Abstract
Background
Deep Brain Stimulation (DBS) surgery can effectively treat many debilitating motor symptoms of Parkinson's disease (PD), but axial symptom improvement is variable. Predictors for post-DBS axial symptom performance have yet to be identified. Pre-surgery ventricle volume may be one predictor, for increasing ventricular size has been associated with worsening gait disturbance. In PD, ventricle size may also increase with the advancement of motor symptoms.
Objective
To examine the hypotheses that 1)lateral ventricular volumes would predict motor and axial motor symptom change from pre to four months post unilateral DBS, and 2) PD patients have larger ventricle volumes to side of symptom onset.
Methods
Idiopathic PD patients (n=37) completed pre-surgery volumetric brain scans and UPDRS motor testing (off-medication), unilateral DBS (Globus Pallidus interna,; n=11; subthalamic nucleus, n=26), and 4 month follow-up motor assessments (on-stimulation). Ventricle volumes were normalized using total intracranial volume.
Results
Total ventricular volume as well as measurements of contralateral/ipsilateral volumes to side of symptom onset or DBS lead placement did not predict outcome motor measures or correlate to axial motor change. Patients improving at least 2 standard errors of measurement (n=6) did not have smaller ventricles relative to those without significant change. Post-operative hemorrhage (n=1) had ventricle volumes similar to the group average. There was no asymmetry in ventricular volume by side of onset or side of lead placement.
Conclusion
Ventricular volume was a poor predictor of acute motor change following DBS. Asymmetrical ventricles may not be a consistent imaging marker for PD motor dysfunction.
Keywords: UPDRS, Idiopathic PD, Deep Brain Stimulation, Axial, Asymmetry
Introduction
Axial disability is a troubling motor manifestation of Parkinson's disease (PD) [1]. Gait dysfunction and postural instability can lead to an increased risk of falls and fall related injuries [2]. Over 68% of PD patients were reported to fall annually with over 51% of patients falling two or more times a year [3]. Gait difficulty and fear of falling are significantly associated with worsened quality of life for individuals with Parkinson's disease [4]. PD patients with both postural instability and gait disturbance early in the course of the illness also have high rates of mortality (up to 80% higher than PD peers without these difficulties [5]).
Deep Brain Stimulation (DBS) can effectively treat many debilitating symptoms of PD [6-8]. DBS outcome data with regard to axial symptom improvement, however, remain variable for both unilateral and bilateral stimulation studies. Using subjective and objective measurements of gait, Kelly and colleagues [9] identified minimal changes in axial symptoms (either increase or decrease) with unilateral subthalamic nucleus (STN) placement, and chance levels of improvement (50% of patients improved) for those with bilateral STN stimulation. Visser and colleagues [10] reported that bilateral STN stimulation failed to improve postural stability over that of optimally dosed levodopa. Okun and colleagues [11] with a randomized unilateral placement study (STN versus globus pallidus internal,GPi) showed that both sites resulted in seven month post-DBS improvement for UPDRS rigidity, bradykinesia, and tremor items, but not postural stability or gait. Walker and colleagues [12], however, reported significant post-STN improvement for UPDRS axial symptoms at three, six, and 12 months post-surgery, although a proportion of the patients also began to develop more gait disturbance and difficulty standing from the seated position at 12 months. Why some individuals demonstrate post-DBS axial improvement while others do not remains unclear in the literature.
Presurgical lateral ventricular size may be an anatomical marker with predictive value for DBS related motor changes and specifically gait and postural instability. Ventricular dilation is hypothesized to impact the integrity of the basal ganglia structures, frontal lobe white matter pathways, and corticospinal tract integrity [13, 14]. Ventricular dilation occurs with normal pressure hydrocephalus, which involves gait disturbance [13] and might be related to gait disturbances in PD [15]. Moreover, lateral ventricle asymmetry is suggested to be an important consideration for motor symptom progression. Lewis and colleagues [14] showed that lateral ventricles contralateral to symptom onset enlarged faster than ipsilateral ventricles in PD with this enlargement associated with motor worsening. Huang and colleagues [16] reported a larger lateral ventricle contralateral to the more symptomatic side in a PD twin, but not a non-PD twin. Moreover, examining ventricular size might have practical DBS surgical implications [17]. Larger ventricles might result in a greater chance of breaching the ventricles and resulting in intraventricular hemorrhage.
For this study, we investigated the role of pre-surgical lateral ventricular volumes on post-DBS motor (specifically gait and postural improvement), and the relative size of lateral ventricles to motor symptom onset and DBS lead placement. We hypothesized that presurgical lateral ventricular volume would predict motor and axial symptom change following DBS surgery with larger volumes predicting poorer motor outcomes. We secondarily examined the hypothesis that ventricular volume contralateral to self-reported side of symptom onset would be larger than the ipsilateral side in our sample, with these volumes predicting outcome. This hypothesis was also examined with ventricles on the same side of DBS lead placement, for DBS placement is often performed contralateral to the side of the body most affected.
Methods
Study Design and Participant Sample
A retrospective study conducted in accordance with the University of Florida's Movement Disorder Center, Institutional Review Board, and the Declaration of Helsinki. Participants completed consent forms to have their baseline, operative, and post-operative data used for research purposes. Chart and MRI data were pulled (years 2002-2007) for consecutive patients who had a diagnosis of “probable” PD, had pre-operative cognitive testing, had completed a pre-surgery MRI on one of two scanners, had completed a unilateral DBS and had 4 month motor outcome data. Probable PD diagnosis was made by one of three fellowship trained Movement Disorder Specialists and determined by: (1) the presence of two out of three motor features of PD (tremor, rigidity, and bradykinesia), (2) and the absence of any features that would suggest other forms of Parkinsonism including lack of a substantial response to levadopa therapy. Exclusion criteria included known secondary causes of PD and prior neurosurgical treatment.
Surgery Protocol
Surgeries were completed by a single surgeon (K.D.F.)/neurologist (M.S.O.) team with multipass electrode mapping [18]. DBS devices were activated one month after intracranial lead implantation. Follow-up evaluations were performed as needed until the optimal chronic stimulation parameters and adjunctive PD medication regimen were determined. All patients were kept on their optimized DBS setting and medication regimen for a minimum of 30 days before repeat motor evaluations were completed.
Imaging Protocol and Variables of Interest
Participants had completed a pre-DBS volumetric brain scan via 1.5 Tesla General Electric (n=10) or Siemens (n=28) MR clinical scanners using a T1-weighted three-dimensional volumetric sequence that allowed reconfiguration into any plane (typical protocol: TR=11ms, TE=3.87ms, Flip Angle=15degrees, Inversion time=600; 1.5mm thick, 120 slices).
Lateral Ventricular Volumes
Were measured from 3D T1-weighted magnetization prepared rapid acquisition gradient echo (MP-RAGE) or spoiled gradient recalled (SPGR) images acquired in the axial plane. For pre-processing the voxel intensity range was restricted to better delineate ventricles from non-ventricle. Trained raters used a semi-automated segmentation method with scans in native space (ITK-SNAP; http://www.itksnap.org [19]; Intra- and inter-rater spatial overlap and reliability were excellent; inter-rater grand Dice Similarity Coefficient (DSC) = .92 ± .04; ICC = .98, Pearson r= .99; intra-rater grand DSC = .96 ± .03, all p<.001). Origin seed bubbles were placed within each lateral ventricle, and an intensity algorithm governed by parameters optimized to minimize error and manual modification expanded the bubbles in 3D space to the periventricular tissue margin. Extraneous voxels were manually removed. Visual inspection confirmed left-right ventricles which were then separated. Variables of interest included 1) total lateral ventricular volume (mm3) and 2) contralateral and ipsilateral volume relative to patient reported onset. Contralateral and ipsilateral volume to lead placement were calculated, for side of symptom onset did not always correspond to side of lead placement due to severity of symptoms at time of surgery.
Intracranial Volume
Volumetric T1 weighted images were processed in BrainSuite by an automated method of quantifying total intracranial volume [20]. A horizontal plane demarcating the superior cerebellar border was applied as a standardized inferior margin for all cranial volumes. Extraneous voxels were manually removed from the 3D volume; visual inspection of the 3D volume confirmed the accuracy of the volumetric assessment.
Corrected Lateral Ventricular volumes
Were calculated by dividing lateral ventricular volumes by intracranial volumes in order to account for intersubject head volume variability [21]. Final primary predictor variables of interest were: 1) Total Lateral Ventricular Volume corrected for intracranial volume (TLVc), 2) Contralateral Ventricle to symptom onset corrected for intracranial volume (CLV symptom_c and Ipsilateral Ventricle Volume to symptom onset corrected for intracranial volume (ILV symptom_c), and 3) Contralateral Ventricle to lead corrected for intracranial volume (CLV lead_c) and Ipsilateral Ventricle to lead corrected for intracranial volume (ILV lead_c).
Motor Outcome Measure: Unified Parkinson's Disease Rating Scale (UPDRS; [22])
Baseline and four-month post DBS UPDRS scores were completed by a fellowship trained movement disorder neurologist. Baseline data were collected “off” medication. Four month post-DBS data were collected “off” medication and optimal “on” electrode stimulation. Primary outcome variables were: UPDRS section III (motor subscale of the Unified Parkinson's Disease Rating Scale; higher=worse), with specific sub-items for axial motor symptoms including “arising from chair” (UPDRS item 27; 0-4), “posture” (UPDRS item 28; 0-4), “gait” (UPDRS item 29; 0-4), and “postural stability” (UPDRS item 30; 0-4).
Covariates Considered
Age, education, duration of PD symptoms (defined as months between first symptoms of PD and baseline evaluation), pre-surgery disease severity (Hoehn and Yahr; H&Y [23]), and baseline general cognitive status as measured by the Mattis Dementia Rating Scale (DRS-2) [24], were considered variables that may alter the expected relationship between lateral ventricular volume and motor scores.
Statistical Analysis
Analyses were based on SPSS v17.0. Imaging variables met normality requirements. Wilcoxon Sign Ranks examined change from baseline to 4-month UPDRS Part III Total Motor and axial subscore motor items. Only significant sub-items were examined in follow-up analyses. Mann-Whitney U tests examined DBS site placement (STN, GPi) on gait change. Two-tailed pairwise t-tests examined differences in contralateral and ipsilateral ventricle volumes. Two-tailed Pearson and Spearman correlations examined potential covariates on ventricle volumes and UPDRS total motor and axial motor items of interest, respectively. Unstandardized residuals were created for significant covariates (e.g., age) that may explain a portion of ventricular volume differences (a residual is the actual value of the dependent variable minus the value predicted by the independent variable). Linear hierarchical regressions (Enter) examined predictive value of baseline corrected ventricle variables on the UPDRS Total Motor score. Here, pre-surgery UPDRS-III Total Motor score was entered in the first step of each model to render the dependent outcome variable (4-month score) as a “residual change score”. Step 2 entered the primary predictor variable (e.g., TLVc). Spearman correlations examined relationship between TLVc and UPDRS ordinal motor sub-item change scores. Independent sample t-tests compared ventricle size for patients who did and did not improve on the UPDRS total motor score (identified using 2 standard errors of measurement (SEM) relative to overall patient change [25]). Restrospective power analysis was conducted using G*Power [26]. Alpha levels were set at .05.
Results
A review of charts between years of 2002 and 2007 identified 53 patients meeting inclusion and exclusion criteria. Of these 54, a set of 37 had MRIs that could be post-processed for total ventricle and intracranial measurements. DBS targets included GPi (n=11) and STN (n=26). Table 1.
Table 1. Participant (n=37) descriptive data for demographic and baseline general cognitive function*.
| Mean | S.D. | Minimum | Maximum | |
|---|---|---|---|---|
| Age | 58.81 | 7.00 | 46.00 | 76.00 |
| Education | 15.22 | 2.51 | 9.00 | 20.00 |
| M/F Ratio | 28:09 | -- | -- | -- |
| Symptom Months | 148.32 | 65.94 | 52.00 | 303.00 |
| H & Y | 3.04 | .63 | 1.50 | 5.00 |
| DRS-2 total score | 137.95 | 5.04 | 123.00 | 144.00 |
Post-surgery changes in motor scores
Table 2. Participants demonstrated significant improvement in overall UPDRS Motor score at four months post-surgery [z= -3.94, p<.001; UPDRS Total Motor Score mean change = 9.93 ± 11.69;], with improvement on the sub-items of Gait [Z= -2.12, p=.03; mean change .46± 1.28;] and Postural Stability [Z= -2.30, p=.02; mean change = .86 ± .92;]. There was no significant change in UPDRS Motor subscale items of Rising from Chair [Z=-1.84, p=.07], or Posture [Z= -1.20, p=.23]. UPDRS axial motor item change scores did not differ significantly for DBS lead placement (STN, GPi;all p>.29).
Table 2. Participant (n=37) Baseline Off-Medication and Post-Deep Brain Stimulation (DBS) Off Medication On Stimulation Unified Parkinson Disease Rating Scale (UPDRS) Motor Mean ± Standard Deviation (S.D.). Scores.
| Baseline | 4 Months Post DBS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | pvalue | |
| Total Motor | 44.19 | 11.95 | 11.00 | 81.00 | 34.27 | 10.57 | 14.00 | 57.00 | < .001 |
| Stability | 1.49 | .96 | 0.00 | 4.00 | .86 | .92 | 0.00 | 4.00 | .02 |
| Gait | 1.86 | .95 | 0.00 | 4.00 | 1.41 | .87 | 0.00 | 4.00 | .03 |
| From Chair | 1.30 | .97 | 0.00 | 4.00 | .96 | 1.03 | 0.00 | 4.00 | .07 |
| Posture | 1.43 | .84s | 0.00 | 3.00 | 1.22 | .67 | 0.00 | 2.00 | .23 |
All p values calculated using Wilcoxon sign ranks tests.
Contralateral to Ipsilateral Ventricular Comparison
Table 3. There were similar contralateral and ipsilateral ventricular volumes to side of symptom onset (t(34)=.65, p=.52) with no change in findings when using corrected values for total intracranial volume (t(34)=.63, p=.53). Uncorrected and corrected volumes were similar for ventricles ipsilateral and contralateral to DBS lead placement (uncorrected = t(36)=.37, .71; corrected= t(36)=.31, p=.76). Ventricle comparisons for lead site subgroups (GPi, STN) were similar (STN subgroup, all p>.18; GPi subgroup, all p>.71).
Table 3. Imaging Variable Mean, Standard Deviation, Minimum and Maximum Values*.
| Mean | S.D. | Minimum | Maximum | |
|---|---|---|---|---|
| TICV | 1315548.71 | 1551333.62 | 1030670.00 | 1653602.00 |
| TLV | 23572.60 | 13948.21 | 7631.27 | 66619.90 |
| TLVc | .0178 | .0098 | .0064 | .0403 |
| CLV symptom | 11560.67 | 7455.65 | 3031.24 | 38750.20 |
| CLV symptom_c | .0088 | .0053 | .0025 | .0234 |
| ILV symptom | 11212.18 | 6437.12 | 3610.00 | 27869.70 |
| ILV symptom_c | .0086 | .0047 | .0030 | .0194 |
| CLV lead | 11763.05 | 6781.67 | 3610.00 | 27869.70 |
| CLV lead_c | .0089 | .0050 | .0030 | .0231 |
| ILV lead | 11972.73 | 7493.53 | 3031.24 | 38750.20 |
| ILV lead_c | .0085 | .0047 | .0030 | .0193 |
NOTE: sample sizes: CLV and ILV symptom variables (n=35; 2 patients reported bilateral onset), remaining variables based on sample of 37 patients. Four decimal places reported for corrected volumes due to resulting small values. TICV = Total Intracranial Volume mm3; TLV = Total Lateral Ventricular mm3 volume; TLVc = corrected Total Lateral Ventricular mm3 volume/TICV; CLV symptom = Contralateral Lateral Ventricle to symptom onset mm3 volume; CLV symptom _c = CLV symptom / TICV; ILV symptom = Ipsilateral lateral ventricle to symptom onset mm3 volume; ILV symptom_c = ILV symptom / TICV; CLV lead = Contralateral Lateral Ventricle to lead mm3 volume; CLV lead _c = CLV lead / TICV; ILV lead = Ipsilateral lateral ventricle to lead mm3 volume; ILV symptom_c = ILV symptom / TICV
Examining Potential Covariates
Age positively associated with ventricle volumes (Pearson correlations: e.g., TLVc, r=.55, p=.001; CLV symptom_c, r= .52, p=.002; ILV symptom_c, r=.51, p=.002). There were no other significant relationships between ventricular volumes and other covariates of concern (symptom duration, H&Y, DRS-2, education; all r<.10, all p >.62). There were no significant relationships between UPDRS motor scores (Part III Total Motor, subscores of Gait, Postural Stability), demographic, symptom duration, H&Y, or pre-surgery general cognition (Spearman Correlations: all ns). The moderate to strong association between age and ventricle volumes indicates that ventricular volume is partially explained (at least 30%) by age in our sample. For this reason, unstandardized residual between age and corrected ventricular volumes were created and used used as the primary predictor for the planned hierarchical analyses.
Lateral Ventricular Volume and Change in UPDRS Motor Score
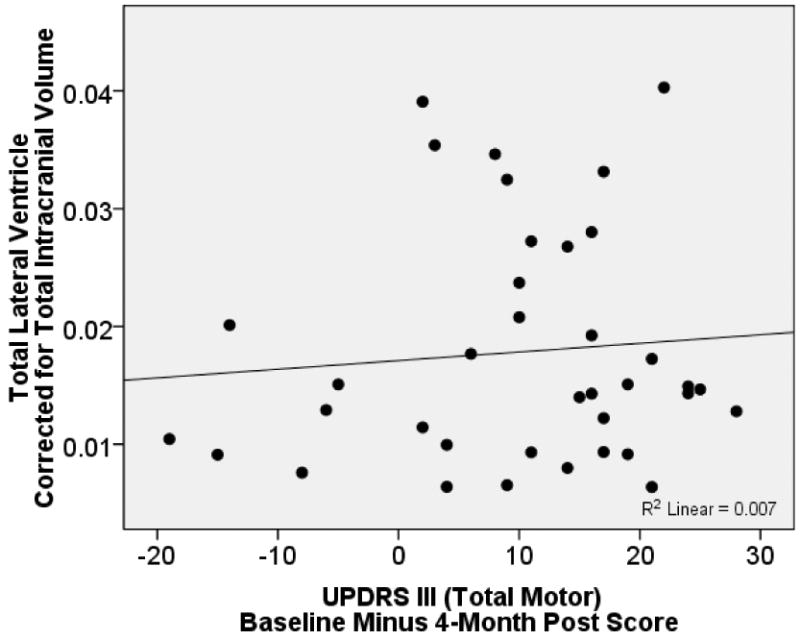
Only baseline UPDRS Total Motor score explained a significant portion (22%) of variance in post DBS total motor scores [F(1, 36)=9.73, p=.004; β=.47, R=.47, R2=.22]. TLVc (residual after controlling for age) did not significantly contribute to the model (β= -.06, Change R2 = .003; F change = .14, p=.72). The same pattern occurred using the residualized CLV_c and ILV_c contralateral/ipsilateral ventricular volumes relative to symptom onset and lead placement (all β's < -.06), and for all raw (non-residualized) lateral ventricle volumes (all β's < .13). Furthermore, there were no significant associations between TLVc (raw or residual) and the UPDRS Total Motor pre-post surgery change score (Figure 1), or UPDRS axial motor subscores (Gait, Postural stability, r's<, 2, ns). Findings were similar for contralateral and ipsilateral analyses.
Figure 1. Scatterplot Depicting Relationship Between Total Lateral Ventricular Volume After Controlling for Total Intracranial Volume (TICV) and Change in UPDRS III Total Motor Score.
Patients with/without UPDRS Motor Change and Ventricular Volume
For the UPDRS III total score, 13.5% (5/37) of patients demonstrated significant improvement using a 2-SEM criterion (requiring change of 21 points). There was no difference in ventricular volume between these two patient groups (mean ± s.d TLV raw: 2 SEM change group = 1308844.50 ± 219779.22; all others = 1316596.24 ± 142669.90, p=.92; mean ± s.d TLV_c: 2SEM group = .0194±.-117; all others = .0175±.0098, p=.71). For the axial sub-items, 5.4% (2/37) demonstrated improvement using a 2-SEM criterion (requiring change of 6 points). Mean ventricle volume similar for those who did versus did not improve on axial items (TLV mean for the 2 SEM group = 1320512.00 ±179722.08 versus TLV mean = 1315265.09 ± 179722.08), this difference was marginalized even less than half a percent after controlling for total intracranial volume (TLVc = 1.4% relative to 1.8%).
Negative Surgery Outcome
One patient experienced an intracranial hemorrhage immediately post GPi placement. The patient's total lateral ventricle and ventricle ipsilateral to lead placement were approximately one standard deviation smaller than that of the other patients (residualized TLVc z score relative to peers = -.91; residualized ILV lead_c z score = -1.13).
Future Sample Size Estimations
Using coefficient of determination reported above, it was identified that identifying a relationship between total lateral ventricle volume and change in UPDRS total motor score at an alpha of .05 and adequate power (0.80), a study would require a sample size of >2440 participants. Similar sample sizes were identified for axial and contralateral/ipsilateral analyses.
Discussion
In our sample of PD patients meeting stringent criteria for unilateral DBS, pre-surgery lateral ventricular volume was not a predictor of acute UPDRS motor score change. Findings were consistent for all ventricular volume analyses; total lateral ventricular volume as well as separate analyses using contralateral and ipsilateral ventricle size did not predict post-DBS outcome. Ventricle variables also did not associate with any UPDRS motor change score. Additionally, there was no significant ventricle size difference for those who showed a marked improvement on the UPDRS motor score or axial sub-items. These findings consistently indicate that lateral ventricle size in our sample was a poor predictor of motor improvement for idiopathic PD patients undergoing STN or GPi placement.
Our sample was well controlled in that each participant went through a two-day rigorous assessment by medical and allied health professionals (i.e., neurology, neurosurgery, psychiatry, speech, neuropsychology). DBS eligibility was also discussed via an interdisciplinary team consensus conference. This screening likely creates a homogeneous or “clean” idiopathic PD DBS sample from which to answer our question. We acknowledge that this rigorous screening might have inadvertently restricted lateral ventricle range, but argue that our findings are worthy of consideration. We show adequate total ventricular volume ranges similar to other published volumes in PD [14]. Our age range was broad (ages 46 to 76) and appropriately correlated with lateral ventricular size [27]. Residuals allowed us to examine the remaining portion of lateral ventricular variance (i.e., not explained by age) on DBS motor outcome. Findings were consistent across all analyses and showed a virtually flat relationship between the remaining variance and motor change in our sample. With a similar participant sample, power analyses estimate that 2440 or more participants would be necessary to show a significant relationship between ventricular volume and pre-post DBS UPDRS total motor change.
Furthermore, the findings suggest that lateral ventricle volume opposite of symptom onset may not be a consistent imaging marker for idiopathic PD. Two investigator groups have recently identified asymmetry in ventricles corresponding to side of onset or side of worsening motor symptoms [14, 15]. These studies involved young onset PD, however. We interpret the collective findings as underscoring the heterogeneity of idiopathic PD and growing knowledge that idiopathic PD involves numerous pathogenic mechanisms [28] that may present differently on imaging.
Regarding the role of ventricular size on DBS complications, we identified one patient with acute intracranial hemorrhage after GPi placement. A comparison of the patient's ventricles to study sample's ventricular means revealed the patient's ventricles were almost one standard deviation smaller than the peers. For this case, ventricle size may not have been a contributor to the negative DBS outcome. As this is only one case, we encourage future studies of ventricular volume in patients with acute hemorrhage following DBS.
Study limitations include restricting our study to unilateral surgery and one acute time, as well as examining axial symptoms with only the UPDRS. Future studies are needed on bilateral and longer time points. While the UPDRS is reliable for assessing PD as a whole, it may not be the most sensitive measure for quantifying inter and intra-individual variability in brain structure and motor outcome, particularly axial symptoms, following DBS. Quantitative posturography and instrumented gait analysis is one potential approach for future studies [29]. We also encourage investigators to examine the role of other neuroanatomical structures on post-DBS outcome. As pointed out by Lang and colleagues [30], our field needs prospectively designed studies that document relationships between neuroanatomical variables and DBS outcome.
In summary, lateral ventricle volume did not predict acute motor improvement following unilateral DBS in our sample of individuals with idiopathic PD. There was no ventricle volume asymmetry with regard to side of symptom onset or lead placement. We encourage more investigations on the role of ventricular volume after bilateral DBS and longer time points, as well a ventricle asymmetry in PD subtypes.
Acknowledgments
Supported in part by NINDS K23NS060660 (CP); NIH T35 07489 (CF), UF National Parkinson Foundation Center of Excellence, UF Foundation. Special thanks to Nadine Schwab, B.S., who assisted with the database on ventricular volumes and John Collazo, B.S., who assisted with MRI parameter coding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boonstra TA, van der Kooij H, Munneke M, Bloem BR. Gait disorders and balance disturbances in Parkinson's disease: clinical update and pathophysiology. Curr Opin Neurol. 2008 Aug;21(4):461–71. doi: 10.1097/WCO.0b013e328305bdaf. [DOI] [PubMed] [Google Scholar]
- 2.Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, et al. A meta-analysis of six prospective studies of falling in Parkinson's disease. Mov Disord. 2007 Oct 15;22(13):1892–900. doi: 10.1002/mds.21598. [DOI] [PubMed] [Google Scholar]
- 3.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002 Jun;72(6):721–5. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson's disease: the relative importance of the symptoms. Mov Disord. 2008 Jul 30;23(10):1428–34. doi: 10.1002/mds.21667. [DOI] [PubMed] [Google Scholar]
- 5.Lo RY, Tanner CM, Albers KB, Leimpeter AD, Fross RD, Bernstein AL, et al. Clinical features in early Parkinson disease and survival. Arch Neurol. 2009 Nov;66(11):1353–8. doi: 10.1001/archneurol.2009.221. [DOI] [PubMed] [Google Scholar]
- 6.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005 Apr;62(4):554–60. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 7.Koller WC, Pahwa R, Lyons KE, Albanese A. Surgical treatment of Parkinson's disease. J Neurol Sci. 1999 Aug 1;167(1):1–10. doi: 10.1016/s0022-510x(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Lozano AM, Kim YJ, Hutchison WD, Sime E, Halket E, et al. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson's disease. Neurology. 1998 Sep;51(3):850–5. doi: 10.1212/wnl.51.3.850. [DOI] [PubMed] [Google Scholar]
- 9.Kelly VE, Samii A, Slimp JC, Price R, Goodkin R, Shumway-Cook A. Gait changes in response to subthalamic nucleus stimulation in people with Parkinson disease: a case series report. J Neurol Phys Ther. 2006 Dec;30(4):184–94. doi: 10.1097/01.npt.0000281255.10174.e2. [DOI] [PubMed] [Google Scholar]
- 10.Visser JE, Allum JH, Carpenter MG, Esselink RA, Speelman JD, Borm GF, et al. Subthalamic nucleus stimulation and levodopa-resistant postural instability in Parkinson's disease. J Neurol. 2008 Feb;255(2):205–10. doi: 10.1007/s00415-008-0636-x. [DOI] [PubMed] [Google Scholar]
- 11.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009 May;65(5):586–95. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker HC, Watts RL, Guthrie S, Wang D, Guthrie BL. Bilateral effects of unilateral subthalamic deep brain stimulation on Parkinson's disease at 1 year. Neurosurgery. 2009 Aug;65(2):302–9. doi: 10.1227/01.NEU.0000349764.34211.74. discussion 9-10. [DOI] [PubMed] [Google Scholar]
- 13.Palm WM, Saczynski JS, van der Grond J, Sigurdsson S, Kjartansson O, Jonsson PV, et al. Ventricular dilation: association with gait and cognition. Ann Neurol. 2009 Oct;66(4):485–93. doi: 10.1002/ana.21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis MM, Smith AB, Styner M, Gu H, Poole R, Zhu H, et al. Asymmetrical lateral ventricular enlargement in Parkinson's disease. Eur J Neurol. 2009 Apr;16(4):475–81. doi: 10.1111/j.1468-1331.2008.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran T, Lang AE. Parkinsonian syndromes associated with hydrocephalus: case reports, a review of the literature, and pathophysiological hypotheses. Mov Disord. 1994 Sep;9(5):508–20. doi: 10.1002/mds.870090503. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Lee YZ, McKeown M, Gerig G, Gu H, Lin W, et al. Asymmetrical ventricular enlargement in Parkinson's disease. Mov Disord. 2007 Aug 15;22(11):1657–60. doi: 10.1002/mds.21626. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Haim S, Asaad WF, Gale JT, Eskandar EN. Risk factors for hemorrhage during microelectrode-guided deep brain stimulation and the introduction of an improved microelectrode design. Neurosurgery. 2009 Apr;64(4):754–62. doi: 10.1227/01.NEU.0000339173.77240.34. discussion 62-3. [DOI] [PubMed] [Google Scholar]
- 18.Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, et al. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol. 1998 Oct;44(4):622–8. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- 19.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006 Jul 1;31(3):1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002 Jun;6(2):129–42. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 21.Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Invest Radiol. 2001 Sep;36(9):539–46. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Fahn S, Elton RL, et al. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson's Disease. New York: Macmillan Publishing Company Inc.; 1987. pp. 153–64. [Google Scholar]
- 23.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 24.Brown GG, Rahill AA, Gorell JM, McDonald C, Brown SJ, Sillanpaa M, et al. Validity of the Dementia Rating Scale in assessing cognitive function in Parkinson's disease. J Geriatr Psychiatry Neurol. 1999 Winter;12(4):180–8. doi: 10.1177/089198879901200403. [DOI] [PubMed] [Google Scholar]
- 25.Dudek FJ. The continuing misinterpretation of the standard error of measurement. Psychological Bulletin. 1979;86(2):335–7. [Google Scholar]
- 26.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41(4):1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 27.Tisserand DJ, Visser PJ, van Boxtel MP, Jolles J. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging. 2000 Jul-Aug;21(4):569–76. doi: 10.1016/s0197-4580(00)00133-0. [DOI] [PubMed] [Google Scholar]
- 28.Gasser T. Genetics of Parkinson's disease. Curr Opin Neurol. 2005 Aug;18(4):363–9. doi: 10.1097/01.wco.0000170951.08924.3d. [DOI] [PubMed] [Google Scholar]
- 29.Rocchi L, Chiari L, Cappello A, Gross A, Horak FB. Comparison between subthalamic nucleus and globus pallidus internus stimulation for postural performance in Parkinson's disease. Gait Posture. 2004 Apr;19(2):172–83. doi: 10.1016/S0966-6362(03)00059-6. [DOI] [PubMed] [Google Scholar]
- 30.Lang AE, Houeto JL, Krack P, Kubu C, Lyons KE, Moro E, et al. Deep brain stimulation: preoperative issues. Mov Disord. 2006 Jun;21 14:S171–96. doi: 10.1002/mds.20955. [DOI] [PubMed] [Google Scholar]


