Abstract
Optimal fibrin balance requires precisely controlled plasmin generation on the surface of endothelial cells, which line the blood vessel wall. As a co-receptor for plasminogen and tissue plasminogen activator (tPA), which are key factors in plasmin generation, the annexin A2 (A2) complex promotes vascular fibrinolysis. The intracellular A2 complex is a heterotetramer of two A2 monomers and two copies of the associated protein, p11. In response to endothelial cell activation, A2 is phosphorylated by src-kinase, and translocated to the cell surface in a highly regulated manner. Over- expression of A2 is seen in acute promyelocytic leukemia during the early hemorrhagic phase, while high titer antibodies to A2, as in antiphospholipid syndrome or cerebral venous thrombosis, are associated with thrombosis. In experimental hyperhomocysteinemia, moreover, derivatization of A2 by homocysteine leads to intravascular fibrin accumulation and dysangiogenesis, features that phenocopy the Anxa2−/− mouse. Exogenous A2 may also offer a novel therapeutic approach to ischemic thrombotic stroke, as administration of A2 in conjunction with conventional tPA-based thrombolytic therapy improved outcome in an animal model. Here, we discuss the role of the A2 system in vascular homeostasis, the molecular interactions that regulate its profibrinolytic activity, and its potential role in the pathogenesis and treatment of vascular disease.
Keywords: annexin A2, fibrinolysis, endothelial cells, thrombosis, angiogenesis
1. Introduction
The efficient circulation of blood is dependent on a stable, patent network of blood vessels (Harvey, 1628). Through the process of hemostasis, vascular injury leads to formation of the primary hemostatic plug, which, as it matures, serves to stem the flow of blood. Formation of clot, or thrombus, reflects activation of the coagulation cascade, generation of thrombin, conversion of soluble circulating fibrinogen to insoluble fibrin, and entrapment of circulating blood cells. Once the injured vessel is repaired, fibrinolysis clears the fibrin containing thrombus, and restores vascular patency.
Although opposing processes, both blood coagulation (hemostasis) and clot dissolution (fibrinolysis) are vital to vascular homeostasis (Hajjar, 2009). In classical fibrinolysis, the sequential activation of specific proteases on the surface of the fibrin-containing thrombus culminates in production of plasmin, a protease that cleaves fibrin into defined soluble degradation products. Plasmin activation results from the cleavage of a single peptide bond at position R560-V561within its inactive zymogen, plasminogen (Plg). In highly regulated reactions, either of two physiologic activators, tissue plasminogen activator (tPA) or urokinase (uPA), can catalyze this reaction. It is interesting to note that, in tPA-dependent plasmin generation, fibrin serves as a potent cofactor for its own destruction
Originally, fibrinolysis was viewed as a process restricted to plasma factors on the surface of a thrombus (Cesarman-Maus and Hajjar, 2005). However, prescient observations made in the late 1950s and early 1960s revealed the presence of small amounts of fibrinolytic activity in association with the vessel wall that were critically dependent upon an intact endothelium (Todd, 1958, 1964). This work foreshadowed the discovery of endothelial cell plasminogen and plasminogen activator receptors decades later.
Several groups have focused on understanding the molecular basis of vascular fibrinolysis, postulating that local generation of plasmin is enabled by receptors that promote fibrinolytic activity on the vessel surface (Hajjar, 2003). These receptors include the urokinase receptor (uPAR) (Blasi et al., 1994), which is expressed primarily on activated, migrating endothelial cells (EC) (Pepper et al., 1993), the annexin A2 complex, which binds both Plg and tPA on both resting and activated EC (Dassah et al., 2009; Kwon et al., 2005), and a series of plasminogen binding proteins expressed on many cell types (Andronicus et al., 2010; Miles et al., 2005).
2. The annexin A2 system in fibrinolysis
2.1 The annexin A2 heterotetramer
Annexin A2 (A2) is a 36-kDa protein member of the annexin superfamily of Ca2+-dependent phospholipid-binding proteins, a dozen of which are expressed in humans (Moss and Morgan, 2004). A2 was originally identified in both avian and mammalian cells as a substrate of the transforming gene product, the pp60src tyrosine kinase, of the avian sarcoma virus (Erikson and Erikson, 1980). Later, it was found to be expressed in a spectrum of cell types. The ~30-kDa core domain of A2 contains four Ca2+-binding “annexin” repeats, which are highly homologous to those of the other 60 annexin family members, while the smaller N-terminal “tail” domain is essentially unique (Gerke et al., 2005; Gerke and Moss, 2002).
In the cytoplasm, A2 exists in soluble, monomeric form. However, in the presence of protein p11, a member of the S100 family of proteins (S100A10), A2 forms a constitutively stable complex comprising two subunits each of A2 and p11 (Gerke and Weber, 1985; Waisman, 1995) (Figure 1). The formation of the (A2•p11)2 complex is largely mediated by hydrophobic contacts between the C-terminal region of p11 and the amino terminal tail of the A2 monomer (Becker et al., 1990; Johnsson et al., 1988; Kube et al., 1992; Rety et al., 1999). The A2 p11-binding region, S1-G14, is closely related to three phosphorylation sites. Phosphorylation of S11, within the p11 binding domain, has been observed to disrupt heterotetramer formation (Jost and Gerke, 1996), thereby constituting a molecular “switch” that might regulate the equilibrium between membrane-associated and cytosolic A2. Recent data suggests that serine phosphorylation of A2 may be regulated by plasmin (He et al., 2011). Whether phosphorylation of S25, also a protein kinase C substrate, serves a similar function is unknown. Phosphorylation of Y23 by pp60src appears to regulate transit of A2 to the cell surface (Deora et al., 2004), and will be discussed in more detail below.
Figure 1. A2 system assembly on the endothelial cell surface.
The A2 heterotetramer is comprised to two p11 subunits and two A2 subunits. A2 consists of four “core” domains (pink) and an N-terminal “tail” domain (yellow). The heterotetramer binds both plasminogen (Plg) and tissue plasminogen activator (tPA), thereby accelerating plasmin (PN) generation. The orientation of carboxy (C)- and amino (N)- termini for A2 and p11 are shown. Adapted from (Gerke et al., 2005).
For most S100 proteins, calcium binding induces a conformational change that places helix III (HIII) in a more perpendicular orientation to helix IV (HIV), forming a cleft that can more readily accept target proteins (Donato, 2001). Protein S100A10 (p11), however, is an exception to the calcium activation rule, as it permanently assumes a “calcium-on” state, due to replacement of the bidentate E65 with S70, and the monodentate D56 with C61 (Burger et al., 1996; Rety et al., 1999). The published crystal structure of p11 in complex with the N-terminal 13 amino acids of A2 (Rety et al., 1999) suggests that the basic unit of p11 structure is a non-covalently-linked homodimer, each component of which can bind the A2 tail peptide to form a heterotetramer. Upon binding, the A2 tail peptide assumes an α-helical conformation that presents key hydrophobic residues (V3, I6, L7, and L10) within a hydrophobic cleft formed by loop L2 and helix HIV of one monomer and helix HI of the other. The C- terminal region of p11, particularly its hydrophobic residues within the C-terminal extension (Y85FVVHM90), such as Y85 and F86, contributes critical contact points with A2.
2.2 Annexin A2-mediated profibrinolytic assembly
The link between A2 and fibrinolysis was discovered by members of our lab while seeking EC surface receptors for Plg and tPA (Hajjar et al., 1994) (Figure 1). Our group discovered that Plg could bind directly to cultured ECs with high affinity (Kd 300 nM) and high specificity (Hajjar et al., 1986). We later found that the circulating form of Plg, N-terminal glutamic acid-plasminogen (Glu-Plg), was converted to a more readily activated form (N-terminal lysine-Plg) upon binding to ECs (Hajjar and Nachman, 1988), a concept that has been reinforced by others (Miles et al., 2003; Silverstein et al., 1988). These findings identified the EC surface as a profibrinolytic microenvironment. Plg binding to ECs can be blocked in the presence of lipoprotein(a) (Lp(a)), a highly atherogenic lipoprotein particle whose apoprotein, apoprotein(a), is structurally homologous to Plg (Hajjar et al., 1989; Miles et al., 1989). This finding suggested a mechanistic explanation for the observation that patients with elevated levels of Lp(a) are highly susceptible to atherosclerosis; inhibition of EC surface fibrinolysis was postulated to favor fibrin deposition and the accumulation of proatherogenic inflammatory cells.
In 1987, we reported that tPA could interact specifically with human ECs (Hajjar et al., 1987). While the higher affinity site possessed features of the physiologic plasminogen activator inhibitor type 1 (PAI-1) and could be blocked by uPA, the lower affinity site appeared to be novel. Ligand blotting of an EC plasma membrane protein extract further distinguished the ~36-kDa, tPA-binding protein from PAI-1 and uPAR (Hajjar and Hamel, 1990). Purification of the candidate tPA receptor from human placental membranes revealed that it interacted specifically, with not only tPA, but also its substrate, Plg (Hajjar, 1991). These findings suggested for the first time that a single EC receptor, much like fibrin, might bind both tPA and Plg.
Identification of the putative tPA-Plg binding protein as annexin A2, then known as “annexin II,” emerged from amino acid sequence studies (Hajjar et al., 1994). We confirmed this designation when cell treatment with antibodies directed against authentic A2 or A2 antisense oligonucleotides each blocked ~50% of Plg and tPA binding. In addition, treatment of A2 with carboxypeptidase B (CPB) canceled its ability to bind Plg, thus implicating a C-terminal lysine or arginine residue. Thrombin activatable fibrinolysis inhibitor (TAFI), a form of CPB that is regulated by thrombin and thrombomodulin, may be an endogenous regulator of the A2 system and Plg binding in vivo (Rijken and Lijnen, 2009). Finally, mutation of K307 to Ala specifically eliminated Plg binding as well, suggesting this interaction requires a proteolytic processing event that liberates K307 by creating new C-terminus.
To study the catalytic effect of A2 on Plg activation, we purified native annexin A2 from human placenta and found that it bound tPA, Plg, and plasmin saturably (Cesarman et al., 1994). This A2 preparation increased the catalytic efficiency (kcat/Km) of tPA-dependent Plg activation by ~60-fold, but had no effect on uPA activation of Plg. The catalytic effect of A2 disappeared in the presence of the lysine analog ε-aminocaproic acid, or by treatment of A2 with CPB, again implicating an interaction between a C-terminal lysine or arginine of A2 and one of the lysine-binding “kringle” domains of Plg. These experiments raised the possibility of a C-terminal Lys residue for Plg binding, and demonstrated the fibrin-like cofactor behavior of A2 with respect to tPA-dependent Plg activation.
We investigated the mechanism by which A2 interacted with the EC surface (Hajjar et al., 1996). A2 was metabolically labeled in cultured human ECs incubated with 35S-radiolabeled methionine, showing its direct synthesis by these cells. Moreover, in the presence of Ca2+, but not other divalent cations, both recombinant and native A2 showed high affinity, equilibrium binding to cultured ECs (Kd ~ 50 nM). This binding could be competed by vesicles containing a series of phospholipids, most prominent among which was phosphatidylserine (Ptd-L-Ser). Binding also diminished in the presence of peptides that mimicked repeat 2 of A2, or upon mutation of the D161 residue that coordinates with the annexin repeat (K118GLGT122) sequence. These results revealed that the interaction of A2 with the EC surface was Ca2+-dependent, targeted Ptd-L-Ser moieties within the plasma membrane, and required anionic phospholipid-binding repeat 2 of A2.
We analyzed the putative tPA-binding domain of A2 (Hajjar et al., 1998). Unlike full-length A2, the core fragment of A2 failed to compete with full-length A2 for binding of tPA, implicating the N-terminal tail domain in this interaction. Indeed, residues L7CKLSL12, and larger peptides containing this fragment, specifically blocked binding of tPA to A2. Mutation of C8, but not C133, C262, or C335 prevented binding of tPA to A2, further implicating the N-terminal region. These data revealed a binding domain for tPA associated with the N-terminal tail of A2.
Based upon these data, we have postulated that both Plg and tPA interact with A2 within the (A2 p11)2 heterotetrameric complex at the EC surface (Fig. 1). Our model suggests that this assembly augments the catalytic efficiency of plasmin activation by 1-to 2-log orders-of-magnitude. It predicts further that the (A2 p11)2 system contributes to blood vessel patency, and that gain-of-function would lead to hemorrhage, while loss-of-function would induce vascular thrombosis.
These data notwithstanding, Waisman and colleagues have proposed an alternative view. They suggest that p11 (S100A10), which possesses a C-terminal lysine residue, may represent the binding site for plasminogen and tPA, and that A2 serves to anchor p11 to the plasma membrane (Kwon et al., 2005; O’Connell et al., 2010). Others have suggested that binding to both A2 and p11 is possible, perhaps depending upon cell type and the local proteolytic milieu (Das et al., 2007). Finally, it should be noted that multiple additional candidate receptors have been proposed for plasminogen, including Plg-RKT (Andronicus et al., 2010), α-enolase (Miles et al., 1991), and histone H2B (Das et al., 2007).
2.3 Cellular trafficking of annexin A2
Upon assembly with p11, the annexin A2 heterotetramer is directed to the inner leaflet of the cell membrane (Zobiack et al., 2001). There, A2 is susceptible to phosphorylation by both serine/threonine and tyrosine protein kinases (Beaton et al., 2002; Bellagamba et al., 1997; Okuse et al., 2002). Previous studies in HEK 293 cells have shown that Y23 of A2 is a target for phosphorylation by the src-family kinase, pp60src (Glenney and Tack, 1985). Src-kinase is activated when Y527 in the C-terminus kinase domain of pp60src is dephosphorylated, and Y416 remains phosphorylated (Cooper and King, 1986). When Y23 of A2 is mutated, preventing tyrosine phosphorylation, translocation of A2 does not occur. Once phosphorylated, the new phospho-(A2 p11)2 heterotetramer is predicted to couple more tightly to inner leaflet anionic Ptd-L-Ser (Montaville et al., 2002), and possibly undergo conformational changes that enhance its translocation to the outer membrane leaflet via an, as yet, undefined mechanism.
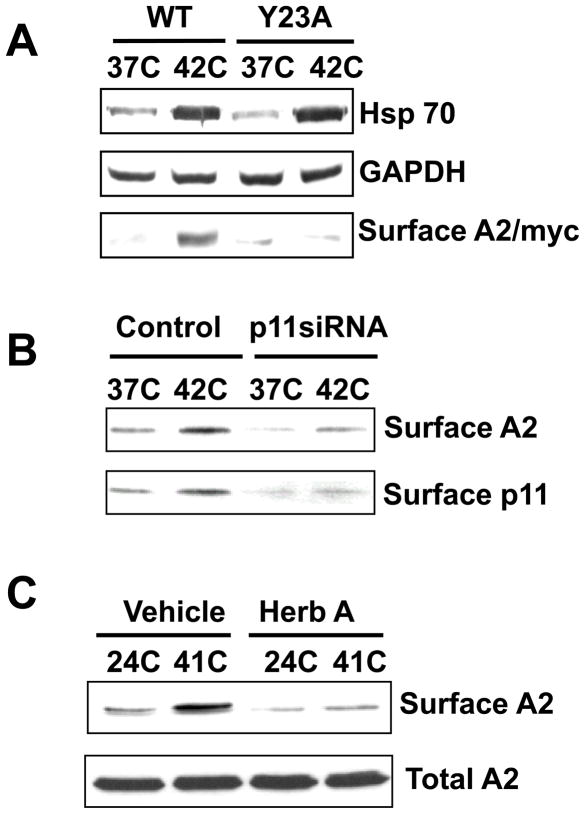
Our group has shown that temperature stress stimulates translocation of A2 to the EC surface, increasing the potential for plasmin generation (Deora et al., 2004) (Figure 2). We microinjected GFP-A2-encoding cDNA into the nuclei of human ECs, and noted that when the tagged cells were exposed to a brief increase in temperature (4h, 42 C), there was a doubling or tripling of the cell surface expression of A2. This process required both tyrosine phosphorylation of A2 at Y23 and the presence of protein p11. Of note, ECs treated with the protein synthesis inhibitor, cyclohexamide, exhibited enhanced, rather than repressed, translocation at both 37 C and 42 C, and there was no change in steady-state mRNA levels during heat stress. In an additional experiment, human ECs treated with brefeldin A, a drug that disrupts the endoplasmic reticulum-Golgi complex, did not interfere with expression of A2 on the cell surface. Thrombin is also known to induce an increase in intracellular calcium, which is a likely early step in the translocation process. Following thrombin stimulation, A2 translocation to the cell surface occurs within minutes (Petersen et al., 2003). We, therefore, concluded that A2 translocation is independent of the classical ER-Golgi pathway, does not require de novo protein synthesis, and occurs rapidly following a variety of cellular stress signals. However, it is currently unknown whether a transporter, vesicle fusion, or a separate process mediates transport across the plasma membrane.
Figure 2. A2 translocation to the endothelial cell surface in vitro and in vivo.
A. When Y23, a phosphorylation target of src kinases, is mutated (Y23A), A2 fails to translocate to the cell surface in response to thermal stress. B. Following knockdown of p11 by RNA silencing, A2 fails to translocate to the cell surface in response to thermal stress. C. Following inhibition of tyrosine kinase activity with herbimycin A, A2 fails to translocate to the cell surface. Reprinted with permission from (Deora et al., 2004).
2.4 Regulation of cell surface annexin A2 expression
p11 directs a wide range of membrane-associated proteins to the plasma membrane in a spectrum of cell types (Svenningsson and Greengard, 2007). These include the tetrodotoxin-resistant sodium channel (Nav 1.8/SNS) (Okuse et al., 2002), the TASK-1 potassium channel (Girard et al., 2002), surface proteins of the blue tongue virus (Beaton et al., 2002), ASIC channels (Donier et al., 2005), TRPV5 and TRPV6 channels (Van de Graaf et al., 2003), and the 5HT-1B serotonin receptor (Svenningsson et al., 2006). This list of diverse binding partners suggests that p11 serves a chaperone-like function, directing receptors and channel proteins to the plasma membrane in many different cell types.
While studying the endothelial cell, we found that, in the absence of A2, p11 is unstable and rapidly degraded through a proteasome-dependent mechanism (He et al., 2008) (Figure 3). When HEK 293 cells, which express very low levels of endogenous p11 and A2, were transfected with FLAG-p11, unbound p11 monomers were observed to be rapidly ubiquitinated and proteolyzed. Co-transfection of A2, however, rescued p11, which bound to the 13 N-terminal amino acids in the A2 tail peptide. This interaction masked an autonomous p11 polyubiquitination signal involving residues Y86-G95, near its A2-binding, C-terminal motif. In a separate series of experiments, siRNA knockdown of p11 in mouse endothelial cells did not affect total expression of A2 (Deora et al., 2004). These studies suggest, first, that A2 directly regulates intracellular levels of p11 in endothelial cells, and, second, that the level of p11 expression in the endothelial cell may be a primary determinant of maximal A2 expression on the cell surface.
Figure 3. p11 expression is regulated by A2.
A. HEK cells, which lack A2 and p11 expression, were transfected with hemaglutinin-tagged ubiquitin, with or without FLAG-tagged p11 and myc-tagged A2. Under these conditions, transfected FLAG-p11 was highly ubiquitinated and degraded in the proteasome (lane 2). The degree of ubiquitination of FLAG-p11 decreased, while total p11 expression increased, in response to incremental increases in A2 expression (lanes 3-6). Additional experiments revealed that A2 masks a polyubiquitination determinant on p11, protecting it from proteasomal destruction. B. In the endothelial cell, p11 can partner either with A2, promoting translocation of the heterotetramer to the cell surface, or with A2. If sufficient A2 is not present, p11 is ubiquitinated and destined for proteasomal destruction. Panel A reprinted with permission from (He et al., 2008).
3. Animal models of annexin A2 system-mediated fibrinolysis
3.1 A2-deficient mice
To gain insight into the physiological role of A2 in vascular homeostasis, our laboratory created mice globally deficient in A2 (Anxa2−/− mice) (Ling et al., 2004). Although Anxa2−/− mice undergo normal development, are fertile, and have a normal lifespan, their tissues display ubiquitously increased deposition of fibrin within the microvasculature (Figure 4A). Interestingly, and for reasons outlined in the previous section, Anxa2−/− mice express reduced levels of endothelial cell p11 (He et al., 2008). Although plasma clotting and clot lysis times are normal in Anxa2−/− mice, their isolated microvascular ECs lack “cofactor activity,” rendering them unable to support tPA- dependent plasminogen activation. Following FeCl3-induced carotid artery injury, moreover, Anxa2−/− mice are more prone to occlusive thrombi and diminished blood flow than their Anxa2+/+ counterparts. Together, these data indicate a defect in fibrin clearance or “surveillance,” especially in smaller blood vessels.
Figure 4. Fibrin accumulation and impaired angiogenesis in Anxa−/− mice.
A. Immunoblot analysis of extracts of five tissues from AnxaA2+/+ or AnxaA2−/− mice (n=4) shows increased fibrin deposition in AnxaA2−/− tissues. Standards (lanes 1–3) represent 2, 5 and 10 ng for lung, spleen, and small intestine, and 1, 2, and 5 ng for liver and kidney. B. Hematoxylin and eosin-stained sections of retinas from AnxaA2+/+ and AnxaA2−/− mice studied in the oxygen-induced retinopathy model of angiogenesis. Neonatal mouse pups were maintained in either room air (RA) or a 75% oxygen atmosphere (O2) from day 7 through day 12 of life. Neoangiogenesis is prominent in AnxaA2+/+ retinas exposed to high oxygen (green arrows), but not in equally treated AnxaA2−/− mice. Green arrowheads indicate dilated intraretinal blood vessels. Reprinted with permission from (Ling et al., 2004).
In addition to attenuated vascular fibrinolysis, Anxa2−/− mice also exhibit impaired neoangiogenesis (Ling et al., 2004) (Figure 4B). Data from multiple in vivo models involving growth factor stimulation (Matrigel implant and corneal pocket) or oxygen priming (oxygen-induced retinopathy) support this concept. In vitro, capillary sprouting from Anxa2−/− aortic explants is reduced, as is activation of matrix metalloproteinases-9 and -13, two plasmin-dependent, angiogenesis-associated proteases. These data suggest that the impaired clearance of fibrin results from deficient tPA-dependent Plg activation on the surface of EC. In addition, they point to a mechanistic link between vascular fibrinolysis and angiogenesis.
3.2 Hyperhomocysteinemia and blockade of A2 function
Homocysteine (HC) is a thiol-containing amino acid, and a metabolic intermediate generated during the biosynthesis of cysteine (Selhub, 1999). In human plasma, HC circulates normally at concentrations of 5–12 μM (Malinow et al., 1999; Nygard et al., 1997). Hyperhomocysteinemia results from mutations in genes encoding cystathionine-β synthase, methionine synthase, and 5,10-methylenetetrahydrofolate reductase, and can also occur in association with environmental factors such as gender, age, and diet, in which deficiency of folate, vitamin B6, and vitamin B12 appear to be primary determinants (Moat et al., 2004; Splaver et al., 2004).
In 1969, McCully reported widespread atherosclerosis-like arterial disease at postmortem in an infant with hyperhomocysteinemia due to an inborn error of cobalamin metabolism (McCully, 1969). Since then, numerous studies, including recent meta-analyses, have confirmed an association between elevated circulating HC levels and macrovascular atherothrombotic disease, including coronary artery occlusion, stroke, and venous thromboembolism (Humphrey et al., 2008). Mild to moderate hyperhomocysteinemia has been linked to macrovascular occlusion related to venous thromboembolism as well as coronary, cerebrovascular, and peripheral atherosclerosis (Nygard et al., 1997). In more recent studies, hyperhomocysteinemia has emerged as an independent risk factor for cerebral small vessel disease, which may lead to lacunar infarction and accounts for about 25% of ischemic strokes (Fassbender et al., 1999; Hassan et al., 2003; Markus, 2007; Wong et al., 2006). In older individuals, several cross-sectional studies have linked hyperhomocysteinemia with depression and with small vessel disease–related dementias, including Alzheimer disease (Folstein et al., 2007; Selhub, 2008; Smith, 2008).
When endothelial cells are treated with micromolar concentrations of HC, tPA-dependent Plg activation is reduced (Hajjar, 1993). In addition, when purified A2 was incubated in vitro with HC, A2 was derivatized by HC through a reduction-sensitive disulfide bond at C8 (Hajjar et al., 1998). This modification prevented binding of tPA to A2. In addition, incubation of cultured ECs with 35S-HC led to reduction-sensitive metabolic labeling of A2, suggesting that the same alteration of A2 can occur in cells exposed to elevated HC levels.
We tested this hypothesis further using an in vivo model of hyperhomocysteinemia (Jacovina et al., 2009). Mice were subjected to a high-methionine, low folate diet that increases HC plasma levels to approximately 70 μM. Under non-reducing conditions, A2 retrieved from these mice failed to bind tPA and did not support Plg activation. Additionally, hyperhomocysteinemic mice exhibited microvascular fibrin accumulation and blunted angiogenic capacity, findings that phenocopied the Anxa2−/− mouse (Figure 5). Notably, the angiogenic defect improved when the mice received intravenous GFP-tagged recombinant A2 (rA2); the infused protein could be tracked to developing corneal neovessels. Because the angiogenic impairment observed in hyperhomocysteinemic mice was not depressed further in Anxa2−/− mice, we concluded that HC exerted its anti-angiogenic effects through an annexin A2-dependent pathway.
Figure 5. Homocysteine disables A2 in vivo.
A. Hyperhomocysteinemia, produced by a high methionine diet, promotes fibrin accumulation and impaired perivascular fibrinolysis in mice. Representative sections of extensively perfused renal tissue from mice on normal chow, glycine-enriched (Gly), or methionine-enriched (Met) diets were stained with rabbit anti-human fibrin(ogen) IgG, followed by CY3-conjugated rabbit IgG (red) and DAPI (blue). Tissue autofluorescence is green. Original magnification, ×400. Scale bars: 100 μm. B. Corneal angiogenesis in response to implantation of bFGF-containing pellets was decreased in mice maintained on a high methionine diet as compared to those on chow or high glycine diets. However, neovascular growth was restored in methionine fed mice upon receiving 5 daily tail vein injections of rA2, but not similar injections of BSA. Shown are representative photographs of corneal neovessels. Reprinted with permission from (Jacovina et al., 2009).
3.3 A2 in models of ischemic cerebral disease
Ischemic stroke is a leading cause of adult morbidity and mortality (Lloyd-Jones et al., 2009). Although tPA is one of the very few drugs currently approved specifically for thrombotic stroke, this agent must be administered within three hours of the onset of symptoms to avoid hemorrhagic transformation and neurotoxicity (Marler and Goldstein, 2003). Therefore, recent efforts have aimed at improving the safety and efficacy of tPA thrombolysis for ischemic stroke.
In preclinical studies, several groups have explored the use of A2 as a potential adjunct to conventional thrombolytic therapy. First, in a rat carotid artery injury model of thrombosis, pretreatment of the animal with intravenous recombinant A2 relieved blood flow impairment, hastened the overall recovery of blood flow, and reduced thrombus size (Ishii et al., 2001). This experiment suggested that prophylactic treatment with A2, even in the absence of thrombolytic therapy, has some efficacy in reducing the sequelae of arterial thrombosis. Second, administration of rA2 prior to embolization of the rat carotid artery with autologous clot, improved patency of thrombosed arteries, increased relative blood flow, and reduced the size of the final infarct in the absence of any exogenous thrombolytic agent (Tanaka et al., 2007). Third, when A2 was delivered in conjunction with tPA in a rat model of focal embolic cerebral stroke, there was enhancement of thrombolytic activity, allowing the use of lower doses of tPA to achieve optimal thrombolysis (Zhu et al., 2010). In addition, overall mortality, brain infarction, and hemorrhage were all reduced, while reperfusion was prolonged; moreover, the therapeutic window for thrombolytic treatment could be extended beyond that assigned to tPA alone. Together, these studies suggest that exogenous A2 may be an effective amplifier of tPA-mediated thrombolysis, permitting lower tPA dosages, extended therapeutic time windows, and fewer neurotoxic and hemorrhagic complications (Fan et al., 2010).
4. Annexin A2 in human hematovascular disease
4.1 Acute promyelocytic leukemia (APL)
Patients with acute promyelocytic leukemia (APL) are predisposed to a hemorrhagic diathesis characterized by hypofibrinogenemia in the setting of both hyperfibrinolysis and disseminated intravascular coagulopathy (Tallman et al., 2007). Leukemic cells from APL patients having the classical balanced translocation involving chromosomes 15 and 17 (t(15;17)), exhibit strikingly increased A2 expression as compared to leukemic cells derived from patients with other forms of leukemia (p<0.01) (Figure 6) (Arbuthnot and Wilde, 2006; Menell et al., 1999; Stein et al., 2009). These cells generate cell surface tPA-dependent plasmin with accelerated efficiency compared with t(15;17)-negative cells, and the observed increase in plasmin generation is attenuated in the presence of anti-A2 antibodies (Menell et al., 1999). Conversely, transfection with A2 cDNA induces plasmin generation in t(15;17)-negative cells. While t(15;17)-positive cells contain abundant A2 mRNA, levels decrease following administration of all-trans-retinoic acid (ATRA), the same treatment used to promote leukemic cell maturation, thereby attenuating hemorrhage in patients with APL. Recent data extend these findings by showing that p11 is also rapidly down-regulated in an APL-like cell line upon treatment with ATRA (O’Connell et al., 2011). These data suggested that the hemorrhagic complications in APL may reflect both disseminated intravascular coagulopathy, with consumption of clotting factors, and overexpression of A2 or its heterotetramer, leading to depletion of the fibrinolytic inhibitor, α2-antiplasmin. These findings provided, moreover, the first evidence that dysregulation of annexin A2, an example of an “annexinopathy” (Rand, 1999), is likely to have clinical relevance.
Figure 6. A2 is overexpressed in APL-positive leukemia cells.

Leukemia cells from patients with (A) t(15;17)-positive acute promyelocytic leukemia and (B) acute myeloid leukemia stained with rabbit anti-A2 followed by FITC-conjugated secondary antibody (green). Cells were counterstained with Evans blue. Original magnification x1000. Reprinted with permission from (Menell et al., 1999).
4.2 Antiphospholipid syndrome (APS)
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by recurrent arterial or venous thrombosis, or fetal loss, in the presence of antiphospholipid antibodies (aPL) (Cohen et al., 2010). Recent evidence has identified A2 as a prominent target for autoantibodies arising in patients with APS (Cesarman-Maus et al., 2006) (Figure 7A). When analyzed by enzyme-linked immunosorbent assay and immunoblot, serum from individuals with APS showed significantly higher levels of anti-A2 antibodies than healthy individuals (P < 0.001), individuals with non-autoimmune thrombosis (P=0.017), or individuals with lupus without thrombosis (P< 0.001) (Cesarman-Maus et al., 2006). In vitro, patient-derived anti-annexin A2 antibodies blocked endothelial surface tPA-dependent plasmin generation, and also “activated” cultured endothelial cells, inducing them to express elevated levels of the prothrombotic agent, tissue factor. These studies suggested that autoantibodies to A2 in the setting of autoimmune disease may have multiple pathogenic effects.
Figure 7. Anti-A2 antibodies are prevalent in patients with anti-phospholipid syndrome and a cohort with cerebral venous thrombosis.
Sera derived from patients with antiphospholipid syndrome (APS) contains higher mean levels of anti-A2 antibodies compared to healthy controls (Healthy), patients with systemic lupus erythematosus without thrombosis (SLE), or patients with thrombosis related to nonimmune risk factors (Non-immune Thrombosis). Results are quantified according to an A2 Index (A.U.), which is the sum of anti-A2 IgG and IgM expressed as subject to mean control levels. Shown in red are the A2 index values for patients with either IgG or IgM titers that exceed the mean control value by more than 3 standard deviations. Bars indicate mean values for each group. Reprinted with permission from (Cesarman-Maus et al., 2006).
In agreement with this concept, a recent study showed that complete activation of endothelial cells with APS-related IgG in vivo requires A2 protein expression (Romay-Penabad et al., 2009). When either Anxa2−/− or Anxa2+/+ mice were injected with IgG isolated from a patient with APS (IgG-APS), or with an anti-β2GPI antibody, post-injury thrombus size was significantly smaller. In addition, tissue factor activity was less in Anxa2−/− mice than in wild type mice. Expression of vascular cell adhesion molecule-1 induced by IgG-APS, moreover, was reduced in aortic tissue derived from Anxa2−/− mice compared to wild type mice. Other groups have noted that A2 can serve as a binding site for β(2)-glycoprotein I (Zhang and McCrae, 2005), a common antigenic target for autoantibodies on APS on cultured endothelial cells (Galli et al., 203). Together, these data implicate A2 in the pathogenesis of APS-associated thrombosis through several possible mechanisms (Krone et al., 2010).
4.3 Cerebral venous thrombosis
A multifactorial disease that affects mainly young adult women and children, cerebral venous thrombosis (CVT) occurs without recognizable risk factors in up to 20% of subjects (Ferro et al., 2004). Although CVT occurs in only 0.7% of the general population, it is seen with increased frequency in individuals with APS (Cervera et al., 2009). In a recent study of a Mexican Mestizo population with CVT, 12.5% exhibited anti-A2 antibody titers that were greater than three standard deviations above the mean value for healthy controls (2.1%; p<0.01) (Figure 7B) (Cesarman-Maus et al., 2011). Among these individuals, no obvious risk factors were detected in 15% of patients, while most had at least one predisposing factor. About 50% were either pregnant or in the puerperal state. A positive aPLA was present in close to 25% of individuals, while hereditary prothrombotic risk factors were present in about 20% of patients. These data are interesting in light of independent data that indicate that cultured human cerebral ECs express higher amounts of A2 and generate more plasmin (P < 0.0001) than ECs from other anatomic sites (Kwaan et al., 2004). Therefore, the development of auto-antibodies to A2 alone may represent a significant risk factor for cerebral thrombosis, independent of the other criteria needed to establish the diagnosis of APS.
5. Concluding remarks
Through evolution, mammals have evolved two opposing, protease-mediated, homeostatic mechanisms, one to convert circulating plasma factors into an insoluble clot, and another to dissolve such a clot and restore the flow of blood (Ratnoff and Forbes, 1984). Inevitably, these two processes, coagulation and fibrinolysis, are inextricably linked. In this article, we have reviewed one of the newer components of the fibrinolytic system, the annexin A2 complex, and described studies published over the last two to three decades that have highlighted its central role in maintaining vascular homeostasis.
The available studies support the concept that annexin A2, with its associated proteins, constitutes a key endothelial cell surface entity that regulates two dynamic processes, fibrin balance and angiogenesis (Figure 8). Originally identified as an endothelial cell surface complex that binds plasminogen and tissue plasminogen activator, the A2 tetramer is now a recognized modulator of vascular physiology in animal models. One challenge for the future will be to ascertain the extent to which A2 and its associated proteins may play an etiologic role in human disorders of hemorrhage and thrombosis. In addition, investigations involving non-endothelial cells, such as neurons (Foulkes et al., 2006), retinal pigment epithelial cells (Law et al., 2009), epithelial cells (Martin-Belmonte et al., 2007), cancer cells (Sharma and Sharma, 2007), and activated macrophages (Brownstein et al., 2004; O’Connell et al., 2010) are intriguing, and are likely to unlock new secrets regarding A2 system function.
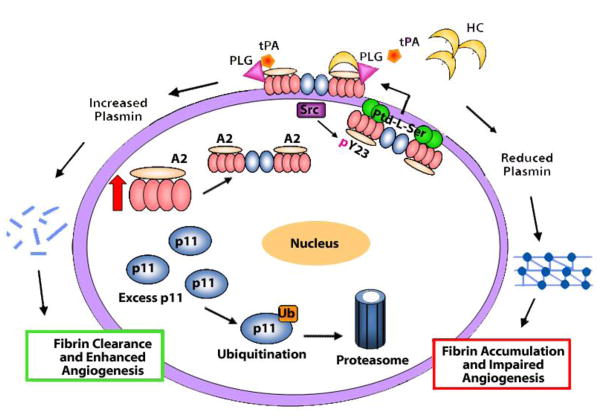
Figure 8. Model for A2 system dynamics in the endothelial cell.
When sufficient A2 is expressed, p11 binds to it, generating the A2 heterotetramer. In the absence of adequate A2, excess p11 is ubiquitinated and targeted for proteasomal degradation. Upon assembly of A2 and p11, the A2 heterotetramer is directed to the inner leaflet of the plasma membrane, where it may be phosphorylated at residue Y23 by activated pp60-c-src kinase (Src). Following phosphorylation, the A2 heterotetramer couples more tightly to inner leaflet anionic phosphatidylserine (Ptd-L-Ser), which may enable further conformational changes promoting translocation to the cell surface. Upon translocation, the A2 heterotetramer may serve as a co-receptor for tPA and plasminogen (Plg), stimulating plasmin production and fibrin clearance. However, in the presence of high levels of homocysteine (HC), A2 is derivatized by HC, which prevents tPA binding. As result, A2 cannot mediate plasmin generation at the endothelial cell surface, resulting in fibrin accumulation and impaired angiogenesis.
Acknowledgments
This work was supported by grants to KAH from the National Heart, Lung, and Blood Institute of the NIH (R01 HL 042093, R01 HL090895, P01 HL046403) and the March of Dimes (6-FY05-94).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andronicus NM, Chen EI, Baik N, Bai H, Parmer CM, Kiosses WB, Kamps MP, Yates JR, Parmer RJ, Miles LA. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood. 2010;115:1319–1330. doi: 10.1182/blood-2008-11-188938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnot C, Wilde JT. Haemostatic problems in acute promyelocytic leukaemia. Blood Rev. 2006;20:289–297. doi: 10.1016/j.blre.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Beaton AR, Rodriguez J, Reddy YK, Roy P. The membrane trafficking protein calpactin forms a complex with bluetongue virus protein NS3 and mediates virus release. Proc Natl Acad Sci USA. 2002;99:13154–13159. doi: 10.1073/pnas.192432299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Weber K, Johnsson N. Protein-protein recognition via short amphiphilic helices; a mutational analysis of the binding site of annexin II for p11. EMBO J. 1990;9:4204–4213. doi: 10.1002/j.1460-2075.1990.tb07868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellagamba C, Hubaishy I, Bjorge JD, Fitzpatrick SL, Fujita DJ, Waisman DM. Tyrosine phosphorylation of annexin II tetramer is stimulated by membrane binding. J Biol Chem. 1997;272:3195–3199. doi: 10.1074/jbc.272.6.3195. [DOI] [PubMed] [Google Scholar]
- Blasi F, Conese M, Moller LB, Pedersen N, Cavallaro U, Cubellis MV, Fazioli F, Hernandez-Marrero L, Limongi P, Munoz-Canoves P, et al. The urokinase receptor: Structure, regulation and inhibitor-mediated internalization. Fibrinolysis. 1994;8:182–188. [Google Scholar]
- Brownstein C, Deora AB, Jacovina AT, Weintraub R, Gertler M, Faisal Khan KM, Falcone DJ, Hajjar KA. Annexin II mediates plasminogen-dependent matrix invasion by human monocytes: Enhanced expression by macrophages. Blood. 2004;103:317–324. doi: 10.1182/blood-2003-04-1304. [DOI] [PubMed] [Google Scholar]
- Burger A, Berendes R, Liemann S, Benz J, Hofmann A, Gottig P, Huber R, Gerke V, Thiel C, Romisch J, et al. The crystal structure and ion channel activity of human annexin II, a peripheral membrane protein. J Mol Biol. 1996;257:839–847. doi: 10.1006/jmbi.1996.0205. [DOI] [PubMed] [Google Scholar]
- Cervera R, Boffa M, Khamashta MA, Hughes GR. The Euro-Phospholipid Project: Epidemiology of the antiphospholipid syndrome in Europe. Lupus. 2009;18:889–893. doi: 10.1177/0961203309106832. [DOI] [PubMed] [Google Scholar]
- Cesarman GM, Guevara CA, Hajjar KA. An endothelial cell receptor for plasminogen/tissue plasminogen activator: II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J Biol Chem. 1994;269:21198–21203. [PubMed] [Google Scholar]
- Cesarman-Maus G, Cantu-Brito C, Barinagarrementeria F, Villa R, Reyes E, Sanchez-Guerrerp J, Hajjar K, Latorre EG. Autoantibodies against the fibrinolytic receptor, annexin A2, in cerebral venous thrombosis. Stroke. 2011;42:501–503. doi: 10.1161/STROKEAHA.110.592121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman-Maus G, Hajjar KA. The molecular basis of fibrinolysis. Br J Haematol. 2005;129:307–321. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- Cesarman-Maus G, Rios-Luna NP, Deora AB, Huang B, Villa R, Cravioto MC, Alarcon-Segovia D, Sanchez-Guerrero J, Hajjar KA. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood. 2006;107:4375–4382. doi: 10.1182/blood-2005-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Berger SP, Steup-Beekman GM, Bloemenkamp KWM, Bajema IM. Diagnosis and management of the antiphosphlipid syndrome. Br Med J. 2010;340:1125–1132. doi: 10.1136/bmj.c2541. [DOI] [PubMed] [Google Scholar]
- Cooper JA, King CS. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986;6:4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Burke T, Plow EF. Histone H2B as a functionally important plasminogen receptor on macrophages. Blood. 2007;110:3763–3772. doi: 10.1182/blood-2007-03-079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassah M, Deora A, He K. The endothelial cell annexin A2 system and vascular fibrinolysis. Gen Physiol Biophys. 2009;28:F20–F28. [PMC free article] [PubMed] [Google Scholar]
- Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates its p11-dependent translocation to the cell surface. J Biol Chem. 2004;279:43411–43418. doi: 10.1074/jbc.M408078200. [DOI] [PubMed] [Google Scholar]
- Donato R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- Donier E, Rugiero F, Okuse K, Wood JN. Annexin II light chain p11 promotes functional expression of acid-sensing ion channel ASICa. J Biol Chem. 2005;280:38666–38672. doi: 10.1074/jbc.M505981200. [DOI] [PubMed] [Google Scholar]
- Erikson E, Erikson RL. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980;21:829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Fan X, Zhanyang Y, Liu J, Liu N, Hajjar KA, Furie KL, Lo EH, Wang X. Annexin A2: A tissue plasminogen activator amplifier for thrombolytic stroke therapy. Stroke. 2010;41:S54–S58. doi: 10.1161/STROKEAHA.110.596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Mielke O, Bertsch T, Nafe B, Froschen S, Hennerici M. Homocysteine in cerebral macroangiography and microangiopathy. Lancet. 1999;353:1586–1587. doi: 10.1016/S0140-6736(99)00309-8. [DOI] [PubMed] [Google Scholar]
- Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F, Investigators I. Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- Folstein M, Liu T, Peter I, Buel J, Arsenault L, Scott T, Qiu WW. The homocysteine hypothesis of depression. Am J Psychiatry. 2007;164:861–867. doi: 10.1176/ajp.2007.164.6.861. [DOI] [PubMed] [Google Scholar]
- Foulkes T, Nassar MA, Lane T, Matthews EA, Baker MD, Gerke V, Okuse K, Dickenson AH, Wood JN. Deletion of annexin 2 light chain p11 in nociceptors causes deficits in somatosensory coding and pain behavior. J Neurosci. 2006;26:10499–19507. doi: 10.1523/JNEUROSCI.1997-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli M, Luciani D, Bertolini G, Barbui T. Anti-beta2-glycoprotein I, antiprothrombin antibodies, and the risk of thrombosis in the antiphospholipid syndrome. Blood. 2003;102:2717–2723. doi: 10.1182/blood-2002-11-3334. [DOI] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: Linking Ca++ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: From structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Gerke V, Weber K. The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells. EMBO J. 1985;4:2917–2920. doi: 10.1002/j.1460-2075.1985.tb04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard C, Tinel N, Terrenoire C, Romey G, Lazdunski M, Borsotto M. p11, an annexin II subunit, an auxiliary protein associated with the background K+ channel, TASK-1. EMBO J. 2002;21:4439–4448. doi: 10.1093/emboj/cdf469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney JR, Jr, Tack BF. Amino-terminal sequence of p36 and associated p10: identification of the site of tyrosine phosphorylation and homology with S-100. Proc Natl Acad Sci U S A. 1985;82:7884–7888. doi: 10.1073/pnas.82.23.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar KA. The endothelial cell tissue plasminogen activator receptor. Specific interaction with plasminogen. J Biol Chem. 1991;266:21962–21970. [PubMed] [Google Scholar]
- Hajjar KA. Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. J Clin Invest. 1993;91:2873–2879. doi: 10.1172/JCI116532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar KA. The Endothelium in Thrombosis and Hemorrhage. In: Loscalzo J, Schaefer AI, editors. Thrombosis and Hemorrhage. Philadelphia: Lippincott Williams and Wilkins; 2003. pp. 206–219. [Google Scholar]
- Hajjar KA. The Molecular Basis of Fibrinolysis. In: Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE, editors. Nathan and Oski’s Hematology of Infancy and Childhood. Philadelphia: Saunders Elsevier; 2009. pp. 1425–1447. [Google Scholar]
- Hajjar KA, Gavish D, Breslow J, Nachman RL. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature. 1989;339:303–305. doi: 10.1038/339303a0. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Guevara CA, Lev E, Dowling K, Chacko J. Interaction of the fibrinolytic receptor, annexin II, with the endothelial cell surface. Essential role of endonexin repeat 2. J Biol Chem. 1996;271:21652–21659. doi: 10.1074/jbc.271.35.21652. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Hamel NM. Identification and characterization of human endothelial cell membrane binding sites for tissue plasminogen activator and urokinase. J Biol Chem. 1990;265:2908–2916. [PubMed] [Google Scholar]
- Hajjar KA, Hamel NM, Harpel PC, Nachman RL. Binding of tissue plasminogen activator to cultured human endothelial cells. J Clin Invest. 1987;80:1712–1719. doi: 10.1172/JCI113262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar KA, Harpel PC, Jaffe EA, Nachman RL. Binding of plasminogen to cultured human endothelial cells. J Biol Chem. 1986;261:11656–11662. [PubMed] [Google Scholar]
- Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator: I. Identity with annexin II. J Biol Chem. 1994;269:21191–21197. [PubMed] [Google Scholar]
- Hajjar KA, Mauri L, Jacovina AT, Zhong F, Mirza UA, Padovan JC, Chait BT. Tissue plasminogen activator binding to the annexin II tail domain. Direct modulation by homocysteine. J Biol Chem. 1998;273:9987–9993. doi: 10.1074/jbc.273.16.9987. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Nachman RL. Endothelial cell-mediated conversion of glu-plasminogen to lys-plasminogen: further evidence for assembly of the fibrinolytic system on the endothelial cell surface. J Clin Invest. 1988;82:1769–1778. doi: 10.1172/JCI113790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W. De Motu cordis: On the motion of the heart and blood in animals. 1910. Vol. 38. New York: P.F. Collier & Son; 1628. [Google Scholar]
- Hassan A, Hunt BJ, O’Sullivan M, Bell R, D’Souza R, Jeffery S, Bamford JM, Markus HS. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain. 2003;127:212–219. doi: 10.1093/brain/awh023. [DOI] [PubMed] [Google Scholar]
- He K, Deora AB, Xiong H, Ling Q, Weksler BB, Niesvizky R, Hajjar KA. Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner, S100A10/p11. J Biol Chem. 2008;283:19192–19200. doi: 10.1074/jbc.M800100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Sui G, Xiong H, Broekman MJ, Huang B, Marcus AJ, Hajjar KA. Feedback regulation of endothelial cell surface plasmin generation by PKC dependent phosphorylation of annexin A2. J Biol Chem. 2011 doi: 10.1074/jbc.M110.185058. epub 11/29/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey LL, Rongwei F, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: A systematic review and meta-analysis. Mayo Clin Proc. 2008;83:1203–1212. doi: 10.4065/83.11.1203. [DOI] [PubMed] [Google Scholar]
- Ishii H, Yoshida M, Hiraoka M, Hajjar KA, Tanaka A, Yasukochi Y, Numano F. Recombinant annexin II modulates impaired fibrinolytic activity in vitro and in rat carotid artery. Circ Res. 2001;89:1240–1245. doi: 10.1161/hh2401.101066. [DOI] [PubMed] [Google Scholar]
- Jacovina AT, Deora AB, Ling Q, Broekman MJ, Almeida D, Greenberg CB, Marcus AJ, Smith JD, Hajjar KA. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2-dependent fibrinolysis. J Clin Invest. 2009;119:3384–3394. doi: 10.1172/JCI39591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N, Marriott G, Weber K. p36, the major cytoplasmic substrate of src tyrosine protein kinase, binds to its p11 regulatory subunit via a short amino-terminal amphipathic helix. EMBO J. 1988;7:2435–2442. doi: 10.1002/j.1460-2075.1988.tb03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M, Gerke V. Mapping of a regulatory important site for protein kinase C phosphorylation in the N-terminal domain of annexin II. Biochim Biophys Acta. 1996;1313:283–289. doi: 10.1016/0167-4889(96)00101-2. [DOI] [PubMed] [Google Scholar]
- Krone KA, Allen KL, McCrae KR. Impaired fibrinolysis in the antiphospholipid syndrome. Curr Rheumatol Rep. 2010;12:53–57. doi: 10.1007/s11926-009-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kube E, Becker T, Weber K, Gerke V. Protein-protein interactions studied by site-directed mutagenesis: Characterization of the annexin II-binding site on p11, a member of the S100 protein family. J Biol Chem. 1992;267:14175–14182. [PubMed] [Google Scholar]
- Kwaan HC, Wang J, Weiss I. Expression of receptors for plasminogen activators on the endothelial cell surface depends on their origin. J Thromb Haemost. 2004;2:306–312. doi: 10.1111/j.1538-7933.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- Kwon M, MacLeod TJ, Zhang Y, Waisman DM. S100A10, annexin A2, and annexin A2 heterotetramer as candidate plasmnogen receptors. Front Biosci. 2005;10:300–325. doi: 10.2741/1529. [DOI] [PubMed] [Google Scholar]
- Law AL, Ling Q, Hajjar KA, Futter C, Greenwood J, Adamson P, Wavre-Shapton ST, Moss SE, Hayes MJ. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol Biol Cell. 2009;20:3896–3904. doi: 10.1091/mbc.E08-12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Q, Jacovina AT, Deora AB, Febbraio M, Simantov R, Silverstein RL, Hempstead BL, Mark W, Hajjar KA. Annexin II is a key regulator of fibrin homeostasis and neoangiogenesis. J Clin Invest. 2004;113:38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: A statement for healthcare professionals from the nutrition committee, American Heart Association. Circulation. 1999;99:178–182. doi: 10.1161/01.cir.99.1.178. [DOI] [PubMed] [Google Scholar]
- Markus HS. Genes, endothelial function and cerebral small vessel disease in man. Exp Physiol. 2007;93:121–127. doi: 10.1113/expphysiol.2007.038752. [DOI] [PubMed] [Google Scholar]
- Marler JR, Goldstein LB. Stroke - tPA and the clinic. Science. 2003;301:1677. doi: 10.1126/science.1090270. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully KS. Vascular pathology of homocysteinemia: Implications for the pathogenesis of atherosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA, Hajjar KA. Annexin II and bleeding in acute promyelocytic leukemia. N Engl J Med. 1999;340:994–1004. doi: 10.1056/NEJM199904013401303. [DOI] [PubMed] [Google Scholar]
- Miles LA, Castellino FJ, Gong Y. Critical role for conversion of Glu-plasminogen to Lys-plasminogen for optimal stimulation of plasminogen activation on cell surfaces. Trends Cardiovasc Med. 2003;13:21–30. doi: 10.1016/s1050-1738(02)00190-1. [DOI] [PubMed] [Google Scholar]
- Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, Plow EF. Role of cell surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry. 1991;30:1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- Miles LA, Fless GM, Levin EG, Scanu AM, Plow EF. A potential basis for the thrombotic risks associated with lipoprotein(a) Nature. 1989;339:301–303. doi: 10.1038/339301a0. [DOI] [PubMed] [Google Scholar]
- Miles LA, Hawley SB, Baik N, Andronicus NM, Castellino F, Parmer RJ. Plasminogen receptors: The sine qua non of cell surface plasminogen activation. Front Biosci. 2005;10:1754–1762. doi: 10.2741/1658. [DOI] [PubMed] [Google Scholar]
- Moat SJ, Lang D, McDowell IFW, Clarke ZL, Madhavan AK, Lewis MJ, Goodfellow J. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem. 2004;15:64–79. doi: 10.1016/j.jnutbio.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Montaville P, Neumann JM, Russo-Marie F, Ochsenbein F, Sanson A. A new consensus sequence for phosphatidylserine recognition by annexins. J Biol Chem. 2002;277:24684–24693. doi: 10.1074/jbc.M109595200. [DOI] [PubMed] [Google Scholar]
- Moss SE, Morgan RO. The annexins. Genome Biol. 2004;5:219, 211–219, 218. doi: 10.1186/gb-2004-5-4-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- O’Connell PA, Madureira PA, Berman JN, Liwski RS, Waisman DM. Regulation of S100A10 by the PML-RARalpha oncoprotein. Blood. 2011 doi: 10.1182/blood-2010-07-298851. ePub 2/10/2011. [DOI] [PubMed] [Google Scholar]
- O’Connell PA, Surette AP, Liwski RS, Svenningsson P, Waisman DM. S100A10 regulates plasminogen-dependent macrophage invasion. Blood. 2010;116:1136–1146. doi: 10.1182/blood-2010-01-264754. [DOI] [PubMed] [Google Scholar]
- Okuse K, Malik-Hall M, Baker MD, Poon WYL, Kong H, Chao MV, Wood JN. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature. 2002;417:653–656. doi: 10.1038/nature00781. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Sappino AP, Stocklin R, Montesano R, Orci L, Vassalli JD. Upregulation of urokinase receptor expression on migrating endothelial cells. J Cell Biol. 1993;122:673–684. doi: 10.1083/jcb.122.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen EA, Sutherland MR, Nesheim ME, Pryzdial EL. Thrombin induces endothelial cell-surface exposure of the plasminogen receptor annexin 2. J Cell Sci. 2003;116:2399–2408. doi: 10.1242/jcs.00434. [DOI] [PubMed] [Google Scholar]
- Rand JH. "Annexinopathies" - A new class of diseases. N Engl J Med. 1999;340:1035–1036. doi: 10.1056/NEJM199904013401310. [DOI] [PubMed] [Google Scholar]
- Ratnoff OD, Forbes CD. Evolution of Knowledge about Hemostasis. In: Ratnoff OD, Forbes CD, editors. Disorders of Hemostasis. New York: Grune & Stratton, Inc; 1984. pp. 1–21. [Google Scholar]
- Rety S, Sopkova J, Renouard M, Osterloh D, Gerke V, Tabaries S, Russo-Marie F, Lewit-Bentley A. The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat Struct Biol. 1999;6:85–89. doi: 10.1038/4965. [DOI] [PubMed] [Google Scholar]
- Rijken DC, Lijnen HR. New insights into teh molecular mechanism of the fibrinolytic system. J Thromb Haemost. 2009;7:4–13. doi: 10.1111/j.1538-7836.2008.03220.x. [DOI] [PubMed] [Google Scholar]
- Romay-Penabad Z, Montiel-Manzano MG, Pappalardo E, Vargas G, Deora AB, Wang M, Jacovina AT, Garcia-Latorre E, Reyes-Maldonado E, Hajjar KA. Pathogenic effects of antiphospholipid antibodies are ameliorated in annexin A2 deficient mice. Blood. 2009;i114:3074–3083. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J. Homocysteine metabolism. Ann Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Selhub J. Public health significance of elevated homocysteine. Food Nutr Bull. 2008;29:S116–S125. doi: 10.1177/15648265080292S116. [DOI] [PubMed] [Google Scholar]
- Sharma MC, Sharma M. The role of annexin II in angiogenesis and tumor progression: A potential therapeutic target. Curr Pharm Des. 2007;13:3568–3575. doi: 10.2174/138161207782794167. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Friedlander RJ, Nicholas RL, Nachman RL. Binding of lys-plasminogen to monocytes and macrophages. J Clin Invest. 1988;82:1948–1955. doi: 10.1172/JCI113814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA. The worldwide challenge of the dementias: A role for B vitamins and homocysteine? Food Nutr Bull. 2008;29:S143–S172. doi: 10.1177/15648265080292S119. [DOI] [PubMed] [Google Scholar]
- Splaver A, Lamas GA, Hennekens CH. Homocysteine and cardiovascular disease: Biological mechanisms, observational epidemiology, and the need for randomized trials. Am Heart J. 2004;148:34–40. doi: 10.1016/j.ahj.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Stein E, McMahon B, Kwaan H, Altman JK, Frankfurt O, Tallman MS. The coagulopathy of acute promyelocytic leukaemia revisited. Best Pract Res Clin Haematol. 2009;22:152–163. doi: 10.1016/j.beha.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, Yacoubi ME, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Greengard P. p11 (S100A10)--an inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Tallman MS, Abutalib SA, Altman JK. The double hazard of thrombophilia and bleeding in acute promyelocytic leukemia. Sem Thromb Hemostasis. 2007;33:330–338. doi: 10.1055/s-2007-976168. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Ishii H, Hiraoka M, Miyasaka N, Kuroiwa T, Hajjar KA, Nagaoka T, Duong TQ, Ohno K, Yoshida M. Efficacy of recombinant annexin 2 for fibrinolytic therapy in a rat embolic stroke model: A magnetic resonance imaging study. Brain Res. 2007;1165:135–143. doi: 10.1016/j.brainres.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AS. Fibrinolysis autographs. Nature. 1958;181:495–496. doi: 10.1038/181495b0. [DOI] [PubMed] [Google Scholar]
- Todd AS. Localization of fibrinolytic activity in tissues. Br Med Bull. 1964;20:210–212. doi: 10.1093/oxfordjournals.bmb.a070333. [DOI] [PubMed] [Google Scholar]
- Van de Graaf SFJ, Hoenderop JGJ, Gkika D, Lamers D, Prenen J, Rescher U, Gerke V, Stub O, Nilius B, Bindels RJM. Functional expression of the epithelial Ca2+ channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J. 2003;22:1478–1487. doi: 10.1093/emboj/cdg162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisman DM. Annexin II tetramer: structure and function. Mol Cell Biochem. 1995;149/150:301–322. doi: 10.1007/BF01076592. [DOI] [PubMed] [Google Scholar]
- Wong A, Mok V, Fan YH, Lam WWM, Liang KS, Wong KS. Hyperhomocysteinemia is associated with volumetric white matter change in patients with small vessel disease. J Neurol. 2006;253:441–447. doi: 10.1007/s00415-005-0022-x. [DOI] [PubMed] [Google Scholar]
- Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-{beta}2-glycoprotein I antibodies. Blood. 2005;105:1964–1969. doi: 10.1182/blood-2004-05-1708. [DOI] [PubMed] [Google Scholar]
- Zhu H, Fan J, Liu J, Murata Y, Yu Z, Hajjar K, Lo EH, Wang X. Annexin A2 combined with low dose tPA improves thrombolytic therapy in a rat model of focal embolic stroke. J Cerebral Blood Flow Metab. 2010;30:1137–1146. doi: 10.1038/jcbfm.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobiack N, Gerke V, Rescher U. Complex formation and submembranous localization of annexin 2 and S100A10 in live HepG2 cells. FEBS Lett. 2001;500:137–140. doi: 10.1016/s0014-5793(01)02604-7. [DOI] [PubMed] [Google Scholar]