Abstract
The hypothesis that the life-extending effect of caloric restriction (CR) is associated with an attenuation of the age-related pro-oxidant shift in the thiol redox state was tested employing a novel experimental design. Amounts of GSH, GSSG and protein mixed disulfides (Pr-SSG) in the skeletal muscle and liver were compared between two strains of mice, which have similar life spans when fed ad libitum (AL), however, under the standard CR regimen, life span of only one strain, C57BL/6, is extended, whereas it remains unaffected in the other strain, DBA/2. Mice were fed AL or 40% less food starting at 4 months and compared at 6 and 24 months of age. The amounts of GSSG and Pr-SSG increased and the GSH:GSSG ratios decreased with age in both strains of AL-fed mice. CR prevented these age-related changes in the C57BL/6, whose life span is extended by CR, but not in the DBA/2 mice, in which it remains unaffected. CR enhanced the activity of glutamate cysteine ligase in the C57BL/6, but not in the DBA/2 mice. Results suggest that longevity extension by CR may be associated with the attenuation of age-related pro-oxidizing shifts in the thiol redox state.
Keywords: aging, dietary restriction, redox state, mixed protein disulfides, glutathione
A long-held generalization in gerontology is that a reduction in the amount of dietary energy intake, also referred to as caloric restriction (CR), extends the life span and diminishes the incidence of a variety of age-related pathological disorders in laboratory rodents and arguably in most other phylogenetic groups. Accordingly, numerous comparisons have been made between the ad libitum-fed (AL) and the respective calorically restricted animals for the identification of alterations that may be responsible for the life span-prolongation effect of CR. However, results of such studies have, in general, indicated that CR impedes the progression of most of the age-associated changes, which occur in the AL-fed animals [1,2], whereby the nature of the alterations that may be responsible for the extension of life span by CR has remained elusive. The main limitation of this conventional experimental design, solely based on comparisons between AL and CR animals, is that the effects of CR emanating from a decrease in the amount of energy intake, that are essentially adaptive, cannot be discerned from those that may be causally or specifically linked to the mechanisms by which CR extends longevity.
An alternative experimental model for understanding the mechanisms of CR was recently suggested by Sohal et al. [3], which advocated the inclusion of a “negative” control, i.e. a genetic strain whose life span is not extended by CR. The rationale was that comparisons between genotypes, whose life span is extended by CR with those where it remains unaffected, could reveal the specific effects of CR that are associated with the prolongation of life span. Contrary to the historical view, recent studies have demonstrated that the effects of CR on life span are genotype-specific rather than being universal. Members of the same species may respond differently to CR. For instance, Forster et al. [4] reported that under the AL regimen, the C57BL/6 and DBA/2 mice consumed the same amount of food throughout life and had a similar life span; however, under the 40% CR regimen, life span of C57BL/6 mice was lengthened by 25-30%, but there was no effect on the longevity in the DBA/2 mice. More recently, Liao et al. [5] found that among 41 recombinant inbred strains of mice, CR shortened the life span of more strains than those where it was extended.
In this context, the main objective of the present study was to test the hypothesis that life span extension by CR depends on its ability to lower the age-associated increase in oxidative stress [6]. Our previous studies have shown that the thiol redox state of the tissues, indicated by the amounts of GSH, GSSG and mixed protein disulfides, becomes more pro-oxidizing with age and CR attenuates the magnitude of these changes [7,8]. Furthermore, the thiol redox state is deemed to be a relatively more sensitive and functionally relevant measure of oxidative stress than the macromolecular structural damage [9]. Accordingly, comparisons of the amounts of glutathione (GSH), glutathione disulfide (GSSG) and protein mixed disulfides (Pr-SSG) were made in the homogenates of hind-leg skeletal muscles and liver of 6- and 24- month-old C57BL/6 and DBA/2 mice that had been fed ad libitum or maintained on a regimen of 40% CR since the age of 4 months. The rationale for the selection of skeltal muscle was that CR has been demonstrated to modulate the age-related mitochondrial changes in this tissue [10]. Liver was chosen because it is the main site for GSH synthesis and supply to other tissues via the vascular system [11]. It was provisionally reasoned that if attenuation of oxidative stress were indeed implicated in the extension of life span, the effects of CR on the thiol redox state would be much more pronounced in the C57BL/6 than in the DBA/2 mice.
Materials and methods
Materials
GSH and GSSG were obtained from Sigma Chemical Co. (St Louis, MO) and used as calibration standards. Acetonitrile, meta-phosphoric acid, THP (Tris(hydroxypropyl)phosphine) and 1-octane sulfonic acid were from EM Science (Gibbstown, NJ). Deionized water was prepared using a Millipore Milli-Q System (Milli-Q Corp., Bedford, MA, USA). All other chemicals were HPLC grade or of the highest purity available.
Animals
All experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals issued by National Institutes of Health and the approval of the University of Southern California (USC) and University of North Texas Health Science Center (UNTHSC) Institutional Animal Care and Use Committees. The effects of long- and short-term CR were conducted in two separate groups of mice. For the long-term study, thirty-nine male DBA/2J and 27 C57BL/6J male mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at 8-9 weeks of age and housed one mouse per cage in polycarbonate cages with mesh tops, under a 12- h light/dark cycle, with the light phase beginning at 0600 h. Mice were fed the NIH-31 diet ad libitum (AL) until 14 weeks of age, whereupon approximately one-half of the mice from each strain were placed gradually under a CR regimen: i.e. a 10% reduction in the amount of food consumed by the AL fed mice during the first week, followed by 20% in the second, and 40% in the third week and thereafter. The CR mice were fed a vitamin-fortified NIH-31 diet [12], designed to equalize per mouse the amount of essential nutrients ingested by the AL mice. The amount of food consumed by the AL mice was monitored throughout the duration of the study in order to maintain the level of food intake by the CR mice at 60% of the AL group. Mice were euthanized at 6 or 24 months of age, i.e., after 3 or 21 months on the 40% CR regimen, and the hind limb skeletal muscles and liver were processed for biochemical studies.
For the short-term study, sixteen male DBA/2JNia and 22 C57BL/6JNia mice were procured from the National Institute on Aging and housed under identical conditions. CR was also implemented gradually, starting at 17 months of age and maintained for 7 weeks, prior to euthanasia and the collection of blood.
Sample preparation
Mice were euthanized by cervical dislocation and decapitation, and the hind limb skeletal muscles and liver were removed and placed in ice-cold antioxidant buffer (150 mM potassium phosphate, 2 mM EDTA, pH 7.4, 100 μM BHT). Tissues were rinsed and finely chopped in the antioxidant buffer and homogenized (5% w/v) in the appropriate isolation buffer (liver: 0.25 M sucrose, 3 mM EDTA, 10 mM Tris buffer, pH 7.4; skeletal muscle: 120 mM KCl, 20 mM HEPES, 2 mM MgCl2, 0.5% (w/v) BSA, 1 mM EGTA, pH 7.4) with a Polytron (PT 1200) (skeletal muscles) and a teflon/glass (liver) homogenizer. Blood was collected from the heart cavity and mixed with 50 μL of 100 mM EDTA/ml blood and centrifuged at 2000g for 3 min to separate serum from the erythrocytes. The pelleted erythrocytes were lysed in 1 ml of ice-cold water for 5 min.
Aliquots (300 μl) of the crude homogenate or red blood cell lysates were mixed with 100 μl of ice-cold 20% (w/v) meta-phosphoric acid, incubated for 30 min in cold and centrifuged for 20 min at 14,000 g at 4°C. Supernatants were transferred to auto-sample vials and injected either immediately or stored at -80°C. For the measurement of the amount of protein-glutathione mixed disulfides (Pr-SSG), the protein pellet from the initial precipitation of homogenates was resuspended and subjected to extensive washing to remove the free (non protein-bound) GSH and GSSG. Washing consisted of three cycles of resuspension of the precipitate in 1.5 ml of 5% (w/v) MPA containing 0.5 mmol/L EDTA, and a 15 s sonication on ice. After each wash, precipitated protein was recovered by centrifugation at 18,000 × g for 20 min at 4°C, and the supernatant was discarded. Protein-bound GSH was released by resuspention/incubation of protein pellets in 300 μl of 10 mM phosphate buffer, pH 8.0, containing 1 mM EDTA and 0.1 mM Tris(hydroxypropyl)phosphine for 1 h at 37°C. Released GSH was analyzed after precipitation of proteins with 100 μl of ice-cold 20% (w/v) meta-phosphoric acid, as described above. The protein content of the homogenates was determined by the BCA protein assay, according to the manufacturer's instructions (Pierce, Rockford, IL).
HPLC-coulometric EC detection of GSH, GSSG and Pr-SSG
The procedure for detection and quantification of GSH and GSSG used here included the previously described precautionary measures to minimize spontaneous GSH oxidation to GSSG [7,8]. Briefly, GSH and GSSG were separated by HPLC, fitted with a Shimadzu Class VP solvent delivery system, using a reverse phase C18 Luna (II) column (3μ; 4.6 × 150 mm), obtained from Phenomenex (Torrance, CA). The mobile phase for isocratic elution consisted of 25 mM monobasic sodium phosphate, 0.5 mM of the ion-pairing agent 1-octane sulfonic acid, 1% (v/v) acetonitrile, pH 2.7, adjusted with 85% phosphoric acid. The flow rate was 0.7 ml/min. Under these conditions, the separation was completed in 35 min; GSSG was the last eluting peak, with a retention time of approximately 25 min. Calibration standards were prepared in 5% (w/v) MPA. GSH and GSSG were detected with a model 5600 CoulArray® electrochemical detector (ESA Inc., Chelmsford, MA), equipped with eight-channel analytical cells, employing potentials from +100 mV to +800 mV (100 mV increment). GSH was monitored at +600 mV and GSSG at +700 mV. Each sample was injected twice, and the average of the peak areas was used for quantification.
Activities of hepatic enzymes
Total glutathione peroxidase activity was measured according to Beutler [13]. Briefly, sonicated homogenate (10 μl) was added to a 1 ml cuvette containing 100 μl of 1 M potassium phoshate buffer, pH 7.0, 10 μl GSH, 20 μl of 0.2 M EDTA, 100 μl of 10 U/ml of glutathione reductase, 10 μl of 0.4 M sodium azide, and 100 μl of 2 mM NADPH, and brought to a final volume of 1 ml with water. After incubation for 10 min at 25 C, 10 μl of 10 mM hydrogen peroxide was added and the reaction followed at 340 nm. Glutathione reductase (GR) activity was determined according to the method described by Carlberg and Mannervik [14]. Ten μl of sonicated homogenate was added to a cuvette containing 50 μl of 2 mM NADPH, 500μl of 0.2 M potassium phosphate buffer containing 2 mM EDTA ( pH 7.0), 50μl of 20 mM GSSG, and water to a final volume of 1 ml. The decrease in absorbance at 340 nm was followed at 25 C. GCL catalytic activity was measured using an HPLC-based assay, as described previously [15].
Statistical analysis of data
Data are presented as the mean ± SE for each tissue for separate groups of mice of each strain/diet condition. To determine the effects of age, mouse strain and dietary regimen on content of GSH, GSSG and Pr-SSG, and GSH:GSSG ratios, data were considered in 3-way analyses of variance, with Age, Strain and Diet as between groups factors. Planned individual comparisons at each of the ages, to assess the effect of diet within each strain, were performed using single degree-of-freedom F tests. Activities of hepatic enzymes as well as concentrations of GSH, GSSG and the GSH:GSSG ratios in erythrocytes were considered in 2-way analyses of variance, with Strain and Diet as between groups factors. For these variables, the effect of age in AL-fed groups of each strain was determined using single degree-of-freedom F tests within a 1-way ANOVA including all six groups within each experiment. Statistical significance was defined as p < 0.05.
Results
Effects of age and caloric restriction on amounts of GSH, GSSG and Pr-SSG in skeletal muscle
Mice were fed ad libitum (AL) or gradually put under a regimen of CR, where the amount of food offered after the age of 4 months was 40% less than the amount consumed by the corresponding AL-fed mice. Comparisons between the AL and CR mice of the two different strains were made at 6 and 24 months of age, that is, after the mice had been put under the 40% CR regimen for 2 and 21 months, respectively.
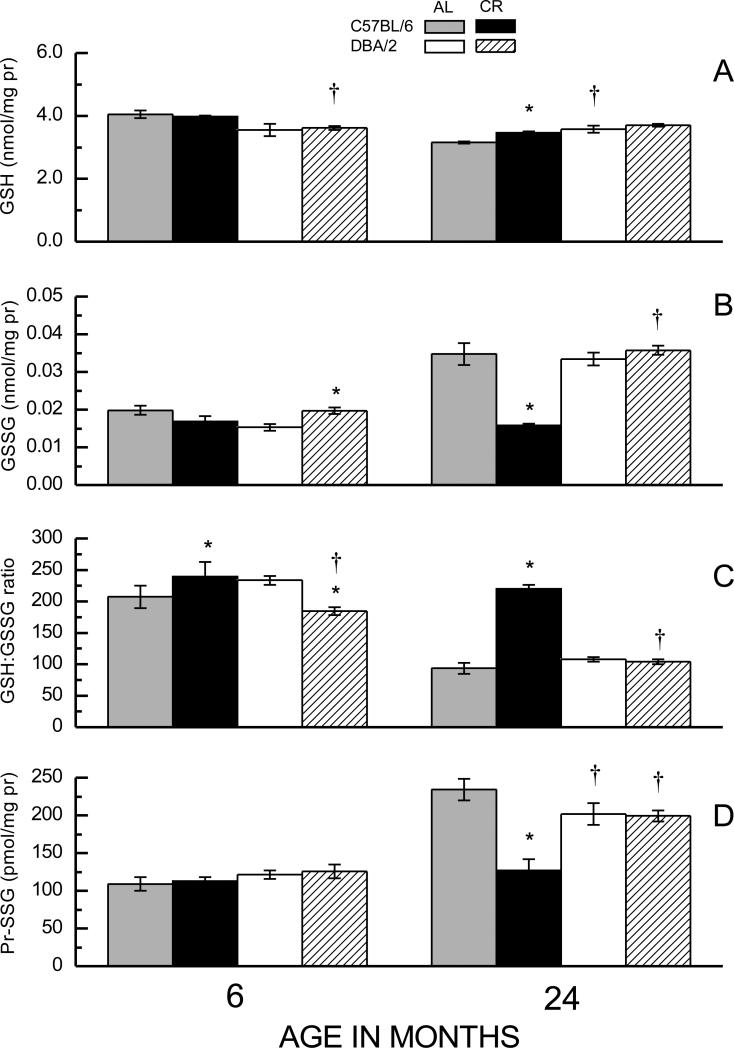
A comparison between 6- and 24-month-old AL fed mice indicated that GSH concentration was 22% lower in the older than the younger C57BL/6 mice, however, there was no age-related difference in the DBA/2 mice (Figure 1A). The GSH content in the latter was 9% lower than in the C57BL/6 mice at 6 months, but 14% higher at 24 months of age. CR attenuated the magnitude of the age-related decrease in the GSH amount in the C57BL/6 mice, but had no effect in the DBA/2 mice. ANOVA on GSH amount indicated a significant main effect of Age as well as an Age × Strain interaction (ps < 0.001), but did not suggest any effect of Diet (ps > 0.137).
Fig. 1.
Effects of age and caloric restriction (CR) on amounts of GSH (A), GSSG (B), the GSH:GSSG ratio (C) and amounts of Pr-SSG (D) in homogenates of hind leg skeletal muscles in C57BL/6 and DBA/2 mice. Mice were fed ad libitum (AL) or gradually put on 40% CR at 4 months of age. Data represent the mean ± SE of 3-10 mice. (* p < 0.05 for planned comparisons of CR with AL of matching age and strain; † for comparison of C57BL/6 to DBA/2 of matching diet and age).
The GSSG amounts in skeletal muscle of AL-fed groups increased during 6 to 24 months of age in both C57BL/6 (75%) and the DBA/2 (118%) mice (Fig. 1B). Notably, CR fully prevented this age-related elevation in GSSG amount in C57BL/6, but had no discernable effect in the DBA/2 mice. Curiously, at 6 months of age, that is 2 months after the imposition of 40% CR, the DBA/2 mice on the CR regimen had 16% higher GSSG content than their AL-fed counterparts; however, CR tended to decrease the GSSG level in the C57BL/6 mice at this age. ANOVA indicated a significant interaction of Age, Strain and Diet on GSSG content (p = 0.004). Thus, the main finding was that CR attenuated the age-related elevation in GSSG concentration in the C57BL/6 mice, whose longevity is extended by CR, but not in the DBA/2 mice, whose life span is not affected by CR.
The GSH:GSSG ratios in the AL-fed mice of both strains of mice decreased 54-55% during 6 to 24 months of age. Again, this decline was prevented by the CR regimen in the C57BL/6, but not in the DBA/2 mice (Fig.1C). At 6 months of age, CR caused a 21% decrease in GSH:GSSG ratio in DBA/2 mice, but had an opposite effect in the C57BL/6 mice. ANOVA of GSH:GSSG ratios indicated a significant Strain × Diet interaction (p<0.001) as well as a nearly significant 3-way interaction (p < 0.08).
The effects of strain, age, and diet on protein mixed disulfide (Pr-SSG) content paralleled those observed for the GSSG levels and GSH:GSSG ratios (Fig. 1D). There was an increase in Pr-SSG content in the AL-fed mice of both strains between 6 and 24 months of age, albeit the increase was greater in C57BL/6 (115%) than in DBA/2 (66%). Long-term CR attenuated the age-related elevation in Pr-SSG in the C57BL/6 mice, but had little effect in the DBA/2. Analysis of the alterations in Pr-SSG content indicated a significant Age × Strain × Diet interaction (p<0.001).
Effects of age and caloric restriction on GSH, GSSG and Pr-SSG in liver
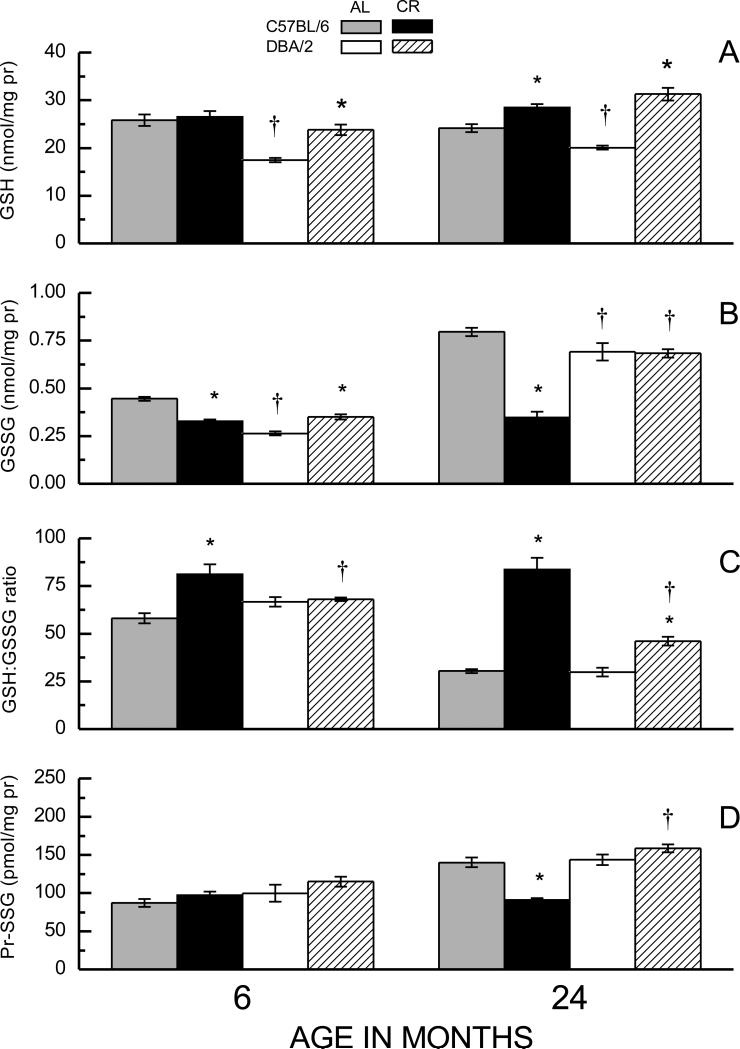
Compared to the skeletal muscle, the amounts of GSH and GSSG in homogenates of 6-month-old AL-fed mice were ~ 5- to 20-fold higher in the liver, which was reflected in relatively lower GSH:GSSG ratios. Furthermore, unlike the skeletal muscle, the GSH amount in liver homogenates of AL fed mice of both strains showed no significant loss during 6 to 24 months of age (Fig. 2A). GSH levels in the liver homogenates of AL-fed DBA/2 mice were 32% and 17% lower than in the AL-fed C57BL/6 mice at the age of 6 and 24 months, respectively. CR caused a 37% increase in GSH content in DBA/2 mice at 6 months of age, but had no effect in the C57BL/6 mice; whereas at 24 months of age, GSH levels were higher under CR in both C57BL/6 (18%) and the DBA/2 (56%) mice. Thus, CR tended to elevate GSH levels in the liver by a greater magnitude in the DBA/2 than in C57BL/6 mice. Analysis of the GSH data indicated significant main effects of Age, Diet, and Strain, as well as a Strain × Diet interaction (ps < 0.001).
Fig. 2.
Effects of CR on amounts of GSH (A) and GSSG (B), the GSH:GSSG ratio (C), and amounts of Pr-SSG (D) in liver homogenates of C57BL/6 and DBA/2 mice at 6 and 24 months of age. Data represent the mean ± SE of 4-8 mice. (* p < 0.05 for planned comparisons of CR with AL of matching age and strain; † for comparison of C57BL/6 to DBA/2 of matching diet and age).
The age-, diet- and strain-related variations in the amounts of GSSG and Pr-SSG in the liver homogenates (Figs. 2B, 2D) paralleled those observed in the skeletal muscle (Fig. 1). In the AL fed mice, the amounts of GSSG were lower in the DBA/2 than in C57BL/6 mice at both 6 and 24 months of age (by 41% and 13%, respectively). The amount of GSSG increased with age in both strains of AL fed mice (79% in C57BL/6; 163% in DBA/2). CR caused a 26% decrease in the level of GSSG in C57BL/6 mice at 6 months of age, whereas it increased GSSG amount by 33% in the DBA/2 mice. Importantly, at 24 months, CR completely prevented the age-related rise in GSSG concentration in C57BL/6 mice, but it had no effect in the DBA/2 mice. Analysis of the GSSG data for the liver homogenates indicated a significant Age × Strain × Diet interaction (p = 0.005).
Under the AL regimen, the GSH:GSSG ratios declined during 6 to 24 months of age by 48% in C57BL/6 and 55% in the DBA/2 mice (Fig. 2C). In the C57BL/6 mice, CR elevated GSH:GSSG ratio by 40% at 6 months and completely prevented the age-related decline observed at 24 months of age. In contrast, in DBA/2 mice, CR had no effect on GSH:GSSG ratio at 6 months of age and failed to prevent the age-related decline at 24 months. Compared to their corresponding 6-month-old AL fed group, CR elevated the GSH:GSSG ratio by 44% in C57BL/6 mice at 24 months of age whereas there was a decrease of 31% in the DBA/2 mice. ANOVA on the GSH:GSSG ratios in the liver yielded significant main effects and 2-way interactions of all factors (ps < 0.001) as well as a 3-way interaction that neared significance (p < 0.07).
The amounts of Pr-SSG in the liver (Fig. 2D) increased during 6 to 24 months of age in the AL-fed mice of both strains, albeit the increase was greater in C57BL/6 (61%) than in DBA/2 mice (44%). CR attenuated the age-related increase in Pr-SSG content in the C57BL/6, but had no effect in the DBA/2 mice. Analysis of the Pr-SSG data showed a significant Age × Strain × Diet interaction (p = 0.008).
Effect of short-term CR on GSH and GSSG in erythrocytes
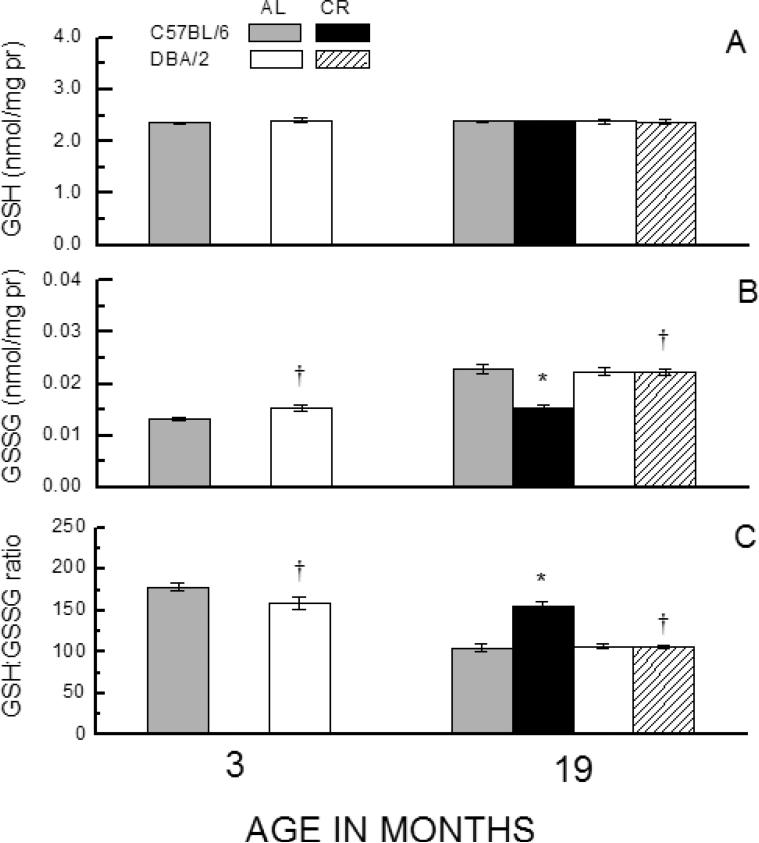
In this experiment, the short-term effects of CR on the thiol redox state were determined in the erythrocytes of the two strains of mice, maintained on AL or CR dietary regimen for 7 weeks, beginning at 17 months of age. In addition, 3-month-old AL-fed mice were used for the determination of age-related effects. The amount of GSH in the erythrocytes (Fig. 3A) did not vary as a function of Age, Strain or Diet (ps > 0.262). However, GSSG and the GSH:GSSG ratios showed significant pro-oxidizing shifts as a function of age (Fig. 3B, C). The 19-month-old AL-fed C57BL/6 mice had a 72% higher GSSG content and a 41% lower GSH:GSSG ratio than the 3-month-old. Strikingly, 40% CR for 7 weeks fully prevented these pro-oxidizing changes in the C57BL/6 mice. In contrast, while the DBA/2 mice showed a 46% increase in GSSG amount and a 33% decrease in GSH:GSSG ratio during 3 to 19 months of age under the AL regimen, CR for 7 weeks had no effect on GSSG content or GSH:GSSG ratio in this strain. It is also worth noting that under the AL regimen, DBA/2 mice had significantly higher GSSG level and a lower GSH:GSSG ratio than the C57BL/6 mice at 3 months of age, however, these inter-strain differences were absent in the 19-month-old mice. Analyses of variance on GSSG content and GSH:GSSG ratios revealed a significant interaction of Strain and Diet (p < 0.015), which conforms with the observed differential effect of CR in the C57BL/6 and the DBA/2 mice.
Fig. 3.
Effect of age and short-term CR on GSH (A), GSSG (B), or GSH:GSSG ratio (C) in erythrocytes of C57BL/6 and DBA/2 mice. Mice were placed on 40% CR for 7 weeks at 15 months of age. Data represent the mean ± SE of 4-10 mice (* p < 0.05 for planned comparisons of CR with AL of matching age and strain; † for comparison of C57BL/6 to DBA/2 of matching diet and age).
Activities of hepatic enzymes associated with glutathione metabolism
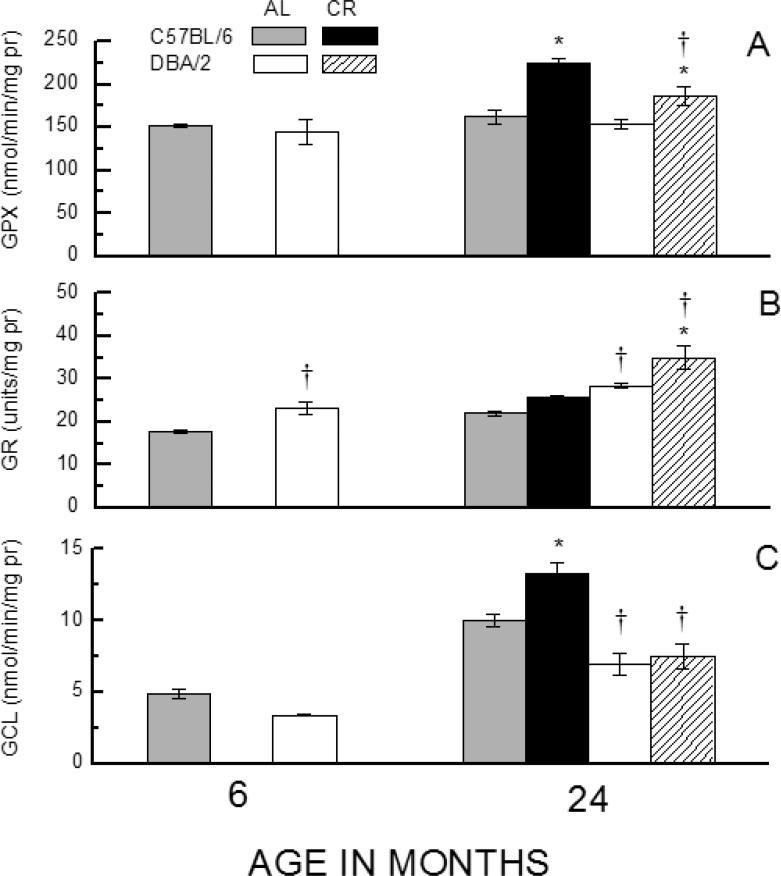
The studies on the skeletal muscle, liver and erythrocytes, described above, suggested that the glutathione redox state is differentially affected by CR in the two strains of mice. To gain an understanding of the possible underlying mechanisms, activities of selected enzymes involved in glutathione metabolism were examined in the liver because it is the main site of the trans-sulfuration pathway [16] and the de novo synthesis of GSH, which is then released into the plasma for translocation to other tissues [17]. Alterations in hepatic GSH metabolism can have a generalized impact on the redox state of extra-hepatic tissues [11]. Accordingly, activities of glutathione peroxidase (GPX), glutathione reductase (GR) and glutamyl cysteine ligase (GCL), which play a direct role in glutathione biosynthesis or oxidation/reduction, were compared between the two strains of mice at 6-months of age under the AL conditions and at 24-months in mice kept under the AL regimen or 40% CR regimen initiated at 4 months of age (Fig. 4). GPx activity utilizes the reductive capacity of GSH to convert hydrogen peroxide to water while simultaneously generating GSSG. There were no notable differences in GPx activity between the two strains at 6- or 24- months of age under the AL regimen; however, the activity was 22-38% higher in the 24-month-old CR mice of both strains than in their age-matched AL counterparts, with C57/BL6 mice showing a 73% greater CR-related increase than the DBA/2 mice. GR activity converts GSSG to GSH using reducing equivalents supplied by NADPH. It was found to increase with age as well as in response to CR in both strains; the activity was 30% higher in the DBA/2 than in C57/BL6 mice at both ages under the AL regimen, and 36% higher than C57BL/6 under the CR regimen.
Fig. 4.
Effects of age and CR on activity of the enzymes GPX (total) (A), GR (B), and GCL (C) in liver homogenates of C57BL/6 and DBA/2 mice. Data represent the mean ± SE of 3-4 mice. (* p < 0.05 for planned comparisons of CR with AL of the same strain; † for comparison of C57BL/6 to DBA/2 of matching diet and age).
GCL is the rate-limiting enzyme in de novo GSH biosynthesis. Its activity exhibited a notable pattern of variations with respect to strain, age, and dietary intake. Under the AL regimen, GCL activity was 70% higher in C57/BL6 than in DBA/2 mice at 6- and 24-months of age. During this period, the activity of GCL increased ~ 2-fold in both strains. Notably, a comparison between the 24-month-old mice, kept under AL or CR regimens, indicated that CR resulted in a 32% increase in GCL activity in C57/BL6 mice, whose life span is extended by CR, but there was no discernable effect in the DBA/2 mice, in which CR has no impact on longevity. Analyses of variance on the data for activity of each of the enzymes indicated a significant main effect of both Strain and Diet (all p<0.031).
Discussion
Results of this study indicated that: (i) in the skeletal muscle and liver of AL-fed mice of both strains, the steady-state amounts of GSSG and Pr-SSG increased and the GSH:GSSG ratios declined as a function of age, suggesting an age-related pro-oxidizing shift in the thiol redox state; (ii) long-term (19 months) CR prevented the age-related increases in the amounts of GSSG and Pr-SSG and the decline in GSH:GSSG ratio in C57BL/6 mice, whose life span is extended by CR, but not in the DBA/2 mice, in which CR has no effect on longevity; and (iii) even relatively short-term (7 weeks) CR also reversed the age-related shift in the glutathione redox state in the erythrocytes in C57BL/6, but not the DBA/2 mice. Furthermore, CR was found to elevate the hepatic GCL activity in the former and not the latter strain
The reason why CR affects the longevity of one particular genotype and not the other is presently unknown, albeit, it has been widely postulated that life span extension by CR may be due to the attenuation of oxidative stress [6]. Although the biochemical manifestations of oxidative stress remain to be clearly defined, one view is that disturbance in the thiol redox homeostasis and the consequent disarray in the signaling networks, rather than the amount of structural damage, is a more relevant indicator of oxidative stress [18-20]. Broadly, the ostensible mechanism is that under physiological conditions, molecules such as H2O2 may react with protein thiolate anions at the active site of certain proteins [21-24], to form cysteinyl sulfenic acid and cause inactivation of the protein activity (reaction 1) [25-26]. Sulfenic acid may react with GSH or a protein sulfhydryl (Pr-SH) generating a mixed disulfide with glutathione (Pr-SSG) or a disulfide bridge (reactions 2, 3), respectively [27-29]. Under conditions of oxidative stress, protein thiols may be progressively hyper-oxidized, forming sulfenic- (-SOH), sulfinic- (-SO2H) and sulfonic acids (-SO3H); the latter two products are deemed to be irreversible [30-31]. The severity of protein cysteinyl thiolate oxidation depends upon the strength (concentration) and the duration of exposure to ROS (H2O2) [32]. Increased H2O2 production may also lead to an elevation in the level of GSSG partly due to the oxidation of GSH during H2O2 reduction by glutathione peroxidase. In turn, GSSG can react with cysteinyl thiolate residues to form Pr-SSG (reaction 4).
| (reaction 1) |
| (reaction 2) |
| (reaction 3) |
| (reaction 4) |
Thus, elevations in the steady-state amounts of GSSG and mixed disulfides of proteins with glutathione may be considered to be important indicators of oxidative stress, emanating from increases in the mitochondrial production of H2O2.
Results of the present study indicated that GSH levels in the skeletal muscle and liver of AL-fed aged mice of both strains remained relatively unaltered as a function of age, whereas GSSG and Pr-SSG amounts increased markedly, resulting in the age-related declines in GSH:GSSG ratios. However, the effect of CR on GSSG and Pr-SSG amounts differed in the two strains of mice. While CR decreased the GSSG and Pr-SSG levels in both tissues of the C57BL/6, it had no effect in the DBA/2 mice. Thus, the ability of the CR regimen to retard or prevent the accumulation of GSSG and Pr-SSG was encountered in the mouse genotype, C57BL6, whose life span is extendable by CR and not in the genotype, DBA/2, where it is not.
A major difference in the response of skeletal muscle and liver to CR was that CR had no effect on the amount of GSH in the skeletal muscle of either mouse strain, while it enhanced GSH level in the liver; albeit this effect was observed only at 24 months of age and the magnitude of the increase was greater in the DBA/2 than C57BL/6 mice. Because CR elevated GSH level in the DBA/2 and this increase was even higher than in the C57BL/6 mice and because CR extends the life span of the latter and not the former genotype, it can be inferred that CR-related increase in GSH amounts is not associated with an extension of life span. Thus, the results demonstrate that the life span prolongation effect of CR, observed in the C57BL/6 but not DBA/2 mice, is associated with the ability of CR to counteract the accumulation of GSSG and Pr-SSG, which can potentially affect the redox-sensitive functional activities of the proteins [20,33,34]. Nevertheless, such correlations are insufficient to establish a cause-and effect relationship between redox state and life span extension by CR.
Though genetically quite dissimilar, the two strains of mice examined here were relatively matched in terms of their median life span under ad libitum conditions (C57BL/6, 26.3 mo; DBA/2, 25.1 mo) [4]. The body weights of the two strains were also similar at 4 months when CR was implemented. In spite of these similarities, the strains exhibit a number of physiological differences that may directly or indirectly have an impact on the redox state. For instance, the amounts of GSH in the heart, liver and skeletal muscle, as well as catalase activity in heart and skeletal muscles, were significantly higher in C57BL/6 than DBA/2 mice [35], suggesting that the former may be relatively more efficient in the elimination of H2O2. The resting rate of oxygen consumption was also lower in the C57BL/6 than DBA/2 mice [3,36]. Another notable difference was that while the two strains consumed the same amount of food throughout adult life, the C57BL/6 gained 20% more body weight and had 160% greater body fat by 24 months of age than the DBA/2, which showed no net gain in body weight or fat during the same period [3,36]. Increases in body weight and adiposity have been shown to elevate the rate of mitochondrial production of H2O2 as well as the level of pro-inflammatory cytokines that are known to exacerbate oxidative stress [37]. Thus, it seems that the attenuation of pro-oxidant changes resulting from CR are observed in the mouse genotype (C57BL/6) that has a relatively low metabolic rate and a propensity to gain body weight when fed ad libitum.
It is widely recognized that the mechanisms involved in the maintenance of the redox state are highly complex, entailing an inter-play between diverse arrays of pro-oxidants and anti-oxidants. The possible involvement of enzymes associated with glutathione metabolism and redox state was explored in the present study. In the DBA/2 mice, CR enhanced the activities of GPx and GR, but not of GCL, whereas in the C57BL/6 mice, it elevated GPx and GCL activities. Thus, elevation of GCL activity by CR occurred in the strain whose life span is also prolonged under the CR regimen. While a cause-and-effect relationship can not be established on the basis of such correlative data, our previous studies on transgenic Drosophila melanogaster have shown that among the various antioxidant enzymes, such as Cu, Zn-SOD, Mn-SOD [38,39], catalase [39,40], intramitochondrial ectopic catalase [41], thioredoxin reductase [39], peroxiredoxins 3 and 5 [42,43] and glucose-6-phosphate dehydrogenase [44], the over-expression of GCL resulted in the most robust extension of life span of these flies [45]. Similarly, it has been experimentally demonstrated that survival under oxidative stress depends more strongly on GCL activity than the steady-state levels of GSH [46,47]. It is well recognized that GCL activity is usually elevated by conditions that increase the level of oxidative stress [48,49]. It is thus intriguing that CR, a regimen known to attenuate oxidative stress, was found here to elevate the activity of GCL. Studies by Langston et al. [50] suggest the existence of a mechanism in which, under conditions of low glucose concentration, a stimulated increase in the insulin levels results in the upregulation of GCL via Nrf2. Recently, Hempenstall et al. [51] reported that CR markedly increases the fasting glucose level in DBA/2, but decreases it in the C57BL/6. It is possible that these opposing effects of CR on glucose levels may play a role in the presence or absence of GCL upregulation observed here.
It should be noted that the C57BL/6 mice used in the current study carry a loss-of–function mutation in the nicotinamide nucleotide transhydrogenase gene (Nnt) that confers a moderate impairment in glucose homeostasis in vivo and an increase in glucose-stimulated production of ROS [52]. It is thus conceivable that the biochemical responses to CR of C57BL/6J mice in these studies could be affected by the mutant Nnt, and will need to be confirmed in C57BL/6 that carry the wild-type allele. Nevertheless, based upon a literature survey of studies in C57BL/6 mice that did or did not carry mutant Nnt, there are no reports suggesting any effect on the ability of CR to increase longevity [53,54] or to elicit a lowering of oxidative stress in various tissues [10,55].
To conclude, results of this study suggest that the life span extension effect of CR may be associated with the attenuation of age-related pro-oxidant shift in the thiol redox state, indicated by the levels of GSSG and Pr-SSG, albeit the nature of the underlying mechanism remains to be elucidated.
Acknowledgements
This study was supported by the grant R01 AG 13563 from the National Institute on Aging-National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Charles C Thomas; Springfield, IL: 1988. [Google Scholar]
- 2.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Sohal RS, Ferguson M, Sohal BH, Forster MJ. Life span extension in mice by food restriction depends on an energy imbalance. J Nutr. 2009;139:533–539. doi: 10.3945/jn.108.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebrin I, Bayne A-CV, Mockett RJ, Orr WC, Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebrin I, Sohal RS. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Deliv Rev. 2008;60:1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radical Biology and Medicine. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson ME, Bridges RJ, Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980;96:848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- 12.Ritz BW, Aktan I, Nogusa S, Gardner EM. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J Nutr. 2008;138:2269–2275. doi: 10.3945/jn.108.093633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beutler E. Red Cell Metabolism: A Manual of Biochemical Methods. Grune and Stratton; New York: 1971. [Google Scholar]
- 14.Carlberg I, Mannervik B. Glutathione reductase. Methods in Enzymology. 1985;113:485–590. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 15.Toroser D, Sohal RS. Kinetic characteristics of native gamma-glutamylcysteine ligase in the aging housefly, Musca domestica L. Biochemical & Biophysical Research Communications. 2005;326:586–593. doi: 10.1016/j.bbrc.2004.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stipanuk MH. Role of the liver in regulation of body cysteine and taurine levels: a brief review. Neurochem Res. 2004;29:105–110. doi: 10.1023/b:nere.0000010438.40376.c9. [DOI] [PubMed] [Google Scholar]
- 17.Anderson ME, Meister A. Dynamic state of glutathione in blood plasma. J Biol Chem. 1980;255:9530–9533. [PubMed] [Google Scholar]
- 18.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 19.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 20.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 22.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 23.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saurin AT, Neubert H, Brennan JP, Eaton P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc Natl Acad Sci USA. 2004;101:17982–17987. doi: 10.1073/pnas.0404762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carballal S, Alvarez B, Turell L, Botti H, Freeman BA, Radi R. Sulfenic acid in human serum albumin. Amino Acids. 2007;32:543–551. doi: 10.1007/s00726-006-0430-y. [DOI] [PubMed] [Google Scholar]
- 27.Gitler C, Zarmi B, Kalef E. General method to identify and enrich vicinal thiol proteins present in intact cells in the oxidized, disulfide state. Anal Biochem. 1997;252:48–55. doi: 10.1006/abio.1997.2294. [DOI] [PubMed] [Google Scholar]
- 28.Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 2004;287:C246–256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- 29.Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- 30.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteinesulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 31.Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal. 2009;11:2655–2671. doi: 10.1089/ars.2009.2728. [DOI] [PubMed] [Google Scholar]
- 32.Maller C, Schroder E, Eaton P. Glyceraldehyde 3-phosphate dehydrogenase is unlikely to mediate hydrogen peroxide signaling: studies with a novel anti-dimedone sulfenic acid antibody. Antioxid Redox Signal. 2010;14:49–60. doi: 10.1089/ars.2010.3149. [DOI] [PubMed] [Google Scholar]
- 33.Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 34.Fratelli M, Goodwin LO, Orom UA, Lombardi S, Tonelli R, Mengozzi M, Ghezzi P. Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc Natl Acad Sci USA. 2005;102:13998–14003. doi: 10.1073/pnas.0504398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferguson M, Rebrin I, Forster MJ, Sohal RS. Comparison of metabolic rate and oxidative stress between two different strains of mice with varying response to caloric restriction. Exp Gerontol. 2008;43:757–763. doi: 10.1016/j.exger.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson M, Sohal BH, Forster MJ, Sohal RS. Effect of long-term caloric restriction on oxygen consumption and body temperature in two different strains of mice. Mech Ageing Dev. 2007;128:539–545. doi: 10.1016/j.mad.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 38.Mockett RJ, Orr WC, Rahmandar JJ, Benes JJ, Radyuk SN, Klichko VI, Sohal RS. Overexpression of Mn-containing superoxide dismutase in transgenic Drosophila melanogaster. Arch Biochem Biophys. 1999;371:260–269. doi: 10.1006/abbi.1999.1460. [DOI] [PubMed] [Google Scholar]
- 39.Orr WC, Mockett RJ, Benes JJ, Sohal RS. Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster. J Biol Chem. 2003;278:26418–26422. doi: 10.1074/jbc.M303095200. [DOI] [PubMed] [Google Scholar]
- 40.Bayne AC, Mockett RJ, Orr WC, Sohal RS. Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and aging in transgenic Drosophila. Biochem J. 2005;391:277–284. doi: 10.1042/BJ20041872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mockett RJ, Bayne AC, Kwong LK, Orr WC, Sohal RS. Ectopic expression of catalase in Drosophila mitochondria increases stress resistance but not longevity. Free Radic Biol Med. 2003;34:207–217. doi: 10.1016/s0891-5849(02)01190-5. [DOI] [PubMed] [Google Scholar]
- 42.Radyuk SN, Michalak K, Klichko VI, Benes J, Rebrin I, Sohal RS, Orr WC. Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem J. 2009;419:437–445. doi: 10.1042/BJ20082003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radyuk SN, Rebrin I, Klichko VI, Sohal BH, Michalak K, Benes J, Sohal RS, Orr WC. Mitochondrial peroxiredoxins are critical for the maintenance of redox state and the survival of adult Drosophila. Free Radic Biol Med. 2010;49:1892–1902. doi: 10.1016/j.freeradbiomed.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Legan SK, Rebrin I, Mockett RJ, Radyuk SN, Klichko VI, Sohal RS, Orr WC. Overexpression of glucose-6-phosphate dehydrogenase extends the life span of Drosophila melanogaster. J Biol Chem. 2008;283:32492–32499. doi: 10.1074/jbc.M805832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orr WC, Radyuk SN, Prabhudesai L, Toroser D, Benes JJ, Luchak JM, Mockett RJ, Rebrin I, Hubbard JG, Sohal RS. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J Biol Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- 46.Moore WR, Anderson ME, Meister A, Murata K, Kimura A. Increased capacity for glutathione synthesis enhances resistance to radiation in Escherichia coli: a possible model for mammalian cell protection. Proc Natl Acad Sci USA. 1989;86:1461–1464. doi: 10.1073/pnas.86.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA. 1992;89:3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krejsa CM, Franklin CC, White CC, Ledbetter JA, Schieven GL, Kavanagh TJ. Rapid activation of glutamate cysteine ligase following oxidative stress. J Biol Chem. 2010;285:16116–16124. doi: 10.1074/jbc.M110.116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langston W, Circu ML, Aw TY. Insulin stimulation of gamma-glutamylcysteine ligase catalytic subunit expression increases endothelial GSH during oxidative stress: influence of low glucose. Free Radic Biol Med. 2008;45:1591–1599. doi: 10.1016/j.freeradbiomed.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hempenstall S, Picchio L, Mitchell SE, Speakman JR, Selman C. The impact of acute caloric restriction on the metabolic phenotype in male C57BL/6 and DBA/2 mice. Mech Ageing Dev. 2010;131:111–118. doi: 10.1016/j.mad.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Freeman H, Shimomura K, Cox RD, Ashcroft FM. Nicotinamide nucleotide transhydrogenase: a link between insulin secretion, glucose metabolism and oxidative stress. Biochem Soc Trans. 2006;34:806–810. doi: 10.1042/BST0340806. [DOI] [PubMed] [Google Scholar]
- 53.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 54.Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- 55.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]