Abstract
The function and longevity of implantable microelectrodes for chronic neural stimulation depends heavily on the electrode materials, which need to present high charge injection capability and high stability. While conducting polymers have been coated on neural microelectrodes and shown promising properties for chronic stimulation, their practical applications have been limited due to unsatisfying stability. Here, poly(3,4-Ethylenedioxythiophene) (PEDOT) doped with pure carbon nanotubes (CNTs) was electrochemically deposited on Pt microelectrodes to evaluate its properties for chronic stimulation. The PEDOT/CNT coated microelectrodes demonstrated much lower impedance than the bare Pt, and the PEDOT/CNT film exhibited excellent stability. For both acute and chronic stimulation tests, there is no significant increase in the impedance of the PEDOT/CNT coated microelectrodes, and none of the PEDOT/CNT films show any cracks or delamination, which have been the limitation for many conducting polymer coatings on neural electrodes. The charge injection limit of the Pt microelectrode was significantly increased to 2.5 mC/cm2 with the PEDOT/CNT coating. Further in vitro experiments also showed that the PEDOT/CNT coatings are non-toxic and support the growth of neurons. It is expected that this highly stable PEDOT/CNT composite may serve as excellent new material for neural electrodes.
1. Introduction
Chronic neural stimulation is utilized in neural prostheses to modify, restore, or bypass a damaged or diseased portion of the nervous system by sensing or delivering electrical pulses to nearby tissue through neural electrodes. Currently, the most clinically successful applications include deep brain stimulators [1, 2] and cochlear implants [3–5], used to reduce symptoms of Parkinson's disease and restore auditory function, respectively. Other medical applications of neural electrodes, such as the treatment of retinitis pigmentosa [6], epilepsy [7], depression [8] and chronic pain [9], have also been reported. With the ever-expanding application of neural electrodes, critical attention must be paid to the safety, function and longevity of these devices, which are ultimately dependent on the stability and biocompatibility of the electrode materials.
Currently, most neural electrodes are made primarily from considerably stable metals, such as platinum, gold, iridium, titanium and stainless steel. As these bare metallic electrodes often suffer from poor performance in long-term stimulation and recording due to poor contact with tissue or scar formation, researchers have developed different surface modification strategies for improving electrode functionality. Applying a thin layer of rough and porous coatings on the neural electrodes can easily increase the effective surface area, improve the efficiency in charge transfer and modulate the electrode biocompatibility. Iridium oxide (IrOx) is the most commonly used coating material for neural electrodes [10–13], as it possesses many unique properties for neural stimulation. IrOx coated electrodes are non-cytotoxic [11], and they have very low impedance and a high charge injection limit, which allows high levels of charge injection without electrode dissolution or water electrolysis [14]. However, IrOx has poor adhesion to underlying substrates, and it may degrade under chronic aggressive stimulations due to its low structural and chemical stability [15, 16].
Recently, conducting polymers, including polypyrrole, polythiophene, and their derivatives, have emerged as new materials for neural interfacing [17–22]. Conducting polymers can be electrochemically deposited on neural electrodes with easy control over their thickness, and different bioactive molecules can be incorporated into the polymers to promote neuronal growth and adhesion to the electrode surface [23, 24]. Poly(3,4-Ethylenedioxythiophene) (PEDOT) has been considered as the most promising conducting polymer because its ordered and well-defined chemical structure offers outstanding conductivity and stability [25]. It has been reported that the electrical properties of neural electrodes can be significantly improved by surface coating with PEDOT [26, 27]. Despite its advantages and promising outlook, conventional PEDOT has yet to be perfected as a coating material for neural electrodes. Our recent study [26] as well as others' [28] have revealed the unsatisfactory long-term stability of PEDOT coatings during chronic electrical stimulation. PEDOT coatings may form cracks or delaminate under stimulation, which may lead to further coating detachment, thus debilitating the function of the electrode.
Here, in order to fulfill biocompatibility and stability requirements for PEDOT as a coating material, we adopt carbon nanotubes (CNTs) as the dopant for PEDOT polymerization to electrochemically deposit PEDOT/CNT coatings on neural electrodes. CNTs are known for their extraordinary strength, electrical conductivity and chemical stability, and have broad bio-related applications [29], which recently have extended into the area of neural electrodes [30]. CNTs have been shown to be able to promote neuron differentiation [31], stimulate neurite outgrowth [32], improve neuronal performance [33] and recording [34], boost neuronal electrical signaling [35] and act as a substrate for neuronal growth [36, 37]. In addition, previous reports have shown that CNTs can be incorporated into conducting polymers, such as polypyrrole [22, 38, 39] and PEDOT [40, 41] to form composite materials with enhanced properties, including stability. Therefore, it is expected that the CNT doped PEDOT (PEDOT/CNT) as a coating material may preserve the biocompatibility of PEDOT and CNTs, and exhibit enhanced long-term stability. In this work, PEDOT/CNT films were electrochemically coated on Pt microelectrode arrays, and their morphology and electrochemical properties were characterized. The stability of the coatings under both strong acute stimulation and long-term chronic stimulation were investigated in detail. And finally, the biocompatibility of the PEDOT/CNT coatings was tested.
2. Materials and Methods
2.1. Materials
Multi-walled CNTs with the length of 10–30 μm and diameter of 20–30 nm were purchased from Cheap Tubes Inc. (Brattleboro, USA). 3,4-Ethylenedioxythiophene, glutaraldehyde (25% in H2O), osmium tetroxide (OsO4, 4 wt.% in H2O) and hexamethyldisilazane (HMDS) were purchased from Sigma-Aldrich. Phosphate buffered saline (PBS, pH 7.4, 10 mM sodium phosphate and 0.9% NaCl) was purchased from Sigma-Aldrich. All other chemicals were of analytical grade, and Milli-Q water from a Millipore Q water purification system was used throughout. The stimulating Pt microelectrode arrays were fabricated by Second Sight Medical Products, Inc., Sylmar, CA. Each array contains 16 electrodes with a circular exposed electrode surface of 200 μm in diameter.
2.2. Electrodeposition of PEDOT/CNT
The CNTs were pretreated by dispersing 200 mg CNTs in 100 mL 1:3 concentrated HNO3 and H2SO4 solution with sonication for 2 hours. The suspension was then kept at room temperature overnight. After the acid treatment, the CNTs were washed with water and separated using ultracentrifugation repeatedly until the pH of the washing solution was neutral. Finally, the CNTs were collected and dried at 60 °C.
PEDOT/CNT films were electrodeposited onto the Pt microelectrodes using a Gamry Potentiostat, FAS2/Femtostat (Gamry Instruments) with Gamry Framework software. A conventional three-electrode system with the Pt microelectrode acting as the working electrode, a platinum wire as the counter electrode, and a silver/silver chloride (Ag/AgCl) reference electrode (CH Instruments) was used. The PEDOT/CNT was electropolymerized from an aqueous solution containing 0.02 M EDOT and 2 mg/mL CNTs. Both constant current (100 nA) and constant potential (1.0 V) were applied for the electrodeposition of PEDOT/CNT, and the film thickness was controlled by adjusting the electrodeposition time. For cell culture tests, plastic microscope cover slips (Fisher Scientific) sputtered with 40 nm Au were used as electrodes, and PEDOT/CNT films were electrodeposited on those electrodes using a constant potential.
2.3. Electrochemical characterization
The Gamry potentiostat was used for both cyclic voltammetry (CV) and electrochemical impedance spectrum (EIS) measurements. The CV was performed by scanning potentials between − 0.6 and 0.7 V at a scan rate of 100 mV/s in PBS. The charge storage capacity was calculated from the time integral of the current in one cycle of the CV waveform (the enclosed area of the CV curve, i.e. the charge passed during one CV cycle). The EIS was measured in PBS in the frequency range from 1 Hz to 100 kHz using an alternating current sinusoid of 5 mV in amplitude with the direct current potential set to 0 V.
2.4. Stimulation
Two types of stimulations were performed to test the stability of the PEDOT/CNT coatings. An aggressive cyclic voltammetric stimulation was performed with the Gamry potentiostat, using a voltage scan from − 0.9 to 0.5 V, at a scan rate of 100 mV/s for 3000 cycles (about 24 hours). The chronic stimulation was performed with a programmable multichannel stimulator made by Multichannel Systems (STG2008), similar to our previous work [26]. A TDS 3014B oscilloscope and the WaveStar program were used to measure and record the voltage excursions. The PEDOT/CNT coated Pt microelectrode arrays were soaked in PBS for about 3 months, and between week 5 and week 7 the electrodes were stimulated under a charge-balanced, cathodic first, biphasic pulse current at 0.35 mC/cm2 at 50 Hz for two weeks. During the 3 month period, the impedance of the electrodes at 1000 Hz and CV was measured periodically to monitor the impedance and electrochemical change over time.
2.5. Primary neuron cell culture
PEDOT/CNT coated cover slips were fixed to the surface of 24-well culture plates with Kwik-Sil (World Precision Instruments, Inc) and sterilized with exposure to UV light for 15 min. Following sterilization, the polymer surfaces were washed with sterile PBS. The surfaces were coated with poly(ethyleneimine) solution (0.05% in borate buffer) followed by laminin (20 mg/mL) to encourage neuron attachment. E18 Sprague/Dawley cortices (Brain Bits, IL) were gently triturated with a 1 mL pipette, removed from Hibernate Media™ by centrifugation at 200 × g and resuspended in Neurobasal media (Invitrogen, 21103-049) supplemented with B27 (Invitrogen 17504-044) and GlutaMax (Invitrogen, 35050-061). Cells were seeded on PEDOT/CNT surfaces at a density of 60k cells per macroelectrode and grown for 3 days.
2.6. Immunocytochemistry
Neurons growing on the PEDOT/CNT surfaces were fixed in 4% paraformaldehyde in PBS for 15 min and washed several times with PBS. The cells were immersed in a blocking buffer (5% goat serum/0.2% triton-X in PBS) for 20 minutes followed by incubation in monoclonal antibody against β-III-tubulin (TuJ1, 1:1000, Sigma) for 1 h. After washing in PBS, the cells were incubated in Alexa Fluor 488 (1:1000, Invitrogen) secondary antibody for one hour, washed in PBS and counterstained for nuclei using Hoechst 33342 (Invitrogen). Immunoreactive cells were imaged using a fluorescence microscope (Axioskop 2 MAT, Carl Zeiss).
2.7. SEM characterization
The surface morphologies and microstructures of the coatings were examined with an XL30 scanning electron microscope (SEM, FEI Company) operated at 10 kV. Cells growing on the coatings were analyzed with the same SEM, but with a lower operating potential of 5 kV. Samples with cells were treated with 2.5% glutaraldehyde and 1% OsO4, both for one hour in sequence, followed by dehydration. The dehydration was performed by soaking the samples in 30% and 50% ethanol in PBS, 70% and 90% ethanol in water, and 100% ethanol in sequence for 15 min each. After a final step of soaking in HMDS for 15 min, the samples with cells were then ready for SEM characterization.
3. Results
3.1. Electrodeposition of PEDOT/CNT
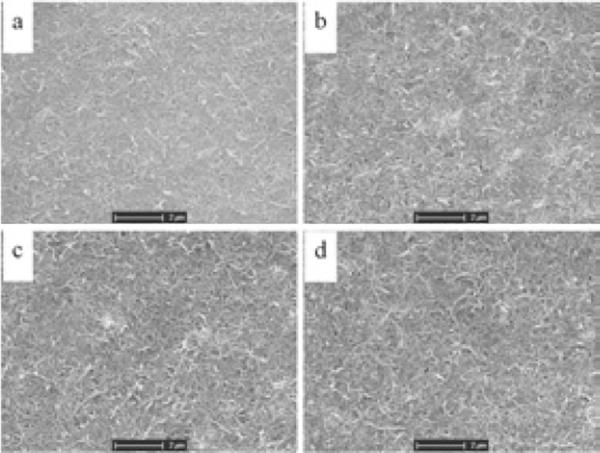
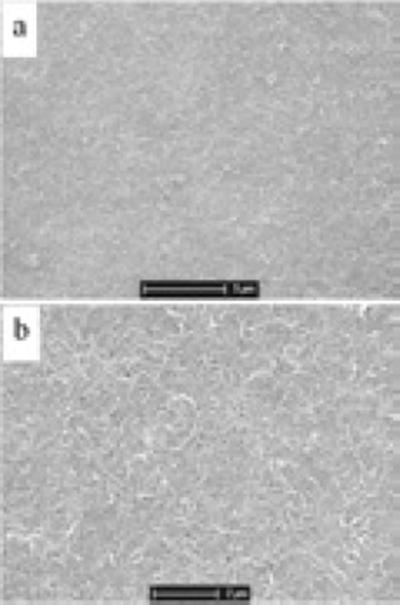
The acid-pretreated CNTs are negatively charged in neutral aqueous solution because they possess many carboxyl groups. During the electropolymerization of PEDOT in aqueous solution containing only EDOT monomer and CNTs, the negatively charged CNTs will act as dopants to balance the positive charge in the backbone of the PEDOT, and be embedded as part of the formed polymer. Figure 1 shows the SEM images of the PEDOT/CNT coatings with different thickness. The CNTs were well dispersed and exhibited a network-like structure. The coatings were generally uniform, with the surface becoming slightly rougher and more porous as the film thickness increased.
Figure 1.
SEM images of PEDOT/CNT coatings on Pt microelectrodes. The films were electrodeposited at a constant current of 100 nA for (a) 100, (b) 200, (c) 300 and (d) 400 seconds.
3.2. Cyclic voltammetry
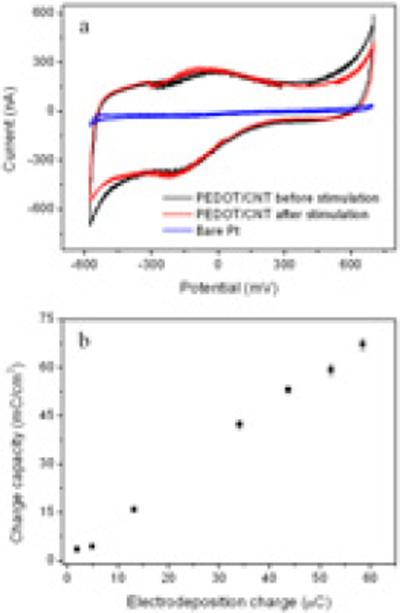
The redox characteristics of the PEDOT/CNT coated electrodes were investigated using CV, as shown in Figure 2a. The PEDOT/CNT undergoes reversible redox reactions as indicated by the oxidation and reduction peaks on the CV curve. The CV curves can also be used to evaluate the charge storage capacity, as the enclosed area of the curve is proportional to the charge storage capacity, i.e. the amount of charge passed during one CV cycle. Clearly, the charge storage capacity of the PEDOT/CNT coated electrode is significantly higher than that of the bare Pt electrode. Such a high charge storage capacity of the coated electrode is associated with the high effective surface area and excellent conductivity of the PEDOT/CNT film. The charge storage capacity of PEDOT/CNT films is increased with the increase in the electrodeposition charge, as shown in Figure 2b. This is in accordance with expectation, because higher electrodeposition charge will lead to thicker and rougher PEDOT/CNT films and presents more electroactive species. Within the tested range, the relationship of the electrodeposition charge and the measured charge storage capacity is clearly linear, even at a very high level of electrodeposition charge. This result is different from previous reports about PEDOT films, where the relationship is only linear for thinner films and the linearity falls off for thicker films due to lower doping level, more defects and denser morphology of the thicker films [26]. For the PEDOT/CNT film, the charge capacity can be linearly increased to more than 70 mC/cm2 by increasing the electrodeposition charge, while the charge capacity of the PEDOT film stops increasing at 7 mC/cm2 [26].
Figure 2.
(a) Cyclic voltammograms of bare Pt electrode and PEDOT/CNT coated electrodes before and after long-term stimulation. (b) The charge capacity of coated electrodes as a function of the PEDOT/CNT electrodeposition charge.
3.3. Electrochemical impedance spectroscopy
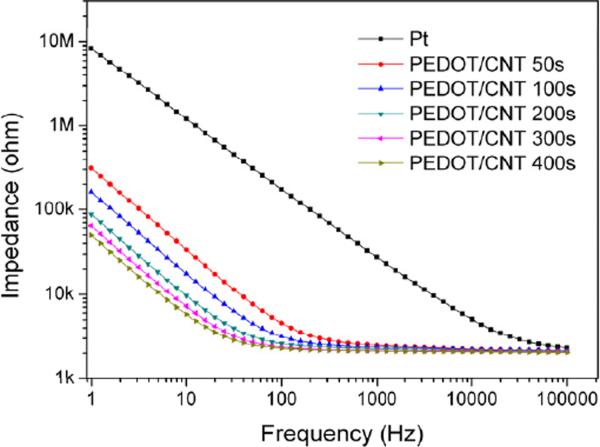
The electrochemical properties of the PEDOT/CNT coated electrodes were further characterized with EIS in PBS. As shown in Figure 3, the impedance modulus of the Pt electrode in the frequency range between 1 and 100,000 Hz sharply decreased after coating with PEDOT/CNT. In addition, the PEDOT/CNT coatings progressively decreased the impedance of the Pt electrode with increasing coating thickness, especially at the lower frequency region.
Figure 3.
Electrochemical impedance spectroscopy of Pt electrodes coated with PEDOT/CNT for 50, 100, 200, 300 and 400 seconds in comparison to the bare Pt electrode.
As discussed elsewhere [42, 43], a circuit model consisting of a solution resistance (RS) in series with a finite-length warburgh diffusion impedance component of bulk film (ZD) and a bulk (electronic) capacitance (Cd) can be used to analyze the measured EIS of conducting polymer coated metal electrodes. Since all the measurements were carried out in the same solution, RS is of a constant value. The finite-length warburgh diffusion impedance ZD is defined as
where τD is a diffusional time constant, CD is the diffusional pseudo-capacitance, and τD/CD can be defined as a diffusional resistance RD [44], which is dependent on the film thickness, and increases with the increase of the film thickness. The electronic capacitance Cd is greatly dependent on the electroactive surface area. In the higher frequency region, the impedance due to Cd is negligible, and the impedance is only affected by ZD. As can be seen in Figure 3, the measured impedance in this region changes negligibly with the increase in film thickness as expected. The negligible impedance change also indicates the good conductivity of the PEDOT/CNT films. While in the lower frequency region, Cd is the dominant contributor for the impedance. As the electroactive surface area becomes larger with the increase of coating thickness, the capacitance Cd becomes bigger, thus leading to an impedance decrease as shown in Figure 3.
3.4. Stimulation
In most neural stimulation applications, a biphasic, cathodic-first current pulse is employed to stimulate the nervous tissues [45]. To simulate the stimulation conditions for practical applications and test the long-term stability, the PEDOT/CNT coated electrodes were soaked in PBS for three months, and between weeks 5 and 7 they were stimulated using a clinically relevant neural stimulation protocol (a biphasic, charge balanced pulse current at 1 mC/cm2 at 50 Hz) for two weeks.
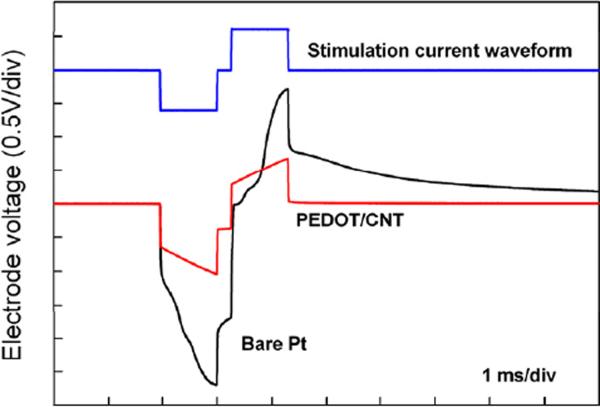
The voltage response (voltage excursion) of an electrode upon current stimulation is a direct indicator of its charge injection capability. The preferred neural electrode should have a higher charge injection capability, because for a given pulse current it generates lower electrode voltage, which is safer for neuronal stimulation. The PEDOT/CNT coated electrodes were examined under pulse stimulation currents to evaluate their charge injection capacity. As shown in Figure 4, under the same stimulation conditions, the PEDOT/CNT coated electrode exhibited a much lower voltage excursion than the bare Pt electrode. The charge injection limit is defined as the maximum cathodic charge that resulted in cathodic or anodic electrode voltage exceeding 0.6 V versus the Ag/AgCl reference electrode. The average charge injection limit measured was 2.5 ± 0.1 mC/cm2 (n = 4), a value much higher than that of Pt [46] and comparable to that of IrOx [14]. The results indicate that the PEDOT/CNT coated electrodes can deliver higher charge densities without generating high voltages that may harm surrounding tissues.
Figure 4.
Voltage excursions of the PEDOT/CNT coated and bare Pt electrodes under the biphasic current stimulation. The electrodes were stimulated in PBS under a charge-balanced, cathodic-first, biphasic pulse current at 1 mC/cm2 at 50 Hz (the top curve).
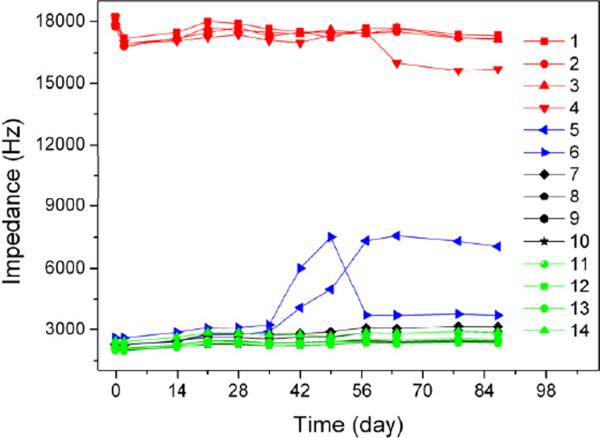
At the end of the three-month immersion in PBS, electrode surface morphology was examined using optical microscopy and SEM. This long-term chronic stimulation didn't cause any noticeable changes to the morphology of the PEDOT/CNT coatings from macro to micro scales, and there was no cracking or delamination on any electrode. Monitoring the electrode impedance can provide useful information about the electrode conditions, as film cracking and delamination will normally result in impedance change. Figure 5 shows representative plots of electrode impedance change over time during the three-month soaking in PBS for different electrodes. Both the stimulated and un-stimulated PEDOT/CNT coated electrodes have much lower impedance than bare Pt electrodes, and their impedance remained unchanged over the three-month period. This result is consistent with the morphology examination that shows that the PEDOT/CNT coatings are highly stable. For some of the ultrathin PEDOT/CNT film coated electrodes, their impedance may increase slightly after the electrical stimulation as shown in Figure 5. This may be ascribed to the fact that the CNT networks that offer the polymer mechanical strength are not well formed in the ultrathin films.
Figure 5.
Monitored electrode impedance changes at 1K Hz over time during the three-month soaking in PBS. Plots 1–4 in red are bare Pt electrodes; plots 5–6 in blue are ultrathin PEDOT/CNT (deposition charge less than 5 μC) coated Pt electrodes with stimulation; plots 7–10 in black and plots 11–14 in green are normal PEDOT/CNT (deposition charge more than 10 μC) coated Pt electrodes with (7–10) and without (11–14) stimulation.
We further checked the CV of the electrodes after stimulation, and no significant changes were observed on the CV curves, as shown in Figure 2a. The unchanged reduction and oxidization peaks in the CV curves indicate that the PEDOT/CNT coatings completely retained their electroactivity after the long-term soaking and stimulation.
To further challenge the stability of the PEDOT/CNT coating, the PEDOT/CNT coated electrodes were subjected to 3000 CV scanning cycles (equal to about 24 hours) in PBS from – 0.9 to 0.5 V, at a scan rate of 100 mV/s. During each CV scan, the polymer will undergo oxidation and reduction accompanied by ion and water movement in and out of the film. Such CV conditions are known to cause delamination and cracking of various conducting polymer films [47]. After such strong stimulation, all the PEDOT/CNT coatings were observed to remain intact on the electrode surface. The stimulated coatings were further characterized with SEM, as shown in Figure 6. The morphology of the PEDOT/CNT coatings exhibits no observable changes after stimulation, compared to that of the un-stimulated ones (Figure 1). The porous network structure persisted the electrode surface, and there is no cracking or delaminating of the film, even at the micro- or nanoscale.
Figure 6.
SEM images of PEDOT/CNT coating on Pt microelectrode after electrical stimulation with (a) lower and (b) higher magnifications.
3.5. Cell culture
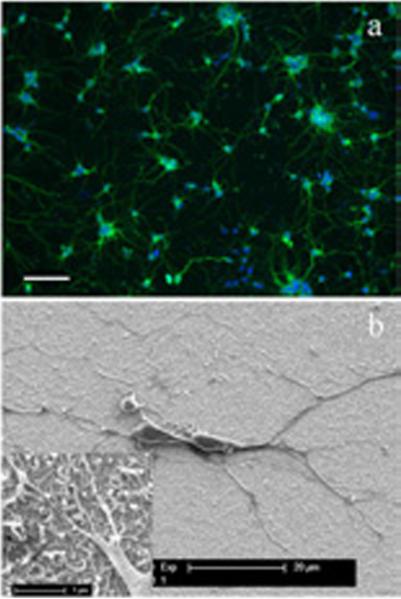
In addition to stability, biocompatibility is another important property that the coating materials for neural electrodes should possess. In order to test the biocompatibility of the PEDOT/CNT, it was electrochemically coated on gold sputtered cover slips, and the resultant PEDOT/CNT coated cover slips were used as substrates for neuron culture. Figure 7a is the fluorescent image of neurons grown on the PEDOT/CNT surface. It is clear that the neurons grow evenly over the whole PEDOT/CNT coating, and neuronal networks were well established, indicating healthy neuronal growth on the PEDOT/CNT substrate. Compared to the cultures on standard glass coverslips, no observable difference was found. Further characterization was carried out with SEM, as shown in Figure 7b. As can be seen, the neurons attached tightly to the PEDOT/CNT surface and exhibited long neurite extensions. Some of the smaller processes intimately grew along or around the nanofibers on the surface. These results suggest that PEDOT/CNT is not toxic and supports healthy cell attachment and neurite outgrowth, implying that the PEDOT/CNT possess excellent biocompatibility.
Figure 7.
Fluorescent (a) and SEM (b) images of neurons cultured on PEDOT/CNT surfaces. For the fluorescent image, the scale bar represents 100μm. The inset in (b) shows the SEM of neurites grown on the PEDOT/CNT surface with high magnification.
4. Discussion
Chronic neural stimulation via microelectrode arrays has tremendous clinical potential in restoring lost or impaired neurological functions. As the current clinically applied electrode materials do not meet the challenge of chronic neural stimulation, the search for alternative materials that have higher charge injection capacity and long-term stability continues. Previously, PEDOT/PSS coating has been demonstrated to be a promising material for stimulation due to its low impedance, high charge injection limit and excellent biocompatibility. However, delamination and cracking have been found under chronic stimulation, especially with thicker films that have better electrical properties. In this work, PEDOT/CNT has been successfully coated on the Pt microelectrode surface using electropolymerization. Similarly to PEDOT/PSS, the resulting film has significantly reduced the impedance of Pt microelectrode, largely due to an increased surface area of the PEDOT/CNT film.
The charge storage capacity of PEDOT/CNT film is 1–2 orders of magnitude higher than that of the uncoated Pt electrode. Higher charge capacity may result in higher charge injection that is desirable for neural stimulation. In addition, the charge storage capacity of PEDOT/CNT films can be increased linearly to more than 70 mC/cm2 as we increase the deposition charge, which is 10 times higher than that of the PEDOT/PSS film [26]. This advantage of the PEDOT/CNT film over PEDOT/PSS can be ascribed to the presence of CNTs, which act as highly conductive dopants for PEDOT. As CNTs possess huge surface area and excellent conductivity, their network-like distribution within the PEDOT film can effectively enlarge the electrode/electrolyte interface and increase the conductivity of the PEDOT/CNT film, thereby making the deposited polymer a very conductive substrate for further PEDOT/CNT deposition.
Another important parameter is the stability of the films. It was found that the PEDOT/CNT coated Pt electrodes were extremely stable during prolonged and aggressive electrical stimulations, suggesting great clinical potential for chronic neural stimulation. Formation of cracks and/or delamination under stimulation is a common failure phenomenon for conventional conducting polymer coatings on neural electrodes [26, 28], while for the proposed PEDOT/CNT film coated electrodes, the coatings remain unchanged after long-term stimulation. The superior structural stability of the PEDOT/CNT films can be related to the network microstructure formed in the presence of CNTs. The mechanically strong CNTs are evenly distributed across the film and function as reinforcing elements that prevent the film from undergoing deformation and cracking. In addition, the expansion and shrinkage of the conducting polymer film [48–50] accompanied with the redox reaction upon stimulation can be greatly reduced (though not eliminated) due to the interdigitated network microstructure, thereby keeping the intimate adhesion of the polymers to the underlying electrode.
As many promising applications of CNTs for clinical use are being explored, specific attention must be paid to the toxicity of CNTs. In our cell culture study we found that neurons grew healthily on the PEDOT/CNT substrate. Most evidence of CNT toxicity was seen in respiratory exposure or tissue accumulation in large doses [51–53]. While in our case, since CNTs are immobilized on the microelectrodes and encapsulated by the PEDOT, there is less possibility for them to diffuse into the surroundings or be directly exposed to the tissue. Furthermore, we pretreated the CNTs with acid and effectively removed the metal catalyst (proved by X-ray photoelectron spectroscopy, XPS, data not shown), which is thought to be the main source of the CNT toxicity in many cases [54].
5. Conclusions
The present study demonstrates that PEDOT/CNT coatings can be electrochemically deposited on the Pt electrode of microelectrode arrays. The coated electrodes exhibited much lower impedance, higher charge storage capacity, and a high charge injection limit of about 2.5 mC/cm2. The PEDOT/CNT coating displayed very high stability under both long-term biphasic pulse stimulation and aggressive cyclic voltammetric stimulation. In addition, it showed excellent in vitro biocompatibility. These excellent characteristics of PEDOT/CNT coating warrant further testing of their chronic stimulation performance in pre-clinical models.
Acknowledgements
This research was financially supported in part by the National Institute of Health R01NS062019, the Department of Defense TATRC grant WB1XWH-07-1-0716, and the National Science Foundation Grants 0748001, 0729869. The authors would like to thank C. Byers for his assistance in performing some electrochemical testing to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Oh MY, Hodaie M, Kim SH, Alkhani A, Lang AE, Lozano AM. Deep brain stimulator electrodes used for lesioning: Proof of principle. Neurosurgery. 2001;49:363–7. doi: 10.1097/00006123-200108000-00018. [DOI] [PubMed] [Google Scholar]
- [2].Okun MS, Rodriguez RL, Foote KD, Sudhyadhom A, Bova F, Jacobson C, et al. A case-based review of troubleshooting deep brain stimulator issues in movement and neuropsychiatric disorders. Parkinsonism Relat D. 2008;14:532–8. doi: 10.1016/j.parkreldis.2008.01.001. [DOI] [PubMed] [Google Scholar]
- [3].Sparreboom M, van Schoonhoven J, van Zanten BGA, Scholten RJPM, Mylanus EAM, Grolman W, et al. The Effectiveness of Bilateral Cochlear Implants for Severe-to-Profound Deafness in Children: A Systematic Review. Otol Neurotol. 2010;31:1062–71. doi: 10.1097/MAO.0b013e3181e3d62c. [DOI] [PubMed] [Google Scholar]
- [4].Johnston JC, Durieux-Smith A, Angus D, O'Connor A, Fitzpatrick E. Bilateral paediatric cochlear implants: A critical review. Int J Audiol. 2009;48:601–17. doi: 10.1080/14992020802665967. [DOI] [PubMed] [Google Scholar]
- [5].Waltzman SB. Cochlear implants: current status. Expert Rev Med Devic. 2006;3:647–55. doi: 10.1586/17434440.3.5.647. [DOI] [PubMed] [Google Scholar]
- [6].Zhou DD, Greenberg R. Microelectronic Visual Prostheses. In: Zhou David, Greenbaum Elias., editors. Implantable Neural Prostheses 1, Devices and Applications, Biological and Medical Physics, Biomedical Engineering. Springer; 2009. pp. 1–42. [Google Scholar]
- [7].Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. 2004;3:111–8. doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]
- [8].Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- [9].Falowski S, Celii A, Sharan A. Spinal cord stimulation: an update. Neurotherapeutics. 2008;5:86–99. doi: 10.1016/j.nurt.2007.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Meyer RD, Cogan SE, Nguyen TH, Rauh RD. Electrodeposited iridium oxide for neural stimulation and recording electrodes. Ieee T Neur Sys Reh. 2001;9:2–11. doi: 10.1109/7333.918271. [DOI] [PubMed] [Google Scholar]
- [11].Weiland JD, Anderson DJ. Chronic neural stimulation with thin-film, iridium oxide electrodes. Ieee T Bio-Med Eng. 2000;47:911–8. doi: 10.1109/10.846685. [DOI] [PubMed] [Google Scholar]
- [12].Niebauer MJ, Wilkoff B, Yamanouchi Y, Mazgalev T, Mowrey K, Tchou P. Iridium oxide-coated defibrillation electrode - Reduced shock polarization and improved defibrillation efficacy. Circulation. 1997;96:3732–6. doi: 10.1161/01.cir.96.10.3732. [DOI] [PubMed] [Google Scholar]
- [13].Negi S, Bhandari R, Rieth L, Solzbacher F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed Mater. 2010;5:015007. doi: 10.1088/1748-6041/5/1/015007. [DOI] [PubMed] [Google Scholar]
- [14].Weiland JD, Liu W, Humayun MS. Retinal prosthesis. Annu Rev Biomed Eng. 2005;7:361–401. doi: 10.1146/annurev.bioeng.7.060804.100435. [DOI] [PubMed] [Google Scholar]
- [15].Cogan SF, Guzelian AA, Agnew WF, Yuen TG, McCreery DB. Over-pulsing degrades activated iridium oxide films used for intracortical neural stimulation. J Neurosci Methods. 2004;137:141–50. doi: 10.1016/j.jneumeth.2004.02.019. [DOI] [PubMed] [Google Scholar]
- [16].Mailley SC, Hyland M, Mailley P, McLaughlin JM, McAdams ET. Electrochemical and structural characterizations of electrodeposited iridium oxide thin-film electrodes applied to neuro stimulating electrical signal. Mat Sci Eng C-Bio S. 2002;21:167–75. [Google Scholar]
- [17].Abidian MR, Ludwig KA, Marzullo TC, Martin DC, Kipke DR. Interfacing Conducting Polymer Nanotubes with the Central Nervous System: Chronic Neural Recording using Poly (3,4-ethylenedioxythiophene) Nanotubes. Adv Mater. 2009;21:3764–70. doi: 10.1002/adma.200900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cui XY, Hetke JF, Wiler JA, Anderson DJ, Martin DC. Electrochemical deposition and characterization of conducting polymer polypyrrole/PSS on multichannel neural probes. Sensor Actuat a-Phys. 2001;93:8–18. [Google Scholar]
- [19].Xiao YH, Martin DC, Cui XY, Shenai M. Surface modification of neural probes with conducting polymer poly(hydroxymethylated-3,4-ethylenedioxythiophene) and its biocompatibility. Appl Biochem Biotech. 2006;128:117–29. doi: 10.1385/abab:128:2:117. [DOI] [PubMed] [Google Scholar]
- [20].Widge AS, Jeffries-El M, Cui XY, Lagenaur CF, Matsuoka Y. Self-assembled monolayers of polythiophene conductive polymers improve biocompatibility and electrical impedance of neural electrodes. Biosensors & Bioelectronics. 2007;22:1723–32. doi: 10.1016/j.bios.2006.08.011. [DOI] [PubMed] [Google Scholar]
- [21].Cui XY, Wiler J, Dzaman M, Altschuler RA, Martin DC. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 2003;24:777–87. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- [22].Lu Y, Li T, Zhao XQ, Li M, Cao YL, Yang HX, et al. Electrodeposited polypyrrole/carbon nanotubes composite films electrodes for neural interfaces. Biomaterials. 2010;31:5169–81. doi: 10.1016/j.biomaterials.2010.03.022. [DOI] [PubMed] [Google Scholar]
- [23].Cui X, Wiler J, Dzaman M, Altschuler RA, Martin DC. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 2003;24:777–87. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- [24].Kim DH, Richardson-Burns SM, Hendricks JL, Sequera C, Martin DC. Effect of immobilized nerve growth factor on conductive polymers: Electrical properties and cellular response. Adv Funct Mater. 2007;17:79–86. [Google Scholar]
- [25].Groenendaal BL, Jonas F, Freitag D, Pielartzik H, Reynolds JR. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv Mater. 2000;12:481–94. [Google Scholar]
- [26].Cui XT, Zhou DD. Poly (3,4-ethylenedioxythiophene) for chronic neural stimulation. IEEE Trans Neural Syst Rehabil Eng. 2007;15:502–8. doi: 10.1109/TNSRE.2007.909811. [DOI] [PubMed] [Google Scholar]
- [27].Hendricks JL, Chikar JA, Crumling MA, Raphael Y, Martin DC. Localized cell and drug delivery for auditory prostheses. Hearing Res. 2008;242:117–31. doi: 10.1016/j.heares.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jan E, Hendricks JL, Husaini V, Richardson-Burns SM, Sereno A, Martin DC, et al. Layered Carbon Nanotube-Polyelectrolyte Electrodes Outperform Traditional Neural Interface Materials. Nano Lett. 2009;9:4012–8. doi: 10.1021/nl902187z. [DOI] [PubMed] [Google Scholar]
- [29].Lu FS, Gu LR, Meziani MJ, Wang X, Luo PG, Veca LM, et al. Advances in Bioapplications of Carbon Nanotubes. Adv Mater. 2009;21:139–52. [Google Scholar]
- [30].Keefer EW, Botterman BR, Romero MI, Rossi AF, Gross GW. Carbon nanotube coating improves neuronal recordings. Nature Nanotechnology. 2008;3:434–9. doi: 10.1038/nnano.2008.174. [DOI] [PubMed] [Google Scholar]
- [31].Chao TI, Xiang S, Chen CS, Chin WC, Nelson AJ, Wang C, et al. Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2009;384:426–30. doi: 10.1016/j.bbrc.2009.04.157. [DOI] [PubMed] [Google Scholar]
- [32].Matsumoto K, Sato C, Naka Y, Whitby R, Shimizu N. Stimulation of neuronal neurite outgrowth using functionalized carbon nanotubes. Nanotechnology. 2010;21:115101. doi: 10.1088/0957-4484/21/11/115101. [DOI] [PubMed] [Google Scholar]
- [33].Cellot G, Cilia E, Cipollone S, Rancic V, Sucapane A, Giordani S, et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol. 2009;4:126–33. doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]
- [34].Keefer EW, Botterman BR, Romero MI, Rossi AF, Gross GW. Carbon nanotube coating improves neuronal recordings. Nat Nanotechnol. 2008;3:434–9. doi: 10.1038/nnano.2008.174. [DOI] [PubMed] [Google Scholar]
- [35].Lovat V, Pantarotto D, Lagostena L, Cacciari B, Grandolfo M, Righi M, et al. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 2005;5:1107–10. doi: 10.1021/nl050637m. [DOI] [PubMed] [Google Scholar]
- [36].Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci. 2000;14:175–82. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- [37].Hu H, Ni Y, Mandal SK, Montana V, Zhao B, Haddon RC, et al. Polyethyleneimine functionalized single-walled carbon nanotubes as a substrate for neuronal growth. J Phys Chem B. 2005;109:4285–9. doi: 10.1021/jp0441137. [DOI] [PubMed] [Google Scholar]
- [38].Chen GZ, Shaffer MSP, Coleby D, Dixon G, Zhou WZ, Fray DJ, et al. Carbon nanotube and polypyrrole composites: Coating and doping. Adv Mater. 2000;12:522–+. [Google Scholar]
- [39].Lee YK, Lee KJ, Kim DS, Lee DJ, Kim JY. Polypyrrole-carbon nanotube composite films synthesized through gas-phase polymerization. Synthetic Met. 2010;160:814–8. [Google Scholar]
- [40].Zou JH, Tran B, Huo Q, Zhai L. Transparent Carbon Nanotube/Poly (3, 4-Ethylenedioxythiophene) Composite Electrical Conductors. Soft Mater. 2009;7:355–65. [Google Scholar]
- [41].Bhandari S, Deepa M, Srivastava AK, Joshi AG, Kant R. Poly(3,4-ethylenedioxythiophene)-Multiwalled Carbon Nanotube Composite Films: Structure-Directed Amplified Electrochromic Response and Improved Redox Activity. J Phys Chem B. 2009;113:9416–28. doi: 10.1021/jp9012976. [DOI] [PubMed] [Google Scholar]
- [42].Bobacka J, Lewenstam A, Ivaska A. Electrochemical impedance spectroscopy of oxidized poly(3,4-ethylenedioxythiophene) film electrodes in aqueous solutions. J Electroanal Chem. 2000;489:17–27. [Google Scholar]
- [43].Cui XY, Martin DC. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sensor Actuat B-Chem. 2003;89:92–102. [Google Scholar]
- [44].Macdonald JR. Impedance spectroscopy : emphasizing solid materials and systems. Wiley; New York: 1987. [Google Scholar]
- [45].Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–98. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- [46].Rose TL, Robblee LS. Electrical stimulation with Pt electrodes. VIII. Electrochemically safe charge injection limits with 0.2 ms pulses. IEEE Trans Biomed Eng. 1990;37:1118–20. doi: 10.1109/10.61038. [DOI] [PubMed] [Google Scholar]
- [47].Liu Y, Gan Q, Baig S, Smela E. Improving PPy adhesion by surface roughening. J Phys Chem C. 2007;111:11329–38. [Google Scholar]
- [48].Jager EWH, Inganas O, Lundstrom I. Perpendicular actuation with individually controlled polymer microactuators. Adv Mater. 2001;13:76–+. [Google Scholar]
- [49].Smela E. Conjugated polymer actuators. Mrs Bull. 2008;33:197–204. [Google Scholar]
- [50].Smela E. Conjugated polymer actuators for biomedical applications. Adv Mater. 2003;15:481–94. [Google Scholar]
- [51].Firme CP, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed-Nanotechnol. 2010;6:245–56. doi: 10.1016/j.nano.2009.07.003. [DOI] [PubMed] [Google Scholar]
- [52].Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- [53].Jain AK, Mehra NK, Lodhi N, Dubey V, Mishra DK, Jain PK, et al. Carbon nanotubes and their toxicity. Nanotoxicology. 2007;1:167–97. [Google Scholar]
- [54].Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Adv Drug Deliver Rev. 2006;58:1460–70. doi: 10.1016/j.addr.2006.09.015. [DOI] [PubMed] [Google Scholar]