Abstract
Dysfunction of the prefrontal cortex (PFC) is a central feature of many psychiatric disorders, such as attention deficit hyperactivity disorder (ADHD), post-traumatic stress disorder (PTSD), schizophrenia and bipolar disorder. Thus, understanding molecular influences on PFC function through basic research in animals is essential to rational drug development. In this review, we discuss the molecular signaling events initiated by norepinephrine and dopamine that strengthen working memory function mediated by the dorsolateral PFC under optimal conditions, and weaken working memory function during uncontrollable stress. We also discuss how these intracellular mechanisms can be compromised in psychiatric disorders, and how novel treatments based on these findings may restore a molecular environment conducive to PFC regulation of behavior, thought and emotion. Examples of successful translation from animals to humans include guanfacine for the treatment of ADHD and related PFC disorders, and prazosin for the treatment of PTSD.
Keywords: prefrontal cortex, spatial working memory, norepinephrine, dopamine, stress
Introduction
Dysfunction of the prefrontal cortex (PFC) is a central feature of many psychiatric disorders (reviewed in Arnsten, 1998; Arnsten, 2007b), and the associated cognitive dysfunction often predicts patients’ functional outcome in society (e.g. Bowie et al., 2010; reviewed in Green, 2006). Thus, it is critical to understand the molecular influences that modulate PFC function in order to develop intelligent medications for psychiatric disorders. Basic research on PFC mechanisms in animals has already led to the successful translation of two treatments for human PFC disorders: guanfacine (Intuniv™) for the treatment of Attention Deficit Hyperactivity Disorder (ADHD), and prazosin for the treatment of Post-Traumatic Stress Disorder (PTSD). This review will briefly describe the molecular influences that strengthen PFC function under optimal conditions, and those that weaken PFC function during uncontrollable stress and in psychiatric disorders, and how understanding these pathways can provide therapeutic targets for drug development.
Higher cognitive functions of the prefrontal cortex
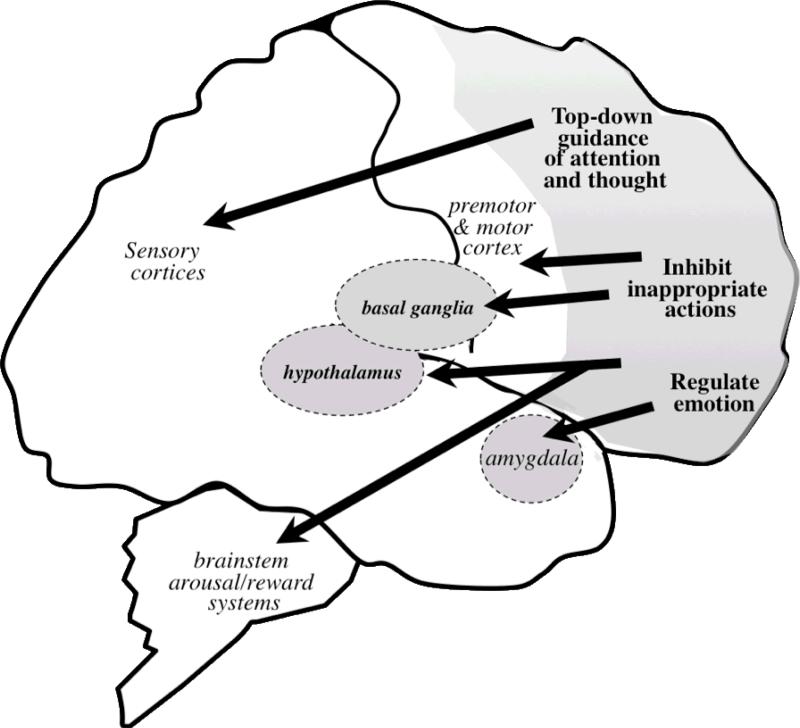
The PFC commands a range of “executive functions” to modulate behavior, thought and affect to produce thoughtful and purposeful actions (Goldman-Rakic, 1995; Goldman-Rakic, 1996). The PFC generates representational knowledge, which allows us to maintain specific information as well as rules and goals in mind to execute cognitive control over our actions and thoughts (Goldman-Rakic, 1995; Goldman-Rakic, 1996; Miller & Cohen, 2001). This “mental sketch pad” also rapidly updates the contents of working memory to remain relevant to the situation at hand (Goldman-Rakic, 1995; Goldman-Rakic, 1996). The PFC is highly interconnected with the rest of the brain (Figure 1), which allows it to receive pertinent information and in turn, appropriately modulate information processing in other regions (reviewed in Arnsten & Castellanos, 2002; Ghashghaei & Barbas, 2002; Goldman-Rakic, 1996; Middleton & Strick, 2000; Selemon & Goldman-Rakic, 1985; Selemon & Goldman-Rakic, 1988). In addition, the PFC projects to subcortical arousal systems, and thus can regulate monoamine and cholinergic inputs to other regions as well as onto itself (Arnsten & Goldman-Rakic, 1984; Aston-Jones, Rajkowski, & Cohen, 2000; Berridge & Waterhouse, 2003; Jodo, Chiang, & Aston-Jones, 1998; Robbins, 2005; Sara & Herve-Minvielle, 1995).
Figure 1.
The prefrontal cortex (PFC) is highly interconnected with the rest of the brain, allowing it to modulate information processing in other regions of the brain, as well as to regulate the arousal and reward systems, to appropriately modulate behavior, thought and affect.
Spatial working memory as a model to study molecular modulation of prefrontal circuits
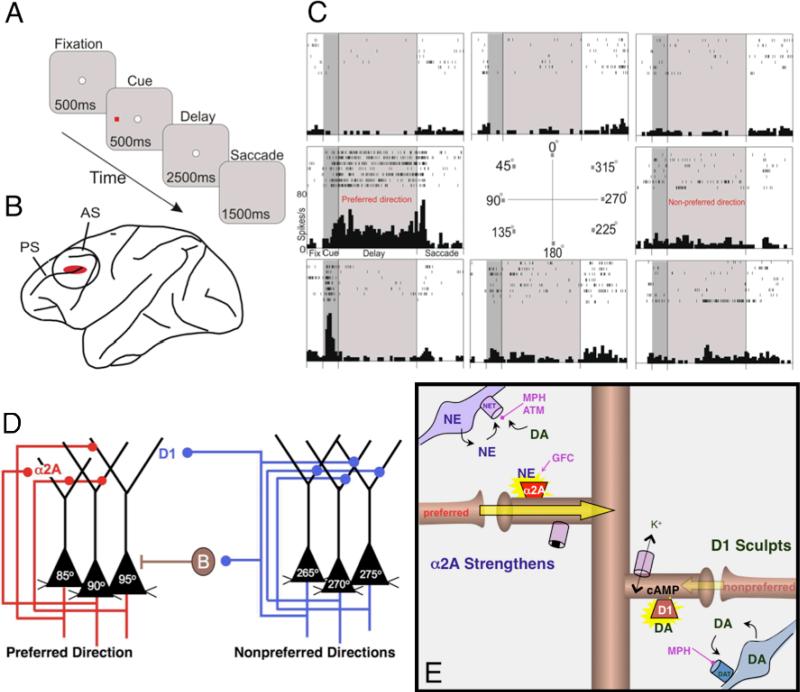
Much of the research on the molecular mechanisms of PFC function has focused on spatial working memory as a model of PFC function. The pioneering work of Goldman-Rakic has delineated the cellular circuitry underlying spatial working memory (Goldman-Rakic, 1995), allowing us to address the molecular mechanisms that strengthen or weaken these networks. Spatial working memory is mediated by the dorsolateral PFC (DLPFC), and specifically, the region surrounding the principal sulcus in monkeys. These studies have been performed in monkeys performing an oculomotor delayed response (ODR) spatial working memory task (Figure 2A), while single units are recorded in the DLPFC (Figure 2B). In this task, the monkeys are required to continually update their working memory for a specific location during the delay periods, when the cues are no longer present in the environment.
Figure 2.
Physiological recordings of PFC microcircuits in the monkey dorsolateral PFC (DLPFC), and their modulation by catecholamine signaling pathways.
A. Monkeys performed the oculomotor delayed response (ODR) task. Each trial begins when the monkey fixates on a central point on a screen (fixation period). Next, a cue briefly appears in 1 of 8 peripheral locations, followed by a 2.5-second delay period, during which the monkey continues to maintain fixation. At the end of the delay period, the monkey makes a memory-guided saccade to the remembered cue location (response period), and is rewarded with juice if correct. Each test session consists of hundreds of trials, in which the cued location randomly changes for each trial, thus requiring the monkey to update his working memory despite extensive, proactive interference.
B. Single-unit recording was performed in the DLPFC, the region associated with spatial working memory in monkeys.
C. The firing patterns of a representative neuron in the monkey DLPFC. Under optimal conditions, neurons show delay-related firing for a preferred direction, but suppress firing for non-preferred directions (Goldman-Rakic, 1995; Goldman-Rakic, 1996). This pattern is considered to be the cellular basis for spatial working memory.
D. Circuit basis for spatial working memory as proposed by Patricia Goldman-Rakic (Goldman-Rakic, 1995). Spatial working memory is maintained in the DLPFC by recurrent excitation among networks of glutamatergic pyramidal cells with shared stimulus inputs, e.g. 90° position in space. Spatial tuning is sharpened by GABAergic interneurons, such as basket cells (B) (Rao et al., 1999; Rao et al., 2000), that suppress firing for dissimilar spatial positions, e.g. 270°. The preferred inputs to the 90° neuron are shown in red; physiological data indicate that these networks are modulated by norepinephrine (NE) alpha-2A adrenoceptor stimulation. In contrast, inputs from non-preferred directions are shown in blue; physiological data indicate that these networks are sculpted by D1 dopamine (DA) receptor stimulation.
E. A working model of catecholamine influences on PFC network connectivity. Under optimal levels of PFC catecholamine release, alpha-2A adrenoceptors strengthen connectivity for the preferred direction of the PFC network by reducing cyclic adenosine monophosphate (cAMP) and closing hyperpolarizing potassium channels. Conversely, D1 DA receptors sharpen spatial tuning by weakening inputs from non-preferred directions by increasing cAMP production. Treatments for ADHD facilitate these actions to restore optimal PFC function, e.g. methylphenidate (MPH) and atomoxetine (ATM) block NE and DA transporters (NET and DAT) and enhance the endogenous stimulation of alpha-2A and D1 receptors, while guanfacine (GFC) directly stimulates alpha-2A adrenoceptors.
While the monkey is performing the ODR task, pyramidal neurons in the DLPFC exhibit persistent firing during the delay periods for cues in a particular direction, called the “preferred direction,” but show no increase or even suppressed firing for other directions, called the “non-preferred directions” (Goldman-Rakic, 1995) (Figure 2C). This pattern of firing is considered to be the cellular basis for spatial working memory. Persistent firing during the delay period is thought to arise from recurrent excitation among networks of PFC pyramidal cells with shared spatial properties, such as those cells receiving inputs for the 90-degree location in space (Goldman-Rakic, 1995; Goldman-Rakic, 1996) (Figure 2D). These cells reside in layer III of the cortex, where pyramidal cells make horizontal connections (Kritzer & Goldman-Rakic, 1995) that are likely mediated predominantly via NMDA receptors with some contribution from AMPA receptors (reviewed in Wang, 2001; Wang, Yang, Gamo & Arnsten, unpublished data). The specificity of the contents of working memory is further refined by lateral inhibition from GABAergic interneurons, such as basket cells, which inhibit networks representing other locations in space (Rao, Williams, & Goldman-Rakic, 1999; Rao, Williams, & Goldman-Rakic, 2000) (Figure 2D). The recurrent firing of pyramidal cell networks depends upon their effective synaptic contacts at dendritic spines; the strength of these connections is dynamically modulated via intracellular signaling events within these spines (e.g. Figure 2E), termed Dynamic Network Connectivity (DNC) (reviewed in Arnsten, Paspalas, Gamo, Yang, & Wang, 2010). While this mechanism allows rapid and precise updating of information according to changing environmental demands, it is also vulnerable to a variety of genetic and environmental insults, such as occur in psychiatric disorders and during exposure to stress.
PFC function varies according to arousal state
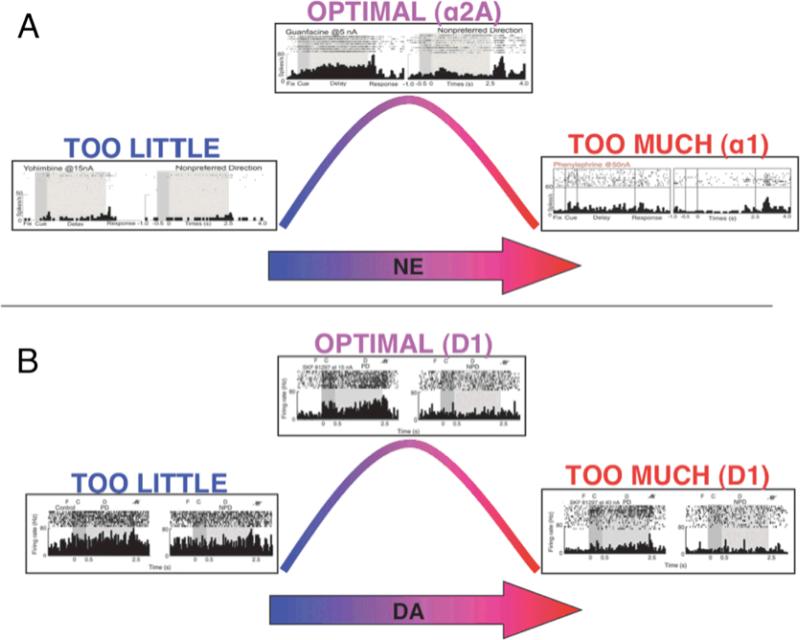
Beginning with the key work by Brozoski et al. (1979), it has been shown that the PFC is powerfully modulated by its neurochemical environment (reviewed in Arnsten, 2007a; Brozoski, Brown, Rosvold, & Goldman, 1979; Robbins & Arnsten, 2009). The DLPFC is especially sensitive to the catecholamines – dopamine (DA) and norepinephrine (NE) – which are released into the PFC according to one's state of arousal or in response to environmental events. Thus, these powerful influences may have evolved to coordinate cognitive state with environmental demands. The catecholamines have an inverted-U-shaped influence on DLPFC function, such that too little or too much impairs function, while moderate levels are required for optimal function (Figure 3).
Figure 3.
NE and DA show inverted-U dose-response curves on delay-related activity in a monkey performing the ODR task.
A. Optimal levels of NE enhance delay-related activity for the preferred direction via alpha-2A adrenoceptors, while excessive levels suppress firing via alpha-1 and beta-1 adrenoceptors (Birnbaum et al., 2004; Wang et al., 2007).
B. Optimal levels of DA enhance spatial tuning by suppressing delay-related firing for the non-preferred direction, while excessive levels suppress firing for both preferred and non-preferred stimuli (Vijayraghavan et al., 2007).
The primate PFC receives NE projections from the locus coeruleus (reviewed in Berridge & Waterhouse, 2003; Levitt, Rakic, & Goldman-Rakic, 1984; Porrino & Goldman-Rakic, 1982). The NE system is thought to be involved in optimizing goal-directed behavior, where NE neurons fire according to one's arousal state and selective attention (reviewed in Aston-Jones, Rajkowski, & Cohen, 1999). They show 1) silence during rapid eye movement sleep, 2) low, tonic firing during slow wave sleep and drowsiness, 3) moderate, tonic firing with phasic responses to relevant stimuli during non-stressed waking, and 4) high tonic firing during stress (reviewed in Aston-Jones et al., 1999). Interestingly, NE neurons can also respond to irrelevant stimuli during fatigue or stress, but inhibit these responses during non-stressed waking (reviewed in Aston-Jones et al., 1999). Appropriate NE firing to environmental stimuli is likely regulated by the PFC (Arnsten & Goldman-Rakic, 1984; Aston-Jones et al., 2000; Berridge & Waterhouse, 2003; Jodo et al., 1998; Sara & Herve-Minvielle, 1995), while high tonic firing during stress exposure requires activation from the amygdala (Goldstein, Rasmusson, Bunney, & Roth, 1996).
The primate PFC receives DA projections from the ventral tegmental area and substantia nigra (Domesick, 1988; Fallon, 1988; Garris, Collins, Jones, & Wightman, 1993; Goldman-Rakic, Lidow, Smiley, & Williams, 1992; Levitt et al., 1984; Lewis, Campbell, Foote, Goldstein, & Morrison, 1987; Lewis, 1992; Lewis, Hayes, Lund, & Oeth, 1992; Porrino & Goldman-Rakic, 1982). Tonic firing of DA neurons may reflect arousal state, while phasic responses are likely associated with reward, for example, during the presentation of a spatial cue in a spatial working memory task (reviewed in Arnsten, Vijayraghavan, Wang, Gamo, & Paspalas, 2009; Schultz, Apicella, & Ljungberg, 1993). In fact, many DA neurons respond phasically to reflect prediction error of rewards (Schultz, 1998). However, a subset of DA neurons fire in response to aversive stimuli (Matsumoto & Hikosaka, 2009), which may increase DA release in the PFC during stressful situations. Rodent studies have also shown catecholamine release to be enhanced in the medial PFC during acute stress (Deutch & Roth, 1990; Finlay, Zigmond, & Abercrombie, 1995; Goldstein et al., 1996; Roth, Tam, Ida, Yang, & Deutch, 1988).
These catecholaminergic projections target the PFC in a lamina and synapse-specific manner, allowing them to precisely modulate PFC network connectivity at those levels. NE and DA neurons target post-synaptic terminals in the superficial and deep layers of the cortex (Aston-Jones et al., 1999; Goldman-Rakic et al., 1992; Levitt et al., 1984; Lewis et al., 1987; Lewis & Morrison, 1989), with DA neurons shown to specifically contact dendritic spines (reviewed in Goldman-Rakic, Leranth, Williams, Mons, & Geffard, 1989; Goldman-Rakic et al., 1992; Smiley & Goldman-Rakic, 1993). These projections target receptors that are also distributed in a lamina and synapse-specific manner, as described below, which further refine the specificity of catecholaminergic modulation and consequent intracellular signaling within PFC circuits.
Once released, the catecholamines initiate a network of intracellular cascades that can rapidly strengthen or weaken the connectivity within PFC networks (Figure 4). Too little release of catecholamines during fatigue or boredom suspends PFC function, likely to conserve energy, as maintenance of working memory is an energy-intensive process (Friedman & Goldman-Rakic, 1994; Swartz, Halgren, Simpkins, & Mandelkern, 1996). PFC function may also be reduced during stressful, dangerous situations when rapid, instinctual or habitual reactions mediated by the amygdala and striatum are enlisted in place of slow, thoughtful responses by the PFC (reviewed in Cahill & McGaugh, 1996; Hu et al., 2007; McEwen, 2004; Wickens, Horvitz, Costa, & Killcross, 2007). In contrast, moderate release of catecholamines occurs during optimal arousal conditions, which would strengthen PFC cognitive abilities (see below; also reviewed in Arnsten, 2009a; Arnsten et al., 2010).
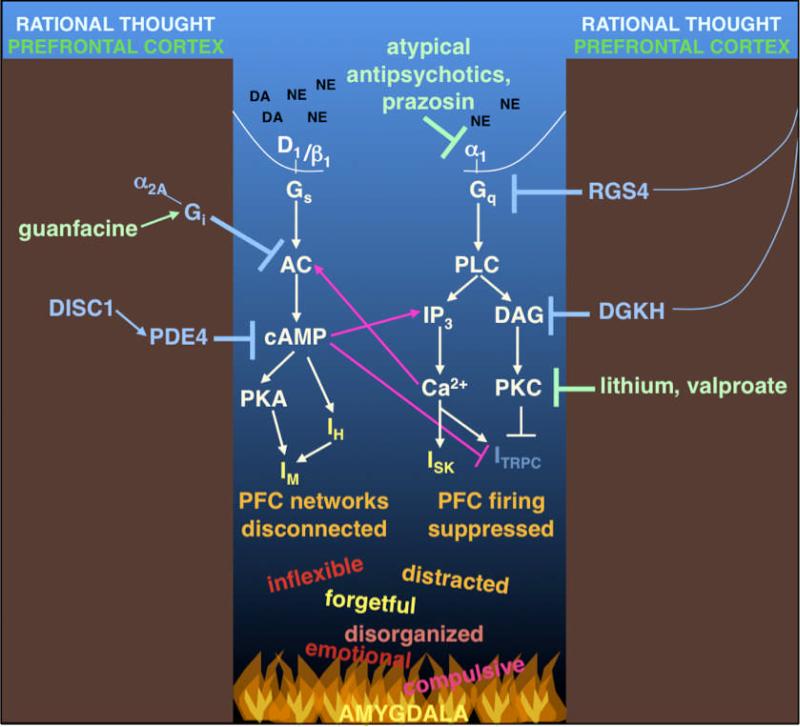
Figure 4.
During exposure to uncontrollable stress, high levels of NE and DA release initiate intracellular signaling events that increase potassium currents (IH, IM and ISK), and decrease depolarizing currents (ICAN, also called ITRPC). This cascade leads to a collapse in PFC network firing and a loss of PFC regulation of behavior. Many of these signaling events appear to have feed-forward interactions, e.g. whereby cAMP facilitates IP3 receptor signaling and vice versa, thus providing the opportunity for rapid changes in cellular physiology. In such a situation, rational control of behavior goes to “hell in a handbasket,” and is replaced by instinctual reactions mediated by subcortical regions such as the amygdala, which are strengthened rather than weakened by PKA and PKC signaling. However, there are signaling pathways in the PFC that serve as “molecular brakes” to inhibit these stress-induced pathways, e.g. DISC1, RGS4 and DGK. Intriguingly, these molecules are often genetically altered in mental illness. Many treatments for psychiatric disorders inhibit these stress responses, e.g. prazosin and atypical antipsychotics block alpha-1 adrenoceptors, while the bipolar agents, lithium and valproate, reduce PKC signaling. These treatments may restore a more optimal signaling environment in the PFC. Abbreviations: AC: adenylyl cyclase; cAMP: cyclic adenosine monophosphate; DA: dopamine; DAG: diacylglycerol; DGKH: DAG kinase-η; DISC1: Disrupted In Schizophrenia 1; IH: h-current mediated by hyperpolarization-activated cyclic nucleotide gated cation (HCN) channels; IM: M-current mediated by KCNQ channels; IP3: inositol triphosphate; ISK: current mediated by small-conductance calcium-activated potassium (SK) channels; ITRPC: current mediated by canonical transient receptor potential channels (TRPC); NE: norepinephrine; PDE4: phosphodiesterase 4; PFC: prefrontal cortex; PKA: protein kinase A; PKC: protein kinase C; PLC: phopholipase C; RGS4: Regulator of G-protein Signaling 4.
Patients with psychiatric disorders are especially vulnerable to stress-induced PFC impairment (e.g. Hammen & Gitlin, 1997), but such impairment occurs under normal circumstances as well, e.g. when getting a call from a doctor with bad news about a loved one. In today's society, it would certainly be useful for PFC function to remain intact during stress, e.g. when restraining ourselves from road rage or remaining calm during a disagreement with a boss. However, this system has evolved to promote survival when one's physical safety is threatened, e.g. when fleeing from an approaching tiger or freezing at the sight of a bear in the woods. Enhancing the functions of the amygdala and hippocampus during such situations may also enhance long-term memories that would promote survival during a similar circumstance in the future.
Modulation of PFC function under optimal arousal conditions
Under optimal arousal conditions, moderate levels of NE and DA regulate PFC network inputs such that signals for relevant information are enhanced, while those for irrelevant noise are suppressed. These actions occur via receptors that influence cyclic adenosine monophosphate (cAMP) production, which in turn alters the open probability of ion channels localized near synaptic inputs on dendritic spines (Wang et al., 2007). NE and DA have complementary actions on PFC network connectivity, with NE stimulation of alpha-2A adrenoceptors increasing “signals” via suppression of cAMP production, and D1 DA receptor stimulation decreasing “noise” by increasing cAMP.
NE strengthens PFC representations by stimulating alpha-2A adrenoceptors in the PFC (Avery, Franowicz, Studholme, van Dyck, & Arnsten, 2000; Birnbaum, Podell, & Arnsten, 2000; Franowicz et al., 2002; Jakala et al., 1999; Li & Mei, 1994; Li, Mao, Wang, & Mei, 1999; Ramos, Stark, Verduzco, van Dyck, & Arnsten, 2006; Sawaguchi, 1998; Tanila, Rama, & Carlson, 1996; Wang et al., 2007) (Figures 2E, 3A). These receptors have higher affinity for NE than the alpha-1 and beta-1 adrenoceptors (O'Rourke, Blaxall, Iversen, & Bylund, 1994), which are discussed below. Much of the previous research has focused on the role of alpha-2 adrenoceptors on presynaptic terminals. However, it is now known that the majority of alpha-2 adrenoceptors in the brain are actually post-synaptic to NE terminals (U'Prichard, Bechtel, Rouot, & Snyder, 1979), and it is these receptors that likely mediate the beneficial effects of NE and alpha-2A agonists on PFC function (Arnsten & Cai, 1993; Arnsten, Steere, & Hunt, 1996; Wang et al., 2007). Alpha-2A adrenoceptors are localized on dendritic spines in the superficial layers of the PFC (Aoki, Go, Venkatesan, & Kurose, 1994; Aoki, Venkatesan, & Kurose, 1998; Goldman-Rakic, Lidow, & Gallager, 1990; Wang et al., 2007), allowing them to modulate PFC circuits in a synapse-specific manner.
Stimulation of alpha-2A adrenoceptors in the PFC improves working memory performance in rodents and monkeys (Arnsten & Goldman-Rakic, 1985; Cai, Ma, Xu, & Hu, 1993; Mao, Arnsten, & Li, 1999; Ramos et al., 2006). At the cellular level, stimulating alpha-2A adrenoceptors in the DLPFC of a monkey engaged in a spatial working memory task enhances recurrent network activity for the relevant cue positions, but not for other positions (Li et al., 1999; Wang et al., 2007) (Figure 3A). Blocking these receptors or depleting NE from the PFC impairs working memory performance and delay-related firing (Arnsten & Goldman-Rakic, 1985; Li & Mei, 1994; Li et al., 1999), likely because there is insufficient encoding of the working memory “signal.”
Alpha-2A adrenoceptors enhance network connectivity by inhibiting cAMP production (Ramos et al., 2006; Wang et al., 2007), which strengthens synaptic connectivity by closing potassium channels, such as the hyperpolarization-activated cyclic nucleotide gated cation (HCN) channels (Wang et al., 2007) and possibly Kv7 (KCNQ) potassium channels (Delmas & Brown, 2005; George, Abbott, & Siegelbaum, 2009; Arnsten, unpublished data) (Figures 2E, 4). Alpha-2A adrenoceptors are colocalized with HCN channels at dendritic spines (Wang et al., 2007), which places them in an ideal location to interact via intracellular pathways within the spines.
Inhibition of cAMP signaling may also enhance connectivity by opening depolarizing TRPC channels (canonical transient receptor potential channels) (Hagenston, Fitzpatrick, & Yeckel, 2008; Partridge, Swandulla, & Muller, 1990) (Figure 4). These actions would depolarize the spine and increase the efficacy of nearby network inputs.
Moderate levels of DA help to “sculpt” the network firing, that is, reduce noise to focus the stimulus selectivity of PFC networks, via moderate simulation of D1/5 receptors (Vijayraghavan, Wang, Birnbaum, Williams, & Arnsten, 2007) (Figures 2E, 3B). (As there are currently no drugs that can distinguish between the D1 and D5 subtypes, we will henceforth refer to the D1/5 receptor family as “D1 receptors.”) Physiological recordings from the PFC of monkeys performing a spatial working memory task show that moderate levels of D1 receptor stimulation suppress network firing for irrelevant cue positions, thus enhancing the signal-to-noise ratio (Vijayraghavan et al., 2007). D1 receptors are localized on dendritic spines of pyramidal cells, allowing DA to modulate PFC circuits in a synapse-specific manner (Bergson et al., 1995; Smiley, Levey, Ciliax, & Goldman-Rakic, 1994). D1 receptors and alpha-2A adrenoceptors appear to be on separate subsets of dendritic spines (Paspalas & Arnsten, unpublished data), which would allow them to simultaneously modulate different PFC networks.
D1 receptors hyperpolarize dendritic spines and reduce connectivity among pyramidal cell networks by increasing cAMP production (Vijayraghavan et al., 2007; Zahrt, Taylor, Mathew, & Arnsten, 1997), which in turn opens potassium channels on dendritic spines (Gamo, Wang & Arnsten, unpublished data) (Figures 2E, 4). DA depletion or insufficient D1 stimulation impairs working memory performance (Sawaguchi & Goldman-Rakic, 1991; Sawaguchi & Goldman-Rakic, 1994; Vijayraghavan et al., 2007; Zahrt et al., 1997), and creates noisy firing patterns, where the neuron fires during the delay period to both relevant and irrelevant information (Vijayraghavan et al., 2007) (Figure 3B). In contrast, moderate levels of D1 receptor stimulation decrease firing to non-preferred directions, narrowing the spatial tuning of the neuron (Figure 3B). It is likely that these sculpting effects on neuronal firing are especially beneficial when a cognitive task requires a narrow focus of memory or attention, such as when remembering a small, specific location in space. In contrast, D1 receptor stimulation may actually be harmful when broader inputs or cognitive flexibility is required, for example, as seen with attentional set-shifting tasks (Crofts et al., 2001).
Treatments that restore optimal levels of alpha-2A and D1 receptor stimulation can help to strengthen PFC function in disorders associated with insufficient catecholamine transmission, such as ADHD. For example, the symptoms of inattentiveness, hyperactivity and impulsivity in ADHD are consistent with PFC dysfunction, and a variety of approaches have implicated weakened PFC function in patients with ADHD. Both structural and functional imaging studies show evidence of smaller PFC volume, reduced PFC activity, weaker PFC white matter connections and weaker resting connectivity in patients (Arnsten, 2006; Castellanos & Tannock, 2002; Dickstein, Bannon, Castellanos, & Milham, 2006; Kieling, Goncalves, Tannock, & Castellanos, 2008; Seidman, Valera, & Makris, 2005; Spencer, 2002). Similarly, many neuropsychological studies have shown evidence of impaired PFC function, especially on tasks that require inhibition of inappropriate actions or sustained regulation of attention (Barkley, 1997).
ADHD can be associated with abnormal catecholamine transmission (reviewed in Arnsten, 2006; Arnsten, 2009b; e.g. Greene, Bellgrove, Gill, & Robertson, 2009; Hess et al., 2009; Kieling, Genro, Hutz, & Rohde, 2008). For example, a subset of ADHD cases is associated with polymorphisms in the gene that encodes for DA beta-hydroxylase, the enzyme that converts DA to NE, which may result in insufficient noradrenergic activity (Daly, Hawi, Fitzgerald, & Gill, 1999; reviewed in Faraone et al., 2005; Kopeckova, Paclt, & Goetz, 2006; Roman et al., 2002). Approved treatments for ADHD enhance catecholamine actions in the PFC, likely restoring catecholamine activity to an optimal range (Arnsten et al., 1996). One such treatment is guanfacine (Intuniv™), an alpha-2A andrenoceptor agonist that mimics NE actions at these receptors (Biederman, Melmed, Patel, McBurnett, Konow et al., 2008; Uhlen & Wikberg, 1991) (Figures 2E, 4).
Originally marketed as an anti-hypertension drug (Sorkin & Heel, 1986), initial studies of guanfacine in animals have illustrated its beneficial effects on PFC function. Guanfacine improved performance in a delayed response task in adult (Avery et al., 2000; Mao et al., 1999; Ramos et al., 2006) and aged (Arnsten, Cai, & Goldman-Rakic, 1988; Rama, Linnankoski, Tanila, Pertovaara, & Carlson, 1996) monkeys, as well as in rats (Ramos et al., 2006). It enhanced delay-related firing in the DLPFC (Wang et al., 2007) and increased the regional cerebral blood flow in this region relative to more posterior cortical regions (Avery et al., 2000) in monkeys performing a spatial working memory task, confirming the importance of this region for the actions of guanfacine. Guanfacine also improved other PFC functions, such as reversal performance in a visual object discrimination task in aged monkeys (Steere & Arnsten, 1997), and performance in a visuomotor association task in adult monkeys (Wang, Tang, & Li, 2004). Furthermore, guanfacine protected cognitive function from distraction by irrelevant stimuli in aged monkeys (O'Neill, Fitten, Siembieda, Ortiz, & Halgren, 2000), and reduced overactivity, impulsiveness and inattentiveness in the spontaneously hypertensive rat, a model of ADHD (Sagvolden, 2006). The range of cognitive functions that benefit from guanfacine illustrate that findings from studies using spatial working memory can be generalized to other PFC functions.
As in animal models, guanfacine has been shown to be effective in improving PFC function in ADHD patients. Guanfacine significantly reduced symptoms in children, adolescents (Biederman, Melmed, Patel, McBurnett, Donahue et al., 2008; Biederman, Melmed, Patel, McBurnett, Konow et al., 2008; Hunt, Arnsten, & Asbell, 1995; Sallee et al., 2009; Scahill et al., 2001) and adults (Hunt et al., 1995; Taylor & Russo, 2001) with ADHD. In children with co-morbid ADHD and tic disorders, it improved sustained visual attention and motor response inhibition, and decreased the severity of tics (Chappell et al., 1995; Scahill et al., 2001). It also improved performance in tests of attention in adults with ADHD (Taylor & Russo, 2001). In addition to ADHD, guanfacine has also improved behavior in autism spectrum disorders (McCracken et al., 2010; Wink, Erickson, & McDougle, 2010). Thus, mimicking the action of NE at alpha-2A adrenoceptors appears to be helpful in many conditions in which top-down PFC guidance of behavior is weakened.
Guanfacine provides an advantage over previously existing alpha-2 agonist treatments, such as clonidine (Catapres™), as it has less sedative and hypotensive side effects due to is greater selectivity for the alpha-2A subtype (Arnsten et al., 1988; Uhlen & Wikberg, 1991). Additional ADHD treatments, methylphenidate (Ritalin™, Concerta™) and atomoxetine (Strattera™), have recently been shown to act via similar mechanisms. These treatments improve delayed response performance in monkeys by increasing endogenous stimulation of alpha-2 adrenoceptors and D1 dopamine receptors (Gamo, Wang, & Arnsten, 2010) (Figure 2E).
Stress signaling pathways take PFC “off-line”
Under uncontrollable stress, excess levels of catecholamines are released in the PFC, and initiate a variety of intracellular pathways that rapidly impair PFC function (Aston-Jones et al., 1999; Deutch & Roth, 1990; Deutch, Clark, & Roth, 1990; Finlay et al., 1995; Roth et al., 1988) (Figure 4). As shown in Figure 4, many of the molecules that inhibit the stress pathways are genetically altered in patients with mental illness, which may explain why these disorders are often precipitated or exacerbated by stress (reviewed in Arnsten, 2009a; Breier, Wolkowitz, & Pickar, 1991; Dohrenwend, Shrout, Link, Skodol, & Stueve, 1995; Hammen & Gitlin, 1997; Mazure, 1995; Mazure & Maciejewski, 2003; Southwick, Rasmusson, Barron, & Arnsten, 2003).
High DA levels impair PFC function via excessive stimulation of D1 DA receptors and widespread increases in cAMP production (Vijayraghavan et al., 2007; Zahrt et al., 1997) (Figures 3B, 4). Physiological studies show that high levels of D1 receptor stimulation suppress all PFC network firing, reducing responses to both preferred and non-preferred inputs (Vijayraghavan et al., 2007) (Figure 3B). These reductions in neuronal firing are mimicked by compounds that increase cAMP signaling, and prevented by cAMP inhibition (Vijayraghavan et al., 2007; Wang et al., 2007). Excess D1 receptor stimulation in the PFC also results in impaired spatial working memory performance in monkeys and rodents (Arnsten & Goldman-Rakic, 1990; Sawaguchi & Goldman-Rakic, 1991; Zahrt et al., 1997). These DA actions are an important part of the stress response, as stress-induced impairments in working memory performance can be prevented by pre-treating the animals with a D1 receptor antagonist (Murphy, Arnsten, Goldman-Rakic, & Roth, 1996).
While D1 DA receptors appear to suppress PFC neuronal activity in a cognitively engaged animal, they have also been shown to enhance excitability in cortical pyramidal cells in vitro (Gorelova & Yang, 2000; Henze, Gonzalez-Burgos, Urban, Lewis, & Barrionuevo, 2000; Seamans, Durstewitz, Christie, Stevens, & Sejnowski, 2001; Wang & O'Donnell, 2001; Yang & Seamans, 1996). It is possible that in a cognitively engaged animal, these basic excitatory influences are saturated, and additional inhibitory effects are seen with higher levels of stimulation (Vijayraghavan et al., 2007), or that the inhibitory effects in vivo override the excitatory influences observed in vitro (reviewed in Arnsten et al., 2009). In this case, having no D1 receptor stimulation would prevent even the basic excitatory influences, thus suppressing all firing. Consistent with this idea, blocking D1 receptors has been shown to reduce DLPFC firing as well as impair performance in monkeys performing a spatial working memory task (Sawaguchi & Goldman-Rakic, 1991; Sawaguchi & Goldman-Rakic, 1994; Sawaguchi, 2000; Sawaguchi, 2001).
High levels of NE release during stress impairs PFC function via lower-affinity alpha-1 (Arnsten, Mathew, Ubriani, Taylor, & Li, 1999; Birnbaum, Gobeske, Auerbach, Taylor, & Arnsten, 1999; Mao et al., 1999; Mohell, Svartengren, & Cannon, 1983) and beta-1 (Aoki et al., 1998; Ramos et al., 2005) adrenoceptors in the PFC (Arnsten, 2000) (Figure 3A). Alpha-1 adrenoceptors are concentrated in superficial layers of the cortex (Goldman-Rakic et al., 1990), while beta receptors are found mainly in the intermediate layers (Aoki et al. 1998). Beta adrenoceptors have been localized on dendritic spines (Aoki et al., 1998), such that they can modulate the PFC circuits in a synapse-specific manner.
Beta-1 adrenoceptors stimulate cAMP production, converging onto the D1 receptor-mediated cAMP signaling to impair PFC function (reviewed in Arnsten, 2007a; Ramos et al., 2005) (Figure 4). In contrast, alpha-1 adrenoceptors activate phosphatidylinositol (PI) signaling, increasing IP3-calcium and protein kinase C (PKC)-mediated pathways via Gq signaling (Figure 4). IP3 receptor stimulation increases calcium release from the endoplasmic reticulum (Soulsby & Wojcikiewicz, 2005), which in turn opens small-conductance calcium-activated potassium (SK) channels (Brennan et al., 2008; Faber, 2010; Hagenston et al., 2008). Calcium can also stimulate some forms of adenylyl cyclase to increase cAMP production (Ferguson & Storm, 2004), further contributing to the collapse of PFC network activity. Finally, calcium is needed for the migration of PKC to the membrane, where it is activated by diacylglycerol (DAG). Activation of this pathway in PFC impairs working memory in rats and monkeys (Birnbaum et al., 2004; Runyan, Moore, & Dash, 2005), and suppresses PFC network firing (Birnbaum et al., 2004).
As with D1 DA receptors, alpha-1 and beta adrenoceptors have also been shown to have excitatory influences on cortical pyramidal cells in vitro (McCormick, Pape, & Williamson, 1991; Mouradian, Sessler, & Waterhouse, 1991; Waterhouse, Moises, & Woodward, 1981). In particular, alpha-1 receptors have been shown to have excitatory effects in the somatosensory cortex (Mouradian et al., 1991; Waterhouse et al., 1981). Again, these basic effects in vitro are likely fully engaged in an awake, behaving animal, and additional inhibitory effects are observed in vivo.
Chronic stress exposure leads to further changes in the cellular architecture of the PFC. There is prominent loss of dendritic spines and atrophy of dendrites in PFC pyramidal cells following chronic stress (Radley & Morrison, 2005; Radley et al., 2006), which correlates with impaired working memory (Hains et al., 2009) and attentional control (Liston, McEwen, & Casey, 2009). Dendritic spine loss appears to involve increased PKC signaling, as daily treatment with a PKC inhibitor prevents the spine loss and cognitive deficits associated with chronic stress (Hains et al., 2009). It is possible that spine loss occurs via PKC-mediated phosphorylation of myristoylated, alanine-rich C-kinase substrate (MARCKS), which leads to actin destabilization and spine collapse (Calabrese & Halpain, 2005). This mechanism may also contribute to the PFC gray matter loss associated with lead poisoning, as lead potently activates PKC by mimicking calcium actions (Markovac & Goldstein, 1988).
While DA and NE neurons project extensively throughout the brain and affect similar receptors in other regions, subsequent signaling cascades and net effects on cognition appear to be unique to the PFC. In the amygdala, stress-induced stimulation of alpha-1 (Ferry, Roozendaal, & McGaugh, 1999) and beta-1 (Cahill & McGaugh, 1996; Debiec & LeDoux, 2006; Ferry & McGaugh, 1999; Introini-Collison, Miyazaki, & McGaugh, 1991; Roozendaal, Quirarte, & McGaugh, 2002) receptors strengthens its function, for example in retaining memory for the inhibitory avoidance task or fear conditioning (reviewed in Wilensky, Schafe, & LeDoux, 2000). Furthermore, D1 receptor stimulation and increase in cAMP help to consolidate long-term memory by facilitating interactions between the hippocampus and PFC (Hopkins & Johnston, 1988; Runyan et al., 2005; Seamans, Floresco, & Phillips, 1998). Such effects may involve D1 receptors that are localized in cellular compartments with few HCN channels to disconnect the synaptic inputs (Arnsten, 2007a). DA also stimulates D2 receptors, which are less abundant than D1 receptors in the PFC and are found mostly in layer V (Lidow, Goldman-Rakic, Gallager, & Rakic, 1991), where cortical outputs to subcortical regions are located. Consistent with this function of layer V, D2 receptors have been shown to modulate response-related firing in the DLPFC of monkeys performing a spatial working memory task (Wang, Vijayraghavan, & Goldman-Rakic, 2004). This activity may serve as a corollary discharge to indicate that the animal has made a response (Arnsten, 2007b). D2 receptors also oppose D1 receptor actions by reducing excitability in PFC pyramidal cells and interneurons (Gulledge & Jaffe, 1998; Seamans et al., 2001; Zheng, Zhang, Bunney, & Shi, 1999). It is currently not known whether mechanisms similar to those in the PFC exist in other association cortices. This unique neurochemical requirement for optimal PFC function is important when developing treatments that target PFC cognitive functions with minimal side effects.
Relationships to genetic alterations in mental illness
There are a variety of mechanisms that serve as “molecular brakes” to protect cognitive function under stress. Figure 4 illustrates some of these molecules: Disrupted in Schizophrenia 1 (DISC1), Regulator of G-protein Signaling 4 (RGS4) and DAG kinase-eta (DGKH). Several psychiatric disorders are associated with genetic alterations of these “molecular brakes,” which may disinhibit the stress signaling pathways and lower the threshold for stress-induced PFC dysfunction.
DISC1, first identified with a translocation mutation in a Scottish family, is now associated with many psychiatric disorders in addition to schizophrenia, and in many populations worldwide (reviewed in Chubb, Bradshaw, Soares, Porteous, & Millar, 2008; Millar et al., 2000). It is a scaffold protein that is found in dendritic spines in the DLPFC of humans (Kirkpatrick et al., 2006; Paspalas & Arnsten, unpublished data), and interacts with many proteins, including phosphodiesterase 4 (PDE4) (Millar et al., 2005; Millar et al., 2007) (Figure 4). DISC1 may activate PDE4 under conditions of high cAMP production (Millar et al., 2005), as occur during stress exposure. Thus, a genetic insult to DISC1 would prevent proper PDE4 function, leading to build-up of cAMP and collapse of PFC function. Consistent with this hypothesis, rodents with Disc1 mutations show impaired working memory function (Gamo et al., 2010; Koike, Arguello, Kvajo, Karayiorgou, & Gogos, 2006; Kvajo et al., 2008).
RGS4 and DGKH have been shown to inhibit Gq-mediated PI-PKC signaling (Figure 4). RGS4, which inhibits Gq signaling (Berman, Wilkie, & Gilman, 1996; Hepler, Berman, Gilman, & Kozasa, 1997; Watson, Linder, Druey, Kehrl, & Blumer, 1996), is greatly reduced in the PFC of patients with schizophrenia (Erdely, Tamminga, Roberts, & Vogel, 2006; Mirnics, Middleton, Stanwood, Lewis, & Levitt, 2001; Volk, Eggan, & Lewis, 2010). Other studies have also found genetic alterations in RGS4 in association with schizophrenia and bipolar disorder (Chowdari et al., 2002; Levitt, Ebert, Mirnics, Nimgaonkar, & Lewis, 2006; Morris et al., 2004; Talkowski et al., 2006), including evidence that RGS4 genotype interacts with PFC volume and connectivity in patients and control subjects (Buckholtz et al., 2007; Prasad et al., 2005). DGKH hydrolyzes DAG and inhibits PKC activation (Figure 4), and mutations that affect its function are strongly associated with bipolar disorder (reviewed in Arnsten & Manji, 2008; Baum et al., 2008). Loss of DGKH function would disinhibit PKC signaling, which may contribute to the loss of PFC gray matter and impaired PFC function observed in bipolar disorder (reviewed in Arnsten & Manji, 2008). Importantly, most treatments for bipolar disorder inhibit PKC signaling, e.g. lithium and valproate (reviewed in Arnsten & Manji, 2008; Manji & Lenox, 1999; Manji, McNamara, Chen, & Lenox, 1999) (Figure 4). Other treatments for psychiatric disorders also target these Gq-mediated pathways. For example, many atypical antipsychotics block alpha-1 and serotonin 5-HT2 receptors, which are both coupled to Gq signaling (reviewed in Arnsten & Manji, 2008; Deutch et al., 1991; Manji & Lenox, 1999), and the alpha-1 adrenoceptor antagonist, prazosin, is used to treat PTSD (Figure 4).
PTSD symptoms involve inappropriate, disinhibited fear responses to trauma-associated stimuli, which are consistent with an impaired ability of the PFC to modulate fear responses via inputs to the amygdala (reviewed in Bremner, 2007; Bremner, Elzinga, Schmahl, & Vermetten, 2008; Elzinga & Bremner, 2002; Milad, Rauch, Pitman, & Quirk, 2006; Morgan, Romanski, & LeDoux, 1993; Southwick et al., 1999). In particular, the medial PFC, which in both humans and rats is reciprocrally connected with they amygdala, is involved in the extinction of fear conditioning (Milad & Quirk, 2002; reviewed in Milad et al., 2006; Morgan et al., 1993), and may be deficient in PTSD patients (reviewed in Milad et al., 2006). Consistent with these hypotheses, studies in PTSD patients show impaired medial PFC activation in response to reminders of traumatic events (Bremner et al., 1999; Bremner, 1999; Bremner et al., 2005; Shin et al., 2004), as well as enhanced amygdala activity (Bremner et al., 2005; Liberzon et al., 1999; Rauch et al., 2000; Shin et al., 1997; Shin et al., 2004) when exposed to negative emotional material relative to normal control subjects. There is further support for impaired PFC function, as PTSD is associated with elevated catecholamine release in response to stress (reviewed in Charney, Deutch, Krystal, Southwick, & Davis, 1993; Southwick et al., 1999), and patients show deficits in working memory (Bremner et al., 1993; Bremner et al., 1995). They are also impaired in the Stroop task, which requires inhibition of habitual responses (McNally, Kaspi, Riemann, & Zeitlin, 1990; McNally, English, & Lipke, 1993; Vasterling, Brailey, Constans, & Sutker, 1998), and in object-alternation and extinction tasks, which depend on the medial PFC (reviewed in Milad et al., 2006).
Prazosin was originally used to treat hypertension and benign prostatic hypertrophy (Hieble & Ruffolo, 1996; Lund-Johansen, Hjermann, Iversen, & Thaulow, 1993), but has since been shown to improve PFC function and weaken amygdala function in animal studies (Ferry et al., 1999). While it did not affect delayed response performance in monkeys under non-stress conditions (Arnsten & Goldman-Rakic, 1985; Li & Mei, 1994), it was able to prevent impairment in spatial working memory induced by stimulation of alpha-1 adrenoceptors as in stress (Arnsten & Jentsch, 1997). Prazosin is now being tested in both civilian (Taylor & Raskind, 2002; Taylor et al., 2006; Taylor et al., 2008) and combat veterans (Raskind et al., 2000; Raskind et al., 2002; Raskind et al., 2003; Raskind et al., 2007) with PTSD, and may also reduce alcohol abuse in veterans with PTSD symptoms (Simpson et al., 2009).
The challenges of translation from animals to humans
It is no accident that the two examples of successful translation discussed above – guanfacine for ADHD and prazosin for PTSD – are drugs that had already been approved for human use to treat other conditions. This fact has allowed creative clinical researchers to directly test ideas emerging from basic research in human patients. The great challenge in drug development is when exciting mechanisms are identified in animals, but no appropriate agents are immediately available for human use. Unfortunately, developing new compounds that are safe for human use involves great expenses in time and money, and relies on the interest of large pharmaceutical companies or small biotechnology firms founded on venture capital. This “valley of death” during the drug development process has slowed or halted the progress of many burgeoning ideas. Studies in monkeys can be particularly helpful in this regard, as a drug that improves cognition following oral administration in monkeys has a high likelihood of succeeding in humans. In fact, the exact same doses of guanfacine and prazosin that have improved PFC function in young monkeys in the above studies are now used in humans. Thus, such studies in primates should help to decide which strategies are worthy of further development, and which doses would be appropriate for human trials.
Conclusion
The rich knowledge of molecular and cellular mechanisms mediating PFC function and dysfunction gained from animal models has allowed the identification of potential drug targets for psychiatric disorders. Elucidating these basic mechanisms is key to understanding the etiology of such disorders, and for the rational translation of basic research to human therapies. The present review has focused on mechanisms underlying spatial working memory. Some of these mechanisms likely extend to other PFC cognitive operations, such as impulse control (Bari, Eagle, Mar, Robinson, & Robbins, 2009; Dalley, Mar, Economidou, & Robbins, 2008) and cognitive flexibility (Kehagia, Murray, & Robbins, 2010). For example, alpha-2A adrenoceptors appear to play an important role in behavioral inhibition (reviewed in Arnsten et al., 1996). Additional neurotransmitters and signaling pathways likely play a significant role in modulating other PFC functions. For example, serotonin is involved in mediating reversal learning in the orbitofrontal cortex (Clarke, Dalley, Crofts, Robbins, & Roberts, 2004; Clarke et al., 2005; Clarke, Walker, Dalley, Robbins, & Roberts, 2007). Investigating the molecular influences on other PFC subregions may help to elucidate the etiology of a variety of neuropsychiatric symptoms, and will be an important arena for future research.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
In preparation for the Special Section on Translating Models of Prefrontal Cortex Function in Behavioral Neuroscience, Mark Baxter, Ed.
Author Note for disclosure of interests: Amy Arnsten and Yale University have a license agreement and receive royalties from Shire Pharmaceuticals for the development of guanfacine (Intuniv™) for the treatment of ADHD and related disorders. Dr. Arnsten also performs consulting, teaching and speaking engagements for Shire Pharmaceuticals, and provides consultation to Lilly Pharmaceuticals.
References
- Aoki C, Go CG, Venkatesan C, Kurose H. Perikaryal and synaptic localization of alpha 2A-adrenergic receptor-like immunoreactivity. Brain Research. 1994;650(2):181–204. doi: 10.1016/0006-8993(94)91782-5. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Kurose H. Noradrenergic modulation of the prefrontal cortex as revealed by electron microscopic immunocytochemistry. Advances in Pharmacology (San Diego, Calif.) 1998;42:777–780. doi: 10.1016/s1054-3589(08)60862-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Castellanos FX. Neurobiology of attention regulation and its disorders. In: Martin A, Scahill L, Charney D, Leckman J, editors. Textbook of child and adolescent pharmacology. Oxford University Press; New York, NY: 2002. pp. 99–109. [Google Scholar]
- Arnsten AFT, Manji HK. Mania: A rational neurobiology. Future Neurology. 2008;3(2):125–131. [Google Scholar]
- Arnsten AFT, Vijayraghavan S, Wang M, Gamo NJ, Paspalas CD. Dopamine's influence on prefrontal cortical cognition: Actions and circuits in behaving primates. In: Iversen L, Iversen S, Dunnett S, Bjorklund A, editors. Dopamine handbook. 1st ed. Oxford University Press; New York, NY: 2009. pp. 230–248. [Google Scholar]
- Arnsten AF. The biology of being frazzled. Science (New York, N.Y.) 1998;280(5370):1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Through the looking glass: Differential noradenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7(1-2):133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: Circuits and pathways. The Journal of Clinical Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: A rational bridge between genetics and the symptoms of mental illness. Cerebral Cortex (New York, N.Y.: 1991) 2007a;17(Suppl 1):i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: A rational bridge between genetics and the symptoms of mental illness. Cerebral Cortex (New York, N.Y.: 1991) 2007b;17(Suppl 1):i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews.Neuroscience. 2009a;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: An important role for prefrontal cortex dysfunction. CNS Drugs. 2009b;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX. Postsynaptic alpha-2 receptor stimulation improves memory in aged monkeys: Indirect effects of yohimbine versus direct effects of clonidine. Neurobiology of Aging. 1993;14(6):597–603. doi: 10.1016/0197-4580(93)90044-c. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: Evidence for alpha-2 receptor subtypes. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1988;8(11):4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Research. 1984;306(1-2):9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science (New York, N.Y.) 1985;230(4731):1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Analysis of alpha-2 adrenergic agonist effects on the delayed nonmatch-to-sample performance of aged rhesus monkeys. Neurobiology of Aging. 1990;11(6):583–590. doi: 10.1016/0197-4580(90)90021-q. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Jentsch JD. The alpha-1 adrenergic agonist, cirazoline, impairs spatial working memory performance in aged monkeys. Pharmacology, Biochemistry, and Behavior. 1997;58(1):55–59. doi: 10.1016/s0091-3057(96)00477-7. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biological Psychiatry. 1999;45(1):26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic network connectivity: A new form of neuroplasticity. Trends in Cognitive Sciences. 2010 doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Steere JC, Hunt RD. The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function. potential significance for attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53(5):448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry. 1999;46(9):1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Progress in Brain Research. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2000;23(3):240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology. 2009;205(2):273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Molecular Psychiatry. 2008;13(2):197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1995;15(12):7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the gi subfamily of G protein alpha subunits. Cell. 1996;86(3):445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Research.Brain Research Reviews. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Donahue J, Lyne A. Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectrums. 2008;13(12):1047–1055. doi: 10.1017/s1092852900017107. [DOI] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121(1):e73–84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: Alpha-1-adrenoceptor mediation in the prefrontal cortex. Biological Psychiatry. 1999;46(9):1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Podell DM, Arnsten AF. Noradrenergic alpha-2 receptor agonists reverse working memory deficits induced by the anxiogenic drug, FG7142, in rats. Pharmacology, Biochemistry, and Behavior. 2000;67(3):397–403. doi: 10.1016/s0091-3057(00)00306-3. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science (New York, N.Y.) 2004;306(5697):882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Depp C, McGrath JA, Wolyniec P, Mausbach BT, Thornquist MH, et al. Prediction of real-world functional disability in chronic mental disorders: A comparison of schizophrenia and bipolar disorder. The American Journal of Psychiatry. 2010;167(9):1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Wolkowitz O, Pickar D. Stress and schizophrenia: Advances in neuropsychiatry and psychopharmacology. In: Tamminga C, Schult S, editors. Schizophrenia research () Raven Press, Ltd.; New York: 1991. [Google Scholar]
- Bremner JD. Alterations in brain structure and function associated with post-traumatic stress disorder. Seminars in Clinical Neuropsychiatry. 1999;4(4):249–255. doi: 10.153/SCNP00400249. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Neuroimaging in posttraumatic stress disorder and other stress-related disorders. Neuroimaging Clinics of North America. 2007;17(4):523–38. ix. doi: 10.1016/j.nic.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Progress in Brain Research. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Capelli S, Delaney R, McCarthy G, et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Research. 1995;59(1-2):97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, et al. Deficits in short-term memory in posttraumatic stress disorder. The American Journal of Psychiatry. 1993;150(7):1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biological Psychiatry. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychological Medicine. 2005;35(6):791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AR, Dolinsky B, Vu MA, Stanley M, Yeckel MF, Arnsten AF. Blockade of IP3-mediated SK channel signaling in the rat medial prefrontal cortex improves spatial working memory. Learning & Memory (Cold Spring Harbor, N.Y.) 2008;15(3):93–96. doi: 10.1101/lm.767408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science (New York, N.Y.) 1979;205(4409):929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A, Honea RA, Straub RE, Pezawas L, Egan MF, et al. Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2007;27(7):1584–1593. doi: 10.1523/JNEUROSCI.5112-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Modulation of memory storage. Current Opinion in Neurobiology. 1996;6(2):237–242. doi: 10.1016/s0959-4388(96)80078-x. [DOI] [PubMed] [Google Scholar]
- Cai JX, Ma YY, Xu L, Hu XT. Reserpine impairs spatial working memory performance in monkeys: Reversal by the alpha 2-adrenergic agonist clonidine. Brain Research. 1993;614(1-2):191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48(1):77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews.Neuroscience. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chappell PB, Riddle MA, Scahill L, Lynch KA, Schultz R, Arnsten A, et al. Guanfacine treatment of comorbid attention-deficit hyperactivity disorder and tourette's syndrome: Preliminary clinical experience. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(9):1140–1146. doi: 10.1097/00004583-199509000-00010. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Archives of General Psychiatry. 1993;50(4):295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- Chowdari KV, Mirnics K, Semwal P, Wood J, Lawrence E, Bhatia T, et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Human Molecular Genetics. 2002;11(12):1373–1380. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Molecular Psychiatry. 2008;13(1):36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science (New York, N.Y.) 2004;304(5672):878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2005;25(2):532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cerebral Cortex (New York, N.Y.: 1991) 2007;17(1):18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cerebral Cortex (New York, N.Y.: 1991) 2001;11(11):1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacology, Biochemistry, and Behavior. 2008;90(2):250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: Preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Molecular Psychiatry. 1999;4(2):192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: Treatment implications for PTSD. Annals of the New York Academy of Sciences. 2006;1071:521–524. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nature Reviews.Neuroscience. 2005;6(11):850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Research. 1990;521(1-2):311–315. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Moghaddam B, Innis RB, Krystal JH, Aghajanian GK, Bunney BS, et al. Mechanisms of action of atypical antipsychotic drugs. implications for novel therapeutic strategies for schizophrenia. Schizophrenia Research. 1991;4(2):121–156. doi: 10.1016/0920-9964(91)90030-u. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Progress in Brain Research. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. discussion 402-3. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: An ALE meta-analysis. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP, Shrout PE, Link BG, Skodol AE, Stueve A. Life events and other possible psychosocial risk factors for episodes of schizophrenia and major depression: A case-control study. In: Mazure CM, editor. Progress in psychiatry. American Psychiatric; Washington, DC: 1995. pp. 43–65. [Google Scholar]
- Domesick VB. Neuroanatomical organization of dopamine neurons in the ventral tegmental area. Annals of the New York Academy of Sciences. 1988;537:10–26. doi: 10.1111/j.1749-6632.1988.tb42094.x. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? Journal of Affective Disorders. 2002;70(1):1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdely HA, Tamminga CA, Roberts RC, Vogel MW. Regional alterations in RGS4 protein in schizophrenia. Synapse (New York, N.Y.) 2006;59(8):472–479. doi: 10.1002/syn.20265. [DOI] [PubMed] [Google Scholar]
- Faber ES. Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+ channels regulates synaptic excitability in the medial prefrontal cortex. The Journal of Physiology. 2010;588(Pt 8):1281–1292. doi: 10.1113/jphysiol.2009.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH. Topographic organization of ascending dopaminergic projections. Annals of the New York Academy of Sciences. 1988;537:1–9. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Storm DR. Why calcium-stimulated adenylyl cyclases? Physiology (Bethesda, Md.) 2004;19:271–276. doi: 10.1152/physiol.00010.2004. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiology of Learning and Memory. 1999;72(1):8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Involvement of alpha1-adrenoceptors in the basolateral amygdala in modulation of memory storage. European Journal of Pharmacology. 1999;372(1):9–16. doi: 10.1016/s0014-2999(99)00169-7. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: Effects of diazepam. Neuroscience. 1995;64(3):619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2002;22(19):8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1994;14(5 Pt 1):2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo NJ, Kata A, Boven L, Bryan C, Lo T, Anighoro K, et al. Society for Neuroscience. San Diego, USA: 2010. Knock-down of disrupted in schizophrenia 1 (DISC1) in the rat prefrontal cortex lowers the threshold for stress-induced cognitive dysfunction. [Google Scholar]
- Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Collins LB, Jones SR, Wightman RM. Evoked extracellular dopamine in vivo in the medial prefrontal cortex. Journal of Neurochemistry. 1993;61(2):637–647. doi: 10.1111/j.1471-4159.1993.tb02168.x. [DOI] [PubMed] [Google Scholar]
- George MS, Abbott LF, Siegelbaum SA. HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K(+) channels. Nature Neuroscience. 2009;12(5):577–584. doi: 10.1038/nn.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions of the Royal Society of London.Series B, Biological Sciences. 1996;351(1346):1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(22):9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1990;10(7):2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Smiley JF, Williams MS. The anatomy of dopamine in monkey and human prefrontal cortex. Journal of Neural Transmission.Supplementum. 1992;36:163–177. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1996;16(15):4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova NA, Yang CR. Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. Journal of Neurophysiology. 2000;84(1):75–87. doi: 10.1152/jn.2000.84.1.75. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. The Journal of Clinical Psychiatry. 2006;67(10):e12. [PubMed] [Google Scholar]
- Greene CM, Bellgrove MA, Gill M, Robertson IH. Noradrenergic genotype predicts lapses in sustained attention. Neuropsychologia. 2009;47(2):591–594. doi: 10.1016/j.neuropsychologia.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. The Journal of Neuroscience. The Official Journal of the Society for Neuroscience. 1998;18(21):9139–9151. doi: 10.1523/JNEUROSCI.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenston AM, Fitzpatrick JS, Yeckel MF. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cerebral Cortex (New York, N.Y.: 1991) 2008;18(2):407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(42):17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Gitlin M. Stress reactivity in bipolar patients and its relation to prior history of disorder. The American Journal of Psychiatry. 1997;154(6):856–857. doi: 10.1176/ajp.154.6.856. [DOI] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. Journal of Neurophysiology. 2000;84(6):2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Hepler JR, Berman DM, Gilman AG, Kozasa T. RGS4 and GAIP are GTPase-activating proteins for gq alpha and block activation of phospholipase C beta by gamma-thio-GTP-gq alpha. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(2):428–432. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess C, Reif A, Strobel A, Boreatti-Hummer A, Heine M, Lesch KP, et al. A functional dopamine-beta-hydroxylase gene promoter polymorphism is associated with impulsive personality styles, but not with affective disorders. Journal of Neural Transmission (Vienna, Austria : 1996) 2009;116(2):121–130. doi: 10.1007/s00702-008-0138-0. [DOI] [PubMed] [Google Scholar]
- Hieble JP, Ruffolo RR., Jr. The use of alpha-adrenoceptor antagonists in the pharmacological management of benign prostatic hypertrophy: An overview. Pharmacological Research : The Official Journal of the Italian Pharmacological Society. 1996;33(3):145–160. doi: 10.1006/phrs.1996.0022. [DOI] [PubMed] [Google Scholar]
- Hopkins WF, Johnston D. Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. Journal of Neurophysiology. 1988;59(2):667–687. doi: 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131(1):160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Hunt RD, Arnsten AF, Asbell MD. An open trial of guanfacine in the treatment of attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(1):50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Miyazaki B, McGaugh JL. Involvement of the amygdala in the memory-enhancing effects of clenbuterol. Psychopharmacology. 1991;104(4):541–544. doi: 10.1007/BF02245663. [DOI] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 1999;20(5):460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83(1):63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Current Opinion in Neurobiology. 2010;20(2):199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kieling C, Genro JP, Hutz MH, Rohde LA. The -1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. American Journal of Medical Genetics.Part B, Neuropsychiatric Genetics : The Official Publication of the International Society of Psychiatric Genetics. 2008;147B(4):485–490. doi: 10.1002/ajmg.b.30636. [DOI] [PubMed] [Google Scholar]
- Kieling C, Goncalves RR, Tannock R, Castellanos FX. Neurobiology of attention deficit hyperactivity disorder. Child and Adolescent Psychiatric Clinics of North America. 2008;17(2):285–307. viii. doi: 10.1016/j.chc.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Xu L, Cascella N, Ozeki Y, Sawa A, Roberts RC. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. The Journal of Comparative Neurology. 2006;497(3):436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(10):3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeckova M, Paclt I, Goetz P. Polymorphisms and low plasma activity of dopamine-beta-hydroxylase in ADHD children. Neuro Endocrinology Letters. 2006;27(6):748–754. [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. The Journal of Comparative Neurology. 1995;359(1):131–143. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Ebert P, Mirnics K, Nimgaonkar VL, Lewis DA. Making the case for a candidate vulnerability gene in schizophrenia: Convergent evidence for regulator of G-protein signaling 4 (RGS4). Biological Psychiatry. 2006;60(6):534–537. doi: 10.1016/j.biopsych.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P, Goldman-Rakic P. Region-specific distribution of catecholamine afferents in primate cerebral cortex: A fluorescence histochemical analysis. The Journal of Comparative Neurology. 1984;227(1):23–36. doi: 10.1002/cne.902270105. [DOI] [PubMed] [Google Scholar]
- Lewis DA. The catecholaminergic innervation of primate prefrontal cortex. Journal of Neural Transmission.Supplementum. 1992;36:179–200. doi: 10.1007/978-3-7091-9211-5_9. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1987;7(1):279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hayes TL, Lund JS, Oeth KM. Dopamine and the neural circuitry of primate prefrontal cortex: Implications for schizophrenia research. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 1992;6(2):127–134. [PubMed] [Google Scholar]
- Lewis DA, Morrison JH. Noradrenergic innervation of monkey prefrontal cortex: A dopamine-beta-hydroxylase immunohistochemical study. The Journal of Comparative Neurology. 1989;282(3):317–330. doi: 10.1002/cne.902820302. [DOI] [PubMed] [Google Scholar]
- Li BM, Mao ZM, Wang M, Mei ZT. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 1999;21(5):601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- Li BM, Mei ZT. Delayed-response deficit induced by local injection of the alpha 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behavioral and Neural Biology. 1994;62(2):134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, et al. Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40(3):657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(3):912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Johansen P, Hjermann I, Iversen BM, Thaulow E. Selective alpha-1 inhibitors: First- or second-line antihypertensive agents? Cardiology. 1993;83(3):150–159. doi: 10.1159/000175963. [DOI] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Ziskind-somerfeld research award. protein kinase C signaling in the brain: Molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biological Psychiatry. 1999;46(10):1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- Manji HK, McNamara R, Chen G, Lenox RH. Signalling pathways in the brain: Cellular transduction of mood stabilisation in the treatment of manic-depressive illness. The Australian and New Zealand Journal of Psychiatry. 1999;33(Suppl):S65–83. doi: 10.1111/j.1440-1614.1999.00670.x. [DOI] [PubMed] [Google Scholar]
- Mao ZM, Arnsten AF, Li BM. Local infusion of an alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biological Psychiatry. 1999;46(9):1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Markovac J, Goldstein GW. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988;334(6177):71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM, editor. Does stress cause psychiatric illness? American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- Mazure CM, Maciejewski PK. A model of risk for major depression: Effects of life stress and cognitive style vary by age. Depression and Anxiety. 2003;17(1):26–33. doi: 10.1002/da.10081. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC, Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: Implications for function of the central noradrenergic system. Progress in Brain Research. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- McCracken JT, Aman MG, McDougle CJ, Tierney E, Shiraga S, Whelan F, et al. Possible influence of variant of the P-glycoprotein gene (MDR1/ABCB1) on clinical response to guanfacine in children with pervasive developmental disorders and hyperactivity. Journal of Child and Adolescent Psychopharmacology. 2010;20(1):1–5. doi: 10.1089/cap.2009.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McNally RJ, English GE, Lipke HJ. Assessment of intrusive cognition in PTSD: Use of the modified stroop paradigm. Journal of Traumatic Stress. 1993;6(1):33–41. [Google Scholar]
- McNally RJ, Kaspi SP, Riemann BC, Zeitlin SB. Selective processing of threat cues in posttraumatic stress disorder. Journal of Abnormal Psychology. 1990;99(4):398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research.Brain Research Reviews. 2000;31(2-3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biological Psychology. 2006;73(1):61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Millar JK, Mackie S, Clapcote SJ, Murdoch H, Pickard BS, Christie S, et al. Disrupted in schizophrenia 1 and phosphodiesterase 4B: Towards an understanding of psychiatric illness. The Journal of Physiology. 2007;584(Pt 2):401–405. doi: 10.1113/jphysiol.2007.140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science (New York, N.Y.) 2005;310(5751):1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Human Molecular Genetics. 2000;9(9):1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Molecular Psychiatry. 2001;6(3):293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- Mohell N, Svartengren J, Cannon B. Identification of [3H]prazosin binding sites in crude membranes and isolated cells of brown adipose tissue as alpha 1-adrenergic receptors. European Journal of Pharmacology. 1983;92(1-2):15–25. doi: 10.1016/0014-2999(83)90103-6. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163(1):109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morris DW, Rodgers A, McGhee KA, Schwaiger S, Scully P, Quinn J, et al. Confirming RGS4 as a susceptibility gene for schizophrenia. American Journal of Medical Genetics.Part B, Neuropsychiatric Genetics : The Official Publication of the International Society of Psychiatric Genetics. 2004;125B(1):50–53. doi: 10.1002/ajmg.b.20109. [DOI] [PubMed] [Google Scholar]
- Mouradian RD, Sessler FM, Waterhouse BD. Noradrenergic potentiation of excitatory transmitter action in cerebrocortical slices: Evidence for mediation by an alpha 1 receptor-linked second messenger pathway. Brain Research. 1991;546(1):83–95. doi: 10.1016/0006-8993(91)91162-t. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(3):1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Fitten LJ, Siembieda DW, Ortiz F, Halgren E. Effects of guanfacine on three forms of distraction in the aging macaque. Life Sciences. 2000;67(8):877–885. doi: 10.1016/s0024-3205(00)00681-0. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Blaxall HS, Iversen LJ, Bylund DB. Characterization of [3H]RX821002 binding to alpha-2 adrenergic receptor subtypes. The Journal of Pharmacology and Experimental Therapeutics. 1994;268(3):1362–1367. [PubMed] [Google Scholar]
- Partridge LD, Swandulla D, Muller TH. Modulation of calcium-activated non-specific cation currents by cyclic AMP-dependent phosphorylation in neurones of helix. The Journal of Physiology. 1990;429:131–145. doi: 10.1113/jphysiol.1990.sp018248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Goldman-Rakic PS. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. The Journal of Comparative Neurology. 1982;205(1):63–76. doi: 10.1002/cne.902050107. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Chowdari KV, Nimgaonkar VL, Talkowski ME, Lewis DA, Keshavan MS. Genetic polymorphisms of the RGS4 and dorsolateral prefrontal cortex morphometry among first episode schizophrenia patients. Molecular Psychiatry. 2005;10(2):213–219. doi: 10.1038/sj.mp.4001562. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Research Reviews. 2005;4(2):271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex (New York, N.Y.: 1991) 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacology, Biochemistry, and Behavior. 1996;55(3):415–422. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AF. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biological Psychiatry. 2005;58(11):894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Stark D, Verduzco L, van Dyck CH, Arnsten AF. Alpha2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learning & Memory (Cold Spring Harbor, N.Y.) 2006;13(6):770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: Evidence for microcolumnar organization in PFC. Journal of Neurophysiology. 1999;81(4):1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2000;20(1):485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: A report of 4 cases. The Journal of Clinical Psychiatry. 2000;61(2):129–133. doi: 10.4088/jcp.v61n0208. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biological Psychiatry. 2007;61(8):928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: A placebo-controlled study. The American Journal of Psychiatry. 2003;160(2):371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Thompson C, Petrie EC, Dobie DJ, Rein RJ, Hoff DJ, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2002;63(7):565–568. doi: 10.4088/jcp.v63n0705. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: Neurochemical modulation of prefrontal cortical function. The Journal of Comparative Neurology. 2005;493(1):140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annual Review of Neuroscience. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman T, Schmitz M, Polanczyk GV, Eizirik M, Rohde LA, Hutz MH. Further evidence for the association between attention-deficit/hyperactivity disorder and the dopamine-beta-hydroxylase gene. American Journal of Medical Genetics. 2002;114(2):154–158. doi: 10.1002/ajmg.10194. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor--cAMP/cAMP/PKA system in influencing memory consolidation. The European Journal of Neuroscience. 2002;15(3):553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Roth RH, Tam SY, Ida Y, Yang JX, Deutch AY. Stress and the mesocorticolimbic dopamine systems. Annals of the New York Academy of Sciences. 1988;537:138–147. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learning & Memory (Cold Spring Harbor, N.Y.) 2005;12(2):103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T. The alpha-2A adrenoceptor agonist guanfacine improves sustained attention and reduces overactivity and impulsiveness in an animal model of attention-Deficit/Hyperactivity disorder (ADHD). Behavioral and Brain Functions : BBF. 2006;2:41. doi: 10.1186/1744-9081-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J, et al. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: A placebo-controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(2):155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Herve-Minvielle A. Inhibitory influence of frontal cortex on locus coeruleus neurons. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(13):6032–6036. doi: 10.1073/pnas.92.13.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T. Attenuation of delay-period activity of monkey prefrontal neurons by an alpha2-adrenergic antagonist during an oculomotor delayed-response task. Journal of Neurophysiology. 1998;80(4):2200–2205. doi: 10.1152/jn.1998.80.4.2200. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T. The role of D1-dopamine receptors in working memory-guided movements mediated by frontal cortical areas. Parkinsonism & Related Disorders. 2000;7(1):9–19. doi: 10.1016/s1353-8020(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T. The effects of dopamine and its antagonists on directional delay-period activity of prefrontal neurons in monkeys during an oculomotor delayed-response task. Neuroscience Research. 2001;41(2):115–128. doi: 10.1016/s0168-0102(01)00270-x. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science (New York, N.Y.) 1991;251(4996):947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. Journal of Neurophysiology. 1994;71(2):515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. The American Journal of Psychiatry. 2001;158(7):1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1993;13(3):900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(1):301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1998;18(4):1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1985;5(3):776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1988;8(11):4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. A positron emission tomographic study of symptom provocation in PTSD. Annals of the New York Academy of Sciences. 1997;821:521–523. doi: 10.1111/j.1749-6632.1997.tb48320.x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, et al. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcoholism, Clinical and Experimental Research. 2009;33(2):255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Heterogeneous targets of dopamine synapses in monkey prefrontal cortex demonstrated by serial section electron microscopy: A laminar analysis using the silver-enhanced diaminobenzidine sulfide (SEDS) immunolabeling technique. Cerebral Cortex (New York, N.Y.: 1991) 1993;3(3):223–238. doi: 10.1093/cercor/3.3.223. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: Predominant and extrasynaptic localization in dendritic spines. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(12):5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin EM, Heel RC. Guanfacine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1986;31(4):301–336. doi: 10.2165/00003495-198631040-00003. [DOI] [PubMed] [Google Scholar]
- Soulsby MD, Wojcikiewicz RJ. The type III inositol 1,4,5-trisphosphate receptor is phosphorylated by cAMP-dependent protein kinase at three sites. The Biochemical Journal. 2005;392(Pt 3):493–497. doi: 10.1042/BJ20051325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick S, Rasmusson AM, Barron X, Arnsten AFT. Neurobiological and neurocognitive alterations in PTSD. In: Vasterling JJ, Brewin CR, editors. Neuropsychology of PTSD: Biological, cognitive and clinical perspectives. Guilford Publications; NY: 2003. pp. 27–58. [Google Scholar]
- Southwick SM, Paige S, Morgan CA, 3rd, Bremner JD, Krystal JH, Charney DS. Neurotransmitter alterations in PTSD: Catecholamines and serotonin. Seminars in Clinical Neuropsychiatry. 1999;4(4):242–248. doi: 10.153/SCNP00400242. [DOI] [PubMed] [Google Scholar]
- Spencer TJ. Attention-deficit/hyperactivity disorder. Archives of Neurology. 2002;59(2):314–316. doi: 10.1001/archneur.59.2.314. [DOI] [PubMed] [Google Scholar]
- Steere JC, Arnsten AF. The alpha-2A noradrenergic receptor agonist guanfacine improves visual object discrimination reversal performance in aged rhesus monkeys. Behavioral Neuroscience. 1997;111(5):883–891. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- Swartz BE, Halgren E, Simpkins F, Mandelkern M. Studies of working memory using 18FDG-positron emission tomography in normal controls and subjects with epilepsy. Life Sciences. 1996;58(22):2057–2064. doi: 10.1016/0024-3205(96)00198-1. [DOI] [PubMed] [Google Scholar]
- Talkowski ME, Seltman H, Bassett AS, Brzustowicz LM, Chen X, Chowdari KV, et al. Evaluation of a susceptibility gene for schizophrenia: Genotype based meta-analysis of RGS4 polymorphisms from thirteen independent samples. Biological Psychiatry. 2006;60(2):152–162. doi: 10.1016/j.biopsych.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H, Rama P, Carlson S. The effects of prefrontal intracortical microinjections of an alpha-2 agonist, alpha-2 antagonist and lidocaine on the delayed alternation performance of aged rats. Brain Research Bulletin. 1996;40(2):117–119. doi: 10.1016/0361-9230(96)00026-3. [DOI] [PubMed] [Google Scholar]
- Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. Journal of Clinical Psychopharmacology. 2002;22(1):82–85. doi: 10.1097/00004714-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Lowe K, Thompson C, McFall MM, Peskind ER, Kanter ED, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biological Psychiatry. 2006;59(7):577–581. doi: 10.1016/j.biopsych.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: A placebo-controlled study. Biological Psychiatry. 2008;63(6):629–632. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology. 2001;21(2):223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- Uhlen S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: Computer modelling reveals that guanfacine is an alpha 2A-selective compound. European Journal of Pharmacology. 1991;202(2):235–243. doi: 10.1016/0014-2999(91)90299-6. [DOI] [PubMed] [Google Scholar]
- U'Prichard DC, Bechtel WD, Rouot BM, Snyder SH. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: Effect of 6-hydroxydopamine. Molecular Pharmacology. 1979;16(1):47–60. [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12(1):125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10(3):376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Volk DW, Eggan SM, Lewis DA. Alterations in metabotropic glutamate receptor 1{alpha} and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. The American Journal of Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, O'Donnell P. D(1) dopamine receptors potentiate nmda-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cerebral Cortex (New York, N.Y.: 1991) 2001;11(5):452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129(2):397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wang M, Tang ZX, Li BM. Enhanced visuomotor associative learning following stimulation of alpha 2A-adrenoceptors in the ventral prefrontal cortex in monkeys. Brain Research. 2004;1024(1-2):176–182. doi: 10.1016/j.brainres.2004.07.062. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science (New York, N.Y.) 2004;303(5659):853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends in Neurosciences. 2001;24(8):455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology. 1981;20(10):907–920. doi: 10.1016/0028-3908(81)90020-4. [DOI] [PubMed] [Google Scholar]
- Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature. 1996;383(6596):172–175. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2007;27(31):8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2000;20(18):7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Current Treatment Options in Neurology. 2010 doi: 10.1007/s11940-010-0091-8. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V-VI rat prefrontal cortex neurons in vitro: Modulation of dendritic-somatic signal integration. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1996;16(5):1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1997;17(21):8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. Opposite modulation of cortical N-methyl-D-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 1999;91(2):527–535. doi: 10.1016/s0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]