Abstract
In humans, the bacterial product lipopolysaccharide (LPS) has been associated with protection from allergic diseases such as asthma. However, in mouse models of allergic asthma, differential effects of LPS have been noted based on the dose. A low dose of LPS promotes Th2 responses and allergic disease but a high dose has been associated with suppression of allergic airway inflammation. Our recent work has described the ability of LPS to increase the frequency of CD11b+Gr1intF4/80+ (abbreviated as Gr1int cells) cells in the lung tissue of mice in a dose-dependent fashion that is dependent on TLR4 and the TLR adaptor protein, MyD88. Both phenotypically and morphologically, the cells were found to have similarities with myeloid-derived suppressor cells. Adoptive transfer of LPS-induced Gr1int cells suppressed allergen-induced airway inflammation suggesting that these myeloid cells may have regulatory functions in allergic asthma. Although the Gr1int cells are readily detectable in the lung tissue of LPS-treated mice, they are barely detectable in the lung-draining lymph nodes (LNs) or in the airway lumen. This causes selective enrichment of these cells over dendritic cells (DCs) in the tissue since migratory DCs are induced by LPS to migrate to the draining LNs for presentation of antigen to LN T cells. The Gr1int cells were found to blunt the ability of lung DCs to upregulate GATA-3 or to promote STAT5 activation in primed Th2 cells, both transcription factors having critical roles in Th2 effector function. Thus, a complete understanding of the generation and regulation of the Gr1int cells would provide new avenues to either promote or delete these cells for disease-specific immunoregulation.
Keywords: CD11b+Gr1intF4/80+ regulatory cells, Dendritic cells, Allergic airway inflammation, LPS
This article highlights our recent finding on the expansion of CD11b+Gr1intF4/80+ myeloid cells by LPS in the lung and their ability to suppress allergic airway inflammation. Additionally, we have reviewed relevant literature.
Endotoxin Exposure and Influence on Incidence of Asthma
Endotoxins are soluble lipopolysaccharide (LPS) fragments of the outer membrane of gram-negative bacteria that aggregate to form micelles. The polysaccharide portion of endotoxin carries an antigenic epitope specific for the organism while the lipid portion is required for binding to cells and activation of processes that provide resistance to infection in a non-specific fashion, i.e. promote innate immunity [1, 2]. Given the fact that endotoxin has adjuvant properties, it made logical sense that a low dose of LPS would promote Th2 immune response [3]. However, if LPS exposure always promotes Th2-mediated allergic disease, it is somewhat counterintuitive that improved hygiene in the industrialized world, which would amount to less LPS exposure, would actually increase the incidence of atopy and asthma. As discussed below, subsequent studies showed that in fact exposure to LPS at high doses suppresses allergic airway inflammation. This provided an explanation for why improved hygiene might have an adverse effect on allergic disease.
To recount a few studies that prompted our own investigations, a study conducted on young children in the rural areas of Europe revealed that a high level of LPS in mattresses is inversely related to the occurrence of hay fever, atopic asthma and atopic sensitization [4]. Although similar epidemiological studies have shown that early-life farm contact protects against allergic diseases, recent studies have shown that farm contact later in life not only reduces the incidence of allergic disease but is also correlated with loss of sensitization [5–7]. All of these studies suggest that continuous exposure to LPS somehow regulates the development and maintenance of asthma. Taken together, these studies show that LPS can have multi-dimensional effects on the development of asthma and can either cause or prevent allergic airway inflammation. However, dose, duration and timing of LPS exposure are all very critical in determining the outcome in allergic airway inflammation. A very low dose of LPS (~1ng) generally induces tolerance, somewhat higher levels of LPS (~100ng) increases the risk of asthma due to its adjuvant effect (Eisenbart, Piggott et al., 2002) whereas a high dose in the microgram range is protective against allergic airway inflammation as shown in multiple studies including our own [3, 8–11].
Although initial studies suggested that Th1 cell development associated with infections might down regulate Th2 cell differentiation, more studies later on suggested that suppression of Th2 development cannot always be explained by a Th1/Th2 balance. The contribution of immunosuppressive mechanisms rather than Th2 to Th1 immune deviation has been lately suggested to be the basis of LPS-mediated suppression of allergic diseases. Most of these studies suggested that exposure to LPS influences development and severity of asthma, however, the mechanisms by which LPS inhibits allergic inflammation were not sufficiently explored.
LPS administration promotes CD11b+ Ly6GintF4/80+ myeloid cell type
In an attempt to understand how LPS inhibits allergic inflammation in the airways, our laboratory has recently shown that repeated exposure to a relatively high dose of LPS (up to 10 μg) temporally promotes an increase in the lung of total lung cells as well as CD11b+ cells in a dose-dependent manner [9]. Further characterization of these CD11b+ cells by flow cytometry revealed a cell population expanded with LPS with a phenotype of CD11b+Gr-1intF4/80+ (Gr1int cells) which distinguished them from neutrophils which are Gr-1highF4/80− but suggested similarities with myeloid derived suppressor cells (MDSCs). Gr1 and CD11b are co-expressed on both neutrophils and on MDSCs. Neutrophils, however, do not express the macrophage-associated molecule F4/80 [12, 13]. MDSCs isolated from tumor sites have been shown to express F4/80 [14, 15]. Other molecules, like CD115 (M-CSF receptor), CD124 (IL-4 receptor α chain) and CD62L, which have been shown to be associated with MDSCs [16] were, however, not detected on the LPS- induced Gr1int cells [9]. Morphological characterization of the LPS-induced Gr1int cells revealed a heterogeneous population of immature myeloid cells which was similar to the Gr1+CD11b+ cells observed in murine models of cancer and trauma [14, 17]. While MDSCs have been largely investigated in the context of tumor growth and development, recent studies have shown that in contrast to their negative role in cancer immunity, MDSC-type cells can play a very important regulatory role in controlling inappropriate inflammatory immune responses in autoimmune diseases [18]. However, no study prior to ours had investigated their role in allergic disease.
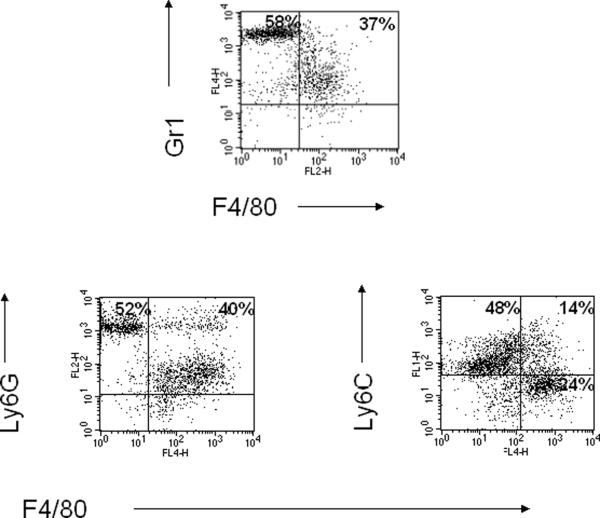
Since Gr-1 recognizes both Ly6G and Ly6C epitopes, we further characterized the Gr1int cells on the basis of expression of Ly6G and Ly6C. Flow cytometric analysis revealed that the LPS-induced Gr1int cells are predominantly Ly6GintF4/80+ cells (Fig.1). Two different subpopulations of MDSCs expressing Ly6G or Ly6C have been identified in the spleens of tumor-bearing mice [14, 19]. The CD11b+Ly6G+Ly6Clow population morphologically resembles polymorphonuclear granulocytes (PMN-MDSC) and uses ROS to mediate T-cell suppression. The CD11b+Ly6G-Ly6Chigh population resembles monocytes (Mo-MDSC) and uses NO to mediate T cell suppression [19].
Fig.1.
LPS administration increases the frequency of CD11b+Ly6G+F4/80+ cells in the lung. LPS (10 μg in 100 μl) was instilled intratracheally into Balb/c mice daily for four consecutive days. 24 h after the final exposure, lung cells were isolated by enzymatic digestion of lung tissue. CD11b+ cells were further purified by positive selection using magnetic bead separation with anti-CD11b microbeads. These cells were then stained with anti-Gr1, anti-Ly6G or anti-Ly6C and F4/80 monoclonal antibodies and were analyzed by flow cytometry.
Expansion of CD11b+Gr1+cells
The induction of MDSC-type cells can be due to either increased myelopoiesis or inhibition of differentiation of myeloid cells or a combination of both. Various factors have been shown to induce the expansion of MDSCs including VEGF, IL-6, GM-CSF, M-CSF, stem cell factor, cycloxygenase-2, prostaglandins, TLR ligands and members of the S100 protein family [20–26]. Our study [9] and those of others [27–29] have shown that repeated exposure to LPS causes expansion of CD11b+Gr1+ cells in different tissues. LPS signaling involves at least two pathways: a MyD88 (a myeloid differentiation primary-response gene 88) -dependent cascade that is essential for production of inflammatory cytokines and a MyD88-independent cascade that mediates the expression of IFN-inducible genes. Our study has recently shown that the generation of LPS-induced CD11b+Gr1int cells in the lung utilizes the TLR4/MyD88 signaling pathway. A study conducted in the context of microbial sepsis has also identified the induction of MyD88-dependent immature CD11b+Gr1+ myeloid cells which was associated with immune suppression [29]. However, a requirement for functional TLR4 was not evident in this particular study in contrast to our observations. Moreover, the Gr1+CD11b+ splenic cells described in the sepsis model do not appear to be the type we have generated in the lung since the splenic cells actually promoted Th2 polarization but suppressed Th1 responses and were detrimental to sepsis [29]. A recent study by Chalmin et al., has shown that Hsp72 from tumor-derived exosomes triggers STAT3-dependent immunosuppressive function of MDSCs in a TLR2/MyD88 dependent manner [30].
In addition to the generation of regulatory Gr1int cells in the lung by LPS exposure, our study has also shown that exposing lineageneg bone marrow progenitor cells to a combination of GM-CSF and LPS can generate CD11b+Gr1+ cells in vitro. Similarly, a study by Greifenberg et al. has also shown that a combination of IFN-γ and LPS can boost the development and activation of bone marrow-derived MDSCs, which blocks further differentiation of bone marrow cells to DCs [31]. Other studies have shown that exposure of precursor cells to a combination of growth factors like GM-CSF and inflammatory cytokines (IL-6 and IL-1β) induces MDSCs with potent T-cell suppressive capacity [20]. S100A9 has been shown to inhibit the differentiation of DCs but to induce the expansion of MDSCs [21].
LPS-induced CD11b+Gr1+ myeloid cells are tissue resident and do not migrate to lymph nodes
Under steady-state conditions, migration of antigen presenting cells to secondary lymphoid organs occurs and it has been shown that inflammatory cytokines or systemic LPS enhances the migration of tissue resident migratory DCs from the periphery to secondary lymphoid organs [32, 33]. Although numerous studies show that inflammatory mediators can promote adaptive immune responses by promoting maturation of DCs, these same mediators can also simultaneously induce the generation of other myeloid cell types by inducing differential programs in precursor/progenitor cells. For example, a study conducted by Massberg et al. showed that migratory hematopoietic stem and progenitor cells (HSPCs) proliferate within extramedullary tissues and give rise to various innate immune effector cells in response to Toll-like receptor agonists [34]. Although approximately 70% of the myeloid cells generated in the presence of LPS expressed the dendritic cell marker CD11c, a small subset (10%) expressed Gr1 at intermediate to high levels. Along the same lines, our study also showed that LPS instillation into lungs after infusion of GFP+ lineageneg (lin−) bone marrow progenitor cells into naïve mice promotes the accumulation of GFP+CD11b+Gr1int cells in the lungs [9].
Our study also suggested that the LPS-induced Gr1int cells lack CCR7 which is essential for the migration to lymph nodes [35]. Since the LPS-induced Gr1int cells do not migrate to LNs and are tissue-dwelling cells, we observed five-to ten fold enrichment of Gr1int cells over DCs in the lung. The study by Massberg et al. also showed that LPS stimulation not only enhances the local proliferation and differentiation of HSPCs but also reduces the migratory capacity of HSPCs within extramedullary tissues by interfering with S1P-S1P1-dependent signaling. It was similarly shown that in vitro incubation of HSPCs with TLR ligands can trigger HSPC proliferation and rapid myeloid differentiation [36]. Another study by Rotta et al., has shown that intradermal injection of Salmonella typhimurium or LPS induces a potent local innate inflammatory response that blocks DC differentiation and migration to the draining LNs [37].
Regulation of allergen-induced allergic inflammation by LPS
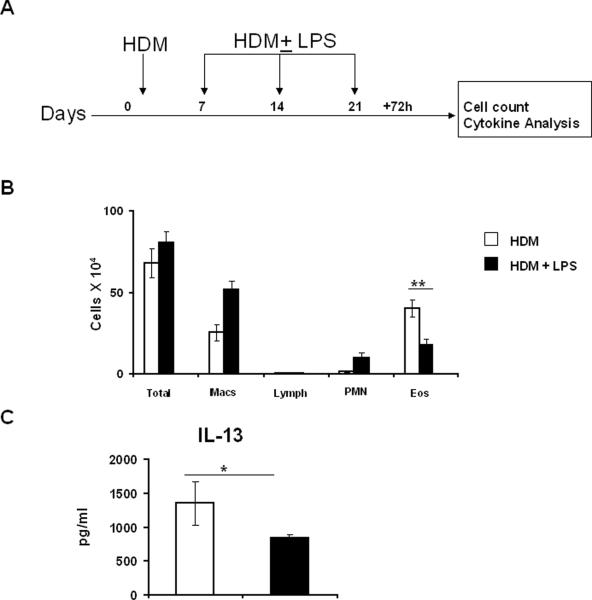
There are currently multiple studies published from different laboratories with seemingly contradictory effects of LPS on allergen-induced airway inflammation. Given that there are multiple variables with regard to the type of allergen instilled, LPS exposure conditions and the variety of models used by different laboratories, it is not surprising that a range of effects of LPS, from promotion to suppression of allergic airway inflammation, has been reported in the literature. A low level of LPS (<100 ng) facilitates the induction of a Th2 response via adjuvant effects on DCs [38]. At relatively higher doses (100ng-1 μg/mouse), administered intraperitoneally (i.p.) before OVA sensitization, LPS reduced OVA-induced eosinophilic inflammation, IgE production, and Th2 cytokines but did not affect AHR, regardless of whether LPS was also instilled during OVA challenge [10]. Along the same lines, pretreatment with LPS plus OVA decreased airway eosinophils and serum OVA-specific IgE with no effect on airway hyperresponsiveness (AHR) [39]. In a previous study by the same investigators, 10 μg of LPS administered systemically before OVA sensitization also significantly reduced eosinophilic inflammation, Th2 cytokine production and serum OVA-specific IgE but without any effect on AHR but when introduced intranasally before OVA sensitization had no effect on either OVA-induced inflammation or AHR [11]. A different study investigated local versus systemic effects of LPS on established airway inflammation. Systemic LPS (20 μg) administration concomitant with the OVA challenge completely suppressed airway eosinophilia, mucus production, airway Th2 cytokine production and AHR [8]. Intranasal LPS administration also suppressed airway eosinophilia, mucus and Th2 cytokine production but failed to suppress AHR [8]. Collectively, these studies demonstrate that the effect of LPS on the outcome of experimental asthma depends on various factors such as timing, dose and route of LPS administration. In our study, concomitant intratracheal delivery of LPS and HDM during sensitization and challenge suppressed eosinophilic airway inflammation and cytokine production and effects on AHR remain to be determined [9]. We have also shown that Th2 cells are unable to induce airway inflammation when adoptively transferred into LPS-treated mice [9]. As shown in Figure 2, LPS instillation post allergen-sensitization also has the similar effects.
Fig.2.
Suppression of HDM-induced eosinophilic inflammation in the airways by LPS post allergen sensitization. (A) HDM (100 μg per mouse) was administered intratracheally for sensitization and mice were challenged with either HDM (100μg per mouse) or HDM ± LPS (LPS at 10 μg per mouse). 72 h after the final instillation, BAL fluid and lung tissue samples were obtained. (B) Differential cell counts in the BAL fluid. (C) IL-13 levels in the lung homogenates. Bars depict the mean ± standard deviation. * P < 0.05 and ** P<.005.
Gr1int cells and Th2 effector functions in the lung
CD4+ T cells producing Th2-type cytokines play a very important role in allergic airways disease [40]. The ability of LPS-induced Gr1int cells to suppress Th2 cell activation was largely unappreciated prior to our study [9]. Since both GATA-3 [41] [42] and STAT5 [43, 44] are required for Th2 cell differentiation and effector function, we also examined the ability of the Gr1int cells to influence DC-mediated stimulation of GATA-3 expression and STAT5 phosphorylation in primed Th2 cells. Inclusion of Gr1int cells in the DC-T cells co-cultures suppressed the ability of DCs to promote Th2 cytokine production, GATA-3 expression and STAT 5 phosphorylation in primed Th2 cells [9].
If the Gr1int cells are able to blunt effector Th2 function, it is possible that this would have consequences in Th2 memory. This is because the size of the effector CD4 T cell population influences the size of the memory cell pool [45]. However, an effector T cell must survive in order to become a memory T cell. It would be interesting to investigate in future studies whether loss of STAT5 phosphorylation and GATA-3 upregulation in effector cells is detrimental to Th2 cell survival given that STAT5 activation has been shown to promote both CD4 [46] and CD8 T cell viability [47].
Mechanism of Gr1int cells-mediated suppression of T cell functions
Various mechanisms have been implicated in immunosuppression of T cells by CD11b+Gr1+ myeloid cells which include expression of Arginase 1, production of nitric oxide, peroxynitrite and reactive oxygen species, T-cell apoptosis and loss of CD3ξ signaling in T cells [16, 48–52]. Our study showed reversal of inhibition of Th2 cytokine production by Gr1int cells by anti-IL-10 antibody or by nor-N(w)-hydroxy-nor-1-arginine (specific inhibitor of Arginase 1) [9]. Since previous studies have suggested induction of Arg1 by IL-10, it is likely that the IL-10/Arg1 axis is involved in the suppressive activity of lung Gr1int cells on Th2 cells. However, in another study, HO-1 was shown to be involved in the suppression of alloreactive responses by LPS-induced MDSCs [28]. The route of exposure and dose of LPS used in that particular study were different from those used in our study. Moreover, the CD11b+Gr1+ cells in the other study were isolated from the spleen. Interestingly, previous studies have suggested promotion of IL-10 expression by HO-1 and in turn IL-10 has been shown to induce HO-1 expression [53, 54].
Concluding Remarks
Th2 cells and their cytokines play a central role in allergen-induced airway inflammation. Our studies show that a high dose of LPS suppresses allergic airways disease in which LPS-induced CD11b+Gr1intF4/80+ cells play an important regulatory role [9]. Future studies are needed to determine whether this suppressive cell type affects memory responses to allergens.
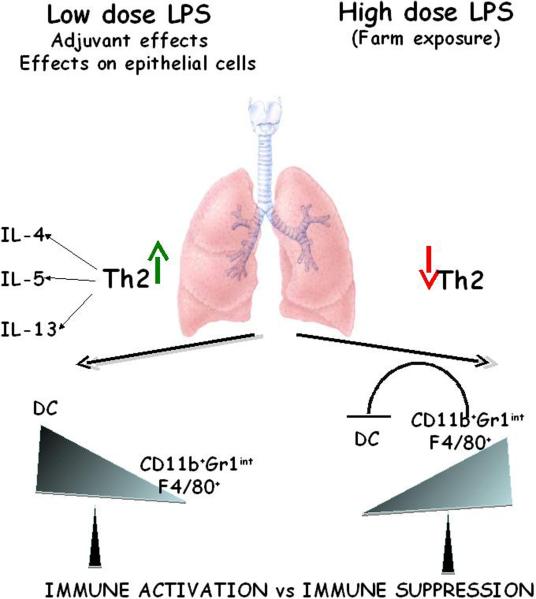
Fig.3.
Schematic depicting immune regulation of asthma by LPS. Inhalation of a low dose of LPS promotes Th2 responses to inhaled antigens due to the effects of LPS on antigen-presenting DCs and epithelial cells. Given that the number of CD11b+Gr1intF4/80+ cells present in the lung is low as compared to DCs when LPS is used at low levels, there is no suppression of effector function of Th2 cells. However, when LPS is used at relatively high doses, two major reasons contribute to blunting of the Th2 phenotype in the lung. First, a high dose of LPS causes a significant increase in the number of CD11b+Gr1intF4/80+ cells in the lung. Second, these cells do not traffic to the draining lymph nodes in contrast to the activated DCs which causes a 5–10-fold enrichment of these cells relative to DCs in the tissue. Molecules expressed by the CD11b+Gr1intF4/80+ cells such as Arginase 1 and IL-10 suppress reactivation of Th2 cells by the resident DCs.
Acknowledgments
This work was supported by US National Institutes of Health grants HL 060207 and HL 069810 (to PR), HL 077430 and AI 048927 (to AR), HL 084932 (to PR and AR), a grant from the American Heart Association, AHA 0865379D (to MA) and T32 HL007563 (to S.S. that funded SP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors declare no conflicts of interest.
References
- [1].Medzhitov R, Janeway C., Jr. Innate immunity. N Engl J Med. 2000;343:338–44. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- [2].Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- [3].Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- [5].Radon K, Ehrenstein V, Praml G, Nowak D. Childhood visits to animal buildings and atopic diseases in adulthood: an age-dependent relationship. Am J Ind Med. 2004;46:349–56. doi: 10.1002/ajim.20000. [DOI] [PubMed] [Google Scholar]
- [6].Prior C, Falk M, Frank A. Longitudinal changes of sensitization to farming-related antigens among young farmers. Respiration. 2001;68:46–50. doi: 10.1159/000050462. [DOI] [PubMed] [Google Scholar]
- [7].Horak F, Jr., Studnicka M, Gartner C, Veiter A, Tauber E, Urbanek R, et al. Parental farming protects children against atopy: longitudinal evidence involving skin prick tests. Clin Exp Allergy. 2002;32:1155–9. doi: 10.1046/j.1365-2745.2002.01448.x. [DOI] [PubMed] [Google Scholar]
- [8].Rodriguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, Lefort J, et al. Bacterial lipopolysaccharide signaling through Toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J Immunol. 2003;171:1001–8. doi: 10.4049/jimmunol.171.2.1001. [DOI] [PubMed] [Google Scholar]
- [9].Arora M, Poe SL, Oriss TB, Krishnamoorthy N, Yarlagadda M, Wenzel SE, et al. TLR4/MyD88-induced CD11b+Gr-1 int F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol. 2010;3:578–93. doi: 10.1038/mi.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Delayre-Orthez C, Becker J, de Blay F, Frossard N, Pons F. Exposure to endotoxins during sensitization prevents further endotoxin-induced exacerbation of airway inflammation in a mouse model of allergic asthma. Int Arch Allergy Immunol. 2005;138:298–304. doi: 10.1159/000088867. [DOI] [PubMed] [Google Scholar]
- [11].Gerhold K, Blumchen K, Bock A, Seib C, Stock P, Kallinich T, et al. Endotoxins prevent murine IgE production, T(H)2 immune responses, and development of airway eosinophilia but not airway hyperreactivity. J Allergy Clin Immunol. 2002;110:110–6. doi: 10.1067/mai.2002.125831. [DOI] [PubMed] [Google Scholar]
- [12].Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–21. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- [13].Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15:384–91. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–9. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–94. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- [18].Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–37. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- [19].Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- [20].Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 185:2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- [23].Liu YY, Sun LC, Wei JJ, Li D, Yuan Y, Yan B, et al. Tumor cell-released TLR4 ligands stimulate Gr-1+CD11b+F4/80+ cells to induce apoptosis of activated T cells. J Immunol. 185:2773–82. doi: 10.4049/jimmunol.1000772. [DOI] [PubMed] [Google Scholar]
- [24].Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–66. [PubMed] [Google Scholar]
- [25].Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- [27].Vaknin I, Blinder L, Wang L, Gazit R, Shapira E, Genina O, et al. A common pathway mediated through Toll-like receptors leads to T- and natural killer-cell immunosuppression. Blood. 2008;111:1437–47. doi: 10.1182/blood-2007-07-100404. [DOI] [PubMed] [Google Scholar]
- [28].De Wilde V, Van Rompaey N, Hill M, Lebrun JF, Lemaitre P, Lhomme F, et al. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant. 2009;9:2034–47. doi: 10.1111/j.1600-6143.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- [29].Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865–76. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- [32].De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–24. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–94. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rotta G, Edwards EW, Sangaletti S, Bennett C, Ronzoni S, Colombo MP, et al. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J Exp Med. 2003;198:1253–63. doi: 10.1084/jem.20030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Eisenbarth SC, Cassel S, Bottomly K. Understanding asthma pathogenesis: linking innate and adaptive immunity. Curr Opin Pediatr. 2004;16:659–66. doi: 10.1097/01.mop.0000145920.00101.e4. [DOI] [PubMed] [Google Scholar]
- [39].Gerhold K, Avagyan A, Reichert E, Blumchen K, Wahn U, Hamelmann E. Lipopolysaccharides modulate allergen-specific immune regulation in a murine model of mucosal tolerance induction. Int Arch Allergy Immunol. 2008;147:25–34. doi: 10.1159/000128583. [DOI] [PubMed] [Google Scholar]
- [40].Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J Clin Invest. 1999;104:985–93. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. The Journal of biological chemistry. 1997;272:21597–603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- [42].Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- [43].Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–48. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- [44].Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nature reviews. 10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hu H, Huston G, Duso D, Lepak N, Roman E, Swain SL. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat Immunol. 2001;2:705–10. doi: 10.1038/90643. [DOI] [PubMed] [Google Scholar]
- [46].Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–11. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A. 107:16601–6. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–8. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- [49].Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–6. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- [50].Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- [51].Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- [52].Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–85. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- [53].Chauveau C, Remy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- [54].Ricchetti GA, Williams LM, Foxwell BM. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol. 2004;76:719–26. doi: 10.1189/jlb.0104046. [DOI] [PubMed] [Google Scholar]