Abstract
Background & Aims
Regulatory T (Treg) cells (CD4+ CD25high FoxP3+) regulate mucosal tolerance; their adoptive transfer prevents or reduces symptoms of colitis in mouse models of inflammatory bowel disease (IBD). T-cell functions are regulated by mesenchymal cells. Colonic CD90+ mesenchymal myofibroblasts and fibroblasts (CMFs) are abundant, non-professional antigen presenting cells in the normal human colonic mucosa that suppress proliferation of activated CD4+ effector T cells. We studied CMF suppressive capacity and evaluated the ability of CMF to induce Treg cells.
Methods
Allogeneic co-cultures of CD4+ T cells and CMFs, derived from normal mucosa of patients undergoing colectomy for colon cancer or inflamed colonic tissues from patients with ulcerative colitis or Crohn’s disease, were used to assess activation of the Treg cells.
Results
Co-culture of normal CMF with resting or naive CD4+ T cells led to development of cells with a Treg phenotype; it also induced proliferation of a CD25+ CD127− FoxP3+ T cells, which expressed CTLA-4, interleukin (IL)-10, and transforming growth factor-β and had suppressive activities. In contrast to dendritic cells, normal CMFs required exogenous IL-2 to induce proliferation of naturally occurring Treg cells. Induction of Treg cells in normal CMFs required MHC class II and prostaglandin E2. CMFs from patients with IBDs had reduced capacity to induce active Treg cells and increased capacity to transiently generate CD4+CD25+/− CD127+ T cells that express low levels of FoxP3.
Conclusions
CMFs suppress the immune response in normal colon tissue and might therefore help maintain colonic mucosal tolerance. Alterations in CMF induction of Treg cells might promote pathogenesis of IBDs.
Keywords: inflammation, TGF, immune regulation, immune response
Introduction
Gut immune responses are normally regulated to maintain a state of mucosal tolerance, which represents a balance between the need to mount protective immunity toward pathogens while not activating damaging inflammatory responses to innocuous luminal antigens (Ag)1. Recent studies suggested that a functionally distinct subset of regulatory T cells, CD4+ CD25high FoxP3+ cells (Treg), is actively involved in the maintenance of immunological tolerance in the gastro-intestinal (GI) tract2–3.
There are at least two types of the Tregs: naturally occurring (nTreg) and inducible (iTreg). nTreg cells develop in the thymus and the majority expresses high levels of cell surface markers associated with an activated/memory T cell phenotype2–4. iTreg cells are elicited in the periphery from naïve T cells under low-dose antigenic stimulation or by several cytokines, although the exact mechanism(s) of iTreg cell induction remains unknown4. Forkhead transcription factor 3, Foxp3, is a master switch that controls Treg cell development and function. Both types of Tregs down regulate immune responses to foreign and self-Ag, and contribute to the suppression of autoimmune disorders3–5. Treg exert their effects by multiple mechanisms (e.g., cytokine deprivation, CTLA-4 signaling, IL-10 or TGB-β-production)4–5. It is not established which of these mechanisms is predominant, but each might have a greater importance under specific physiological/pathological conditions.
Recent studies suggest that Treg cells play a major role in murine models of inflammatory bowel disease (IBD). Adoptive transfer of CD4+CD45RBhigh T cells depleted of the Treg cells into immunodeficient mice resulted in development of IBD-like chronic colitis7–8. In contrast, transfer of Treg cells into animals with chronic colitis led to the amelioration of the disease7–8. Adecrease in Treg number in active IBD in humans has been documented9. Despite advances in understanding the role of the Treg cells in the maintenance of peripheral tolerance in the intestinal mucosa, the mechanisms involved in their regulation in the intestine and what factors contribute to the alteration of the Treg numbers in IBD remains unclear. It has been recently reported that small intestinal GALT associated CD103+ dendritic cells (DCs), as well as small bowel epithelial cells can induce Tregs10–11. However, the origin and possibility of the expansion of the Tregs in the colon remain obscure.
We recently reported that colonic myofibroblasts/fibroblasts (CMFs) are novel non professional APCs and are abundant in the normal human colonic mucosa12. CMFs are a distinct population of mesenchymal stromal cells that are positive for CD90. Activated CMFs express α-smooth muscle actin (α-SMA, myofibroblast marker), but are negative for other cell surface markers that define conventional APCs. CMFs form a network throughout the colonic lamina propria and have been implicated in the regulation of mucosal inflammation12–16. Recent data from our laboratory indicates that CMFs may have a dual regulatory role on CD4+ T cell activity. They induce proliferation of resting CD4+ T cells in a MHC class II-dependent manner12, but suppressing proliferation of activated CD4+ effector T cells via mechanisms involving the B7-related co-inhibitors PD-L1 and PD-L215. Importantly, it has been recently reported that intestinal myofibroblasts taken from chronically inflamed tissues display a fundamentally altered phenotype compared to their counterparts extracted from normal tissues at the same anatomical site13,16. CMFs are actively involved in the progression of the IBD associated inflammation via altered deposition of matrix, upregulation of proinflammatory cytokine production, and changes in the array of secreted, soluble immunoregulatory molecules and mediators13,16.
In this study, we have further analyzed interactions between CMFs and CD4+ T cells to determine if CMFs stimulate expansion of the Treg cells. We found that CMFs contribute to the maintenance of FoxP3+ phenotype of the nTreg and induce generation of iTreg cells from naïve CD4+ T cells via mechanisms that involve MHC class II and PGE2 signals. Moreover, we demonstrated that IBD-derived CMFs, when compared to normal CMFs, have a decreased ability to induce cells bearing the Treg phenotype. Thus, our data suggests that CMFs might play a prominent role in mucosal tolerance via regulation of the Treg number, and disruption of CMF mediated regulation of the Treg may contribute to IBD progression.
Materials & Methods
Antibodies and Reagents
Please see Supplemental information online at www.gastrojournal.org
Human colonic tissue and primary CMF culture
For CMF isolation, fresh human colonic mucosal sections were obtained from discarded surgical tissue in compliance with protocols approved by the UTMB Institutional Review Board. Areas of uninvolved colonic tissue from patients undergoing colectomy for colon cancer were used as the source for normal CMFs (N-CMFs). CMFs were also isolated from inflamed colonic tissues from patients with ulcerative colitis (UC) or Crohn’s disease (CD) undergoing colonic resections for IBD (IBD-CMFs, CD-CMFs or UC-CMFs). Primary cultures of CMFs were generated according to the method described by Mahida et al.17 and routinely used in our laboratory as previously described12,15–16.
Generation of human DCs from peripheral blood mononuclear cells
Please see Supplemental information online at www.gastrojournal.org
CMF: T cell allogeneic cocultures
Unless otherwise indicated, peripheral blood mononuclear cells (PBMC) were prepared from the blood of healthy donors by density gradient centrifugation over Ficoll-Paque™ Plus according to the manufacturer’s instructions. Naïve, resting and regulatory human CD4+ T cells were purified from these PBMC using commercially available kits that negatively select CD4+ CD45RA+ T cell, human CD4+ resting T cell and positively select CD4+ CD25+ regulatory T cells, respectively (Miltenyi Biotec., Auburn, CA). The purity of isolated T cells (>98%) was confirmed by flow cytometry. CMF primary cultures were stimulated with IFN-γ (100 U/ml) for 7 days prior to study in order to induce optimal MHC class II expression as previously described12. Cells were then rested for at least 24 h prior to use in the experiments. CMFs or freshly generated DC (obtained as described above) were cocultured with allogeneic T cells at a ratio 10:1, respectively, and incubated for 5–7 days at 37°C with 5% CO2. In some CFSE proliferation experiments, human recombinant IL-2 (eBioscience) was added to the CMFs: T co-cultures at a concentration of 10 ng/mL.
Flow cytometry
Please see Supplemental information online at www.gastrojournal.org
IL-10, TGF-β and PGE2 ELISA
Conditioned media were collected from the CMF: T cell, CMF or T cell culture wells at 24h, 5 and 7 days and analyzed for the production of the IL-10 and TGF-β using ELISA kits (BD Bioscience) and PGE2 using a R&D Systems kit, according to the manufacturer’s instructions.
Real Time RT-PCR
The real time RT-PCR was carried out as described previously15. Please see Supplemental information online at www.gastrojournal.org.
CFSE proliferation assays
Please see Supplemental information online at www.gastrojournal.org
T Cell Suppression Assays
The T cell suppression experiments were performed as described previously24. For details, please see Supplemental information online at www.gastrojournal.org.
Statistical analysis
Unless otherwise indicated, the results were expressed as the mean ± SE of data obtained from at least three independent experiments done with triplicate sets in each experiment. Differences between means were evaluated by ANOVA using Student’s t-test for multiple comparisons. Values of P <0.05 were considered statistically significant.
Results
CMFs stabilize FoxP3 expression in nTreg and induce their proliferation in presence of IL-2
We showed previously that CMFs induce proliferation of resting CD4+ T cells isolated from peripheral blood 12, which is also known to contain CD4+ CD25high FoxP3+ nTreg cells (nTreg). Thus, we investigated the interaction of the Treg and CMFs isolated from normal colonic mucosa (N-CMFs).
Previously, we reported that, in culture, MHC class II expression by CMF drastically decreases when compare to that on acutely isolated cells and the high levels demonstrated in situ12. Thus, in all experiments primary CMFs were stimulated with IFN-γ (100 U/ml) prior to use in order to restore optimal MHC class II expression as described in the Methods. Theability of N-CMFS to induce generation of Treg in seven day allogeneic co-cultures of the CMFs with CFSE-labeled resting CD4+ T cells were studied. A significant increase in the percentage of the CD25highFoxP3+ T cells in the dividing fraction of CD4+ T cells co-cultured with N-CMFs was observed (Gate P3, Figure 1A) and represented ~31.4 ± 5.8 % of the dividing T cells (Figure S1, see supplement online at www.gastrojournal.org). This coincides with increased expression of the suppressive cytokines IL-10 and TGF-β1 by T cells co-cultured with N-CMFs (Figure 1B). The majority of proliferating CD4+CD25high T cells derived from CMFs-T cell co-cultures that were positive for FoxP3 did not express CD127, the IL-7 α chain receptor, and, thus, correspond to the true Treg phenotype (Figure 1B). A moderate increase in of the FoxP3+CD127+ T cell fraction corresponding to the FoxP3 transiently expressing CD4+ effector T cells was also noted in the CMF-T cell co-cultures (Figure 1B). In contrast to Treg cells the expression of FoxP3 by T effector cells reported to be low, and was not sufficient to suppress expression of CD127 maker and increase the production of suppressive cytokines produced by the Treg18.
Figure 1.
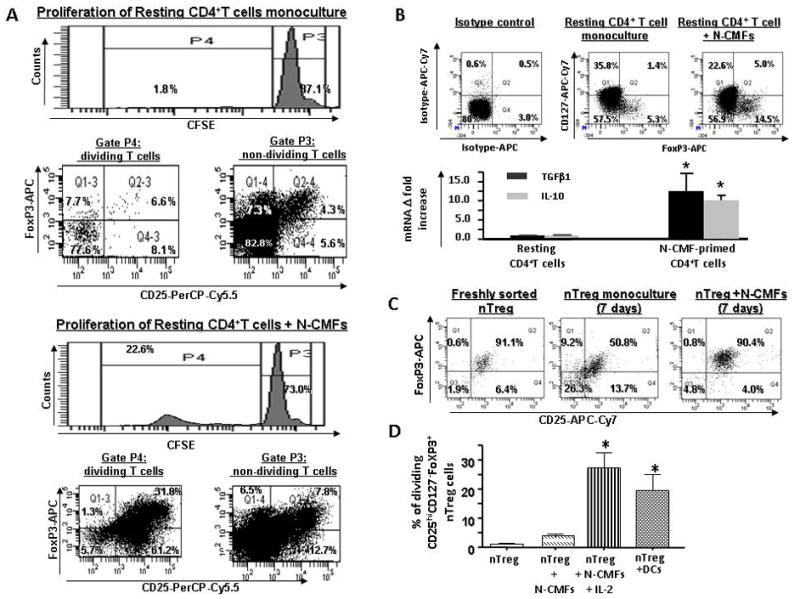
Normal (N) CMFs contribute to the maintenance of nTreg phenotype. CFSE-labeled resting CD4+ T cells were cultured without or with allogeneic N-CMFs at a ratio 1:10 for 7 days in 24 well plates. T cell from these co-cultures were subjected to surface CD4 and CD25, CD127 and intracellular FoxP3 staining following by flow cytometry. (A) Live events were gated in P1 and percentage of CD25+FoxP3+ T cells in non dividing (P3) and dividing (P4) fractions of CD4+ resting T cells growing in monoculture or cocultured with N-CMFs was evaluated. A representative experiment is shown (n=5 allogeneic donor pair, two experimental replicate each) (B) Distribution of CD127 and FoxP3 (FACS analysis) and expression of TGFβ1 and IL-10 (real-time RT-PCR) in the CD4+ resting T cells growing in monoculture or cocultured with N-CMFs was evaluated. A representative experiment is shown (n=4 allogeneic donor pair, two experiment replicate each) (D) The surface CD25 and intracellular FoxP3 expression in the freshly isolated and seven day monoculture of nTreg, and N-CMF primed nTreg was analyzed. A representative experiment is shown (n=5 allogeneic donor pair, two experiment replicate each). (D) Percentage of the dividing CD4+CD25highCd127−FoxP3+ nTreg in response to the allogeneic stimulation with N-CMF in presence/absence of IL-2 (10 ng/mL) or BM-derived DC was measured in 7 day co-culture as described above. Results are calculated as the mean value of the percentage of the dividing cells for three independent allogeneic pair of N-CMFs and CD4+ T cells healthy donors ± SD. Each assay was conducted in duplicate. * p < 0.05.
Next, we analyzed how N-CMFs affect FoxP3 expression and proliferation of nTreg purified from peripheral mononuclear cells. When purified nTreg were cultured alone, their FoxP3 expression was reduced, whereas those in co-culture with N-CMFs maintained FoxP3 expression (Figure 1C). Analysis of purified nTreg induced by CMFs demonstrated that, in contrast to classical APCs such as BM-derived DCs, co-culturing of N-CMFs with nTreg did not induce significant proliferation of nTreg cells (Figure 1D). IL-2 is reported to be essential for the physiological expansion of nTreg in humans and rodents19–20. Thus, we analyzed whether addition of IL-2 to the N-CMFs-nTreg co-cultures resulted in proliferation of the nTreg. Figure 1D demonstrates that addition of IL-2 to these co-cultures resulted in strong proliferation of the nTreg comparable to that induced by BM-derived DCs.
CMFs induce generation of iTreg cells from naïve CD4+ CD45RA+ T cells
Next, we sought to determine the capacity of CMFs to generate iTreg cells from naïve CD4+ CD45RA+ T (Th0) cells. Allogeneic Th0 cells were incubated with MHC class II expressing CMFs at ratios of 10:1 for up to 12 days. A significant increase in the expression of FoxP3 mRNA was observed at day seven of the co-cultures (Figure 2A) and in the frequency of the iTreg cells (Figure 2B). The percentage of the induction of FoxP3+ T cells in CMF primed Th0 cells (10.2±3.6%) was comparable to that in the cocultures with DCs (12.3 +− 3.9 %) (Figure 2B). The iTregs generated from CMFs-primed Th0 cells did not express surface CD127 (Figure 3A). Since downregulation of the CD127 is associated with acquisition of regulatory function by T cells and inversely correlated with FoxP3 expression21–23, our data suggests that normal CMFs can contribute to the generation of the iTreg from naive CD4+ T cells at a capacity comparable to that of classical APCs, such as DC.
Figure 2.
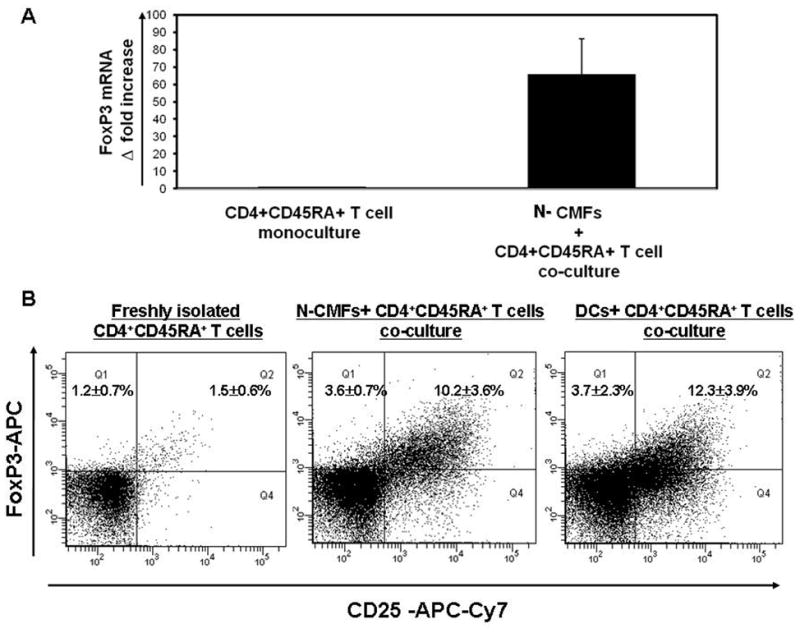
CD4+ CD25high FoxP3+ Treg cells (iTreg) are induced from naive CD4+CD45RA+ T helper (Th0) cells primed with N-CMFs. Th0 cells were cultured without or with allogeneic N-CMFs at a ratio 1:10 for one week. (A) The RNA from the N-CMF primed Th0 cells was analyzed for FoxP3 mRNA expression using real time RT-PCR. The mRNA levels for FoxP3 was normalized to 18S. Data represent mean of mRNA Δ fold increase ± SEfrom duplicates in four experiments (n=8) *P<0.01. (B) The surface CD25 and intracellular FoxP3 expression in the allogeneic N-CMF or BM-derived DC primed CD4+ T cells was analyzed using flow cytometry.. The appropriate isotype controls were included in the experiments. A representative experiment is shown (n=5). *p<0.01.
Figure 3.
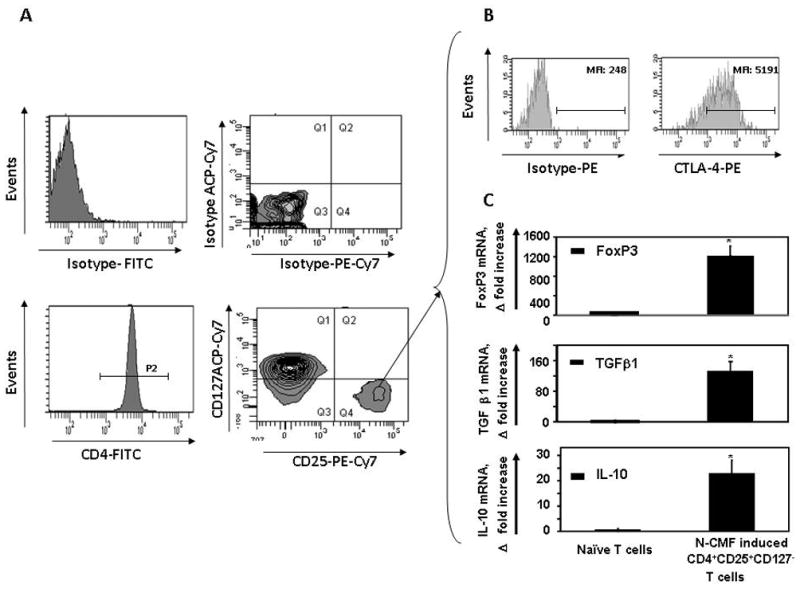
CD4+ CD25high FoxP3+ iTreg induced by allogeneic N-CMFs exhibit an activated phenotype. Th0 cells were cultured with allogeneic N-CMFs at ratio 1:10. Harvested T cells (day 7) were stained with anti-CD4 FITC, anti-CTLA-PE, anti-CD25PE-Cy7 and anti-CD127 mAbs or appropriate isotype controls. Live events were gated in P1, then (A) CD4+ T cells were gated in P2 and analyzed for CD25 and CD127 expression. The CD4+ T cells bearing CD25+CD127− phenotype (Q4 quadrant) were (B) analyzed for CTLA-4 expression and sorted for further analysis. A representative experiment is shown (n=5). The RNA was extracted from sorted CD4+ CD25+CD127− T cells was analyzed for (C) FoxP3, TGF-β1 and IL10 mRNA expression using real time RT-PCR. The mRNA levels for each gene of interest were normalized to 18S. Data represent mRNA Δ fold increase mean ± standard errors from duplicates in five experiments (n=10) *p<0.01.
The Treg cells induced by CMFs exhibit an activated phenotype
Increased expression of CTLA-4 is associated with an activated phenotype of Treg cells and is involved in Treg suppressive function4–5. Thus, we analyzed whether CMF-induced iTreg cells express CTLA-4. The CD4+ CD25high CD127− T cells sorted from the CMFs:Th0 cell co-cultures. (Figure 3A) demonstrated that the cells express both intracellular CTLA-4 (Figure 3B) and FoxP3 mRNA (Figure 3C). Since production of IL-10 and TGF-β1 by Treg cells is central to their suppressive function4–5, expression of these cytokines was measured by real-time RT-PCR in CD4+ CD25high CD127− T cells (iTreg) sorted from the seven day Th0:CMFs cell co-cultures. CMF-induced iTreg cells express moderate levels of the IL-10 and high levels of TGF-β1 (Figure 3C), but not TGF-β2 and TGF-β3 (data not shown). Taken together these results indicate that the CMF-induced iTreg cells have the elements needed to exert suppressive function.
The iTreg cells induced by CMFs inhibit activated T effector cell proliferation
The main function of Treg cells is to negatively regulate responses of immune cells, including activated T effector cells2,4–5. Thus, we analyzed the ability of CMF-induced Treg to suppress proliferation of CD3/CD28 preactivated syngeneic CD45RA+CD4+ naïve T cells (T effector, Teff). T cells from the seven day CMF:Th0 cell cocultures were sorted into CD4+ CD25high and CD4+CD25low populations (Figure 4A). These populations were incubated separately for 96 h with syngeneic, preactivated Teff in various Treg:Teff ratios. The CMF-induced CD4+ CD25high T cells induced a significant (60%) decrease in the proliferation of preactivated CD45RA+CD4+ naïve T helper cells (Figure 4B) at a 1: 8 Treg:Teff ratio, which was comparable with the percentage of suppression by circulating CD4+ CD25high T cells isolated from PBMC of the same donors (Figure 4C). Thus, our data demonstrated that CMFs induce functionally active iTreg. Surprisingly, a minor level of suppression by CMF-induced CD4+ CD25low T cell fraction has been also observed (Figure 4C), perhaps due to the production of the immunoregulatory cytokines by CD4+CD25low T cell fraction sorted from CMF-CD4+ T cell co-cultures.
Figure 4.
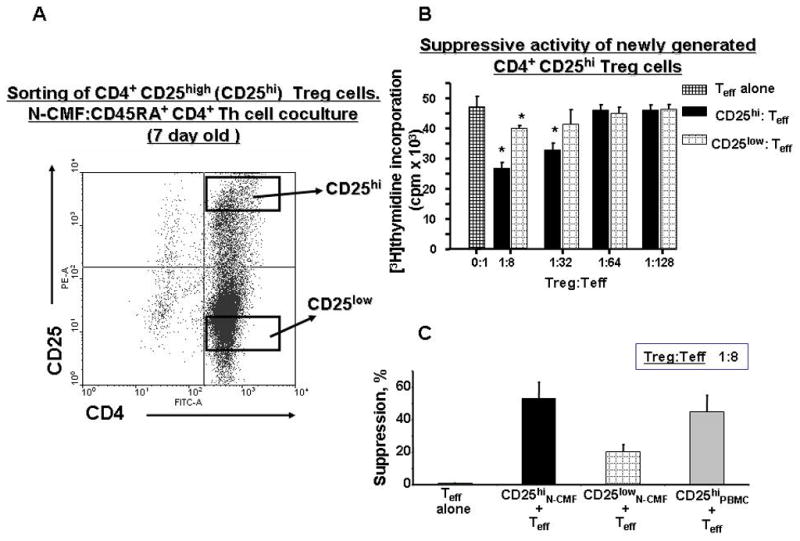
CD4+CD25high iTreg cells induced by N-CMFs suppress proliferation of syngeneic, activated CD4+ T effector cells. (A) CD4+CD25high T cells induced by co-culturing of Th0 with CMFs for 7 days were sorted by FACSAria sorter and CD4+CD25high or CD4+CD25−T cells were co-cultured with CD2/CD3/CD28-activated CD45RA+CD4+CD25− effector (Teff) cells for 4 days. Eighteen hours prior to the end of the co-culture, the cells were pulsed with [3H]-methyl-thymidine. (B) Mean counts per minute (c.p.m) ± standard error (SE) of triplicate cultures of CD4+ T cells isolated from one donor are shown here in a representative experiment of three. (C) The percent suppression of Teff is shown and represents a decrease in the proliferative response (c.p.m.) of CD4+ Teff cells when compared to activated Teff cells alone. The means are shown as the results of triplicates in 3 experiments, n=9 (* p < 0.05).
De novo generation of CD4+ CD25high FoxP3+ Treg cells by CMFs is MHC class II- and PGE2- dependent
We have previously shown that MHC class II is essential for CMF-induced resting CD4+ T cell proliferation12. Others have demonstrated that MHC class II-TcR interactions are involved in the development of the FoxP3+ polyclonal Treg11. Thus, we examined the role of MHC class II expression by human CMFs in the induction of iTreg cells. Allogeneic co-cultures of CMFs with Th0 cells were established in the presence of the anti-class II MHC antibodies (anti- HLA-DR, -DQ and -DP cocktail, clones L243 and IVA12). A significant decrease in FoxP3 mRNA expression and CMF induction of iTreg cells in the CMF:Treg cell co-cultures (up to 50%) was observed in the presence of anti-MHC class II Abs, but not isotype controls (Figure 5A–B). The fact that CMF-induced iTregs were only partially reduced in the presence of anti-MHC class II Abs suggested that this induction requires other mediators as well.
Figure 5.
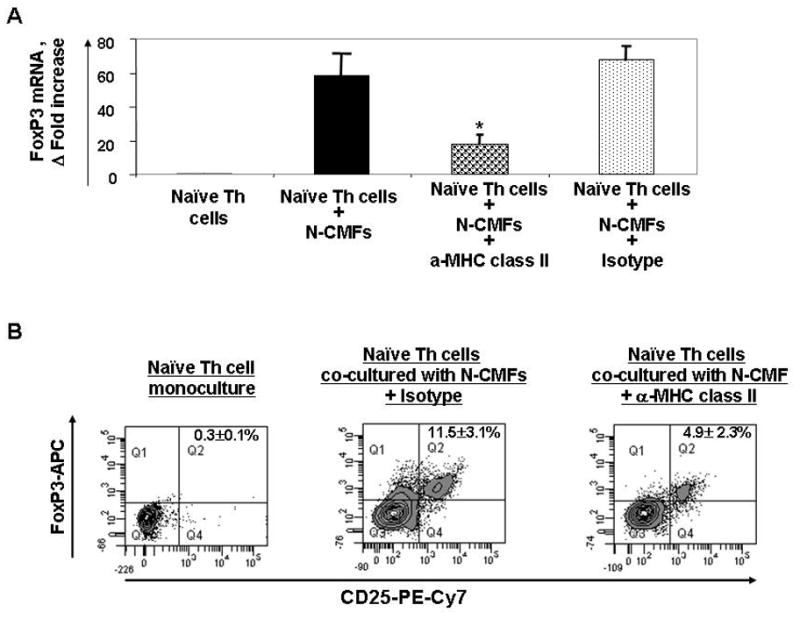
Induction of the iTreg from naïve CD4+CD45RA+ T cells (Th0) by allogeneic N-CMFs depends on MHC class II. T cells were cultured without or with allogeneic CMFs at a ratio 1:10 for 7 days in 24 well plates in presence/absence of anti-MHC class II mAb mix (anti- HLA-DR, -DQ and -DP cocktail, clones L243 and IVA12) or isotype mix control. (A) The RNA from the N-CMF primed T cells was analyzed for FoxP3 mRNA expression by using real time RT-PCR. The mRNA level for FoxP3 was normalized to 18S. Data represent mean of mRNA Δ fold increase ± SEfrom duplicates in three experiments (n=6) *P<0.01. (B) The surface CD25 and intracellular FoxP3 expression in the N-CMF primed T cells was analyzed using flow cytometry. Harvested T cells were stained with anti-CD4 FITC, anti-CD25PE-Cy7, and anti-FoxP3-APC mAbs. The appropriate isotype controls were included in the experiments. A representative experiment is shown (n=5). *p<0.01.
Since it has been demonstrated that peripheral induction of regulatory T cells may involve PGE224 and because CMFs are avid producers of these molecules 16–17, we investigated involvement of this mediator in CMF-induced generation of the Tregs. Addition of a PGE2 synthesis inhibitor indomethacin (INT 10 μM) to the CMF: Th0 cell co-culture decreased in the induction of iTregs (5.92±1.8 %), when compared to 10.86±2.6% in the controls (Figure 6A). Additionally, a strong upregulation of indomethacin-inhibitable PGE2 production was observed in the CMF: naïve T helper cell co-culture (Figure 6B). Moreover, a significant decrease of the FoxP3 mRNA expression in the presence of INT was observed (Figure 6C). Taken together these data suggest the importance of the MHC class II- and PGE2 mediated signals in the colonic induction of the Treg cells.
Figure 6.
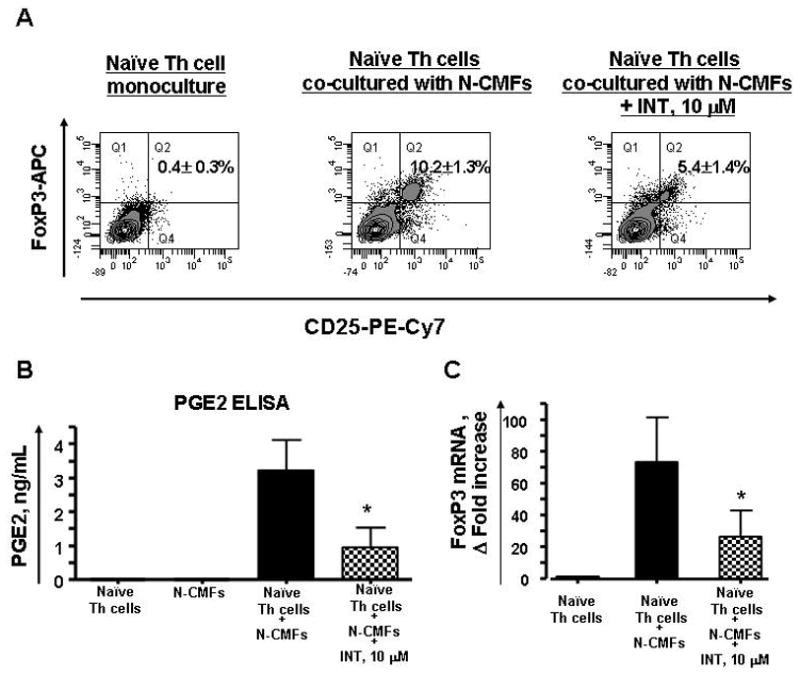
Induction of iTreg from naïve CD4+CD45RA+ T (Th0) cells by allogeneic N-CMFs involves PGE2. T cells were cultured without or with allogeneic N-CMFs at a ratio 1:10 for 7 days in 24 well plates in presence/absence of indomethacin, 10 μM. (A) The surface CD25 and intracellular FoxP3 expression in the allogeneic N-CMF primed T cells was analyzed using flow cytometry. The appropriate isotype controls were included in the experiments. A representative experiment is shown (n=6). *P<0.05. (B) The PGE2 production in culture supernatant was measured using ELISA. Data represent mean ± SEfrom duplicates in three experiments (n=6). (C) The RNA from the N-CMF primed T cells were analyzed for FoxP3 mRNA expression using real time RT-PCR. The mRNA level for FoxP3 was normalized to 18S. Data represent mean of mRNA Δ fold increase ± SEfrom duplicates in three experiments (n=6). * P< 0.05.
IBD-derived CMFs have a decreased capacity to induce Treg cells
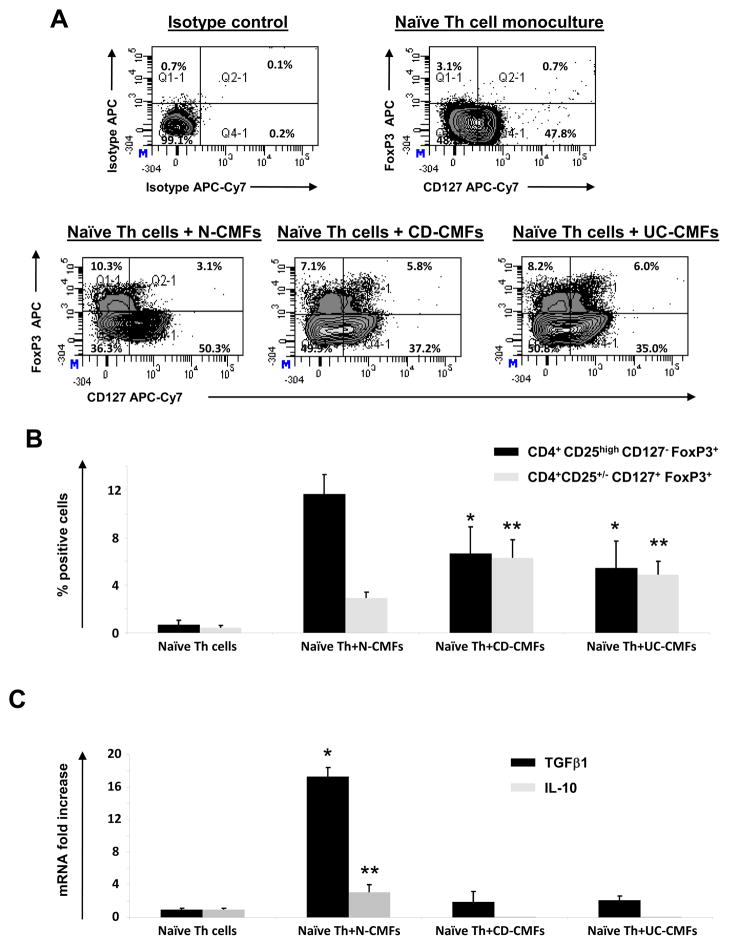
CMFs are important contributors to the progression of IBD and, when isolated from chronically inflamed IBD tissues, they display a fundamentally altered phenotype/function compared with cells extracted from normal tissues13,16. Thus, we compared the capacity of IBD- and N- CMFs to induce a Treg cell phenotype from Th0 cells. Allogeneic Th0 cells were co-cultured with MHC class II expressing CD-, UC- or N-CMFs at a ratio of 10:1 for up to 12 days. No significant difference in the generation of the FoxP3+CD25high cells by CMF derived from IBD vs those derived from normal controls was observed (Figure S2 at www.gastrojournal.org). IL-7 receptor α-chain or CD127 has been shown to be inversely correlated with the suppressive activity of the CD4+CD25high T cells21–22. Moreover, IL-7/IL-7R signaling has been suggested to be implicated in the development and persistence of murine chronic colitis23. Thus, we analyzed whether the combination of CD127 with CD4, FoxP3 and CD25 identified by multi-color flow cytometry analysis would lead to the identification of a difference in the generation of the T reg by CMFs derived from IBD compared to those isolated from normal controls (Figure 7A and B). A decrease in the generation of the CD4+CD127−FoxP3+ T cells and increase CD4+CD127+FoxP3+ T was observed when Th0 cells were primed with IBD-derived CMFs when compare to N-CMFs (Figure 7A). Up to 50% of IBD-induced FoxP3+ T cells were positive for CD127. A significant decrease in the induction of the true T cells bearing the true Treg phenotype was observed (a.k.a. FoxP3+ CD25high, but negative for the CD127) as shown on Figure 7B. Instead we found increased generation of CD4+CD25+/− FoxP3+ cells that were positive for CD127 when Th0 cells were primed by IBD-derived CMFs. T cells derived from the co-culture of IBD-CMFs with Th0 cells had reduced expression of TGF-β1 and did not produce IL-10, cytokines associated with active Treg (Figure 7C). While the mechanisms implicated in this impairment of IBD-derive CMF are currently unknown, our data suggest that in contrast to normal CMFs, IBD-derived CMFs have diminished capacity to induce “true” Treg phenotype generation from Th0 cells.
Figure 7.
IBD-derived CMFs have reduced capacity to induce the Treg cells from CD4+CD45RA+ T helper (Th0) cells and promote the expression of FoxP3 by CD127+ T cells. Th0 cells were cultured without or with allogeneic CMFs at a ratio 1:10 up to 12 days in 24 well plates. (A) The surface CD127 and intracellular FoxP3 expression in the N-, CD or UC-CMF primed CD4+ T cells was analyzed using flow cytometry. The appropriate isotype controls were included in the experiments. A representative experiment is shown (n=9). (B) Distribution of the iTreg (CD4+CD25highCD127−FoxP3+) and transitory FoxP3 expressing cells (CD4+CD25+/−CD127+FoxP3+) in the N-, CD or UC -primed T cells. The results are shown as mean ± standard errors from nine independent experiments. The ANOVA one way variance analysis combined with Bonferroni’s multiple comparison post test was used to analyze the significance of the variation between the experimental groups. *A significant decrease (p<0.05) in the generation of the iTreg (CD4+CD25highCD127−FoxP3+) by CD- and UC-CMFs when compare to N-CMF was observed. **A significant increase (p<0.05) in the generation of the CD4+CD25+/−CD127+FoxP3+ by CD- and UC-CMFs when compare to N-CMF was observed. (C) TGF-β1 and IL-10 mRNA expression by T cells derived from the N-, CD or UC-CMF primed T cells was analyzed using relative quantitative real time RT-PCR. The mRNA levels for TGF-β1 and IL-10 was normalized to 18S. Data represent mean of mRNA Δ fold increase ± SEfrom duplicates in four experiments (n=8). The Bonferroni-corrected ANOVA variance analysis revealed a significant increase in TGF-β1 and IL-10 production when Th0 cells were primed by N-CMFs (*p<0.001 and **p<0.05, respectively), but not when the Th0 cells were primed with CD or UC-CMFs.
Discussion
APCs play a critical role in maintaining the balance between tolerance and inflammation in the gut1,10–12. Role of professional APCs, such as DCs and macrophages, in the differentiation of T cells and regulation of their activity has been well investigated1,10–12,25. Little is known about the role of intestinal stromal cells in these processes. It has been established that stromal cells are important contributors to immune homeostasis and function12–16,26–27. Despite the fact that the term “stromal” encompasses a broad range of stationary cells with various developmental origins, the CD90+ fibroblast-like cells which are replenished in part from bone marrow mesenchymal stem cells form a major stromal component that is ubiquitously found in a close association with T cells in peripheral lymphoid organs, including mesenteric lymph nodes26,27.
We recently reported that human colonic stromal CD90+ myofibroblast/fibroblast cells are abundant in the normal human colonic lamina propria and can act as non professional APCs12. Low level expression of B7.1/2 molecules and relatively higher expression of B7-related co-inhibitor B7-H1 (PD-L1) and B7-DC(PD_L2)15 led us to hypothesize that CMFs are local “suppressors” of activated T cell responses in normal colon contributing to mucosal tolerance. The present study supports our hypothesis and suggests that in the normal colon, CMFs can also indirectly contribute to the suppression of active inflammation by supporting expansion of the Treg cells.
As has been previously reported for the mesenchymal stem cells28 (probable progenitor cells for CMFs), our experiments indicate that N-CMFs may contribute to the maintenance of FoxP3+ phenotype of nTreg. Although the exact mechanisms involved in CMF’s beneficial effect on the maintenance of nTreg phenotype are still to be determined, an effect on both survival and FoxP3 expression stability is likely. Stromal cells are known to produce IL-7, Il-15, retinoic acid and TGFβ16,29 and these mediators contribute to Treg maintenance4,16,29. We demonstrated that CMFs can support nTreg proliferation, but in contrast to professional APCs such as DCs, N-CMFs require the presence of exogenous IL-2. IL-2 is essential for the physiological expansion the nTreg cells in humans and rodents. TCR engagement on the nTreg cells in combination with IL-2 was shown to be sufficient to overcome anergic properties of these cells resulting in the nTreg proliferation19–20,30. Activated CD4+ T effector cells were demonstrated to contribute to the proliferation/survival of nTreg by secreting IL-2 in vitro and in vivo31. Neutralization of the IL-2 reduces nTreg numbers in vivo20.
While this is the first report demonstrating that human normal colonic CD90+ stromal cells support nTreg expansion, it is not without precedent. Other mesenchymal cells such as dermal fibroblasts32 and rheumatoid synovial fibroblasts29 have been shown to induce proliferation of nTreg by an IL-15-dependent mechanism. Further, other members of the fibroblast family such as hepatic stellate cells have been shown to be capable of expanding nTreg when supplemented with IL-233.
Our data indicate that priming of naïve CD4+ T cells with allogeneic N-CMFs can induce generation of iTreg cells at a rate comparable with BM-derived DCs. Although FoxP3 expression is mainly associated with regulatory T cell phenotype, FoxP3 may possibly be transiently expressed by activated CD4+ T cells34. We demonstrate here that FoxP3+ iTreg induced by human CMFs have an activated phenotype with strong expression of CTLA-4, TGF-β1 and IL-10, and are capable of suppressing the proliferation of activated syngeneic effector T cells. In contrast to rodent studies, it has been difficult to convert human peripheral blood derived naïve CD4+ T cells into FoxP3+ iTreg cells with potent and stable suppressive ability; only transient FoxP3 expression in the TCR activated human naïve T cells occurred in the presence of TGFβ135. Further, this type of stimulation was not sufficient to confer to these FoxP3+ cells significant anergic and suppressive capacities. More recently it have been reported that the presence of additional factors, such as trans-retinoic acid, PGE2, PD-L1 signaling, might be required for the generation of active iTreg with suppressive capacity10,24,36–387. All of these factors are known to be produce by human fibroblasts/myofibroblasts14–16. We demonstrate herein that induction of iTreg from N-CMF primed naive CD4+ T cells depends on both cell-contact mediated interactions (MHC class II-TCR signaling) and production of a soluble factor (PGE2). PGE2 reported topromote the conversion of CD4+CD25− cells to Treg cells24. However, to our knowledge, this is the first report suggesting the importance of the CMF-derived PGE2 in the regulation of Treg cells and, consequently, effector T cell behavior in the colon. It is not clear which of the various potential iTreg induction mechanisms is of greater importance under specific physiological conditions, and this will be an important question for future.
It has been suggested that fibroblast-like stromal cells can modify the quality, quantity and duration of inflammatory responses38. The direct immunosuppressive, PD-L1-mediated effect of CMFs has been previously demonstrated by us15. It is still unclear whether PD-L1 or Treg mediated immune suppressive mechanisms predominate during a particular inflammatory event. However, it is likely that disruptions in these anti-inflammatory, immunoregulatory functions may prevent the proper transition from acute to resolving inflammation leading to the establishment of chronic inflammation. There is ample substantiation that intestinal fibroblasts/myofibroblasts taken from the chronic disease tissues display a fundamentally altered phenotype compared with these cells from normal tissues at the same anatomical site: changes in TNF-α, TGFβ and MMP production were identified in the IBD-derived myofibroblasts and it is thought that these cells contribute to the IBD-associated fibrosis and disruption of immunoregulation39–41. Here we present additional evidence that CMFs may play a role in immunopathogenesis of IBD: IBD-derived CMFs have a decreased capacity to induce active Treg cells capable of producing suppressive cytokines TGFβ1 and IL-10. Moreover, a shift in the balance in IBD-CMF – primed T cells from “true” Treg phenotype (CD4+CD25highCD127− FOXP3+) toward FoxP3 expressing CD127+ T cells (CD4+CD25highCD127+ FOXP3+) suggests that during IBD progression CMFs may promote the persistence of CD127+ colitogenic CD4+ T cells that only transiently express FoxP3. It is unclear how the disruption of this immunoregulatory function of the CMFs occurs during the switch from acute to chronic inflammation associated with IBD, but in rheumatoid arthritis an alteration in synovial fibroblasts capacity to regulate T responder/Treg balance has been recently reported to implicate IL-15 expression by the disease derived fibroblasts29. Further studies are clearly necessary to understand the role of the CMF-diminished capacity to induce Treg in the progression of IBD.
Our study adds human CMFs to the list of mesenchymal stromal cells (e.g., dermal and synovial fibroblasts, hepatic stellate cells and mesenchymal stem cells) 40–42,45, that may contribute to the regulation of the Treg cells and, thus, to the maintenance of peripheral tolerance. Further, CMFs appear to be local contributors to the maintenance of mucosal tolerance in the colon via at least two independent mechanisms: (1) expression of co-inhibitors of the B7 family (reference) and (2) support of Treg cells expansion. These findings taken together with previous reports12,15 indicate that CMF are relevant to normal inflammatory responses and that alteration of their regulatory function might be involved in IBD immunopathogenesis.
Supplementary Material
Acknowledgments
Supported by grants from the NIDDK (DK55783), American Gastroenterology Association, the John Sealy Memorial Endowment Fund, the UTMB Gastrointestinal Research Interdisciplinary Program, the James W. McLaughlin Endowment Fund, and Crohn’s & Colitis Foundation of America
Footnotes
No conflicts of interest exist
Author’s contribution to the submitted manuscript
Irina V. Pinchuk, PhD
Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; obtained funding.
Ellen J. Beswick, PhD
Acquisition of data; analysis and interpretation of data; material support; critical revision of the manuscript for important intellectual content.
Jamal I. Saada, MS
Acquisition of data; technical support; analysis and interpretation of data.
Gushyalatha Boya, MD
Acquisition of data.
David Schmitt, PhD
Acquisition of data.
Gottumukkala S. Raju, MD
Material support, critical revision of the manuscript for important intellectual content.
Julia Brenmoehl, PhD
Acquisition of data, material support, critical revision of the manuscript for important intellectual content.
Gerhard Rogler, MD, PhD
Material support; critical revision of the manuscript for important intellectual content.
Victor E. Reyes, PhD
Study concept and design; Analysis and interpretation of data; material support; critical revision of the manuscript for important intellectual content; obtained funding, study supervision.
Don W. Powell, MD
Study concept and design; analysis and interpretation of data; material support; critical revision of the manuscript for important intellectual content; obtained funding, study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Annacker O, Powrie F. Homeostasis of intestinal immune regulation. Microbes Infect. 2002;4:567–574. doi: 10.1016/s1286-4579(02)01574-5. [DOI] [PubMed] [Google Scholar]
- 2.Makita S, Kanai T, Nemoto Y, et al. Intestinal lamina propria retaining CD4+CD25+ regulatory T cells is a suppressive site of intestinal inflammation. J Immunol. 2007;178:4937–4946. doi: 10.4049/jimmunol.178.8.4937. [DOI] [PubMed] [Google Scholar]
- 3.Allez M, Mayer L. Regulatory T cells: peace keepers in the gut. Inflamm Bowel Dis. 2004;10:666–676. doi: 10.1097/00054725-200409000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 6.Gad M. Regulatory T cells in experimental colitis. Curr Top Microbiol Immunol. 2005;293:179–208. doi: 10.1007/3-540-27702-1_9. [DOI] [PubMed] [Google Scholar]
- 7.Powrie F, Leach MW, Mauze S, et al. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 8.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 9.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui KR, Powrie F. CD103+ GALT DCs promote Foxp3+ regulatory T cells. Mucosal Immunol. 2008;(Suppl1):S34–S38. doi: 10.1038/mi.2008.43. [DOI] [PubMed] [Google Scholar]
- 11.Westendorf AM, Fleissner D, Groebe L, et al. CD4+Foxp3+ regulatory T cell expansion induced by antigen-driven interaction with intestinal epithelial cells independent of local dendritic cells. Gut. 2009;58:211–219. doi: 10.1136/gut.2008.151720. [DOI] [PubMed] [Google Scholar]
- 12.Saada JI, Pinchuk IV, Barrera CA, et al. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol. 2006;177:5968–5979. doi: 10.4049/jimmunol.177.9.5968. [DOI] [PubMed] [Google Scholar]
- 13.Andoh A, Bamba S, Brittan M, et al. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther. 2007;114:94–106. doi: 10.1016/j.pharmthera.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Pinchuk IV, Beswick EJ, Saada JI, et al. Monocyte chemoattractant protein-1 production by intestinal myofibroblasts in response to staphylococcal enterotoxin a: relevance to staphylococcal enterotoxigenic disease. J Immunol. 2007;178:8097–8106. doi: 10.4049/jimmunol.178.12.8097. [DOI] [PubMed] [Google Scholar]
- 15.Pinchuk IV, Saada JI, Beswick EJ, et al. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology. 2008;135:1228–1237. doi: 10.1053/j.gastro.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinchuk IV, Mifflin RC, Saada JI, et al. Intestinal mesenchymal cells. Curr Gastroenterol Rep. 2010;12:310–318. doi: 10.1007/s11894-010-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahida YR, Beltinger JJ, Makh S, et al. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273:G1341–G1348. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- 18.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 19.Wuest TY, Willette-Brown J, Durum SK, et al. The influence of IL-2 familycytokines on activation and function of naturally occurring regulatory T cells. J Leukoc Biol. 2008;84:973–80. doi: 10.1189/jlb.1107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setoguchi R, Hori S, Takahashi T, et al. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel L, Berthelot L, Pettré S, et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest. 2008;118:3411–3419. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Totsuka T, Kanai T, Nemoto Y, et al. IL-7 Is essential for the development and the persistence of chronic colitis. J Immunol. 2007;178:4737–4748. doi: 10.4049/jimmunol.178.8.4737. [DOI] [PubMed] [Google Scholar]
- 24.English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandtzaeg P. Nature and function of gastrointestinal antigen-presenting cells. Allergy. 2001;56 (Suppl67):16–20. doi: 10.1034/j.1398-9995.2001.00903.x. [DOI] [PubMed] [Google Scholar]
- 26.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–29. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haniffa MA, Wang XN, Holtick U, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 28.Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–18. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Benito-Miguel M, García-Carmona Y, Balsa A, et al. A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25- responder T cells. J Immunol. 2009;183:8268–79. doi: 10.4049/jimmunol.0900007. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann P, Eder R, Kunz-Schughart LA, et al. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 31.Yu A, Malek TR. Selective availability of IL-2 is a major determinantcontrolling the production of CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2006 Oct 15;177(8):5115–21. doi: 10.4049/jimmunol.177.8.5115. [DOI] [PubMed] [Google Scholar]
- 32.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang G, Yang HR, Wang L, et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86 :1492–502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merlo A, Tagliabue E, Menard S, et al. Matured human monocyte-derived dendritic cells (MoDCs) induce expansion of CD4(+)CD25(+)FOXP3(+) T cells lacking regulatory properties. Immunol Lett. 2008;117:106–113. doi: 10.1016/j.imlet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive humanCD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007 Oct 15;110(8):2983–90. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beswick EJ, Pinchuk IV, Das S, et al. Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect Immun. 2007;75:4334–4341. doi: 10.1128/IAI.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol. 2009;183:4119–26. doi: 10.4049/jimmunol.0901065. [DOI] [PubMed] [Google Scholar]
- 38.Flavell SJ, Hou TZ, Lax S, Filer AD, Salmon M, Buckley CD. Fibroblasts as novel therapeutic targets in chronic inflammation. Br J Pharmacol. 2008;153 (Suppl 1):S241–S246. doi: 10.1038/sj.bjp.0707487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKaig BC, Hughes K, Tighe PJ, et al. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol. 2002;282:C172–C182. doi: 10.1152/ajpcell.00048.2001. [DOI] [PubMed] [Google Scholar]
- 40.Di Sabatino A, Pender SL, Jackson CL, et al. Functional modulation of Crohn’s disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology. 2007;133:137–149. doi: 10.1053/j.gastro.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 41.Di Sabatino A, Jackson CL, Pickard KM, et al. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut. 2009;58:777–789. doi: 10.1136/gut.2008.149096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.