Abstract
Nuclear Pore Complexes (NPCs) are highly selective transport gates that enable the bidirectional traffic of macromolecules across the nuclear envelope (NE). NPCs are located at the fusion pores between the inner and outer membranes of the NE and are built from a common set of ~30 different proteins, nucleoporins. Remarkably, recent proteomic, bioinformatic and structural studies have provided firm evidence that key structural nucleoporins share common ancestry with elements of coated vesicles, indicating an evolutionary link between these structures. This has provided novel insight into the origin of NPCs and may help us to functionally characterize these fundamental components of eukaryotic cells.
Introduction
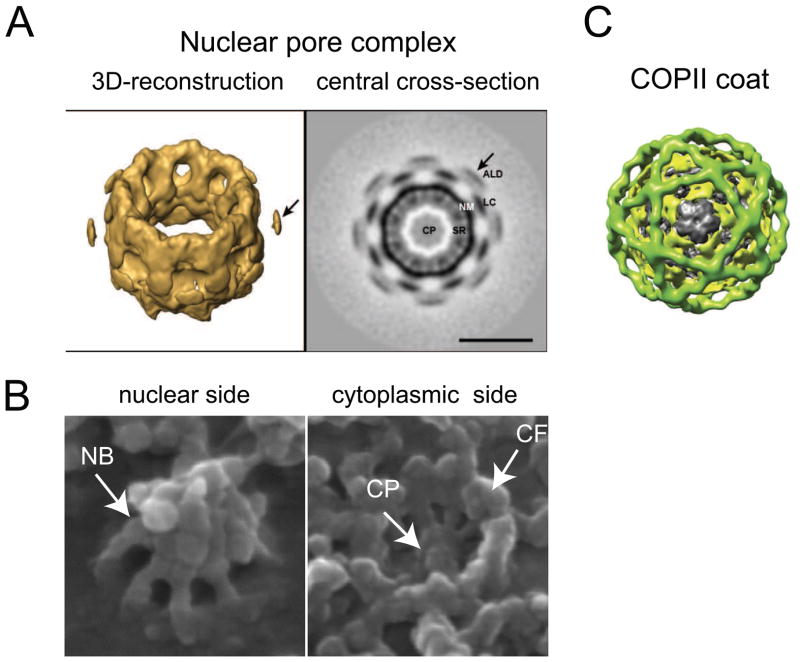
NPCs are uniquely eukaryotic structures required for the functional separation of the cytoplasm and the nucleus by the NE. Analyses of NPCs from a range of species revealed that they are assembled from ~30 nucleoporins, the majority of which are highly conserved [1–4•,5]. Due to an inherent 8-fold rotational symmetry of NPCs all nucleoporins are present in multiple copies accounting for ~ 500 individual polypeptides per pore. The general organization of NPCs in various organisms is very similar consisting of a ring-like channel with 8 identical subunits or “spokes” [6–8]. The NPC ring has an outer diameter of ~ 120 nm and is almost symmetrical with respect to the NE (Fig 1A). The central transport channel is filled with unstructured material often referred to as the “central plug” or “transporter” [9,10•] (Fig 1A left panel, Fig 1B). In addition, the NPC contains asymmetrical nuclear and cytoplasmic extensions, known as “nuclear basket” and “cytoplasmic filaments” [8,9,11–13] (Fig 1B).
Figure 1.
Structural highlights of NPCs and COP/clathrin coats. (A) A 3D-reconstruction (left) or a central cross-section (right) of the NPC structure acquired by cryo-EM tomography using Xenopus NPCs. Note the characteristic 8-fold symmetry of the NPC. The image is adapted from [10•]. CP, central plug; SR, spoke ring complex; NM, nuclear membrane; LC, luminal connection; ALD, additional luminal density. (B) Surface views of the nuclear (left) and the cytoplasmic sides (right) of Xenopus NPC acquired using high-resolution scanning EM. The images highlight the nuclear basket (NB), cytoplasmic filaments (CF) and the central plug (CP). These structures are not clearly visible with cryo-EM tomography (Fig. 1A) due to their dynamic nature. Images were kindly provided by Dr. Elena Kiseleva. (C) Architecture of the COPII coat visualized by EM single particle reconstruction. Note the spherical architecture and highly symmetrical arrangement of coatomers within the coat structure (adapted from [30]).
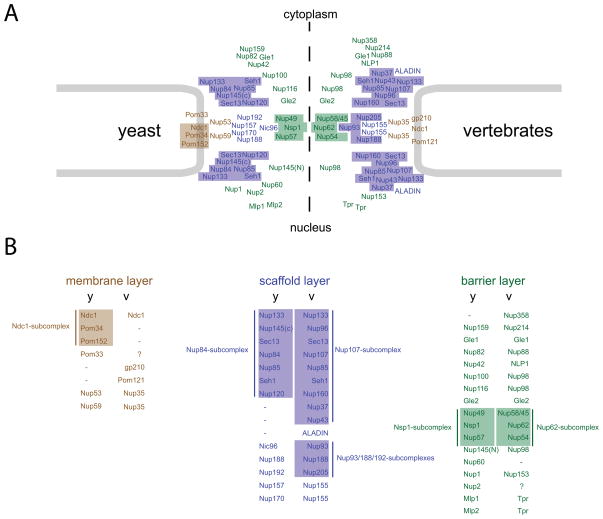
NPC components can be broadly grouped into three categories: (a) membrane nucleoporins, which are integrated in the pore membrane; (b) scaffold nucleoporins involved in forming the NPC framework; and (c) barrier nucleoporins critical for the selective permeability of NPCs. These three classes appear to occupy distinct zones or layers within the NPC [14••,15••]. The first layer is formed by membrane nucleoporins, which help to anchor the scaffold layer nucleoporins. The scaffold layer in turn attaches barrier nucleoporins that face the central channel and form the asymmetric NPC extensions (Fig. 2 and 1A–B). Although this classification is somewhat arbitrary, it reflects the general functional specialization observed among nucleoporins.
Figure 2.
NPC composition in yeast and vertebrates. (A) Panel displays the distribution and approximate location of yeast nucleoporins (left) and corresponding vertebrate nucleoporins (right) within the NPC. Based on their functional properties and localization nucleoporins can be classified into 3 functional layers: the membrane layer (brown) consisting of transmembrane and membrane-associated nucleoporins, the scaffold layer (blue) composed of core structural nucleoporins, and the barrier layer (green) containing nucleoporins involved in the selective NPC permeability and other functions. (B) The correspondence between yeast (y) and vertebrate (v) nucleoporins. Colored boxes depict biochemically stable nucleoporin subcomplexes.
It is an intriguing problem how a highly complex structure, such as the NPC, co-evolved with the nuclear envelope. Interestingly, Sec13, a key component of the COPII membrane coat, involved in ER-to-Golgi transport, is also found to be a stable NPC component [16]. Moreover, scaffold nucleoporins generally display a simple and characteristic structural composition containing either a beta-propeller fold (Sec13 and Seh1), a predominantly alpha-helical fold (Nup84, Nup85, Nup145(C), Nic96, Nup188, Nup192) or a combination of these two fold types (Nup170/157, Nup120 and Nup133) [2,17]. The same features also characterize components of the evolutionary related endomembrane trafficking coats, COPI, COPII and clathrin, leading to the hypothesis that the NPC scaffold and these membrane coats originated from a common ancestral structure during the evolution of the endomembrane system [2,18]. In this review, we highlight recent progress in our understanding of NPC architecture and assembly, and discuss parallels in the organization of NPCs and COP/clathrin coats.
Structural similarities between nuclear pore complex and membrane coat components
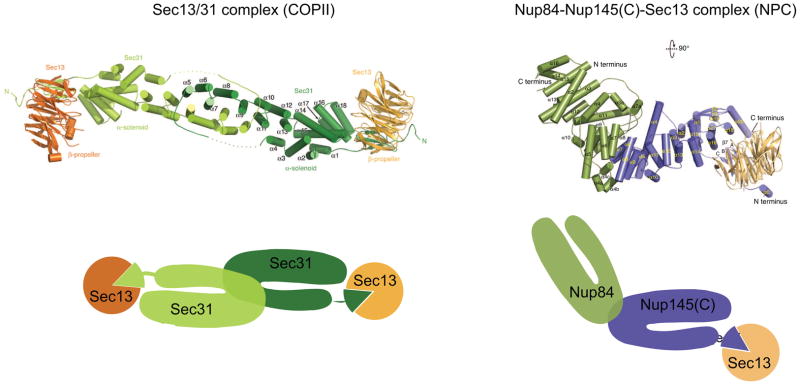
In the last several years, considerable progress has been made in determining the atomic structures of multiple scaffold nucleoporins. Remarkably, at least six of them display similarities in their structural organization with elements of the COPII coat. Despite extremely limited sequence homology, Nup84, Nup85, Nup145(C) and Nic96 share a common U-shaped architecture with Sec31, a component of the Sec13/31 COPII coatomer complex [19• •,20•,21–24] (Fig 3). Furthermore, a characteristic in-trans insertion of Sec31 into the propeller blades of Sec13 is found in both Nup145(C)-Sec13 and Nup85-Seh1 hetero-dimers [19••,24–26] (Fig 3). Finally, the anti-parallel arrangement of the Nup84-Nup145(C) complex also resembles the organization of a Sec31 dimer within the Sec13/31 structure [23,26] (Fig 3). Therefore it seems likely that at least six scaffold nucleoporins (corresponding to ~60% of the scaffold layer) share ancestry with the COPII coatomer element.
Figure 3.
Structural similarities between COPII coat elements and scaffold nucleoporins as exemplified by the Nup84-Nup145(C)-Sec13 complex structure. The top panels show structures of the Sec13/31 COPII coatomer complex (left) and the Nup84-Nup145(C)-Sec13 nucleoporin complex (right). The bottom panels display schematic representations of these complexes highlighting (a) the characteristic U-shaped organization of Nup84 and Nup145(C), which is similar to the arrangement of the Sec31 alpha-helical domain, (b) the anti-parallel mode of interaction between Nup84 and Nup145(C) and two Sec31 molecules, and (c) the in-trans blade insertions of Nup145(C) or Sec31 into the Sec13 beta-propeller. For a detailed description of these similarities see [19••,23] and main text. The structures were adapted from [23,26].
General comparison of nuclear pore complex and membrane coat organization
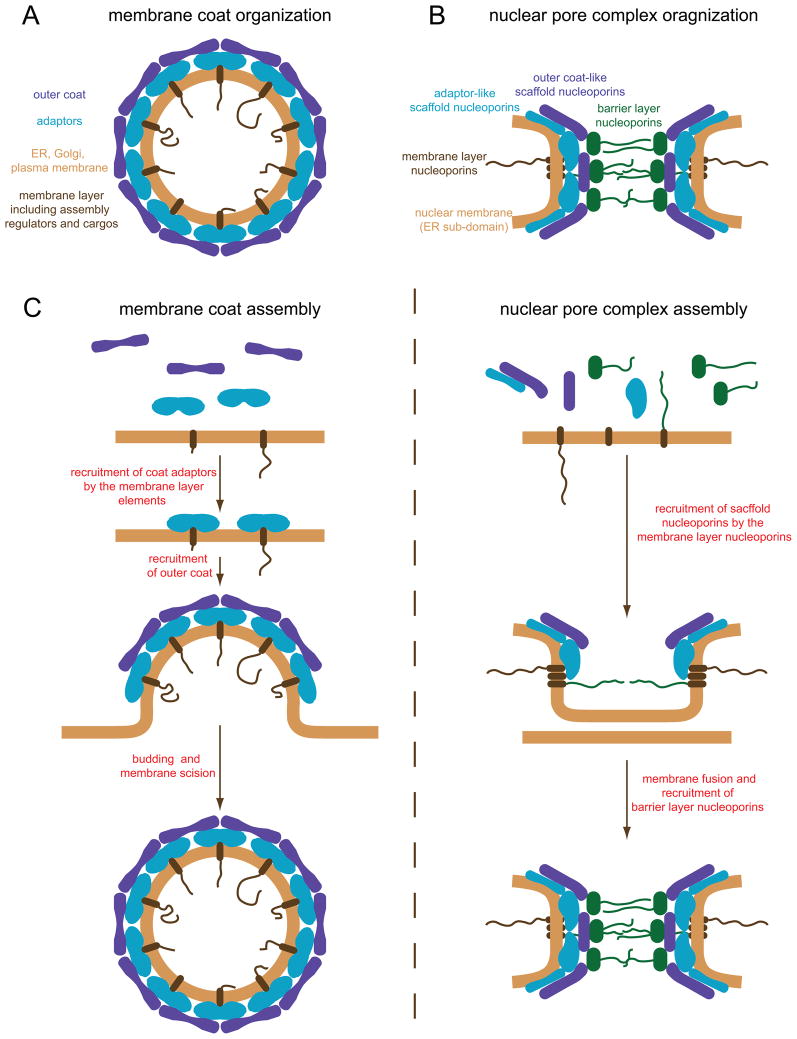
The structural similarities between some of the scaffold nucleoporins and membrane coat elements raise the intriguing question of how much resemblance these multiprotein structures have to one another? It is well established that COP and clathrin-type coats form through polymerization of coatomer subunits and package membrane cargos into transport vesicles. All these coats are comprised of a membrane layer, which contains cargos and regulators of coat assembly, followed by a double layer formed by adaptor proteins and outer coat proteins [27] (Fig 4A). The adaptor proteins couple coat assembly with cargo packaging by direct interactions with membrane cargos. In contrast, the outer coat is primarily responsible for polymerization into a higher-order structure, which curves the membrane and shapes the transport vesicle [28]. At first glance, this arrangement looks remarkably similar to the layered NPC organization described above. Whereas, the membrane layer of the NPC can be compared to the membrane layer of the COP/clathrin coats, the NPC scaffold layer corresponds to the adaptor/outer coat layer (Fig 2 and Fig 4A–B).
Figure 4.
(A and B) Models of the membrane coat (A) and NPC organization (B). Functionally similar parts of NPCs and membrane coats are shown in the same color. (C) Corresponding steps in the assembly of membrane coats (left) and NPCs (right).
However, in spite of these parallels, the endomembrane coating systems and NPCs also exhibit significantly different properties. For example, endomembrane coats have a spherical geometry stabilizing positive membrane curvatures (Fig 1C), and are naturally dynamic [28–30]. In contrast, NPCs are arranged in a donut-like shape (Fig 1), giving rise to both positive and negative membrane curvatures, and their scaffold elements form extremely stable assemblies. Furthermore, NPCs function as highly selective transport gates while there is no obvious barrier layer-like structure present in COP/clathrin coats. Therefore, it is still uncertain to which extent the assembly mechanisms and organization principles of the COP/clathrin coats can be extrapolated to NPCs. In the following parts we discuss in more detail corresponding aspects of these eukaryotic membrane-deforming systems.
The membrane layer in nuclear pore complexes and membrane coats
In yeast, the membrane layer includes four transmembrane nucleoporins: Ndc1, Pom152, Pom34, which form a biochemically stable subcomplex [3,31–33•], and Pom33 [34]. Transmembrane nucleoporins are functionally redundant [33•,35,36] and evolutionary flexible [1,2]. For example, vertebrates express three transmembrane nucleoporins vNdc1, Pom121 and gp210 but only Ndc1 appears to have a clear yeast ortholog [37–40]. In addition to these transmembrane nucleoporins, two yeast paralogs Nup53 and Nup59 can also be grouped into the membrane layer. Nup53 and Nup59 are functionally redundant with Pom152 and Pom34 and contain C-terminal amphipathic helices, which in the case of Nup53 allows for direct membrane insertion [33 ,35,36][41,42]. Nup53/59 is widely conserved in evolution (Nup35 in vertebrates) although it was not detected in some fungal species, like Aspergillus [1,43]
The evolutionary roots of membrane nucleoporins cannot be clearly traced. However, it is interesting that the luminal domain of gp210 shares similarity with the extracellular part of intimin-like proteins, a family of prokaryotic transmembrane proteins [2,44]. Additionally, the luminal domain of yeast Pom152 displays similarity to cadherins, transmembrane proteins located in the plasma membrane [17]. It is therefore possible that membrane layer nucleoporins originated from membrane cargos initially sorted to the cell surface by membrane coats, which would make them functionally homologous to coat cargos.
Scaffold nucleoporins and coatomers
As discussed above, six scaffold nucleoporins share similarity with elements of the Sec13/31 COPII coatomer complex. In contrast, the evolutionary origin of the remaining scaffold nucleoporins is less clear. Structures of Nup170, Nup133 and Nup120 [45–48] revealed weak similarities amongst these nucleoporins [49], but did not uncover obvious homologies to any known coat proteins except for a tandem arrangement of beta-propeller and alpha-helical domains. However, there is a very rich interaction network between these nucleoporins and the membrane layer. Yeast Nup170, its paralog Nup157 and vertebrate Nup155 all make multiple direct contacts to membrane layer nucleoporins [33•,50•,51,52•]. Moreover, yNup120, vNup133 and yNup170 contain amphipathic helices known to mediate direct membrane binding [53•,54]. The tight interaction of the beta-propeller/alpha-helical scaffold nucleoporins with the membrane layer is reminiscent to the direct binding of adaptors of COP/clathrin coats to membrane cargo suggesting that they may share functional homology. Thus, scaffold nucleoporins might be functionally divided into adaptor-like (beta-propeller/alpha-helical proteins) and outer coat-like (Sec13/31-like) elements (Fig 4A and B).
Given the parallels between NPCs and COP/clathrin coats can we safely conclude that these structures all follow the same organizational principles and that NPCs also form regular lattices via anti-parallel arrangement of their scaffold subunits [29]? Current nucleoporin structures and NPC models do not give a clear answer. For example, various, mutually exclusive NPC arrangements were proposed for the Nup84 complex, the largest and the most structurally characterized NPC subcomplex, which accounts for almost a third of the total NPC mass [14••,15••,23,24,46,55•,56,57]. Therefore, the currently available data do not appear to be sufficient to accurately predict the three-dimensional organization of NPCs suggesting that further structural analyses of higher-order nucleoporin complexes will be needed.
The barrier layer: a unique functional feature of the nuclear pore complex?
A key role of the NPC is to function as a barrier enabling the highly selective and regulated exchange of macromolecules between the cytoplasm and the nucleus in eukaryotes. This function is brought about by a set of nucleoporins all containing phenylalanine-glycine-rich (FG)-repeats, which are part of the barrier layer. The mechanistic role of FG nucleoporins in NPC selectivity remains somewhat controversial but specific interactions between FG nucleoporins and soluble nuclear transport receptors or karyopherins are a critical feature for NPC selectivity [58].
How did the filtering function of the NPC evolve? Given the unique role of the NPC as a selective transport channel, barrier FG nucleoporins might represent an NPC-specific invention. A structure with obvious homology to the FG barrier layer cannot be found on top of COP/clathrin coats. However, it is intriguing that the barrier function of the NPC is not only restricted to soluble molecules but also extends to transmembrane proteins [59,60], which would resemble the function of COP/clathrin coats that act as filtering devices to selectively retain and enrich for membrane cargos. Furthermore, membrane layer nucleoporins (yNup53, yNup59, vPom121) also contain FG-repeats [39,61], and there is evidence for interactions between scaffold nucleoporins and FG-domains [20•]. Finally, structural similarities between scaffold nucleoporins, coat proteins and soluble transport receptors/karyopherins were also noted [2,17].
Together, this might suggest that the evolutionary relationships with the COP/clathrin coat elements may extend beyond the NPC scaffold structure and may include the NPC barrier function or even the entire nucleocytoplasmic transport system.
Assembly mechanics of nuclear pore complexes and membrane coats
COP and clathrin coats form de novo and assemble from a soluble pool of coatomers initiated by the membrane recruitment of adaptor proteins (Fig 4C left). One mechanism of adaptor recruitment involves Arf1-like GTPases, which in their GTP-bound state bind to membranes and subsequently recruit adaptor proteins [28]. GTPase-independent mechanisms involve recruitment of adaptors via membrane cargos or direct membrane interactions [62]. The initial membrane recruitment is followed by a deposition of outer coat proteins, which induces polymerization of a regular lattice structure, membrane deformation and ultimately the production of a transport vesicle (Fig 4C left). NPCs also assemble de novo in interphase cells [63,64•] but the mechanism of this process is only beginning to emerge. As discussed above, multiple physical links between the membrane and scaffold layer nucleoporins exist and components of this interaction network play an important role in early steps of interphase pore assembly [33•,35,36,50•,52•,53•,65]. Additional studies will be necessary to dissect early steps of NPC biogenesis, but the role of interactions between membrane and scaffold nucleoporins may mimic adaptor-cargo interactions in early steps of COP/clathrin coat assembly (Fig 4C).
While formation of a COP/clathrin coat ultimately results in production of a transport vesicle, NPC assembly gives rise to a fusion pore between the inner and outer NE membranes (Fig 4C). Thus both processes rely on a membrane fusion event albeit with rather different topological outcomes. The scission of coated vesicles is complex and relies on the coat itself and, in addition, can depend on various activities including Sar1, dynamin or the actin cytoskeleton [66]. The mechanism of nuclear pore fusion is not understood and it is not known whether intrinsic NPC components are sufficient or whether non-nucleoporin co-factors are needed to complete the fusion step. Interestingly, several studies point towards a critical role for the lipid composition or the membrane properties during early steps of NPC assembly prior to the incorporation of barrier nucleoporins [67•,68,69].
Another unresolved question is, how NPC assembly is specifically targeted to the NE. The GTPase Ran is the only well-established regulatory factor linked to the NPC [70]. Similar to Arf1-like GTPases, Ran belongs to the superfamily of small GTP-binding proteins, which act as regulators of molecular interactions by switching between GDP and GTP bound states often at specific subcellular locations. Unlike Arf1, however, Ran is not membrane-associated and regulates protein-protein interactions by triggering associations and dissociation between nucleo-cytoplasmic transport receptors and their cargos [70,71]. However, there is evidence that the Ran GTPase cycle is necessary for NPC biogenesis [72] and a role in NPC assembly was reported for the major Ran effector importin-beta, [63,73]. Furthermore, the membrane layer nucleoporins, yNup53 and vPom121, contain NLS sequences, which interact with transport receptors and are necessary for NPC incorporation [53•,74•,75]. The Ran-pathway might function in NPC biogenesis by affecting the interactions or localization of membrane layer nucleoporins. In addition to Ran-pathway components, the DNA-binding protein Mel28/ELYS has been implicated in targeting NPC assembly to the NE. However, its function might be restricted to post-mitotic NPC reassembly in higher eukaryotes. [53•,76–78].
Conclusions
In the last several years, considerable progress has been made in our understanding of the biochemical and structural composition of NPCs. Key questions, which remain to be answered are related to mechanistic aspects of NPC assembly and to principles of the three-dimensional organization of NPCs. It has become clear that NPCs and other membrane coating systems have at least in part a common evolutionary origin that dates back to the last eukaryotic common ancestor. The evolutionary ties between components of the membrane coating systems in eukaryotes may extend far beyond the structural resemblance of their key components. This may help us not only to get insight into aspects of NPC function but also to better understand the origins of modern eukaryotic complexity.
Acknowledgments
We would like to thank Ohad Medalia, Elena Kiseleva, Bill Balch, Jonathan Goldberg and Thomas Schwartz for providing figures. We are also grateful to members of our lab, in particular to Elisa Dultz, Ben Monpetit and Ryan Joyner, for discussions and for comments on the manuscript. This work was supported by NIH grants R01GM058065 and RC1GM091533.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
• of outstanding interest
- 1.Neumann N, Lundin D, Poole AM. Comparative genomic evidence for a complete nuclear pore complex in the last eukaryotic common ancestor. PLoS One. 2010;5:e13241. doi: 10.1371/journal.pone.0013241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mans BJ, Anantharaman V, Aravind L, Koonin EV. Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex. Cell Cycle. 2004;3:1612–1637. doi: 10.4161/cc.3.12.1316. [DOI] [PubMed] [Google Scholar]
- 3.Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol. 2000;148:635–51. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.DeGrasse JA, DuBois KN, Devos D, Siegel TN, Sali A, Field MC, Rout MP, Chait BT. Evidence for a shared nuclear pore complex architecture that is conserved from the last common eukaryotic ancestor. Mol Cell Proteomics. 2009;8:2119–2130. doi: 10.1074/mcp.M900038-MCP200. This paper describes the proteomic analysis of NPCs from Trypanosoma brucei, a highly divergent eukaryote, evolutionarily distant from species whose NPCs have been analyzed previously. The authors find that in spite of low sequence homology the majority of experimentally identified nucleoporins in trypanosomes have clear orthologs in yeast and vertebrates. Intriguingly, NPCs from T. brucei contain both scaffold and barrier layer nucleoporins, but transmbrane nucleoporins were not identified. Together these results suggest that a common NPC core structure was already present in the last eukaryotic common ancestor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol. 2002;158:915–27. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gall JG. Octagonal nuclear pores. J Cell Biol. 1967;32:391–399. doi: 10.1083/jcb.32.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maul GG. On the octagonality of the nuclear pore complex. J Cell Biol. 1971;51:558–563. doi: 10.1083/jcb.51.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Q, Rout MP, Akey CW. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Mol Cell. 1998;1:223–234. doi: 10.1016/s1097-2765(00)80023-4. [DOI] [PubMed] [Google Scholar]
- 9.Beck M, Lucic V, Forster F, Baumeister W, Medalia O. Snapshots of nuclear pore complexes in action captured by cryo-electron tomography. Nature. 2007;449:611–615. doi: 10.1038/nature06170. [DOI] [PubMed] [Google Scholar]
- 10•.Frenkiel-Krispin D, Maco B, Aebi U, Medalia O. Structural analysis of a metazoan nuclear pore complex reveals a fused concentric ring architecture. J Mol Biol. 2010;395:578–586. doi: 10.1016/j.jmb.2009.11.010. This paper describes a refined NPC structure from isolated Xenopus laevis nuclear envelopes. Using cryo-electron tomography and symmetry independent analysis methods the authors obtain an NPC model at 6.4 nm resolution, which reveals details of the spoke-ring architecture and a fused ring arrangement of NPC subunits. [DOI] [PubMed] [Google Scholar]
- 11.Kiseleva E, Goldberg MW, Allen TD, Akey CW. Active nuclear pore complexes in Chironomus: visualization of transporter configurations related to mRNP export. J Cell Sci. 1998;111 ( Pt 2):223–236. doi: 10.1242/jcs.111.2.223. [DOI] [PubMed] [Google Scholar]
- 12.Akey CW, Radermacher M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol. 1993;122:1–19. doi: 10.1083/jcb.122.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiseleva E, Allen TD, Rutherford S, Bucci M, Wente SR, Goldberg MW. Yeast nuclear pore complexes have a cytoplasmic ring and internal filaments. J Struct Biol. 2004;145:272–288. doi: 10.1016/j.jsb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 14••.Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. Determining the architectures of macromolecular assemblies. Nature. 2007;450:683–694. doi: 10.1038/nature06404. [DOI] [PubMed] [Google Scholar]
- 15••.Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. doi: 10.1038/nature06405. This and the previous paper [14• •] present the most comprehensive analysis of nucleoporin interactions and localization data as of yet. The authors utilize a novel computational approach to model the three-dimensional architecture of yeast NPCs combining various experimental data sets including protein interactions, immuno-EM localization and nucleoporin size estimates. The authors come to several intriguing conclusions predicting (a) a separation of nucleoporins into distinct functional layers within the NPC (b) a concentric ring architecture of the NPC (c) a pseudo 16-fold symmetry of the NPC scaffold where each of 8 “spokes” consist of two almost identical parts. [DOI] [PubMed] [Google Scholar]
- 16.Siniossoglou S, Wimmer C, Rieger M, Doye V, Tekotte H, Weise C, Emig S, Segref A, Hurt EC. A novel complex of nucleoporins, which includes Sec13p and a Sec13p homolog, is essential for normal nuclear pores. Cell. 1996;84:265–75. doi: 10.1016/s0092-8674(00)80981-2. [DOI] [PubMed] [Google Scholar]
- 17.Devos D, Dokudovskaya S, Williams R, Alber F, Eswar N, Chait BT, Rout MP, Sali A. Simple fold composition and modular architecture of the nuclear pore complex. Proc Natl Acad Sci U S A. 2006;103:2172–7. doi: 10.1073/pnas.0506345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2004;2:e380. doi: 10.1371/journal.pbio.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Brohawn SG, Leksa NC, Spear ED, Rajashankar KR, Schwartz TU. Structural evidence for common ancestry of the nuclear pore complex and vesicle coats. Science. 2008;322:1369–1373. doi: 10.1126/science.1165886. This article describes high-resolution structures of Nup145(C)-Sec13 and Nup85-Seh1 complexes, components of the evolutionary conserved yeast Nup84-subcomplex. The study reveals a structural homology with the Sec13/31 complex of COPII coats and the authors experimentally demonstrate that characteristic protein interaction sites are conserved between the Nup84 and COPII complexes. Furthermore they provide evidence that, in addition to Nup145(C) and Nup85, two other alpha-helical scaffold nucleoporins Nic96, and Nup84 share structural homology with each other and with the COPII coatamer element, Sec31. This work presents strong experimental evidence for an evolutionary link between NPCs and COP/clatrin-like membrane coats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Schrader N, Stelter P, Flemming D, Kunze R, Hurt E, Vetter IR. Structural basis of the nic96 subcomplex organization in the nuclear pore channel. Mol Cell. 2008;29:46–55. doi: 10.1016/j.molcel.2007.10.022. This paper describes the high-resolution structure of the C-terminal domain of the scaffold nucleoporin Nic96. Among other findings the authors show that Nic96 can bind to FG-repeats, a property previously found only in soluble nucleocytoplasmic transport receptors. [DOI] [PubMed] [Google Scholar]
- 21.Jeudy S, Schwartz TU. Crystal structure of nucleoporin Nic96 reveals a novel, intricate helical domain architecture. J Biol Chem. 2007 doi: 10.1074/jbc.M705479200. [DOI] [PubMed] [Google Scholar]
- 22.Nagy V, Hsia KC, Debler EW, Kampmann M, Davenport AM, Blobel G, Hoelz A. Structure of a trimeric nucleoporin complex reveals alternate oligomerization states. Proc Natl Acad Sci USA. 2009;106:17693–17698. doi: 10.1073/pnas.0909373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brohawn SG, Schwartz TU. Molecular architecture of the Nup84-Nup145C–Sec13 edge element in the nuclear pore complex lattice. Nat Struct Mol Biol. 2009;16:1173–1177. doi: 10.1038/nsmb.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debler EW, Ma Y, Seo HS, Hsia KC, Noriega TR, Blobel G, Hoelz A. A fence-like coat for the nuclear pore membrane. Mol Cell. 2008;32:815–826. doi: 10.1016/j.molcel.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Hsia KC, Stavropoulos P, Blobel G, Hoelz A. Architecture of a coat for the nuclear pore membrane. Cell. 2007;131:1313–1326. doi: 10.1016/j.cell.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fath S, Mancias JD, Bi X, Goldberg J. Structure and organization of coat proteins in the COPII cage. Cell. 2007;129:1325–1336. doi: 10.1016/j.cell.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 27.McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Pucadyil TJ, Schmid SL. Conserved functions of membrane active GTPases in coated vesicle formation. Science. 2009;325:1217–1220. doi: 10.1126/science.1171004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison SC, Kirchhausen T. Structural biology: Conservation in vesicle coats. Nature. 2010;466:1048–1049. doi: 10.1038/4661048a. [DOI] [PubMed] [Google Scholar]
- 30.Stagg SM, LaPointe P, Razvi A, Gurkan C, Potter CS, Carragher B, Balch WE. Structural basis for cargo regulation of COPII coat assembly. Cell. 2008;134:474–484. doi: 10.1016/j.cell.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wozniak RW, Blobel G, Rout MP. POM152 is an integral protein of the pore membrane domain of the yeast nuclear envelope. J Cell Biol. 1994;125:31–42. doi: 10.1083/jcb.125.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chial HJ, Rout MP, Giddings TH, Winey M. Saccharomyces cerevisiae Ndc1p is a shared component of nuclear pore complexes and spindle pole bodies. J Cell Biol. 1998;143:1789–1800. doi: 10.1083/jcb.143.7.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Onischenko E, Stanton LH, Madrid AS, Kieselbach T, Weis K. Role of the Ndc1 interaction network in yeast nuclear pore complex assembly and maintenance. J Cell Biol. 2009;185:475–491. doi: 10.1083/jcb.200810030. This work characterizes interactions between membrane and scaffold layer nucleoporins and demonstrates their importance in the biogenesis and maintenance of NPCs. In addition, an in vivo NPC assembly assay is introduced that takes advantage of the photoconvertable fluorescent protein Dendra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chadrin A, Hess B, San Roman M, Gatti X, Lombard B, Loew D, Barral Y, Palancade B, Doye V. Pom33, a novel transmembrane nucleoporin required for proper nuclear pore complex distribution. J Cell Biol. 2010;189:795–811. doi: 10.1083/jcb.200910043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aitchison JD, Rout MP, Marelli M, Blobel G, Wozniak RW. Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J Cell Biol. 1995;131:1133–1148. doi: 10.1083/jcb.131.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miao M, Ryan KJ, Wente SR. The integral membrane protein Pom34p functionally links nucleoporin subcomplexes. Genetics. 2006;172:1441–57. doi: 10.1534/genetics.105.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stavru F, Hulsmann BB, Spang A, Hartmann E, Cordes VC, Gorlich D. NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes. J Cell Biol. 2006;173:509–19. doi: 10.1083/jcb.200601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wozniak RW, Bartnik E, Blobel G. Primary structure analysis of an integral membrane glycoprotein of the nuclear pore. J Cell Biol. 1989;108:2083–2092. doi: 10.1083/jcb.108.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallberg E, Wozniak RW, Blobel G. An integral membrane protein of the pore membrane domain of the nuclear envelope contains a nucleoporin-like region. J Cell Biol. 1993;122:513–21. doi: 10.1083/jcb.122.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansfeld J, Guttinger S, Hawryluk-Gara LA, Pante N, Mall M, Galy V, Haselmann U, Muhlhausser P, Wozniak RW, Mattaj IW, et al. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol Cell. 2006;22:93–103. doi: 10.1016/j.molcel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Marelli M, Lusk CP, Chan H, Aitchison JD, Wozniak RW. A link between the synthesis of nucleoporins and the biogenesis of the nuclear envelope. J Cell Biol. 2001;153:709–24. doi: 10.1083/jcb.153.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel SS, Rexach M. Discovering novel interactions at the nuclear pore complex using Bead Halo: A rapid method for detecting molecular interactions of high and low affinity at equilibrium. Mol Cell Proteomics. 2007 doi: 10.1074/mcp.M700407-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Osmani AH, Davies J, Liu HL, Nile A, Osmani SA. Systematic deletion and mitotic localization of the nuclear pore complex proteins of Aspergillus nidulans. Mol Biol Cell. 2006;17:4946–61. doi: 10.1091/mbc.E06-07-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Y, Frey EA, Pfuetzner RA, Creagh AL, Knoechel DG, Haynes CA, Finlay BB, Strynadka NC. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature. 2000;405:1073–1077. doi: 10.1038/35016618. [DOI] [PubMed] [Google Scholar]
- 45.Leksa NC, Brohawn SG, Schwartz TU. The structure of the scaffold nucleoporin Nup120 reveals a new and unexpected domain architecture. Structure. 2009;17:1082–1091. doi: 10.1016/j.str.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo HS, Ma Y, Debler EW, Wacker D, Kutik S, Blobel G, Hoelz A. Structural and functional analysis of Nup120 suggests ring formation of the Nup84 complex. Proc Natl Acad Sci US A. 2009;106:14281–14286. doi: 10.1073/pnas.0907453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berke IC, Boehmer T, Blobel G, Schwartz TU. Structural and functional analysis of Nup133 domains reveals modular building blocks of the nuclear pore complex. J Cell Biol. 2004;167:591–7. doi: 10.1083/jcb.200408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boehmer T, Jeudy S, Berke IC, Schwartz TU. Structural and functional studies of Nup107/Nup133 interaction and its implications for the architecture of the nuclear pore complex. Mol Cell. 2008;30:721–731. doi: 10.1016/j.molcel.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittle JR, Schwartz TU. Architectural nucleoporins Nup157/170 and Nup133 are structurally related and descend from a second ancestral element. J Biol Chem. 2009;284:28442–28452. doi: 10.1074/jbc.M109.023580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Makio T, Stanton LH, Lin CC, Goldfarb DS, Weis K, Wozniak RW. The nucleoporins Nup170p and Nup157p are essential for nuclear pore complex assembly. J Cell Biol. 2009;185:459–473. doi: 10.1083/jcb.200810029. This article shows that the paralogous scaffold nucleoporins Nup157 and Nup170 play a redundant but essential role in the assembly of new NPCs in yeast. Together with [33 •] this work demonstrates that interactions between the NPC scaffold and membrane layers are critical for NPC biogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawryluk-Gara LA, Platani M, Santarella R, Wozniak RW, Mattaj IW. Nup53 is required for nuclear envelope and nuclear pore complex assembly. Mol Biol Cell. 2008;19:1753–1762. doi: 10.1091/mbc.E07-08-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Mitchell JM, Mansfeld J, Capitanio J, Kutay U, Wozniak RW. Pom121 links two essential subcomplexes of the nuclear pore complex core to the membrane. J Cell Biol. 2010;191:505–521. doi: 10.1083/jcb.201007098. Herein the authors demonstrate that, like in yeast [33 •] [50 •], vertebrate scaffold nucleoporins are directly connected to the NPC membrane layer. Moreover, this paper shows that these connections are functionally important for NPC biogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Doucet CM, Talamas JA, Hetzer MW. Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell. 2010;141:1030–1041. doi: 10.1016/j.cell.2010.04.036. This paper analyzes NPC assembly in interphase vertebrate cells. It identifies specific roles for Pom121 and ELYS in interphase and post-mitotic NPC assembly, respectively. Furthermore, the authors characterize a membrane-interaction motif in Nup133 critical for NPC biogenesis in interphase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha–helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–46. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- 55•.Kampmann M, Blobel G. Three-dimensional structure and flexibility of a membrane-coating module of the nuclear pore complex. Nat Struct Mol Biol. 2009;16:782–788. doi: 10.1038/nsmb.1618. In this article, the authors report a detailed EM structure of the heptameric Nup84 subcomplex from yeast. The paper confirms that this complex assembles into a Y-shaped structure and reveals structural details that are reminiscent of interactions previously described in other membrane coating elements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lutzmann M, Kunze R, Buerer A, Aebi U, Hurt E. Modular self-assembly of a Y-shaped multiprotein complex from seven nucleoporins. Embo J. 2002;21:387–97. doi: 10.1093/emboj/21.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siniossoglou S, Lutzmann M, Santos-Rosa H, Leonard K, Mueller S, Aebi U, Hurt E. Structure and assembly of the Nup84p complex. J Cell Biol. 2000;149:41–54. doi: 10.1083/jcb.149.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weis K. The nuclear pore complex: oily spaghetti or gummy bear? Cell. 2007;130:405–7. doi: 10.1016/j.cell.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 59.King MC, Lusk CP, Blobel G. Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature. 2006;442:1003–7. doi: 10.1038/nature05075. [DOI] [PubMed] [Google Scholar]
- 60.Theerthagiri G, Eisenhardt N, Schwarz H, Antonin W. The nucleoporin Nup188 controls passage of membrane proteins across the nuclear pore complex. J Cell Biol. 2010;189:1129–1142. doi: 10.1083/jcb.200912045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen NP, Huang L, Burlingame A, Rexach M. Proteomic analysis of nucleoporin interacting proteins. J Biol Chem. 2001;276:29268–74. doi: 10.1074/jbc.M102629200. [DOI] [PubMed] [Google Scholar]
- 62.Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 63.D'Angelo MA, Anderson DJ, Richard E, Hetzer MW. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 2006;312:440–443. doi: 10.1126/science.1124196. [DOI] [PubMed] [Google Scholar]
- 64•.Dultz E, Ellenberg J. Live imaging of single nuclear pores reveals unique assembly kinetics and mechanism in interphase. J Cell Biol. 2010;191:15–22. doi: 10.1083/jcb.201007076. Using fluorescently labeled nucleoporins, the authors analyze the assembly kinetics of NPCs during interphase in mammalian cells. This revealed a correlation between nuclear growth and NPC assembly and identified important differences in the order of nucleoporin assembly between interphase and post-mitotic NPC biogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madrid AS, Mancuso J, Cande WZ, Weis K. The role of the integral membrane nucleoporins Ndc1p and Pom152p in nuclear pore complex assembly and function. J Cell Biol. 2006;173:361–71. doi: 10.1083/jcb.200506199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antonny B. Membrane deformation by protein coats. Curr Opin Cell Biol. 2006;18:386–394. doi: 10.1016/j.ceb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 67•.Fichtman B, Ramos C, Rasala B, Harel A, Forbes DJ. Inner/Outer Nuclear Membrane Fusion in Nuclear Pore Assembly: Biochemical Demonstration and Molecular Analysis. Mol Biol Cell. 2010 doi: 10.1091/mbc.E10-04-0309. In this article, the authors take advantage of the Xenopus in vitro nuclear assembly system to manipulate the membrane composition. They show that the recruitment of transmembrane and scaffold nucleoporins can be uncoupled from the formation of the NPC diffusion channel and the recruitment of bulk FG-nucleoporins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scarcelli JJ, Hodge CA, Cole CN. The yeast integral membrane protein Apq12 potentially links membrane dynamics to assembly of nuclear pore complexes. J Cell Biol. 2007;178:799–812. doi: 10.1083/jcb.200702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneiter R, Hitomi M, Ivessa AS, Fasch EV, Kohlwein SD, Tartakoff AM. A yeast acetyl coenzyme A carboxylase mutant links very-long-chain fatty acid synthesis to the structure and function of the nuclear membrane-pore complex. Mol Cell Biol. 1996;16:7161–7172. doi: 10.1128/mcb.16.12.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–51. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 71.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 72.Ryan KJ, McCaffery JM, Wente SR. The Ran GTPase cycle is required for yeast nuclear pore complex assembly. J Cell Biol. 2003;160:1041–1053. doi: 10.1083/jcb.200209116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harel A, Chan RC, Lachish-Zalait A, Zimmerman E, Elbaum M, Forbes DJ. Importin beta negatively regulates nuclear membrane fusion and nuclear pore complex assembly. Mol Biol Cell. 2003;14:4387–96. doi: 10.1091/mbc.E03-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Yavuz S, Santarella-Mellwig R, Koch B, Jaedicke A, Mattaj IW, Antonin W. NLS-mediated NPC functions of the nucleoporin Pom121. FEBS Lett. 2010;584:3292–3298. doi: 10.1016/j.febslet.2010.07.008. In this paper, the authors identify functional nuclear localization signals in the vertebrate transmembrane nucleoporin Pom121 recognized by transport receptors. Moreover, they demonstrate that these sequences are required for physical interaction of Pom121with a set of nucleoporins. [DOI] [PubMed] [Google Scholar]
- 75.Lusk CP, Makhnevych T, Marelli M, Aitchison JD, Wozniak RW. Karyopherins in nuclear pore biogenesis: a role for Kap121p in the assembly of Nup53p into nuclear pore complexes. J Cell Biol. 2002;159:267–278. doi: 10.1083/jcb.200203079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasala BA, Orjalo AV, Shen Z, Briggs S, Forbes DJ. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc Natl Acad Sci USA. 2006;103:17801–17806. doi: 10.1073/pnas.0608484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rasala BA, Ramos C, Harel A, Forbes DJ. Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol Biol Cell. 2008;19:3982–3996. doi: 10.1091/mbc.E08-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Franz C, Walczak R, Yavuz S, Santarella R, Gentzel M, Askjaer P, Galy V, Hetzer M, Mattaj IW, Antonin W. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 2007;8:165–172. doi: 10.1038/sj.embor.7400889. [DOI] [PMC free article] [PubMed] [Google Scholar]