Hovatta O, Lindroth L. Vitamin E levels in seminal plasma can be elevated by oral administration of vitamin E in infertile men. Int J Androl. 1993;16:165–6. doi: 10.1111/j.1365-2605.1993.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez C, Ghyselinck NB, Depeiges A, Dufaure JP. Immunochemical localization and association with spermatozoa of androgen-regulated proteins of MR 24000 secreted by the mouse epididymis. Biol Cell. 1990;68:171–4. doi: 10.1016/0248-4900(90)90304-l. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987;8:338–48. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewis SE, Boyle PM, McKinney KA, Young IS, Thompson W. Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil Steril. 1995;64:868–70. doi: 10.1016/s0015-0282(16)57870-4. [DOI] [PubMed] [Google Scholar]
- 12.Vernet P, Aitken RJ, Drevet JR. Antioxidant strategies in the epididymis. Mol Cell Endocrinol. 2004;216:31–9. doi: 10.1016/j.mce.2003.10.069. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell WM, Stojanov T. Liquid storage of ram semen in the absence or presence of some antioxidants. Reprod Fertil Dev. 1996;8:1013–20. doi: 10.1071/rd9961013. [DOI] [PubMed] [Google Scholar]
- 14.Lenzi A, Picardo M, Gandini L, Dondero F. Lipids of the sperm plasma membrane: from polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum Reprod Update. 1996;2:246–56. doi: 10.1093/humupd/2.3.246. [DOI] [PubMed] [Google Scholar]
- 15.Mazzilli F, Rossi T, Sabatini L, Pulcinelli FM, Rapone S, Dondero F, et al. Human sperm cryopreservation and reactive oxygen species (ROS) production. Acta Eur Fertil. 1995;26:145–8. [PubMed] [Google Scholar]
- 16.Chatterjee S, Gagnon C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev. 2001;59:451–8. doi: 10.1002/mrd.1052. [DOI] [PubMed] [Google Scholar]
- 17.Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl. 2000;21:895–902. [PubMed] [Google Scholar]
- 18.Baumber J, Ball BA, Linfor JJ, Meyers SA. Reactive oxygen species and cryopreservation promote DNA fragmentation in equine spermatozoa. J Androl. 2003;24:621–8. doi: 10.1002/j.1939-4640.2003.tb02714.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004;2:12. doi: 10.1186/1477-7827-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarason RL, VandeVoort CA, Mader DR, Overstreet JW. The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques. J Med Primatol. 1991;20:122–5. [PubMed] [Google Scholar]
- 21.Biggers JD, Whittingham WWKDG. Methods in Mammalian Embryology. San Francisco: W.H. Freeman & Co Ltd; 1971. [Google Scholar]
- 22.Tollner TL, VandeVoort CA, Overstreet JW, Drobnis EZ. Cryopreservation of spermatozoa from cynomolgus monkeys (Macaca fascicularis) J Reprod Fertil. 1990;90:347–52. doi: 10.1530/jrf.0.0900347. [DOI] [PubMed] [Google Scholar]
- 23.De Iuliis GN, Wingate JK, Koppers AJ, McLaughlin EA, Aitken RJ. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J Clin Endocrinol Metab. 2006;91:1968–75. doi: 10.1210/jc.2005-2711. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Lin Q, Liu R, Xiao W, Liu W. Protective Effects of Ascorbate and Catalase on Human Spermatozoa During Cryopreservation. J Androl. 2009 doi: 10.2164/jandrol.109.007849. [DOI] [PubMed] [Google Scholar]
- 25.Michael A, Alexopoulos C, Pontiki E, Hadjipavlou-Litina D, Saratsis P, Boscos C. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology. 2007;68:204–12. doi: 10.1016/j.theriogenology.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 26.Cervato G, Cazzola R, Cestaro B. Studies on the antioxidant activity of milk caseins. Int J Food Sci Nutr. 1999;50:291–6. doi: 10.1080/096374899101175. [DOI] [PubMed] [Google Scholar]
- 27.Ricker JV, Linfor JJ, Delfino WJ, Kysar P, Scholtz EL, Tablin F, et al. Equine sperm membrane phase behavior: the effects of lipid-based cryoprotectants. Biol Reprod. 2006;74:359–65. doi: 10.1095/biolreprod.105.046185. [DOI] [PubMed] [Google Scholar]
- 28.Dong Q, Correa LM, VandeVoort CA. Rhesus monkey sperm cryopreservation with TEST-yolk extender in the absence of permeable cryoprotectant. Cryobiology. 2009;58:20–7. doi: 10.1016/j.cryobiol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumber J, Ball BA, Linfor JJ. Assessment of the cryopreservation of equine spermatozoa in the presence of enzyme scavengers and antioxidants. Am J Vet Res. 2005;66:772–9. doi: 10.2460/ajvr.2005.66.772. [DOI] [PubMed] [Google Scholar]
- 30.Sariozkan S, Bucak MN, Tuncer PB, Ulutas PA, Bilgen A. The influence of cysteine and taurine on microscopic-oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology. 2009;58:134–8. doi: 10.1016/j.cryobiol.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Santos MR, Martinez-Pastor F, Garcia-Macias V, Esteso MC, Soler AJ, Paz P, et al. Sperm characteristics and DNA integrity of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa frozen in the presence of enzymatic and nonenzymatic antioxidants. J Androl. 2007;28:294–305. doi: 10.2164/jandrol.106.000935. [DOI] [PubMed] [Google Scholar]
- 32.Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129:357–67. [PubMed] [Google Scholar]
- 33.Storey BT. Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int J Dev Biol. 2008;52:427–37. doi: 10.1387/ijdb.072522bs. [DOI] [PubMed] [Google Scholar]
- 34.Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays. 1994;16:259–67. doi: 10.1002/bies.950160409. [DOI] [PubMed] [Google Scholar]
- 35.Griveau JF, Le Lannou D. Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl. 1997;20:61–9. doi: 10.1046/j.1365-2605.1997.00044.x. [DOI] [PubMed] [Google Scholar]
- 36.Meluzzi A, Sirri F, Manfreda G, Tallarico N, Franchini A. Effects of dietary vitamin E on the quality of table eggs enriched with n-3 long-chain fatty acids. Poult Sci. 2000;79:539–45. doi: 10.1093/ps/79.4.539. [DOI] [PubMed] [Google Scholar]
- 37.Sugino H, Ishikawa M, Nitoda T, Koketsu M, Juneja L, Kim M, et al. Antioxidative Activity of Egg Yolk Phospholipids. Journal of Agricultural and Food Chemistry. 1997;45:4. [Google Scholar]
- 38.Jia W, Slominski BA, Guenter W, Humphreys A, Jones O. The effect of enzyme supplementation on egg production parameters and omega-3 fatty acid deposition in laying hens fed flaxseed and canola seed. Poult Sci. 2008;87:2005–14. doi: 10.3382/ps.2007-00474. [DOI] [PubMed] [Google Scholar]
- 39.Kuksis A. Yolk lipids. Biochim Biophys Acta. 1992;1124:205–22. doi: 10.1016/0005-2760(92)90132-f. [DOI] [PubMed] [Google Scholar]
- 40.Grobas S, Mendez J, Lopez BC, De BC, Mateos GG. Effect of vitamin E and A supplementation on egg yolk alpha;-tocopherol concentration. Poult Sci. 2002;81:376–81. doi: 10.1093/ps/81.3.376. [DOI] [PubMed] [Google Scholar]
- 41.Hargitai R, Matus Z, Hegyi G, Michl G, Toth G, Torok J. Antioxidants in the egg yolk of a wild passerine: differences between breeding seasons. Comp Biochem Physiol B Biochem Mol Biol. 2006;143:145–52. doi: 10.1016/j.cbpb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Thuwanut P, Chatdarong K, Techakumphu M, Axner E. The effect of antioxidants on motility, viability, acrosome integrity and DNA integrity of frozen-thawed epididymal cat spermatozoa. Theriogenology. 2008;70:233–40. doi: 10.1016/j.theriogenology.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Askari HA, Check JH, Peymer N, Bollendorf A. Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process. Arch Androl. 1994;33:11–5. doi: 10.3109/01485019408987797. [DOI] [PubMed] [Google Scholar]
- 44.Satorre MM, Breininger E, Beconi MT, Beorlegui NB. alpha;-Tocopherol modifies tyrosine phosphorylation and capacitation-like state of cryopreserved porcine sperm. Theriogenology. 2007;68:958–65. doi: 10.1016/j.theriogenology.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 45.de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997;2:48–54. doi: 10.1530/ror.0.0020048. [DOI] [PubMed] [Google Scholar]