Abstract
The discovery of induced pluripotent stem cells (iPSC) has, in the short time since their discovery, revolutionized the field of stem cell biology. This technology allows the generation of a virtually unlimited supply of cells with pluripotent potential similar to that of embryonic stem cells (ESC). However, in contrast to ESC, iPSC are not subject to the same ethical concerns and can be easily generated from living individuals. For the first time, patient-specific iPSC can be generated and offer a supply of genetically identical cells that can be differentiated into all somatic cell types for potential use in regenerative therapies or drug screening and testing. As the techniques for generation of iPSC lines are constantly evolving, new uses for human iPSC are emerging from in-vitro disease modeling to high throughput drug discovery and screening. This technology promises to revolutionize the field of medicine and offers new hope for understanding and treatment of numerous diseases.
Introduction
The goal of regenerative therapies, namely to regenerate organs or entire parts of the human body has been significantly advanced in recent years by new advances in stem cell biology. While the concept of organ regeneration has existed for millennia in Greek mythology, the ability to generate pluripotent stem cells from somatic cells has dramatically narrowed the gap between myth and reality. In 1963, the self-renewing abilities of transplanted mouse bone marrow stem cells were quantified and documented for the first time1. Another major advance in stem cell biology came with the isolation and propagation of mouse ESC2. These new stem cells represented the first immortal cells capable of self renewal and possessing pluripotent properties with the ability to differentiate into all cell types of the adult mouse. The groundbreaking creation of iPSC in 2006 provided a pluripotent cell type that is ethically unburdened, potentially autologous and easily generated and propagated, which is already affecting our approach to regenerative therapies.
The iPSC field has benefited tremendously from the advances and discoveries in the ESC field as the knowledge and protocols for human ESC have been translated into the iPSC field in an almost parallel manner, catapulting it forward. Although in its infancy, the field of iPSC technology has grown beyond simply their potential application in regenerative therapies. As will be discussed in this review, human iPSC are being used in reverse translational medicine to model human disease ex-vivo, something not possible in the era preceding the advent of iPSC technology. These “disease in a dish models” have provided a potent tool to study human disease in-vitro. Furthermore, iPSC technology has provided a new platform for drug discovery and safety screening in a high throughput manner. Thus, iPSC represent a new and exciting model for both human disease research and clinical therapeutic application.
A Brief History of Nuclear Reprogramming
The ability to reprogram a somatic cell into a pluripotent cell has been a goal of regenerative research for many decades. The progress made developing this technology and the lessons learned have evolved into a body of knowledge that was crucial for the eventual discovery of iPSC. Initial attempts to reprogram cells involved transplanting a somatic nucleus into an enucleated oocyte, called somatic cell nuclear transfer (SCNT). More than four decades ago, the first successful attempt at reprogramming cells was reported by Briggs and colleagues in frogs3. In 1997, Sir Ian Wilmut and his team surprised the world by presenting the first cloned mammal, Dolly the sheep, engineered via SCNT4. After Dolly was cloned, it was believed that SCNT would become the method of choice for generating patient-specific pluripotent cells5. However, the process is very inefficient and the reprogramming incomplete leading to Dolly’s premature death and early onset of a number of degenerative diseases. Thus, more than a decade later, the ability to utilize this technology for generating human pluripotent stem cells remains elusive despite successes in other species including primates6. Furthermore, the use of SCNT to generate patient-specific pluripotent stem cells would be ethically challenging, as it requires generation of human blastocysts.
Mouse ESC were first derived in the early 1980’s simultaneously by two independent groups2,7, and because of their plasticity and potentially unlimited capacity for self-renewal, they were predicted to transform research in mammalian development, genetics, stem cell biology and regenerative medicine. A major breakthrough in the field of human stem cells occurred when Thompson’s group isolated and cultured human embryonic stem cells8. However, it quickly became apparent that although human ESC would become an invaluable tool to study development and stem cell biology, translating their promise into the clinics has been problematic. After over twenty years of research there is currently only one Food and Drug Agency (FDA) approved clinical trial using human ESC9. While overcoming the potential oncogenic risk of pluripotent stem cells continues to be an area of intense research, investigators have long sought to overcome the ethical and immunologic issues surrounding ESC by creating autologous pluripotent stem cells. In search for factors that could reprogram somatic cells to a pluripotent state, investigators identified four transcription factors whose retroviral overexpression enabled the induction of a pluripotent state in murine fibroblasts10. Simultaneous ectopic expression of Oct4, Sox2, c-Myc, and Klf4 led to generation of iPSC that were very similar to murine ES cells in morphology, proliferation, and teratoma formation. Human somatic cell reprogramming has also been reported by several groups producing human iPSC that are morphologically and phenotypically similar to human ES cells10,11. Since then, the field has seen an exponential growth in the field of reprogramming with a progressive refinement and reduction in the number of factors required to achieve reprogramming. Human iPSC have been created using non-integrating adenoviruses12, piggyBAC models13, combining reprogramming factors with small molecules14 to minimize the number of reprogramming factors required, and most recently, repeated administration of synthetic mRNA15. These new methodologies raise the hope for the creation of iPSC without genetic modification or need for host DNA integration, which is likely to facilitate their translation into the clinics.
Advances in iPSC Technology and Potential for Clinically Useable iPSC Lines
For clinical use, iPSC would have to pass the strictest of criteria for safety and efficacy; however, great concern exists over the oncogenic potential of pluripotent cells, particularly iPSC. The first iPSC were created using lentiviral over-expression of the four reprogramming factors including the oncogenic factor Myc. Both Myc, secondary to its progrowth properties, and the integrating lentiviral system due to the potential for insertion mutations can be oncogenic16,17. Chimeric mice derived from iPSC obtained from lentiviral reprogrammed cells displayed a higher tendency for tumor formation18. Numerous approaches have been used to successfully generate iPSC without genome manipulation or integration12 but perhaps the most exciting is the use of small molecules to reprogram somatic cells into iPSC. Although, reprogramming by chemicals alone has yet been reported, many groupss have progressively reduced the number of reprogramming factors required and now only Oct3/4 along with chemical compounds is required14.
Another issue that will need to be resolved before iPSC are used clinically is the functional consequences of reprogramming and how similar iPSC are to other pluripotent cells like ESC. There is accumulating evidence that there are distinct differences between iPSC and ESC and that current reprogramming strategies do not completely reset the epigenetic landscape. Human iPSC have significant epigenetic differences when compared to human ESC leading to divergent gene expression prompting speculation that iPSC represents a new class of pluripotent cells19. A comparison of global gene expression patterns between iPSC and ESC revealed that iPSC may retain a common gene expression signature with the donor cells used to derive them especially during the initial passages20. Early passage iPSC cells retain residual DNA methylation signatures characteristic of their tissue of origin, which appears to result in preferential differentiation to the lineage from which they were derived21. These residual epigenetic marks of the donor tissue could be reset by prolonged culture, serial reprogramming or by treatment of iPSCs with chromatin-modifying drugs. This concept of “epigentic memory” where iPSC derived from different tissues retain patterns of gene expression reminiscent of the donor tissue has also been reported by others22. The biological significance of this phenomena remains unclear. Clearly, it affects differentiation potential21 whether it also affects safety will need to be determined. iPSC cells derived from different cell types demonstrated different rates of refractory cells post reprgramming and varying teratoma forming potentials23. Although the explanation for this is unknown and thus far no direct evidence exists that the malignant potential of refractory cells is related to epigenetic memory, our knowledge of the role of epigenetics in cancer suggests it may play an important role. Therefore, iPSC lines derived from different tissue types are subtly distinct and will likely have varying differentiation potentials and oncogenic risks associated with them. This will be an important consideration when determining what patient donor cell types to use for deriving iPSC.
For the cell progeny derived from human iPSC to be used clinically they will need to be genetically stable and functional in the long term. Therefore, full epigenetic screening and standardized assays to define the results of reprogramming will need to de developed prior to use in any large scale clinical trials24. Investigators have also discussed incorporating safety features such as a suicide gene that can be activated in the event of tumor growth or any unwanted effects of the transplanted cells to ensure a mechanism exists to remove these cells if necessary. One proposed mechanism is to stably transfect the iPSC with thymidine kinase that would render the transplanted cells and all of their progeny susceptible to gancyclovir25,26. Nonetheless, even if these issues are resolved, creation of clinical grade autologous iPSC for every patient may not be economically feasible. A potential solution may be the creation of meticulously derived and thoroughly assessed human iPSC banks of multiple human lymphocyte antigen (HLA) haplotypes and of different genetic backgrounds to avoid immune rejection for use in an off-shelf manner. These human iPSC lines would be mass produced in FDA approved Good Medical Practice (GMP) facilities to be distributed and used when called upon for cell therapies for patients after matching HLA types and genetic background to minimize the chance of rejection of transplanted tissue and the need for immunosuppression. Although considerable technical issues still face the field, great progress has already been made to address these concerns of bringing iPSC to the clinic.
Clinical Application of Human iPSC
Human iPSC have the potential to revolutionize regenerative medicine, human disease modeling, as well as drug safety and discovery studies. Since human iPSC can differentiate into any cell type and the supply is virtually unlimited, they are an ideal source of cells for transplantation to treat end stage organ failure. Furthermore, unlike human ESC that are derived from discarded human embryos, iPSC are ethically acceptable by advocates of adult and embryonic stem cells alike27,28. Human iPSC also offer the potential for autologous cell transplantation without concerns for haplotype matching and immune suppression therapy that would be required if human ESC are used for cell replacement therapies.
Correcting Genetic Disorders
The first demonstration of the therapeutic potential of iPSC to treat disease was shown in a “humanized” mouse model of sickle cell anemia29. In this model, the mouse α-globin genes have been replaced with human α-globin genes, and the mouse β-globin genes have been replaced with human Aγ and βS (sickle) globin genes. Mice homozygous for the human βS allele develop typical sickle cell disease symptoms including severe anemia. Mouse iPSC were generated from adult h βS/hβS male mice. The resultant hβS/hβS iPSC were genetically rescued by recombination with a human βA wild type globin gene. Hematopoietic progenitors were obtained in-vitro from the genetically engineered βA iPSC line and re-injected into the humanized mutant mice. There was functional correction of the sickle cell defect after stem cell transplantation and marked increases in RBC counts, hemoglobin, and packed cell volume levels. This general approach has now been tested in other animal models of human disease including Parkinson’s disease30, hemophilia type A31 and heart disease32. Efforts are now underway to develop large animal disease models with species-specific iPSC, which will facilitate testing of iPSC derivatives in large animal models such as the pig that is physiologically similar to humans33.
Treating Heart Disease
Cardiovascular disease is a leading cause of mortality and morbidity and there are few, if any options, to reverse or repair damage after a myocardial infarction. Many investigators have attempted to restore cardiac function after myocardial infarction by transplanting adult stem cell types, particularly bone marrow-derived cells34,35. Although initial results have been promising, there has been no significant demonstration of new cardiac myogenesis with the use of adult type stem cells. Functional improvement demonstrated with adult stem cells has been variable and transient and likely secondary to paracrine effects of the injected cells by modulating inflammatory responses, reducing myocyte apoptosis, and improving vasculogenesis to the affected territory. The growing consensus in the field is that there is little if any true cardiac regeneration with adult stem cell therapy35,36. Ideally, a multipotent cell with the ability to form cardiomyocytes, smooth muscle cells, and endothelial cells when transplanted would be optimal given the engrafted cells must integrate with native tissue and form viable electromechanically coupled myocardial tissue to avoid transplant cell-induced arrhythmias37,38. The initial attempts to use pluripotent stem cells for myocardial regeneration were done with injecting undifferentiated ESC directly into the injured heart39,40. Although they demonstrated improvement in myocardial function, this is not a feasible option for clinical use as more recent reports suggest that the transplanted undifferentiated cells form teratomas in the wall of the heart41,42. One approach to overcome this teratogenic risk is to pre-differentiate these embryonic stem cells prior to transplantation into more lineage-committed cells43. This potentially would negate the risk of teratoma formation. Nonetheless, the use of embryonic stem cells in itself is still problematic in clinical trials, not only due to ethical concerns, but also due to the immunogenicity of allogeneic non-haplotype matched transplanted cells and the potential for immune rejection. The issue of immunogenicity of transplanted allogeneic ESC and their progeny remains a problem as these cells induce a host immune response leading to rejection of transplanted cells and tissue. The original belief that embryonic stem cells are immune privileged and do not lead to immune rejection of haplotype unmatched embryonic stem cells has not been the case41. It has been demonstrated that embryonic stem cells upregulate histocompatibility antigens type I and II as they progressively differentiate thus increasing the potential for immune rejection44. This would necessitate long term immunosuppression and all the unwanted side effects and risks associated with the therapy.
The advent of human iPSC has been tremendously exciting for the field of cardiovascular regeneration. This technology offers the potential for autologous or haplotype-matched iPSC to be used for clinical regenerative therapies as clinical grade iPSC lines are generated without retroviral transduction of reprogramming factors and without the need for the oncogenic factor Myc. Several groups have demonstrated that mouse iPSC share developmental pathways with ESC and can differentiate into all three cardiovascular cell types typically found in the heart45,46. This capacity for cardiovascular differentiation has also has been demonstrated in human iPSC as well47,48. Recently, cardiac myocytes were generated from iPSC reprogrammed without the use of the oncogenic reprogramming factor Myc bringing the potential of iPSC for clinical therapies a step closer to reality49. Not only was Myc unnecessary for reprogramming of fibroblasts to iPSC, Myc-free iPSC lines demonstrated more efficient cardiogenesis and ability integrate into host heart tissue when compared to Myc-derived iPSC lines49. This was the case as well in-vitro where robust and sustained beating activity was documented in cardiomyocytes derived from Myc-free iPSC49. Transplantation of iPSC-derived cardiac myocytes improved left ventricular function after myocardial infarction in animal models50. iPSC treatment restored post-ischemic contractile performance, ventricular wall thickness, and electric stability while achieving in situ regeneration of cardiac, smooth muscle, and endothelial tissue50. While exciting, extending these results into humans would require derivation of clonal lineage committed cardiac cells or very efficient purification protocols to minimize any teratoma potential. Furthermore, the epigenetic profiles of different iPSC lines are quite varied, whether due to different donor cell types or the reprogramming methods used to generate the cells, which affects the differentiation potential of these iPSC22. Highly varied cardiac differentiation potentials of different human ESC and iPSC lines of greater than a hundred fold have been described48, 51. Therefore, detailed characterization of the human iPSC lines to be used clinically would be required and their differentiation potential quantified prior to use. Finally, more efficient differentiation protocols are required, as the process is currently quite inefficient and the cardiomyocytes generated are phenotypically and electrophysiologically quite heterogeneous and more similar to fetal cells47. It is possible that the in-vivo environment may lead to maturation of iPSC derived cardiomyocytes into mature and electromechanically coupled cardiomyocytes, but further work on maturing these progeny cells would be required. Human iPSC generation by reprogramming of differentiated human somatic cells into a pluripotent state may someday eliminate the need for controversial human ESC and provide a mechanism to generate customized, patient-specific pluripotent cells for cardiac regenerative therapies and cardiovascular tissue engineering.
Human iPSC: Beyond Regenerative Therapies
Diagnosis and Disease Pathophysiology
Although research into the potential use of iPSC for regenerative therapy has been a primary focus of the field, many other applications for human iPSC are also being explored. One of the most advanced is “reverse translation” studies using iPSC created from patients with genetic disorders to perform mechanistic studies into disease pathophysiology. Prior attempts to model human genetic diseases have mainly been limited to small animal models such as the mouse or using human ESC derived from discarded embryos after pre-implantation genetic diagnosis in Cystic Fibrosis and Huntington’s diseas52,53. Although promising, this approach has been limited by the relatively few diseases that can be screened prenatally and has faced the same ethical controversies that surround human ESC more generally. To circumvent these issues, investigators have attempted to create in-vitro disease models by genetically modifying currently available human ESC lines via homologous gene recombination. However, genetically modifying human ESC has encountered many technical difficulties and achieving the desired homologous recombination has been challenging. The advent of human iPSC reprogramming and protocols to differentiate them into the cell type of interest has made creating disease in a dish models a viable option to study human disease in-vitro. A number of diseased patient-derived human iPSC lines have been generated and the resulting differentiated cells have been shown to recapitulate the respective disease phenotype including Long QT Syndrome (LQTS)54, ALS55, spinal muscular atrophy56. Conversely, these disease-specific iPSC can be used as a diagnostic tool. Phenotypic characterization of abnormal currents in myocytes created from an individual predisposed to arrhythmias could point to both the genetic mutation and treatment.
Although tremendously exciting, using human iPSC for reverse translation is still in its infancy and application outside the laboratory is limited by the expense of creating the patient-specific iPSC and the labor-intensive characterization required. Further, many of the cell types derived from human ESC and iPSC to date are phenotypically more similar to fetal rather than adult cells, which may represent a significant problem as many diseases would require a more adult phenotype to be manifested to truly recreate the disease in-vitro sufficiently to be able to study. Another challenge facing the disease in a dish model is that, to demonstrate a disease-related phenotype in progeny cells from patient-derived iPSC, addition of stressors may be required to induce the phenotype such as age, oxidative stress, environmental or even unknown factors. Furthermore, certain diseases are not cell autonomous but require contact and interaction with other cell types as in the example of ALS where disease in neurons is related to secretions from adjacent Glial cells, not innate to the neurons themselves. Although it appears that disease modeling using human iPSC would be better suited to highly penetrant disorders of genetic origin, investigators have also used human iPSC to model myeloproliferative disorders, which typically develop later in life and are strongly affected by environmental issues57. The ability of human iPSC technology to provide pluripotent stem cells from a wide array of genotypes and clinical phenotypes will undoubtedly prove to be an invaluable tool for the study of human disease in the future.
Drug Discovery
Another evolving application for human iPSC is their potential use for new drug discovery. Given the high costs incurred in development of new candidates and the high rate of drug candidate attrition that occurs late in development, pharmaceutical companies have become increasingly wary of embarking on new drug development. Thus, the last decade has witnessed a significant decrease in new drug discovery, with only half as many new candidates reported compared to the previous decade58. The reason for this is likely multifactorial but the costs of drug development has increased exponentially59. Of particular concern, forty percent of the late attrition of drugs in development has been attributed to unforeseen cardiotoxic side effects of the drugs being tested in humans that was not realized in preclinical animal models60. The application of human iPSC technology may address some of the issues pharmaceutical companies face with low research and development productivity and high costs by offering a new and more efficient way to screen large libraries of small molecules. The advantages that this approach offers are multifold. First, not only can drug discovery be automated to efficiently screen thousands of small molecules, this can be done on different lines of patient-specific iPSC-derived somatic cells to assess the possible effects of small molecules on different cell types from different ethnic and genetic backgrounds. A major criticism and limitation of many clinical trials is the under representation of women and minorities, which could be conceivably addressed with diverse panels of iPSC. A common cardiotoxicity of new drugs is lethal arrhythmias secondary to QT prolongation and ventricular tachycardia, which may deteriorate into ventricular fibrillation and death. These arrhythmias are precipitated by the prolongation of myocardial repolarization as measured by the QT segment of the electrocardiogram. This led the FDA to define QT prolongation as one of the major toxicities to be screened for as part of the safety profile during drug development. Currently drugs are screened for potential QT prolongation by patch clamping or ligand binding assays using isolated cardiac tissue and in-vivo studies for arrhythmias in large animal models. Although these animal models are the best we currently have available, they do not accurately predict the propensity of drugs to induce QT prolongation in humans with an unacceptably high rate of false negatives61. Therefore a more robust and sensitive screening modality to assess drugs for potential cardiac toxicities is required and human iPSC-derived cardiomyocytes may provide a very reliable and sensitive model for high throughput screening of drugs. The reliability of in-vitro models of human cardiac myocytes has been validated by several studies assessing the electrophysiologic response to well characterized drugs and their effect on the QT segment62,63. Human ESC-derived cardiomyocytes responded to the drugs in the anticipated fashion as would be expected from in-vivo studies. Thus, using human iPSC-derived cardiac myocytes from different genders and ethnic backgrounds to screen for drug toxicities could dramatically improve the safety of new drugs and increase the efficiency of drug development.
Conclusion
With an aging population, ever-increasing costs of healthcare and a decrease in new drug discoveries, new and innovative ways of accelerating and streamlining human disease study and treatment are required. Human iPSC technology offers an unprecedented opportunity to develop patient-specific and disease-specific iPSC lines to be used for regenerative therapies, disease study, drug safety and discovery. The prospect of cellular and tissue regenerative therapies offers tremendous hope for the transplant field that finds itself in a supply-demand crisis with a constant shortage of donor organs and a rising number of patients requiring transplants. Although the field is still in its early stages, the transformative power of this new technology appears unlimited and should spawn novel therapies for years to come.
Figure 1.
Differentiated somatic cells (fibroblasts) can be reprogrammed into induced pluripotent stem cells (iPSC) with retroviral over-expression of the four factors Oct4, Sox2, Klf4, c-Myc. iPSC have the potential to differentiate into all somatic cell types.
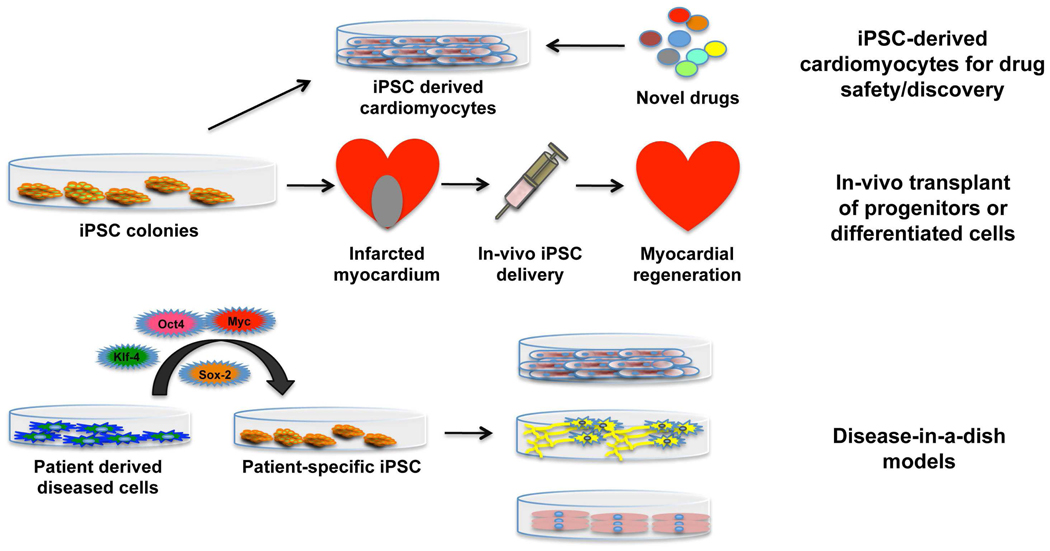
Figure 2.
Schematic outline of potential therapeutic applications of iPSC. iPSC can be differentiated into cardiomyocytes for drug screening and discovery. Autologous or haplotype matched iPSC-derived cells can be used for myocardial regenerative therapies post myocardial infarction. iPSC can be derived from specific patients to recreate the disease-in-a-dish models in-vitro for study.
Acknowledgements
This work was supported by the Alberta Heritage Foundation for Medical Research Fellowship to A.N. and the NIH (HL080111 and HL094941), CIRM (RB1-01354), Laubisch and Cardiovascular Development Funds to WRM.
Abbreviations
- ESC
embryonic stem cells
- iPSC
induced pluripotent stem cells
- CPC
cardiac progenitor cells
- SCNT
somatic cell nuclear transfer
- CM
cardiac myocytes
- SMC
smooth muscle cells
- EC
endothelial cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Becker AJ, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 2.Evans MJ. Establishment in culture of pluripotential cells from mouse embryos. Proc. Natl. Acad. Sci. U. S. A. 1981;78(12):7634–7638. [Google Scholar]
- 3.King TJ. Transplantation of living nuclei from blastula cells into enucleated frogs' eggs. Proc. Natl. Acad. Sci. U. S. A. 1952;38:455–463. doi: 10.1073/pnas.38.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnieke AE, Ritchie WA, Mycock K, Scott AR, Ritchie M, Wilmut I, Colman A, Campbell KH. Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science. 1997;278(5346):2130–2133. doi: 10.1126/science.278.5346.2130. [DOI] [PubMed] [Google Scholar]
- 5.Gurdon JB, Simonsson S. Nuclear reprogramming and stem cell creation. Proc. Natl. Acad. Sci. U. S. A. 2003;100(1):11819–11822. doi: 10.1073/pnas.1834207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne JA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- 7.Martin G. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U. S. A. 1981;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, Jones J. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 9.Geron Corporation. World's first clinical trial of human embryonic stem cell therapy cleared. Regen. Med. 2009;4(2):161. [PubMed] [Google Scholar]
- 10.Takahashi K. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 12.Hochedlinger K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136(4):509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woltjen K, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;489(7239):766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, Zhu S, Joo JY, Do JT, Xiong W, Kim JB, Zhang K, Schöler HR, Ding S. Conversion of mouse epiblast stem cells to an earlier pluripotency state by small molecules. J. Biol. Chem. 2010;285(39):29676–29680. doi: 10.1074/jbc.C110.150599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren L, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Boland Michael J, Nazor Kristopher L, Rodriguez Alberto R, Gifford Wesley, Martin Greg, Kupriyanov Sergey, Baldwin Kristin K. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- 19.Deng J, Xie B, Gore A, LeProust EM, Antosiewicz-Bourget J, Egli D, Maherali N, Park IH, Yu J, Daley GQ, Eggan K, Hochedlinger K, Thomson J, Wang W, Gao Y, Zhang K. Targeted bisulfite sequencing reveals changes in DNA methylation associated with nuclear reprogramming. Nat. Biotechnol. 2009;27:353–360. doi: 10.1038/nbt.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin MH, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh Z, Wu Y, Hu S, Quertermous T, Wu JC. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS ONE. 2010;5(2):e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura K, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, Ogawa D, Ikeda E, Okano H, Yamanaka S. Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 24.Park IH, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 25.Ciceri F, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, Turchetto L, Colombi S, Bernardi M, Peccatori J, Pescarollo A, Servida P, Magnani Z, Perna SK, Valtolina V, Crippa F, Callegaro L, Spoldi E, Crocchiolo R, Fleischhauer K, Ponzoni M, Vago L, Rossini S, Santoro A, Todisco E, Apperley J, Olavarria E, Slavin S, Weissinger EM, Ganser A, Stadler M, Yannaki E, Fassas A, Anagnostopoulos A, Bregni M, Stampino CG, Bruzzi P, Bordignon C. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet. Oncol. 2009;10(5):489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 26.Menzel O, Wildhaber BE, Jond C, Lasne F, Habre W, Trono D, Nguyen TH, Chardot C. Biosafety in ex vivo gene therapy and conditional ablation of lentivirally transduced hepatocytes in nonhuman primates. Mol. Ther. 2009;17(10):1754–1760. doi: 10.1038/mt.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick JB. Stem cells and ethics: current issues. J. Cardiovasc. Transl. Res. 2010;3(2):122–127. doi: 10.1007/s12265-009-9155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer J. The significance of induced pluripotent stem cells for basic research and clinical therapy. J. Med. Ethics. 2008;34(12):849–851. doi: 10.1136/jme.2008.024786. [DOI] [PubMed] [Google Scholar]
- 29.Hanna J, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 30.Wernig M, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5656–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu D, Fink LM, Adcock DM, Yang J, Ward DC, Ma Y. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:808–813. doi: 10.1073/pnas.0812090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson TJ, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of Acute Myocardial Infarction by Human Stemness Factors Induced Pluripotent Stem Cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts RM, Ezashi T. Induced pluripotent stem cells from swine (Sus scrofa): why they may prove to be important. Cell Cycle. 2009;8(19):3078–3081. doi: 10.4161/cc.8.19.9589. [DOI] [PubMed] [Google Scholar]
- 34.Hutcheson KA, Hueman MT, Hopkins MB, Glower DD, Taylor DA. Comparison of benefits on myocardial performance of cellular cardiomyoplasty with skeletal myoblasts and fibroblasts. Cell Transplant. 2009;9:359–368. doi: 10.1177/096368970000900307. [DOI] [PubMed] [Google Scholar]
- 35.Galli D, Staszewsky L, Zanetta L, Sampaolesi M, Bai A, Martinoli E, Carlo E, Balconi G, Fiordaliso F, Chimenti S, Cusella G, Dejana E, Cossu G, Latini R. Mesoangioblasts, vessel-associated multipotent stem cells, repair the infracted heart by multiple cellular mechanisms: a comparison with bone marrow progenitors, fibroblasts, and endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2005;25:692–697. doi: 10.1161/01.ATV.0000156402.52029.ce. [DOI] [PubMed] [Google Scholar]
- 36.Laflamme MA, Epstein SE, Murry CE. Cell-Based Therapy for Myocardial Ischemia and Infarction: Pathophysiological Mechanisms. Annu. Rev. Pathol. 2007;2:307–339. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa SI, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+ VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 38.Menasché P. Stem Cell Therapy for Heart Failure. Are Arrhythmias a Real Safety Concern? Circulation. 2009;119:2735–2740. doi: 10.1161/CIRCULATIONAHA.108.812693. [DOI] [PubMed] [Google Scholar]
- 39.Min JY, Sullivan MF, Ke Q, Converso KL, Chen Y, Morgan JP, Xiao YF. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J. Thorac. Cardiovasc. Surg. 2003;125:361–369. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 40.Singla DK, Ma L, Douglas PS, Sullivan R, Lyons GE, Kamp TJ. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J. Mol. Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Nussbaum J, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 42.Behfar A, Faustino RS, Arrell DK, Hodgson DM, Yamada S, Puceat M, Niederlander N, Alekseev AE, Zingman LV, Terzic A. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J. Exp.Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caspi O, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J. Am. Coll. Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 44.Swijnenburg RJ, Vogel H, Baker J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL, Fedoseyeva EV, Robbins RC. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:166–172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 45.Schenke-Layland K, Angelis E, Butylkova Y, Heydarkhan-Hagvall S, Gekas C, Zhang R, Goldhaber JI, Mikkola HK, Plath K, MacLellan WR. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells. 2008;26(6):1537–1546. doi: 10.1634/stemcells.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narazaki G, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118(5):498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 47.Zwi L, Arbel G, Huber I, Gepstein A, Park IH, Gepstein L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120(15):1513–1523. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Research. 2009;104(4):e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez-Fernandez A, Yamada S, Reyes S, Alekseev AE, Perez-Terzic C, Ikeda Y, Terzic A. iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circ. Research. 2009;105(7):648–656. doi: 10.1161/CIRCRESAHA.109.203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson M Timothy J, PhD, Martinez-Fernandez Almudena, PharmD, Yamada Satsuki, MD, PhD, Perez-Terzic Carmen, MD, PhD, Ikeda Yasuhiro, DVM, PhD, Terzic Andre., MD, PhD Repair of Acute Myocardial Infarction by Human Stemness Factors Induced Pluripotent Stem Cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osafune K, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 2008;26(3):313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 52.Pickering SJ, Patel M, Taylor H, Black C, Burns CJ, Ekonomou A, Braude PR. Generation of a human embryonic stem cell line encoding the cystic fibrosis mutation deltaF508, using preimplantation genetic diagnosis. Reprod. Biomed. Online. 2005;10(3):390–397. doi: 10.1016/s1472-6483(10)61801-9. [DOI] [PubMed] [Google Scholar]
- 53.Stephenson EL, Braude PR. Preimplantation genetic diagnosis as a source of human embryonic stem cells for disease research and drug discovery. BJOG. 2009;116(2):158–165. doi: 10.1111/j.1471-0528.2008.02009.x. [DOI] [PubMed] [Google Scholar]
- 54.Moretti A, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127(6):1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 55.Dimos JT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1169–1170. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 56.Ebert AD, Rose FF, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):227–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye Zhaohui, Mali Prashant, Dowey Sarah, Williams Donna M, Jang Yoon-Young, Dang Chi V, Spivak Jerry L, Moliterno Alison R, Cheng Linzhao. Human induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114(27):5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hughes B. 2008 FDA drug approvals. Nat. Rev. Drug Discov. 2009;8(93) doi: 10.1038/nrd2813. [DOI] [PubMed] [Google Scholar]
- 59.Hu Michael, Sheu Jack, Tschopp Daniel. The Innovation Gap in Pharmaceutical Drug Discovery and New Models for R&D Success. Kellogg School of Management. 2007 [Google Scholar]
- 60.Kola I. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004;3:711–716. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 61.Pugsley MK, Curtis MJ. Principles of Safety Pharmacology. Br. J. Pharmacol. 2008;154(7):1382–1399. doi: 10.1038/bjp.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braam SR. Human stem cell models for predictive cardiac safety pharmacology. Stem Cell Res. 2010;4(3):155–156. doi: 10.1016/j.scr.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Otsuji TG, Kurose Y, Yamauchi K, Tada M, Nakatsuji N. Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: Qualitative effects on electrophysiological responses to drugs. Stem Cell Res. 2010;4(3):201–213. doi: 10.1016/j.scr.2010.01.002. [DOI] [PubMed] [Google Scholar]