Abstract
Epidemiological and recent prospective analyses of long febrile seizures (FS) and febrile status epilepticus (FSE) support the idea that in some children, such seizures can provoke temporal lobe epilepsy (TLE). Because of the high prevalence of these seizures, if epilepsy was to arise as their direct consequence, this would constitute a significant clinical problem. Here we discuss these issues, and describe the use of animal models of prolonged FS and of FSE to address the following questions: Are long FS epileptogenic? What governs this epileptogenesis? What are the mechanisms? Are there any predictive biomarkers of the epileptogenic process, and can these be utilized, together with information about the mechanisms of epileptogenesis, for eventual prevention of the TLE that results from long FS and FSE.
Introduction
Febrile seizures (FS) are defined as seizures taking place during fever, but which are not a result of an invasive infection of the central nervous system. These seizures occur in infants and young children, with a median age of 11–18 months [42,88,107,112]. Fever-related seizures are very rare in normal adults, so that the ability of fever to generate seizures is generally considered a characteristic of the developing brain. In addition, the reason that FS are the most common of all childhood seizures may derive from the fact that infants and children sustain > 6 febrile episodes per year, so that fever is more common than other potential seizure-provoking insults such as trauma or hyponatremia.
FS, both short and long, may occur in both normal children and those with a predisposition to the seizures and to the development of epilepsy, such as ion channel mutations or cortical dysplasia. However, studies indicate that even identical twins may diverge in the presence of long FS and the development of temporal lobe epilepsy (TLE), suggesting that the occurrence of FSE in itself might be epileptogenic in the non-predisposed brain [60]. However, whereas twin studies provide a valuable tool, it is difficult to control clinical studies for host-brain variability. Therefore, discovering if long FS or FSE are sufficient to provoke epilepsy requires controlled experimental models. Here we focus on the following points:
Are prolonged FS and febrile status epilepticus (FSE) epileptogenic?
What is the role of predisposing elements of the host brain, such as gene mutations, cortical dysplasia, in FS-induced epileptogenesis?
What parameters of the FS themselves (duration, severity) govern the development of epilepsy?
How does epilepsy arise after FS? Several mechanisms (cell loss, inflammation and altered patterns of gene expression) have been implicated in epileptogenesis.
Are there predictive biomarkers of epileptogenesis?
What therapeutic strategies can be used for preventing and/or reversing FS-induced epileptogenesis?
For each of these points, we briefly describe available information from clinical studies, followed by contributions of experimental approaches.
A. Are prolonged FS and FSE epileptogenic?
FS lasting less than 10 or 15 minutes [2,14,89] have not been associated with subsequent epilepsy or cognitive deficits in prospective or retrospective studies [12,121,122]. However, the consequences of long FS, one of the forms of complex FS, are controversial [2,13]. Retrospective studies have linked a history of long FS and subsequent TLE [26,50,54,116]. Prospective studies generally failed to implicate long FS as causing TLE (see [105], for review), although careful review suggests that up to 40% of individual with long/focal FS developed epilepsy ([2]; Hesdorffer et al., personal communication). More recently, the FEBSTAT study, focusing on FSE, has begun to define abnormal EEGs, MRI changes and the development of TLE in children who have sustained FSE [72,93,107]. These findings, because of their prospective and careful design, are the strongest evidence to date about a causal relationship of FSE with TLE. However, children enter the study upon development of FSE. Therefore, predisposing factors of FSE, which might also predispose to TLE (or will affect the probability of FSE resulting in TLE) cannot be excluded. In other words, whereas FEBSTAT will answer the question of the development of epilepsy after FSE, and will delineate clear pre-existing factors such as cortical dysplasia or gene mutations, it will be difficult to establish a direct causal effect. To control for predisposing factors in the host brain requires animal models. Such models have been created for both individuals with predisposing factors (brain injury, dysplasia, ion channel mutations) and for a ‘non-predisposed’ host brain, and are described below.
B. What is the role of predisposing elements of the host brain (gene mutations, cortical dysplasia) on epileptogenesis?
A large body of literature has addressed the potential genetic basis of FS [11,35,46,55,75,128], and the hypothesis that characteristics of the brain of the child who has a long FS govern if the child will develop epilepsy. FS run in families [15,16] but are also more common in children in day-care centers [17], and their generation is likely a result of both genetic and environmental causes that vary in each individual [11,25,47]. In several clear instances, specific mutations predispose to both FS development and subsequent epilepsy, including sodium and chloride channel mutations [3,46,101,128]. These mutations generate the “generalized epilepsy with febrile seizures plus” syndrome (GEFS+) of familial FS and/or several types of epilepsy. Cortical dysplasia has also been implicated in the development of epilepsy after FS [111].
Similarly, animal models of cortical dysplasia [51] and prior injury [99] suggest that FS affect the injured brain differently, including the probability of generation of epilepsy. Elegant studies in mice genetically engineered to express human mutations such as of sodium channels involved in the GEFS+ syndrome and its extreme form, severe myoclonic epilepsy of infancy (SMEI) [77,78,90], demonstrated increased sensitivity to hyperthermia-induced seizures. Additional in vitro studies have begun to provide the underlying mechanisms [4,20,65,66,109,110,117,129]. Thus, both human and animal data are consistent with the idea that characteristics of the host brain contribute to the development of FS and to their consequences.
However, FS occurs throughout the world in generally ‘non-predisposed’ children who have no evidence of preexisting injury, brain-affecting gene mutations, or cortical dysplasia. Therefore, an important clinical question is whether FS can generate TLE in generally ‘normal’ children. If so, then the potential specific characteristics of the seizures that predict epileptogenesis should be studied.
C. What parameters of the FS themselves (duration, severity) govern the development of epilepsy?
In children, simple FS are defined as short (< 10 or 15 minutes), and without focal features. The vast majority of epidemiological studies suggest that these FS are not associated with epileptogenesis [2,12,89]. Complex FS are defined as seizures that are long (> 10–15 minutes), or with focal features (e.g. involvement of one side of the body), or recur within 24 hours of the first episode [2,88] or within the same febrile illness [23]. In addition FSE is generally defined as FS longer than 30 minutes [93,107]. (Note: Scott et al. [102,103] define seizures lasting more than 30 minutes in normal children without intracranial infection as prolonged FS rather than FSE). It is these long and focal FS that are statistically associated with epileptogenesis, and the major correlation has been with the duration of the seizures [87,93,102,103,107,120]. However, the duration of FS in itself might also be an indication of a subtle abnormality of the host brain that interferes with stopping of the seizure [105]. Thus, in children, it is difficult to study if the duration of the FS itself is important for epileptogenesis. Duration of seizure is a crucial parameter in the adult brain; SE results in major changes in neuronal physiology (e.g., [53,74]). Thus, duration of FS is a logical candidate to contribute to the initiation of an epileptogenic process.
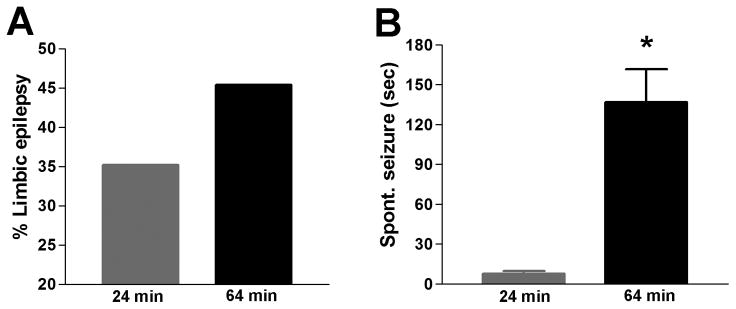
In animal models, duration of FS can be controlled experimentally. Dubé et al. initially used seizures of ~20 minutes, recapitulating “complex FS” [36,40]. More recently, the group used FSE-like seizures, and found that seizure duration influenced the incidence of limbic epilepsy and also governed the severity of the resulting spontaneous seizures (Figure 1a,b and [44]). Whereas shorter FS led to spontaneous seizures lasting seconds, experimental FSE led to longer seizures with robust motor phenomena (Figure 2). Other groups also employed models generating seizures lasting either ~20 minutes [31,32,61,70,71], or shorter single seizures [33,62,100], or a series of nine short FS [27,28]. The subsequent incidence of limbic epilepsy has not been examined in most of these studies. Scantlebury et al. [100] investigated this outcome in normal versus injured brains, and found that 86% of the rats that experienced cortical focal lesions and FS became epileptic in adulthood.
Fig. 1.
The duration of FS influenced the probability of developing limbic epilepsy and the severity of the resulting spontaneous recurrent seizures. (A) The percentage of rats developing limbic epilepsy increased by 30% after a 64 minute FS compared to a 24 minute FS. (B) The duration of FS affected the duration and the severity of the resulting spontaneous seizures: Mean duration of seizures was significantly longer (136.7≤25 sec, n = 18) after a 64 minute FS compared to a 24 minute FS (7.8≤0.3 sec, n = 57; median durations, 91 vs 7 sec). Modified from [44], with permission.
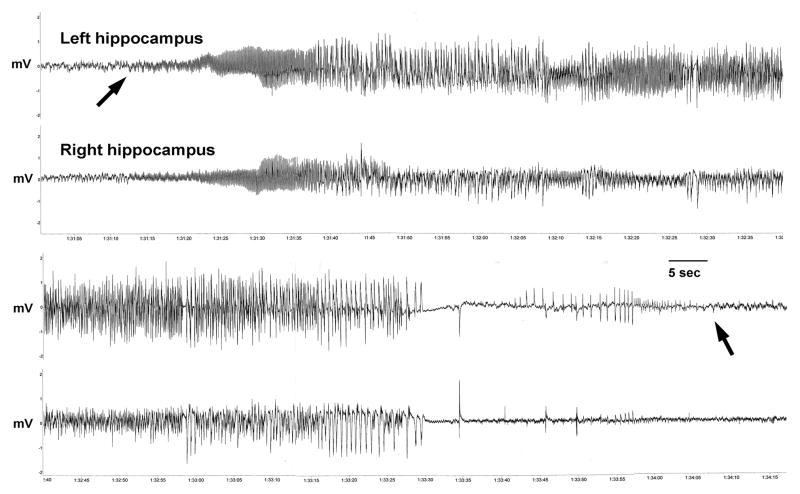
Fig. 2.
Example of spontaneous electrographic seizure recorded from hippocampal bipolar electrodes in adult rats that experienced a 64 minute FS. Arrows point to onset and end of epileptiform discharges. Calibration: 5 sec. Dube et al., unpublished; please see [44] for methods.
In conclusion, a body of clinical work spanning decades has suggested that FS duration might contribute to the probability of epileptogenesis. The FEBSTAT study, that includes children with FS longer than 30 minutes, has to date found epileptogenesis only in children whose seizures were ~an hour long (Shinnar, personal communication). Animal models have controlled for potential host brain confounders and established that FS duration is an important parameter for epileptogenesis. Thus, a preventive approach to FS-provoked epileptogenesis involves prevention of long FS by aborting the seizures with methods such as using benzodiazepines. This is reasonable for a second or third seizure, but is obviously not feasible for the first FS.
D. How does epilepsy arise after FS? The role of cell loss, inflammation and altered patterns of gene expression
Is epileptogenesis associated with cell loss?
One of the structural hallmarks in patients with mesial TLE and a history of long FS is a specific pattern of cell loss in hippocampus, i.e mesial temporal sclerosis (MTS), and a reorganization of the remaining circuit [34,59,79,85,114]. These changes are considered by many to be required for epileptogenesis [7,91,108]. The nature of the relationship between cell loss and epileptogenesis in humans after long FS remains unclear. It has been widely hypothesized that FS cause MTS and the development of TLE is a consequence of MTS [93,103,120]. However, the clinical literature also supports the alternative view that TLE after FS might precede MTS, and the latter results from the epileptic seizures [67,86,96,106].
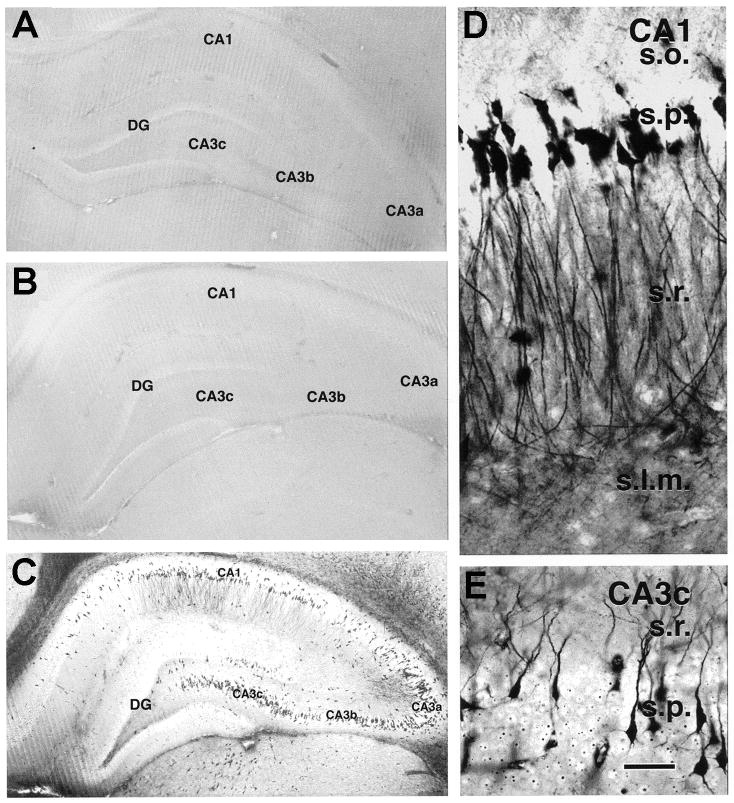
In animal models, early injury of neurons in hippocampus was found, that mirrored the distribution of cells that are lost or damaged in TLE/MTS (Figure 3). Interestingly, these cells seemed to recover over time, so that a significant loss of neuronal populations in hippocampi of rats was not found following single [9,100] or repeated episodes of FS [27], including in rats that became epileptic [40,44]. Although the methods employed (cell counts) cannot exclude subtle loss of some hilar interneuron populations, these data support the notion that cell loss is not a prerequisite for epileptogenesis. These findings are consistent with the presence of epileptogenesis without significant cell loss in other models in immature and adult rats [8,69,94,131]. In addition, Dubé et al., found increased hippocampal MRI signals in a subpopulation of rats that experienced FS [37,43]. These T2 changes were not associated with increased water content (Dubé, unpublished observations) or accompanied by neuronal loss in the hippocampus. These findings support three concepts: First, functional alterations of neuronal and network properties might take place in the absence of long-lasting structural changes (e.g. cell loss, sprouting), and result in epilepsy. Second, the MTS found in individuals with TLE and a history of FSE might not necessarily be a cause of the epilepsy, but a consequence [67,81,96]. Third, the acute increased T2 relaxation time reported in children with FSE within days of the seizures [93,103,120] might not indicate acute cell loss.
Fig. 3.
Cell injury in the same distribution as found in MTS is transiently provoked by experimental long FS. Using a modified Golgi stain, silver uptake was found in populations of hippocampal neurons in rats that experienced FS (C) compared with normothermic (A) and hyperthermic (same duration of hyperthermia but no seizures; B) controls. Note abundant stained neurons in CA1 (Sommer’s section) (D), CA3 and the hilus (E), as demonstrated here. Serial evaluation of hippocampi between 24 hours and 4 weeks demonstrated gradual resolution of this cell injury without apparent cell loss [from 119, with permission], Scale bar: 50 μm.
Does inflammation contribute to epileptogenesis that follows FSE?
Inflammation is emerging as a major mechanism that contributes to epileptogenesis in a number of clinical settings [18,19,82,124,125]. In the context of febrile seizures, fever not only increases brain temperature, but also involves the release of inflammatory mediators, particularly cytokines [1,24,95,123,124] such as interleukin-1β (IL-1β), within the brain. In children, higher levels of this cytokine in cerebrospinal fluid and/or plasma have been detected in individuals with FS by some groups [56,126] but not by others [68,118]. Some have implicated IL-1β in TLE with MTS [64], including a mutation in the IL-1β gene promoter [64,127]. It is intriguing that fever of specific infectious etiologies, and specifically human herpes virus 6 (HHV6) might influence the probability of generation of FS [5,130]. Whether this virus leads to higher levels or a unique profile of cytokine induction in the child’s brain compared with other pathogens has not been studied, though evidence of HHV infection has been found in tissue from individuals with TLE [48].
In animal models, the involvement of endogenous IL-1β in the generation of FS was supported by the increased threshold temperature required to elicit experimental FS in mice lacking the IL-1β receptor type 1 [39] and by the increased proportion of rats developing FS after lipopolysacharide and kainic acid treatment [58]. As mentioned above, IL-1β may contribute to the generation of FS in human infants. However, whether or not IL-1β contributes to the epileptogenic process that is triggered by FS remains unclear. Dubé et al., found that IL-1β expression was induced in reactive astrocytes for at least 24 hours after FSE and returned to basal levels within 72 hours [44]. Interestingly, when FS-experiencing rats that became epileptic were compared to those in which the inciting FS did not lead to spontaneous seizures, hippocampal IL-1β levels were higher only in rats that developed epilepsy (ibid).
Coordinate changes in the expression of numerous genes may contribute to the epileptogenic process
Experimental FSE induces numerous molecular changes (reviewed in [41]; [83]). Among them, lasting changes in the expression of specific genes such ion channels and endocannabinoid receptors have been explored to date. Notably, hyperpolarization-activated cation channels (HCN) have been implicated not only in FS [29,30], but in other models as well [45,63,76,92,97]. Seizures induce a long-lasting reduction of HCN1 isoform expression [21,22]. The precise mechanisms by which alterations in HCN channels and Ih contribute to human epileptogenesis are not fully known; however, HCN1 channel expression was altered in a subset of resected hippocampi from patients with TLE and MTS, often with a history of early life seizures [10]. Mutations in HCN channel genes have recently been discovered in individuals with epilepsy [35], further supporting the role of these channels in the epileptogenic process.
Similarly, increased endocannabinoid receptor levels inducing a short-term plasticity phenomenon has been described [31,32]. Altered expression of other genes may also play a role [27,38,57].
Whereas the changes in the expression of the above specific genes have been studied in detail, recent evidence supports massive, coordinate transcriptional regulation of hundreds of genes to be involved in the epileptogenic process that follows FSE. These changes may underlie the mechanisms by which FSE initiate the transformation of ‘normal’ neurons and neuronal circuits into epileptic ones. Activation of specific transcription factors by seizures which regulate select gene clusters, may contribute to epileptogenesis. One candidate is the neuron-restrictive silencer factor (NRSF) that was recently shown to be involved in FS and other models [6,52,84,115]. Studies are underway to determine which genes are regulated by NRSF and the role of this transcription factor in epileptogenesis that follows FS. Therefore, identifying key genes that may contribute to the development of epileptogenesis and delineating the mechanisms that regulate these genes may provide molecular targets for the development of novel therapeutic strategies for preventing the development of TLE after FS.
E. Predictive biomarkers of epileptogenesis
If FS lead to TLE, this process arises only in a subset of children. Defining predictive biomarkers to identify the individuals experiencing long FS and/or FSE that are risk for epilepsy is critical and should provide a powerful tool for testing of potential interventions. MRI changes and EEG activity alterations could constitute excellent biomarkers because they can be quantified and repeated.
Early MRI changes, specifically, increased T2 signal arising within days after long FS in children, have been described [93,103,120]. In early studies of EEG that were obtained within a week after FS, Frantzen et al. [49] described abnormalities, mainly focal or global slowing, in about a third of the EEGs. However, the presence of an abnormal EEG did not correlate with seizure recurrence [49,113] or with the subsequent development of epilepsy [49]. More recently, the FEBSTAT study, focusing on FSE, has begun to define MRI changes (increased T2 signal) and abnormal EEGs (focal slowing) in children who have sustained FSE. The study plans to correlate these changes (as well as subsequent hippocampal volume loss) with the development of TLE. These are important and powerful clinical approaches; however it would be difficult to exclude factors that predispose to the occurrence of FSE and/or to the development of TLE in human studies. In addition, the development of TLE after FSE often involves time periods lasting a decade or more [50,80].
In this context, predictive animal models of FS-provoked epileptogenesis are useful. Using MRI, Jansen et al. found increased T2 values early (within 24 hours) after experimental FS, as did Dubé et al. [37]. Reduced apparent diffusion coefficients in hippocampus and other limbic structures 24 hours after FS were also apparent in the Jansen study [61]. The MRI alterations in that study persisted up to 2 months after FS, but the group did not correlate the MRI changes with epileptogenesis. Direct correlation of early FSE-induced MRI changes with subsequent epileptogenesis was attempted by Dubé et al. [43,44] who imaged controls and rats that experienced FSE one month later, and found increased hippocampal T2 relaxation times in a subset of FS rats [44]. However, T2 values did not distinguish the rats that developed limbic epilepsy from those that did not, though these T2 values correlated with interictal activity. Interestingly, the same group found that augmented T2 values one month after the FS correlated with hippocampal dysfunction manifested as spatial memory deficits [43]. Another potential biomarker, interictal activity, was investigated in the models of FS with a prior lesion [100] and of long FS and FSE [40,44]. Whereas Scantlebury and colleagues detected the presence of interictal activity (spikes and epileptic discharges) only in rats that developed limbic epilepsy (86%), Dubé et al. found that interictal activity arose in ~90% of the rats after FSE, while ~45% of the rats became epileptic. No rat became epileptic without interictal activity; however, the presence of such activity did not predict epileptogenesis. Interestingly, EEG spectrum analysis revealed that a significant reduction of energy in the low frequencies (delta and theta range) distinguished epileptic rats from controls and those with interictal activity alone [40].
The possibility that inflammatory molecules might constitute biomarkers for epileptogenesis has been investigated by several groups. Sharp (e.g., [73,104]) investigated the gene expression profile in peripheral white blood cells one day after kainic acid seizures, as well as after other insults, and found unique gene-expression patterns for each of the experimental conditions. In the context of FS, Sasaki et al. [98] examined the induction of genes related to inflammatory mediators and ion channels in leukocytes of a small number of children with FS and controls. They stimulated these cells with a Toll-like receptor agonist, synthetic double strand RNA. The expression of a number of inflammatory genes, among them IL-1β, was enhanced in children with FS compared to controls, suggesting a modified inflammatory response. It would be interesting to examine if differences in gene expression or induction in peripheral white blood cells (especially inflammatory mediators) distinguish individuals (rats and children) that develop epilepsy after FSE from those that would not. In a rat model, significantly higher levels of IL-1β were detected in the hippocampi of rats with FSE compared to controls 24 hours after the seizures, and, as mentioned, high IL-1β levels distinguished FSE rats that became epileptic from control rats [44]. These data lead to the speculation that if feasible and validated, detection of IL-1β in the periphery might provide an interesting and potentially clinically relevant biomarker.
F. What therapeutic strategies can be used for preventing and/or reversing FS-induced epileptogenesis?
The evidence summarized here indicates that long FS and FSE may provoke epilepsy. In addition, the duration of the FSE seems to be an important determinant of the development of subsequent limbic epilepsy in the non-predisposed brain (Figure 1). These findings suggest that preventing long FS should be a therapeutic goal. In addition, because it is clinically not feasible to abort all long FS and FSE, identification of children at risk for epileptogenesis should lead to preventive measures. At the present time, no single mechanism has emerged to account for FSE-induced epilepsy in the non-predisposed brain. As discussed above, inflammation is implicated and will likely be a subject of preclinical and clinical studies [124].
In summary, much has been learned over the past decade about the epileptogenesis that follows FS. Both clinical and experimental studies have addressed several key questions, and established that very long FS and FSE provoke changes in the brain that promote epilepsy. These changes take place at multiple levels, are likely driven by transcriptional mechanisms, and are potentially induced by inflammatory mediators. The discovery of early predictive biomarkers is crucial; these biomarkers, together with elucidation of the epileptogenic mechanisms will provide avenues for prevention and intervention.
Acknowledgments
The authors thank Mrs. Barbara Cartwright for excellent editorial help. Supported by NIH grant R37 NS35439 and NS35439-S1 (ARRA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alheim K, Bartfai T. The interleukin-1 system: receptors, ligands, and ICE in the brain and their involvement in the fever response. Ann N Y Acad Sci. 1998;840:51–58. doi: 10.1111/j.1749-6632.1998.tb09548.x. [DOI] [PubMed] [Google Scholar]
- 2.Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493–498. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- 3.Audenaert D, Schwartz E, Claeys KG, Claes L, Deprez L, Suls A, Van Dyck T, Lagae L, Van Broeckhoven C, Macdonald RL, De Jonghe P. A novel GABRG2 mutation associated with febrile seizures. Neurology. 2006;67:687–690. doi: 10.1212/01.wnl.0000230145.73496.a2. [DOI] [PubMed] [Google Scholar]
- 4.Barela AJ, Waddy SP, Lickfett JG, Hunter J, Anido A, Helmers SL, Goldin AL, Escayg A. An epilepsy mutation in the sodium channel SCN1A that decreases channel excitability. J Neurosci. 2006;26:2714–2723. doi: 10.1523/JNEUROSCI.2977-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barone SR, Kaplan MH, Krilov LR. Human herpesvirus-6 infection in children with first febrile seizures. J Pediatr. 1995;127:95–97. doi: 10.1016/s0022-3476(95)70263-6. [DOI] [PubMed] [Google Scholar]
- 6.Bassuk AG, Wallace RH, Buhr A, Buller AR, Afawi Z, Shimojo M, Miyata S, Chen S, Gonzalez-Alegre P, Griesbach HL, Wu S, Nashelsky M, Vladar EK, Antic D, Ferguson PJ, Cirak S, Voit T, Scott MP, Axelrod JD, Gurnett C, Daoud AS, Kivity S, Neufeld MY, Mazarib A, Straussberg R, Walid S, Korczyn AD, Slusarski DC, Berkovic SF, El-Shanti HI. A homozygous mutation in human PRICKLE1 causes an autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome. Am J Hum Genet. 2008;83:572–581. doi: 10.1016/j.ajhg.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- 9.Bender RA, Dubé C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or alteredneurogenesis,, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:399–412. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;23:6826–6836. doi: 10.1523/JNEUROSCI.23-17-06826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg AT, Shinnar S, Shapiro ED, Salomon ME, Crain EF, Hauser WA. Risk factors for a first febrile seizure: a matched case-control study. Epilepsia. 1995;36:334–341. doi: 10.1111/j.1528-1157.1995.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 12.Berg AT, Shinnar S. Unprovoked seizures in children with febrile seizures: short-term outcome. Neurology. 1996;47:562–568. doi: 10.1212/wnl.47.2.562. [DOI] [PubMed] [Google Scholar]
- 13.Berg AT, Shinnar S. Complex febrile seizures. Epilepsia. 1996;37:126–133. doi: 10.1111/j.1528-1157.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 14.Berg AT, Shinnar S, Darefsky AS, Holford TR, Shapiro ED, Salomon ME, Crain EF, Hauser AW. Predictors of recurrent febrile seizures: a prospective cohort study. Arch Pediatr Adolesc Med. 1997;151:371–378. doi: 10.1001/archpedi.1997.02170410045006. [DOI] [PubMed] [Google Scholar]
- 15.Berg AT, Shinnar S, Levy SR, Testa FM. Childhood-onset epilepsy with and without preceding febrile seizures. Neurology. 1999;53:1742–1748. doi: 10.1212/wnl.53.8.1742. [DOI] [PubMed] [Google Scholar]
- 16.Berkovic S, Scheffer I. Febrile seizures: genetics and relationship to other epilepsy syndromes. Curr Opin Neurol. 1998;11:129–134. doi: 10.1097/00019052-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Bethune P, Gordon K, Dooley J, Camfield C, Camfield PI. Which child will have a febrile seizure? Am J Dis Child. 1993;147:35–39. doi: 10.1001/archpedi.1993.02160250037013. [DOI] [PubMed] [Google Scholar]
- 18.Bien CG, Urbach H, Schramm J, Soeder BM, Becker AJ, Voltz R, Vincent A, Elger CE. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69:1236–1244. doi: 10.1212/01.wnl.0000276946.08412.ef. [DOI] [PubMed] [Google Scholar]
- 19.Boer K, Crino PB, Gorter JA, Nellist M, Jansen FE, Spliet WG, van Rijen PC, Wittink FR, Breit TM, Troost D, Wadman WJ, Aronica E. Gene expression analysis of tuberous sclerosis complex cortical tubers reveals increased expression of adhesion and inflammatory factors. Brain Pathol. 2010;20:704–719. doi: 10.1111/j.1750-3639.2009.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowser DN, Wagner DA, Czajkowski C, Cromer BA, Parker MW, Wallace RH, Harkin LA, Mulley JC, Marini C, Berkovic SF, Williams DA, Jones MV, Petrou S. Altered kinetics and benzodiazepine sensitivity of a GABAA receptor subunit mutation [gamma 2(R43Q)] found in human epilepsy. Proc Natl Acad Sci USA. 2002;99:15170–15175. doi: 10.1073/pnas.212320199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brewster AL, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200–207. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camfield P, Camfield C. Febrile seizures. In: Shinnar S, Amir N, Branski D, editors. Childhood Seizures. Basel: Karger; 1995. pp. 32–38. [Google Scholar]
- 24.Cartmell T, Luheshi GN, Rothwell NJ. Brain sites of action of endogenous interleukin-1 in the febrile response to localized inflammation in the rat. J Physiol. 1999;518:585–594. doi: 10.1111/j.1469-7793.1999.0585p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassano PA, Koepsell TD, Farwell JR. Risk of febrile seizures in childhood in relation to prenatal maternal cigarette smoking and alcohol intake. Am J Epidemiol. 1990;132:462–473. doi: 10.1093/oxfordjournals.aje.a115681. [DOI] [PubMed] [Google Scholar]
- 26.Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, Olivier A, Andermann E, Robitaille Y, Lopes-I Cendes I, et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- 27.Chang YC, Huang AM, Kuo YM, Wang ST, Chang YY, Huang CC. Febrile seizures impair memory and cAMP response-element binding protein activation. Ann Neurol. 2003;54:706–718. doi: 10.1002/ana.10789. [DOI] [PubMed] [Google Scholar]
- 28.Chang YC, Kuo YM, Huang AM, Huang CC. Repetitive febrile seizures in rat pups cause long-lasting deficits in synaptic plasticity and NR2A tyrosine phosphorylation. Neurobiol Dis. 2005;18:466–475. doi: 10.1016/j.nbd.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen K, Ratzliff A, Hilgenberg L, Gulyás A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen K, Neu A, Howard AL, Földy C, Echegoyen J, Hilgenberg L, Smith M, Mackie K, Soltesz I. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chisholm J, Kellogg C, Franck JE. Developmental hyperthermic seizures alter adult hippocampal benzodiazepine binding and morphology. Epilepsia. 1985;26:151–157. doi: 10.1111/j.1528-1157.1985.tb05399.x. [DOI] [PubMed] [Google Scholar]
- 34.de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 35.Dibbens LM, Reid CA, Hodgson B, Thomas EA, Phillips AM, Gazina E, Cromer BA, Clarke AL, Baram TZ, Scheffer IE, Berkovic SF, Petrou S. Augmented currents of an HCN2 variant in patients with febrile seizure syndromes. Ann Neurol. 2010;67:542–546. doi: 10.1002/ana.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubé C, Chen K, Eghbal-Ahmad M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long-term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- 37.Dubé C, Yu H, Nalcioglu O, Baram TZ. Serial MRI after experimental febrile seizures: altered T2 signal without neuronal death. Ann Neurol. 2004;56:709–714. doi: 10.1002/ana.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubé C, Brunson KL, Eghbal-Ahmadi M, Gonzalez-Vega R, Baram TZ. Endogenous neuropeptide Y prevents recurrence of experimental febrile seizures by increasing seizure threshold. J Mol Neurosci. 2005;25:275–284. doi: 10.1385/JMN:25:3:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1β contributes to the generation of experimental febrile seizures. Ann Neurol. 2005;57:152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubé C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubé CM, Brewster AL, Richichi C, Zha Q, Baram TZ. Fever, febrile seizures and epilepsy. Trends Neurosci. 2007;30:490–496. doi: 10.1016/j.tins.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubé CM, Brewster AL, Baram TZ. Febrile seizures: mechanisms and relationship to epilepsy. Brain Dev. 2009;31:366–371. doi: 10.1016/j.braindev.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubé CM, Zhou JL, Hamamura M, Zhao Q, Ring A, Abrahams J, McIntyre K, Nalcioglu O, Shatskih T, Baram TZ, Holmes GL. Cognitive dysfunction after experimental febrile seizures. Exp Neurol. 2009;215:167–177. doi: 10.1016/j.expneurol.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubé CM, Ravizza T, Hamamura M, Zha Q, Keebaugh A, Fok K, Andres AL, Nalcioglu O, Obenaus A, Vezzani A, Baram TZ. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci. 2010;30:7484–7494. doi: 10.1523/JNEUROSCI.0551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dugladze T, Vida I, Tort AB, Gross A, Otahal J, Heinemann U, Kopell NJ, Gloveli T. Impaired hippocampal rhythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proc Natl Acad Sci USA. 2007;104:17530–17535. doi: 10.1073/pnas.0708301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, Buresi C, Malafosse A. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- 47.Forsgren L, Sidenvall R, Blomquist HK, Heijbel J, Nyström L. An incident case-referent study of febrile convulsions in children: genetical and social aspects. Neuropediatrics. 1990;21:153–159. doi: 10.1055/s-2008-1071484. [DOI] [PubMed] [Google Scholar]
- 48.Fotheringham J, Donati D, Akhyani N, Fogdell-Hahn A, Vortmeyer A, Heiss JD, Williams E, Weinstein S, Bruce DA, Gaillard WD, Sato S, Theodore WH, Jacobson S. Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med. 2007;5:e180. doi: 10.1371/journal.pmed.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frantzen E, Lennox-Buchthal M, Nygaard A. Longitudinal EEG and clinical study of children with febrile convulsions. Electroencephalogr Clin Neurophysiol. 1968;24:197–212. doi: 10.1016/0013-4694(68)90001-1. [DOI] [PubMed] [Google Scholar]
- 50.French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 51.Germano IM, Zhang YF, Sperber EF, Moshé SL. Neuronal migration disorders increase susceptibility to hyperthermia-induced seizures in developing rats. Epilepsia. 1996;37:902–910. doi: 10.1111/j.1528-1157.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 52.Gillies SG, Haddley K, Vasiliou SA, Jacobson GM, von Mentzer B, Bubb VJ, Quinn JP. Distinct Gene Expression Profiles Directed by the Isoforms of the Transcription Factor Neuron-Restrictive Silencer Factor in Human SK-N-AS Neuroblastoma Cells. J Mol Neurosci. 2010 Jul 23; doi: 10.1007/s12031-010-9420-3. [DOI] [PubMed] [Google Scholar]
- 53.Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamati-Haddad A, Abou-Khalil B. Epilepsy diagnosis and localization in patients with antecedent childhood febrile convulsions. Neurology. 1998;50:917–922. doi: 10.1212/wnl.50.4.917. [DOI] [PubMed] [Google Scholar]
- 55.Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haspolat S, Mihçi E, Coskun M, Gümüslü S, Ozben T, Yegin O. Interleukin-1beta, tumor necrosis factor-alpha, and nitrite levels in febrile seizures. J Child Neurol. 2002;17:749–751. doi: 10.1177/08830738020170101501. [DOI] [PubMed] [Google Scholar]
- 57.Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat. Neuroscience. 2000;101:571–580. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heida JG, Pittman QJ. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia. 2005;46:1906–1913. doi: 10.1111/j.1528-1167.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 59.Houser CR. Neuronal loss and synaptic reorganization in temporal lobe epilepsy. Adv Neurol. 1999;79:743–761. [PubMed] [Google Scholar]
- 60.Jackson GD, McIntosh AM, Briellmann RS, Berkovic SF. Hippocampal sclerosis studied in identical twins. Neurology. 1998;51:78–84. doi: 10.1212/wnl.51.1.78. [DOI] [PubMed] [Google Scholar]
- 61.Jansen JF, Lemmens EM, Strijkers GJ, Prompers JJ, Schijns OE, Kooi ME, Beuls EA, Nicolay K, Backes WH, Hoogland G. Short- and long-term limbic abnormalities after experimental febrile seizures. Neurobiol Dis. 2008;32:293–301. doi: 10.1016/j.nbd.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Jiang W, Duong TM, de Lanerolle NC. The neuropathology of hyperthermic seizures in the rat. Epilepsia. 1999;40:5–19. doi: 10.1111/j.1528-1157.1999.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 63.Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D'Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanemoto K, Kawasaki J, Miyamoto T, Obayashi H, Nishimura M. Interleukin (IL)1beta, IL-1alpha, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol. 2000;47:571–574. [PubMed] [Google Scholar]
- 65.Kang JQ, Shen W, Macdonald RL. Why does fever trigger febrile seizures? GABAA receptor gamma2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies. J Neurosci. 2006;26:2590–2597. doi: 10.1523/JNEUROSCI.4243-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang JQ, Shen W, Macdonald RL. The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. J Neurosci. 2009;29:2845–2856. doi: 10.1523/JNEUROSCI.4772-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapur J. Is mesial temporal sclerosis a necessary component of temporal lobe epilepsy? Epilepsy Curr. 2006;6:208–209. doi: 10.1111/j.1535-7511.2006.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lahat E, Livne M, Barr J, Katz Y. Interleukin-1beta levels in serum and cerebrospinal fluid of children with febrile seizures. Pediatr Neurol. 1997;17:34–36. doi: 10.1016/s0887-8994(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 69.Lee CL, Hannay J, Hrachovy R, Rashid S, Antalffy B, Swann JW. Spatial learning deficits without hippocampal neuronal loss in a model of early-onset epilepsy. Neuroscience. 2001;107:71–84. doi: 10.1016/s0306-4522(01)00327-x. [DOI] [PubMed] [Google Scholar]
- 70.Lemmens EM, Lubbers T, Schijns OE, Beuls EA, Hoogland G. Gender differences in febrile seizure-induced proliferation and survival in the rat dentate gyrus. Epilepsia. 2005;46:1603–1612. doi: 10.1111/j.1528-1167.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 71.Lemmens EM, Aendekerk B, Schijns OE, Blokland A, Beuls EA, Hoogland G. Long-term behavioral outcome after early-life hyperthermia-induced seizures. Epilepsy Behav. 2009;14:309–315. doi: 10.1016/j.yebeh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Lewis DV. MRI Abnormalities Following Febrile Status Epilepticus: Preliminary Findings from the FEBSTAT Study. Abstract; American Epilepsy Society Meeting; 2009. p. IW.03. [Google Scholar]
- 73.Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- 75.Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol. 2010;588:1861–1869. doi: 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marcelin B, Chauvière L, Becker A, Migliore M, Esclapez M, Bernard C. h channel-dependent deficit of theta oscillation resonance and phase shift in temporal lobe epilepsy. Neurobiol Dis. 2009;33:436–447. doi: 10.1016/j.nbd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 77.Martin MS, Dutt K, Papale LA, Dubé CM, Dutton SB, de Haan G, Shankar A, Tufik S, Meisler MH, Baram TZ, Goldin AL, Escayg A. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010;285:9823–9834. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mashimo T, Ohmori I, Ouchida M, Ohno Y, Tsurumi T, Miki T, Wakamori M, Ishihara S, Yoshida T, Takizawa A, Kato M, Hirabayashi M, Sasa M, Mori Y, Serikawa T. A missense mutation of the gene encoding voltage-dependent sodium channel (Nav1.1) confers susceptibility to febrile seizures in rats. J Neurosci. 2010;30:5744–5753. doi: 10.1523/JNEUROSCI.3360-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mathern GW, Pretorius JK, Babb TL. Influence of the type of initial precipitating injury and at what age it occurs on course and outcome in patients with temporal lobe seizures. J Neurosurg. 1995;82:220–227. doi: 10.3171/jns.1995.82.2.0220. [DOI] [PubMed] [Google Scholar]
- 81.Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer’s hypothesis revisited. Prog Brain Res. 2002;135:237–251. doi: 10.1016/s0079-6123(02)35023-4. [DOI] [PubMed] [Google Scholar]
- 82.Mazarati AM, Pineda E, Shin D, Tio D, Taylor AN, Sankar R. Comorbidity between epilepsy and depression: role of hippocampal interleukin-1beta. Neurobiol Dis. 2010;37:461–467. doi: 10.1016/j.nbd.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McClelland S, Flynn C, Dubé C, Richichi C, Mundy J, Petrosyan R, Zha Q, Bernard C, Baram TZ. The transcriptional repressor NRSF/REST mediates acquired HCN channelopathy in the epileptogenic process, Abstract, Society for Neuroscience meeting. Neuroscience. 2009 number #147.7/I9. [Google Scholar]
- 84.McClelland S, Flynn C, Dubé C, Yang J, Mundy J, Petrosyan NgKR, Bernard C, Baram TZ. NRSF / REST dependent and independent gene pathways contribute to the generation of a hyperexcitable hippocampal network, Abstract. Society for Neuroscience meeting, Neuroscience; 2010. Session number 41. [Google Scholar]
- 85.Mikkonen M, Soininen H, Kälviänen R, Tapiola T, Ylinen A, Vapalahti M, Paljärvi L, Pitkänen A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol. 1998;44:923–934. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- 86.Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006;47:900–907. doi: 10.1111/j.1528-1167.2006.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Natsume J, Bernasconi N, Miyauchi M, Naiki M, Yokotsuka T, Sofue A, Bernasconi A. Hippocampal volumes and diffusion-weighted image findings in children with prolonged febrile seizures. Acta Neurol Scand Suppl. 2007;86:25–28. [PubMed] [Google Scholar]
- 88.Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med. 1976;295:1029–1033. doi: 10.1056/NEJM197611042951901. [DOI] [PubMed] [Google Scholar]
- 89.Nelson KB, Ellenberg JH. Prognosis in children with febrile seizures. Pediatrics. 1978;61:720–727. [PubMed] [Google Scholar]
- 90.Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci USA. 2009;106:3994–3999. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pitkänen A, Nissinen J, Nairismägi J, Lukasiuk K, Gröhn OH, Miettinen R, Kaupinen R. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog Brain. 2002;135:67–83. doi: 10.1016/S0079-6123(02)35008-8. [DOI] [PubMed] [Google Scholar]
- 92.Powell KL, Ng C, O'Brien TJ, Xu SH, Williams DA, Foote SJ, Reid CA. Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia. 2008;49:1686–1695. doi: 10.1111/j.1528-1167.2008.01593.x. [DOI] [PubMed] [Google Scholar]
- 93.Provenzale JM, Barboriak DP, VanLandingham K, MacFall J, Delong D, Lewis DV. Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis. AJR Am J Roentgenol. 2008;190:976–983. doi: 10.2214/AJR.07.2407. [DOI] [PubMed] [Google Scholar]
- 94.Raol YS, Budreck EC, Brooks-Kayal AR. Epilepsy after early-life seizures can be independent of hippocampal injury. Ann Neurol. 2003;53:503–511. doi: 10.1002/ana.10490. [DOI] [PubMed] [Google Scholar]
- 95.Reid AY, Galic MA, Teskey GC, Pittman QJ. Febrile seizures: current views and investigations. Can J Neurol Sci. 2009;36:679–686. doi: 10.1017/s0317167100008246. [DOI] [PubMed] [Google Scholar]
- 96.Salmenperä T, Könönen M, Roberts N, Vanninen R, Pitkänen A, Kälviäinen R. Hippocampal damage in newly diagnosed focal epilepsy: a prospective MRI study. Neurology. 2005;64:62–68. doi: 10.1212/01.WNL.0000148643.36513.2A. [DOI] [PubMed] [Google Scholar]
- 97.Santoro B, Lee JY, Englot DJ, Gildersleeve S, Piskorowski RA, Siegelbaum SA, Winawer MR, Blumenfeld H. Increased seizure severity and seizure-related death in mice lacking HCN1 channels. Epilepsia. 2010 Apr 2; doi: 10.1111/j.1528-1167.2010.02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sasaki K, Matsuo M, Maeda T, Zaitsu M, Hamasaki Y. Febrile seizures: characterization of double-stranded RNA-induced gene expression. Pediatr Neurol. 2009;41:114–118. doi: 10.1016/j.pediatrneurol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Scantlebury MH, Ouellet PL, Psarropoulou C, Carmant L. Freeze lesion-induced focal cortical dysplasia predisposes to atypical hyperthermic seizures in the immature rat. Epilepsia. 2004;45:592–600. doi: 10.1111/j.0013-9580.2004.51503.x. [DOI] [PubMed] [Google Scholar]
- 100.Scantlebury MH, Gibbs SA, Foadjo B, Lema P, Psarropoulou C, Carmant LL. Febrile seizures in the predisposed brain: a new model of temporal lobe epilepsy. Ann Neurol. 2005;58:41–49. doi: 10.1002/ana.20512. [DOI] [PubMed] [Google Scholar]
- 101.Schlachter K, Gruber-Sedlmayr U, Stogmann E, Lausecker M, Hotzy C, Balzar J, Schuh E, Baumgartner C, Mueller JC, Illig T, Wichmann HE, Lichtner P, Meitinger T, Strom TM, Zimprich A, Zimprich F. A splice site variant in the sodium channel gene SCN1A confers risk of febrile seizures. Neurology. 2009;72:974–978. doi: 10.1212/01.wnl.0000344401.02915.00. [DOI] [PubMed] [Google Scholar]
- 102.Scott RC, Gadian DG, King MD, Chong WK, Cox TC, Neville BG, Connelly A. Magnetic resonance imaging findings within 5 days of status epilepticus in childhood. Brain. 2002;125:1951–1959. doi: 10.1093/brain/awf202. [DOI] [PubMed] [Google Scholar]
- 103.Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126:2551–2557. doi: 10.1093/brain/awg262. [DOI] [PubMed] [Google Scholar]
- 104.Sharp FR, Lit L, Xu H, Apperson M, Walker W, Wong B, Gilbert DL, Hershey A, Glauser TA. Genomics of brain and blood: progress and pitfalls. Epilepsia. 2006;47:1603–1607. doi: 10.1111/j.1528-1167.2006.00809.x. [DOI] [PubMed] [Google Scholar]
- 105.Shinnar S, Berg AT, Moshe SL, Shinnar R. How long do new-onset seizures in children last? Ann Neurol. 2001;49:659–664. [PubMed] [Google Scholar]
- 106.Shinnar S. Febrile Seizures and Mesial Temporal Sclerosis. Epilepsy Curr. 2003;3:115–118. doi: 10.1046/j.1535-7597.2003.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shinnar S, Hesdorffer DC, Nordli DR, Jr, Pellock JM, O'Dell C, Lewis DV, Frank LM, Moshé SL, Epstein LG, Marmarou A, Bagiella E FEBSTAT Study Team. Phenomenology of prolonged febrile seizures: results of the FEBSTAT study. Neurology. 2008;71:170–176. doi: 10.1212/01.wnl.0000310774.01185.97. [DOI] [PubMed] [Google Scholar]
- 108.Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- 109.Spampanato J, Escayg A, Meisler MH, Goldin AL. Functional effects of two voltage-gated sodium channel mutations that cause generalized epilepsy with febrile seizures plus type 2. J Neurosci. 2001;21:7481–7490. doi: 10.1523/JNEUROSCI.21-19-07481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spampanato J, Kearney JA, de Haan G, McEwen DP, Escayg A, Aradi BT, MacDonald I, Levin SI, Soltesz I, Benna P, Montalenti E, Isom LL, Goldin AL, Meisler MH. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J Neurosci. 2004;24:10022–10034. doi: 10.1523/JNEUROSCI.2034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spreafico R, Tassi L, Colombo N, Bramerio M, Galli C, Garbelli R, Ferrario A, Lo Russo G, Munari C. Inhibitory circuits in human dysplastic tissue. Epilepsia. 2000;41(Suppl 6):S168–173. doi: 10.1111/j.1528-1157.2000.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 112.Stafstrom CE. The incidence and prevalence of febrile seizures. In: Baram TZ, Shinnar S, editors. Febrile Seizures. Academic Press; San Diego: 2002. pp. 1–25. [Google Scholar]
- 113.Stores G. When does an EEG contribute to the management of febrile seizures? Arch Dis Child. 1991;66:554–557. doi: 10.1136/adc.66.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- 115.Tapia-Ramirez J, Eggen BJ, Peral-Rubio MJ, Toledo-Aral JJ, Mandel G. A single zinc finger motif in the silencing factor REST represses the neural-specific type II sodium channel promoter. Proc Natl Acad Sci USA. 1997;94:1177–1182. doi: 10.1073/pnas.94.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Theodore WH, Bhatia S, Hatta J, Fazilat S, DeCarli C, Bookheimer SY, Gaillard WD. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology. 1999;52:132–136. doi: 10.1212/wnl.52.1.132. [DOI] [PubMed] [Google Scholar]
- 117.Thomas EA, Hawkins RJ, Richards KL, Xu R, Gazina EV, Petrou S. Heat opens axon initial segment sodium channels: a febrile seizure mechanism? Ann Neurol. 2009;66:219–226. doi: 10.1002/ana.21712. [DOI] [PubMed] [Google Scholar]
- 118.Tomoum HY, Badawy NM, Mostafa AA, Harb MY. Plasma interleukin-1beta levels in children with febrile seizures. J Child Neurol. 2007;22:689–692. doi: 10.1177/0883073807304007. [DOI] [PubMed] [Google Scholar]
- 119.Toth Z, Yan XX, Haftoglou S, Ribak CE, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. 1998;43:413–426. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 121.Verity CM, Butler NR, Golding J. Febrile convulsions in a national cohort followed up from birth. II--Medical history and intellectual ability at 5 years of age. Br Med J. 1985;290:1311–1315. doi: 10.1136/bmj.290.6478.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verity CM, Greenwood R, Golding J. Long-term intellectual and behavioral outcomes of children with febrile convulsions. N Engl J Med. 1998;338:1723–1728. doi: 10.1056/NEJM199806113382403. [DOI] [PubMed] [Google Scholar]
- 123.Vezzani A, Baram TZ. New roles for interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Curr. 2007;7:45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2010 doi: 10.1038/nrneurol.2010.178. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vincent A, Irani SR, Lang B. The growing recognition of immunotherapy-responsive seizure disorders with autoantibodies to specific neuronal proteins. Curr Opin Neurol. 2010;23:144–150. doi: 10.1097/WCO.0b013e32833735fe. [DOI] [PubMed] [Google Scholar]
- 126.Virta M, Hurme M, Helminen M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia. 2002;43:920–923. doi: 10.1046/j.1528-1157.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- 127.Virta M, Hurme M, Helminen M. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr Neurol. 2002;26:192–195. doi: 10.1016/s0887-8994(01)00380-0. [DOI] [PubMed] [Google Scholar]
- 128.Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 129.Xu R, Thomas EA, Gazina EV, Richards KL, Quick M, Wallace RH, Harkin LA, Heron SE, Berkovic SF, Scheffer IE, Mulley JC, Petrou S. Generalized epilepsy with febrile seizures plus-associated sodium channel beta1 subunit mutations severely reduce beta subunit-mediated modulation of sodium channel function. Neuroscience. 2007;148:164–174. doi: 10.1016/j.neuroscience.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 130.Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, Rhoads MP, Nguy L, Bornemann R, Morrow RA, Corey L. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 131.Zhang X, Cui SS, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, Honer WG, Corcoran ME. Relations between brain pathology and temporal lobe epilepsy. J Neurosci. 2002;22:6052–6061. doi: 10.1523/JNEUROSCI.22-14-06052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]