Abstract
Summary
The association between follicle-stimulating hormone (FSH) and bone density was tested in 111 postmenopausal women aged 50–64 years. In the multivariable analysis, weight and race were important determinants of bone mineral density. FSH, bioavailable estradiol, and other hormonal variables did not show statistically significant associations with bone density at any site.
Introduction
FSH has been associated with bone density loss in animal models and longitudinal studies of women. Most of these analyses have not considered the effect of weight or race.
Methods
We tested the association between FSH and bone density in younger postmenopausal women, adjusting for patient-related factors. In 111 postmenopausal women aged 50–64 years, areal bone mineral density (BMD) was measured at the lumbar spine, femoral neck, total hip, and distal radius using dual-energy X-ray absorptiometry, and volumetric BMD was measured at the distal radius using peripheral quantitative computed tomography (pQCT). Height, weight, osteoporosis risk factors, and serum hormonal factors were assessed.
Results
FSH inversely correlated with weight, bioavailable estradiol, areal BMD at the lumbar spine and hip, and volumetric BMD at the ultradistal radius. In the multivariable analysis, no hormonal variable showed a statistically significant association with areal BMD at any site. Weight was independently associated with BMD at all central sites (p<0.001), but not with BMD or pQCT measures at the distal radius. Race was independently associated with areal BMD at all sites (p≤0.008) and with cortical area at the 33% distal radius (p=0.004).
Conclusions
Correlations between FSH and bioavailable estradiol and BMD did not persist after adjustment for weight and race in younger postmenopausal women. Weight and race were more important determinants of bone density and should be included in analyses of hormonal influences on bone.
Keywords: Bone densitometry, Bone QCT, Hormones and receptors, Menopause, Osteoporosis
Introduction
A 2006 study by Sun et al. suggested that high circulating concentrations of follicle-stimulating hormone (FSH) significantly contributed to the bone loss seen in hypogonadal mouse models [1]. Several basic science and clinical studies have offered further support for this hypothesis. Liu et al. demonstrated that an FSH inhibitor had protective effects on alveolar bone resorption [2], and Rendina identified an allele of the FSH receptor that was associated with lower bone mineral density (BMD) in postmenopausal women aged 50–75 years [3]. A 2006 longitudinal cohort study of pre- and perimenopausal women showed that bone turnover and spine and hip bone mineral density losses were more strongly associated with longitudinal FSH changes than with estradiol (E2) or other hormonal variables [4]. In 2008, Riggs et al. published a prospective, population-based assessment of rates of bone loss at multiple skeletal sites measured by quantitative computed tomography (QCT) of the lumbar spine and peripheral QCT (pQCT) of the distal radius and tibia [5]. In postmenopausal women who were not being treated with estrogen, selective estrogen receptor modulators, or bisphosphonates, multiple significant correlations of FSH, bioavailable E2, and bioavailable testosterone with both cortical and trabecular bone loss were found at all sites studied. A cross-sectional study of 699 healthy Chinese women found that serum FSH and LH levels were significantly negatively correlated with areal BMD [6]. Collectively, the above studies suggested that FSH might have a role independent of estrogen in controlling bone mass and might be a useful predictor of osteoporosis risk.
However, other studies have shown opposing findings and a more complex picture. Gao et al. demonstrated lack of a direct effect of FSH in causing bone loss in the FSH receptor null mouse [7]. Similarly, in 2010, Drake et al. demonstrated in a prospective study that GNRH suppression of FSH secretion in postmenopausal women increased levels of bone resorption markers; they concluded that FSH does not regulate bone resorption [8]. In a 2004 cross-sectional study of women under age 40, Devleta et al. found greater bone loss in hypergonadotropic amenorrheic women than in hypogonadotropic amenorrheic women [9]. Importantly, they found a positive correlation between BMI and lumbar spine bone density (P=0.025) in the amenorrheic women, and that BMI was more strongly associated with bone density (P=0.032) than duration of amenorrhea. Although the authors speculated about a possible direct effect of FSH on bone metabolism, the lower BMI of the hypergonadotropic amenorrheic women compared with the hypogonadotropic group likely influenced the bone density findings. The conflicting results among these analyses warrant a more thorough investigation of potential confounding factors such as weight in the apparent association between FSH and low bone density.
We conducted a cross-sectional study to test associations between serum FSH and measures of bone strength in 111 postmenopausal women aged 50–64 years, some with past or current hormone use. The study population represented patients too young for routine BMD screening, but at an appropriate age for osteoporosis risk factor assessment. This age range of women has also been exposed to persistently high FSH concentrations for years, and so would be more likely than premenopausal women to show low bone density if FSH is associated with bone loss. In addition to dual-energy X-ray absorptiometry (DXA), we used pQCT at the distal radius to detect differences in the trabecular and cortical bone compartments that might be missed by routine clinical imaging techniques. We hypothesized that after adjustment for anthropomorphic and demographic measures, endogenous and exogenous estrogen, and other hormonal factors that might affect bone density, higher serum FSH concentrations would be associated with lower DXA and distal radial pQCT bone density measures. Lack of such findings could indicate patient-related variables such as weight and race provide important information in analyses of hormonal influences on bone in postmenopausal women.
Methods
Study participants
Participants were 111 community-dwelling postmenopausal women aged 50–64 years of mixed ethnic background, recruited between 2005 and 2010 from appointment rosters of university-based primary care clinics or from the community by university e-mail listservs, posters, and referrals by past study participants. Postmenopausal status was defined as the absence of menstrual bleeding or spotting for >12 consecutive months prior to eligibility screening. We included past or current hormone users because these women would be considered for osteoporosis risk assessment and screening, and because we could adjust for hormone use in our multivariable analysis. Women were excluded if they had ever taken an osteoporosis treatment agent (bisphosphonate, selective estrogen receptor modulator, calcitonin, or teriparatide) or prescription vitamin D or vitamin D derivative, had a contraindication to DXA bone density testing, weighed greater than 305 lb, or could not provide blood for laboratory testing. The research protocol was reviewed and approved by the Institutional Review Board of the University of North Carolina and the UNC Clinical Translational Research Center. Informed consent was obtained from all participants at the time of the study visit.
Measurements
Participants were instructed to fast and to void no more than once after midnight before attending a morning study visit in the UNC Clinical Translational Research Center. All blood and urine was collected before 10 a.m. Specimen collection and bone imaging were completed while the participants were fasting.
Areal bone density measures
Bone mineral density was measured at the femoral neck, total hip, lumbar spine (L1–L4), and distal radius in all participants by a trained and certified radiologic technologist using DXA (Hologic Discovery densitometer, Hologic, Inc., Bedford, MA). The technician was blinded regarding the specific study hypotheses. Quality control was maintained by daily scanning of an anthropomorphic spine phantom. The coefficient of variation for the densitometer is 0.6% and the reference limits for variation are ±1.5%. In vivo precision was not measured because the State of North Carolina Department of Environment, Health and Safety does not allow performance of DXA on healthy volunteers for the purpose of precision testing unless it is written as part of a protocol. DXA results were recorded in grams per square centimeter, and as T-scores ([BMD of participant – mean BMD of reference population]/SD of BMD of reference population) based on Hologic reference norms for white and nonwhite women for the lumbar spine, total hip, femoral neck and distal radius. In accordance with World Health Organization diagnostic criteria, patients were considered to have osteoporosis if their T-score was −2.5 or below at any central (spine or hip) anatomical site [10]. A research radiologist (JBR) in the UNC Department of Radiology reviewed the DXA reports.
Peripheral QCT measures
We included pQCT imaging at the distal radius because of the high concentration of trabecular bone at the 4% site, which would allow more sensitive measurements of bone density changes in response to hormonal influences compared to DXA. We measured total and trabecular cross-sectional area (mm2) and volumetric bone density (mg/cm3) at the ultradistal radius by pQCT using an XCT2000 scanner (Stratec, Orthometrix). Total and cortical area and volumetric density were also assessed at the 33% distal radius. These sites were chosen according to the 2007 International Society of Clinical Densitometry Official Positions [11] that recommend scan slices at the 4% site, which contains up to 80% trabecular bone [12], and a shaft location (15–65% of radius length) consisting predominantly of cortical bone. Based on an analysis of the last 140 phantom scans performed on the XCT2000 sn-91255 at UNC between January 31, 2007 and January 14, 2010, the accuracy was −0.20% for measures of trabecular bone density (analysis by Dan Schiferl, Bone Diagnostic Inc., January 22, 2010). In addition, as a measure of precision, the standard deviation of the same scans was 1.67 mg/cm3.
Biochemical analyses
Samples of fasting serum were centrifuged within 3 h of collection and refrigerated before submission to an on-site laboratory. On the day of the study visit, FSH, luteinizing hormone (LH), and E2≥20 pg/ml were measured using chemiluminescence-based immunoassays (OrthoClinical Diagnostics, Raritan, NJ), and albumin was measured using a bromocresol green spectrophotometric method (OrthoClinical Diagnostics, Raritan, NJ). The remaining serum was frozen in aliquots at −30°C for short-term storage and −80°C for long-term storage for additional testing. One sample which had an FSH >200 mIU/ml required quantification using an immunoenzymatic assay (Beckman-Coulter, Inc, Brea, CA). E2 concentrations below 20 pg/ml were measured with one of two assays: (1) a no-extraction double antibody 125I RIA (Siemens, Los Angeles, CA), performed at the Research Triangle Institute International Laboratory of Reproductive and Endocrine Toxicology (Research Triangle Park, NC) for 12 samples, or (2) liquid chromatography, tandem mass spectrometry performed at Esoterix, Inc. Endocrinology Laboratory Services for 99 samples. We used bioavailable (free or associated with albumin in the circulation) sex steroid concentrations because they better reflect the fractions of these factors available to tissues [13]. The bioavailable E2 (bio E2) concentration was calculated by multiplying the total E2 by the fraction that was non-SHBG bound as per Khosla et al. [13]. Bioavailable testosterone (bio T, differential precipitation method) was measured and reported by Mayo Medical Laboratories.
Assays for the following were also conducted: thyroid stimulating hormone (TSH, (OrthoClinical Diagnostics, Raritan, NJ), free thyroxine (OrthoClinical Diagnostics, Raritan, NJ), parathyroid hormone (PTH, Roche Diagnostics, Corp, Indianopolis, IN), sex-hormone binding globulin (SHBG, Siemens Healthcare Diagnostics, Deerfield, IL), dehydroepiandrosterone sulfate (DHEAS, Siemens Health-care Diagnostics, Deerfield, IL), bone specific alkaline phosphatase (BSAP, Beckman-Coulter, Inc., Brea, CA), and serum 25-OH vitamin D (liquid chromatography tandem-mass spectrometry, Mayo Medical Laboratories).
Other independent variables
Demographic information and risk factors for osteoporosis and fracture (including age, years since menopause, use of hormone therapy, hysterectomy with or without ovariectomy) were recorded by trained research assistants. Anthropometric measurements were taken by a research nurse in the UNC Clinical Translational Research Center.
Statistical analysis
Descriptive statistics
In the univariate analysis, means and standard deviations were calculated for demographic and anthropomorphic measures, hormonal variables, and DXA and pQCT measures. Spearman rank correlations, which are not sensitive to outliers, were used to analyze unadjusted relationships among FSH, bio E2, and other independent variables and outcomes.
Multivariable analysis
Separate multiple linear regression models were constructed for these dependent variables: areal BMD (g/cm2) at the lumbar spine, femoral neck and total hip, total bone area (mm2) and total volumetric bone density (mg/cm3) at the 4% and 33% distal radius, trabecular area and volumetric density at the ultradistal radius, and cortical area and volumetric density at the 33% distal radius. These independent variables were initially considered for each model: age, weight, race (nonwhite vs. white), years since menopause, ever use of hormone therapy, FSH, bio E2, bio T, LH, SHBG, DHEA-S, TSH, PTH, BSAP, and serum 25-OH vitamin D. We used simple (single variable) linear regression to select into the multivariable models for BMD outcomes those independent variables with at least one p value for unadjusted associations with the BMD outcomes less than 0.05; we repeated this variable selection process for the pQCT outcomes. To test for multi-collinearity of the explanatory variables in the models, variance inflation factors were calculated and ranged from 1.08 (BSAP) to 3.19 (FSH) [VIF <10 indicates no extreme multi-collinearity]. Results from the models were expressed in beta coefficients, which represent the mean change in the dependent variable for a 1 unit change in the independent variable, adjusted for the other covariates. For example, a beta coefficient (β) of −0.0003 for FSH vs. femoral neck BMD would mean for a 1-unit (1 mIU/ml) increase in FSH, the femoral neck BMD was 0.0003 g/cm2 lower, adjusted for the other independent variables in the model. Age, weight, and years since menopause were divided by 10 before inclusion in the models; after this transformation, a β of −0.005 meant for a 10-year increase in age, the femoral neck BMD was 0.005 g/cm2 lower, adjusted for the other independent variables. Race was coded as 0= nonwhite, 1=white; thus, β<0 for the race variable meant that bone density was lower in white women compared with nonwhite women. Statistical analyses were performed using the Stata SE 9.2 software [14].
We used the Bonferroni correction for adjusting the significance levels for p values to correct for multiple comparisons [15]. Using this method, p values ≤0.0125 and ≤0.00625 were considered statistically significant for DXA bone density outcomes (four) and pQCT outcomes (eight), respectively. p values greater than these thresholds but less than 0.05 were considered nearly significant.
Sample size calculations
The planned sample size of 111 women provided 80% power to detect a (population) correlation coefficient between two continuous-scaled variables as small (in absolute terms) as 0.26 with two-sided tests of statistical significance at the 0.05 level (e.g., a correlation between FSH and trabecular volumetric BMD of −0.26). In comparison, an unadjusted r value of 0.44 was found in a correlation of bioavailable estradiol and trabecular volumetric bone mineral density in a population-based cohort of women aged 40–59 years [16]. For a correlation of this magnitude, the study provided 99% power.
Results
Study population, anthropomorphic, FSH, and sex steroid measures
Participants had a mean age of 57.5 years, were predominantly white (80.0%), and were overweight on average (mean weight 79.7 kg, mean BMI 29.6; Table 1). Fifty-two percent of the women had used hormone therapy, including 16 (14.4%) current users. Mean FSH was 59.4 mIU/ml with values varying over a wide range (1.6–270 mIU/mL). FSH was significantly higher in normal weight women compared with overweight and obese women (80.0 vs. 49.5 mIU/ml, p<0.001) and in past/never estrogen users vs. current estrogen users (62.4 vs. 41.3 mIU/ml, p=0.02). Similarly, bio E2 (0.76 vs. 1.11 ng/dl, p=0.45) and bio T (2.35 vs. 3.06 ng/dl, p=0.29) were lower in normal weight women compared with heavier women, but these differences did not reach statistical significance. Mean BMD was in the normal range (T-score≥−1 at all sites).
Table 1. Subject characteristics (n=111).
| Characteristic | Mean (SD) or n (%) |
|---|---|
| Age, years | 57.5 (3.7) |
| Weight, kg | 79.7 (20.1) |
| Range | 46.8–137.2 |
| BMI (kg/m2) | 29.6 (74) |
| Range | 18.7–49.2 |
| Ethnicity/race, n (%) | |
| White | 80 (72.1) |
| Black | 27 (24.3) |
| Other | 4 (3.6) |
| Years since menopause | 11.3 (9.0) |
| Had a hysterectomy, n (%) | 38 (43.7) |
| Ever use of hormone therapy, n (%) | 58 (52.3) |
| Current use of hormone therapy, n (%) | 16 (14.4) |
| FSH, mIU/ml | 59.4 (34.8) |
| Range | 1.6–270 |
| Total serum E2, pg/mL | 13.0 (28.8) |
| Bio E2, ng/dl | 0.99 (2.25) |
| Range | 0–19.55 |
| Bio T, ng/dl | 2.54 (2.71) |
| SHBG, nmol/L | 47.8 (32.8) |
| Lumbar spine DXA | |
| BMD, g/cm2 | 1.014 (0.143) |
| T-score | −0.49 (1.22) |
| Z-score | 0.79 (1.23) |
| Femoral neck DXA | |
| BMD, g/cm2 | 0.772 (0.134) |
| T-score | −0.87 (1.02) |
| Z-score | 0.23 (0.98) |
| Total hip DXA | |
| BMD, g/cm2 | 0.889 (0.135) |
| T-score | −0.58 (0.94) |
| Z-score | 0.20 (0.92) |
| Distal radius DXA | |
| BMD, g/cm2 | 0.702 (0.062) |
| T-score | 0.13 (1.04) |
Normal values for postmenopausal women: FSH 21.5–131 mIU/ml, total E2 <15 pg/ml, bio E2 not available from reference laboratories, bio T not established, SHBG 20–130 nmol/L
Unadjusted Spearman's correlations
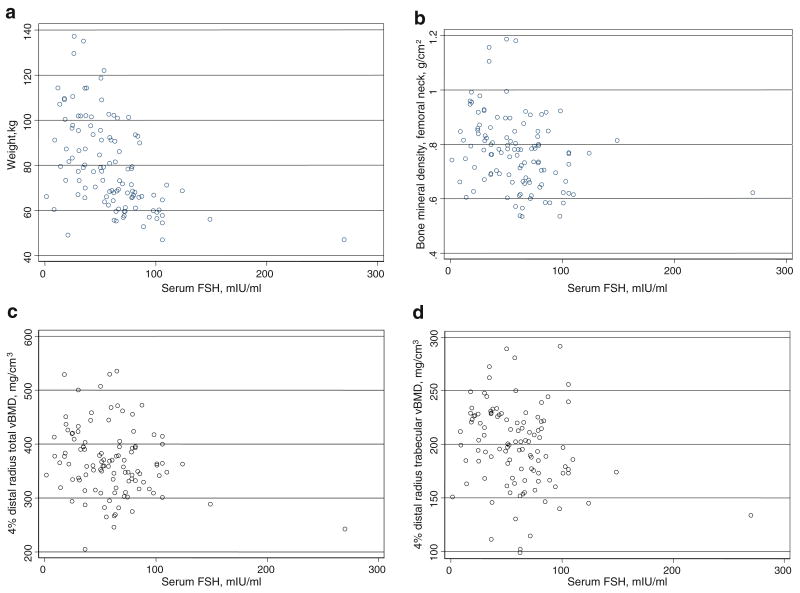
Rank correlations among selected patient-related factors and hormonal variables are shown in Table 2. FSH was strongly correlated with LH (r=0.81, p<0.001) and inversely correlated with weight (r=−0.57, p<0.001, Fig. 1a) and bio E2 (r=−0.50, p<0.001). White women weighed less than nonwhite women (r=−0.35, p<0.001). Weight was moderately correlated with bio E2 (r=0.42, p< 0.001) and less strongly with bio T (p=0.25, p=0.007).
Table 2. Unadjusted Spearman's rank correlation coefficients between selected independent variables and areal BMD.
| Age | Weight | Race | FSH | Bio E2 | BioT | LS BMD |
FN BMD |
TH BMD |
DR BMD |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Age | −0.10 | −0.24* | −0.20* | −0.34*** | ||||||
| Weight | −0.01 | 0.60*** | 0.58*** | 0.58*** | 0.29** | |||||
| Race | 0.17 | −0.35** | −0.35*** | −0.44*** | −0.38*** | −0.42*** | ||||
| FSH | −0.02 | −0.57*** | 0.36*** | −0.39*** | −0.36*** | −0.38*** | −0.21* | |||
| Bio E2 | −0.05 | 0.42*** | −0.21* | −0.50*** | 0.25** | 0.30** | 0.29** | 0.21* | ||
| Bio T | 0.16 | 0.25** | −0.11 | −0.17 | 0.35*** | 0.28** | 0.24* | 0.23* | 0.23* | |
| LH | −0.06 | −0.51*** | 0.32*** | 0.81*** | −0.30** | −0.11 | −0.39*** | −0.35*** | −0.34*** | −0.17 |
BMD bone mineral density, LS lumbar spine, FN femoral neck, TH total hip, DR distal radius
p<0.05;
p<0.01;
p<0.001
Fig. 1.
a Scatterplot of weight vs. serum FSH. b Scatterplot of femoral neck areal BMD vs. serum FSH. c Scatterplot of total vBMD at ultradistal radius vs. serum FSH. d Scatterplot of trabecular vBMD at ultradistal radius vs. serum FSH
FSH was inversely correlated with areal bone density sites (r=−0.39 to −0.21, p≤0.028 for all sites; Table 2, Fig. 1b). Weight (r=0.29 to 0.60, p≤0.0024 for all sites) and race (r=−0.44 to −0.35, p≤0.0002 for all sites) were moderately correlated with areal BMD.
Correlations with pQCT measures are shown in Table 3. FSH was inversely correlated with total (r=−0.22, p=0.02) and trabecular (r=−0.27, p=0.005) vBMD at the ultradistal radius [Figs. 1c and d], and with total bone area (r=−0.23, p=0.02) and cortical area (r=−0.21, p=0.04) at the 33% site. Weight showed statistically significant associations with the same pQCT sites as FSH. Bio E2 and bio T showed statistically significant associations with total and trabecular vBMD values at the ultradistal site. Race was associated with total vBMD (r=−0.21, p=0.03) at the ultradistal site and was moderately and inversely correlated with total bone area (r=−0.31, p=0.001) and cortical area (r=−0.39, p<0.001) at the 33% distal radius.
Table 3. Unadjusted Spearman's rank correlation coefficients between selected independent variables and distal radius pQCT measures.
| 4% Total bone area | 4% Total vBMD | 4% Trab area | 4% Trab vBMD | 33% Total bone area | 33% Total vBMD | 33% Crt area | 33% Crt vBMD | |
|---|---|---|---|---|---|---|---|---|
| Age | 0.02 | −0.21* | −0.07 | −0.10 | −0.09 | −0.20* | −0.26** | −0.13 |
| Weight | 0.16 | 0.25** | 0.06 | 0.29** | 0.27** | 0.04 | 0.35*** | −0.08 |
| Race | −0.03 | −0.21* | 0.03 | −0.18 | −0.31** | 0.04 | −0.39*** | 0.02 |
| FSH | 0.01 | −0.22* | 0.06 | −0.27** | −0.23* | 0.06 | −0.21* | 0.01 |
| Bio E2 | −0.04 | 0.21* | −0.07 | 0.32*** | 0.02 | 0.19 | 0.12 | 0.24* |
| Bio T | −0.07 | 0.26** | −0.10 | 0.32*** | −0.01 | 0.17 | 0.11 | 0.05 |
| LH | −0.06 | −0.13 | −0.02 | −0.18 | −0.24* | 0.11 | −0.19* | 0.08 |
vBMD volumetric bone mineral density, trab trabecular, crt cortical
p<0.05;
p<0.01;
p<0.001
Multivariable analysis with areal BMD outcomes
After adjustment for the other independent variables in the models, FSH, bio E2, and bio T were not significantly associated with areal BMD at any site. Weight was independently associated with BMD of the lumbar spine (β=0.033, p=0.007), femoral neck (β=0.028, p<0.001), and total hip (β=0.027, p<0.001); this indicates that for each 10 kg increase in weight, areal BMD was 0.027– 0.033 g/cm2 greater. Race was independently associated with lumbar spine (β=−0.074, p=0.008), femoral neck (β=−0.108, p<0.001), total hip (β=−0.087, p=0.001), and distal radial (β=−0.057, p<0.001) BMD (Table 4). Age (β=−0.043, p=0.003) and BSAP (β=−0.004, p=0.001) were significantly associated with distal radial BMD.
Table 4. Beta coefficients for independent associations in adjusted models of BMD (n= 111).
| Independent variablea | Beta coefficient (SE) | |||
|---|---|---|---|---|
| Lumbar spine BMD | Femoral neck BMD | Total hip BMD | Distal radius BMD | |
| Age, years | −0.003 (0.031) | −0.054 (0.028) | −0.033 (0.029) | −0.0428 (0.014)* |
| Weight, kg | 0.033 (0.007)* | 0.028 (0.006)* | 0.027 (0.006)* | 0.005 (0.003) |
| Race | −0.074 (0.028)* | −0.108 (0.025)* | −0.087 (0.026)* | −0.057 (0.012)* |
| FSH, mIU/ml | 0.00001 (0.001) | −0.0003 (0.001) | −0.0006 (0.0005) | −0.0002 (0.0003) |
| Bioavailable estradiol, ng/dl | 0.007 (0.005) | 0.002 (0.005) | 0.002 (0.005) | 0.003 (0.002) |
| Bioavailable testosterone, ng/dL | 0.003 (0.004) | −0.002 (0.003) | 0.00006 (0.003) | 0.002 (0.002) |
| LH, mIU/ml | 0.0001 (0.001) | 0.001 (0.001) | 0.001 (0.001) | 0.0008 (0.0005) |
| TSH, microIU/ml | −0.008 (0.009) | −0.007 (0.008) | −0.011 (0.008) | 0.001 (0.004) |
| SHBG, nmol/L | −0.0002 (0.0004) | −0.0006 (0.0004) | −0.0007 (0.0004) | −0.00001 (0.0002) |
| BSAP | 0.005 (0.003) | −0.004 (0.002) | −0.003 (0.003) | −0.004 (0.001)* |
BMD bone mineral density, SE standard error
p<0.0125
Age and weight divided by 10; other independent variables were ever use of hormone therapy, years since menopause, PTH, DHEAS, and 25-OH vitamin D, all nonsignificant
Multivariable analysis with pQCT outcomes at the 4% and 33% distal radius
Compared to the findings for areal BMD at weight bearing sites, fewer significant associations were found for distal radial pQCT outcomes. In the adjusted models, no hormonal variable showed any significant associations with pQCT outcomes (Tables 5 and 6). Only race was significantly associated with cortical area (β=−7.822, p= 0.001) at the 33% distal radius. Several associations were near statistical significance (0.00625<p<0.05): at the 4% site, weight (β=5.268, p=0.045) and bio T (β=−3.553, p= 0.011) were associated with total bone area and FSH (β=−0.411, p=0.020) and LH (β=0.725, p=0.033) were associated with trabecular vBMD. At the 33% site, race (β=−7.836, p=0.026) was associated with total bone area and age (β=−55.790, p=0.039), bio E2 (β=8.421, p= 0.048), and BSAP (β=−5.936, p=0.014) were associated with total vBMD. Age, weight, and BSAP were associated with cortical area, and bio E2 and BSAP were associated with cortical vBMD at the 33% site. Other analyzed measures including years since menopause, ever use of hormone therapy, TSH, PTH, SHBG, DHEAS, and 25-OH vitamin D showed no statistically significant nor near significant associations with distal radial pQCT measures in the adjusted models.
Table 5. Beta coefficients for independent associations in adjusted models of pQCT measures at the 4% (ultradistal) radius.
| Independent variablea | Beta coefficient (SE) | |
|---|---|---|
| Total bone area | Trabecular vBMD | |
| Age, years | 0.346 (11.298) | −9.752 (9.787) |
| Weight, kg | 5.268 (2.597)* | 3.938 (2.250) |
| Race | −0.698 (10.103) | −10.998 (8.752) |
| FSH, mIU/ml | 0.185 (0.200) | −0.411 (0.173)* |
| Bioavailable estradiol, ng/dl | 0.337 (1.840) | −0.609 (1.594) |
| Bioavailable testosterone, ng/dL | −3.553 (1.378)* | 0.784 (1.194) |
| LH, mIU/ml | 0.010 (0.386) | 0.725 (0.334)* |
| TSH, microIU/ml | −3.199 (3.167) | −.338 (2.743) |
| SHBG, nmol/L | −0.168 (0.162) | −0.116 (0.140) |
| BSAP, ug/L | −0.677 (0.987) | −0.770 (0.855) |
| DHEAS, mcg/dL | −0.037 (0.094) | 0.153 (0.082) |
SE standard error
Age and weight divided by 10; other independent variables were ever use of hormone therapy, years since menopause, PTH, and 25-OH vitamin D, all nonsignificant. All results nonsignificant for total bone volumetric BMD and trabecular area at the 4% site (data not shown).
0.00625<p<0.05
Table 6. Beta coefficients for independent associations in adjusted models of pQCT measures at the 33% distal radius.
| Independent variable* | Beta coefficient (SE) | |||
|---|---|---|---|---|
| Total bone area | Total vBMD | Cortical area | Cortical vBMD | |
| Age, years | −2.222 (3.892) | −55.790 (26.642)* | −6.045 (2.484)* | −10.874 (8.836) |
| Weight, kg | 0.983 (0.864) | 2.681 (5.914) | 1.165 (0.551)* | −1.308 (1.961) |
| Race | −7.836 (3.459)* | −18.070 (23.677) | −7.822 (2.207)** | −5.904 (7.853) |
| FSH, mIU/ml | −0.027 (0.067) | −0.010 (0.457) | −0.019 (0.043) | −0.0109 (0.152) |
| Bioavailable estradiol, ng/dl | −0.39 (0.613) | 8.421 (4.200)* | 0.237 (0.391) | 3.327 (1.393)* |
| Bioavailable testosterone, ng/dL | −0.35 (0.457) | 2.575 (3.127) | −0.104 (0.291) | 0.655 (1.037) |
| LH, mIU/ml | −0.063 (0.128) | 1.118 (0.880) | 0.037 (0.0820) | 0.219 (0.292) |
| TSH, microIU/ml | −1.924 (1.057) | 8.361 (7.234) | −0.815 (0.674) | 0.723 (2.399) |
| SHBG, nmol/L | −0.032 (0.055) | .080 (0.374) | −.022 (0.035) | 0.141 (0.124) |
| BSAP, ug/L | −0.098 (0.345) | −5.936 (2.360)* | −0.459 (0.220)* | −1.806 (0.783)* |
| DHEAS, mcg/dL | −0.023 (0.032) | 0.119 (0.218) | −0.008 (0.020) | 0.058 (0.072) |
vBMD volumetric bone mineral density, SE standard error
0.00625<p<0.05;
p<0.00625
Age and weight divided by 10; other independent variables were years since menopause, PTH, and 25-OH vitamin D, all nonsignificant
Results of a post hoc sensitivity analysis for FSH vs. BMD and pQCT outcomes that omitted the observation with outlying FSH value shown in Fig. 1 did not qualitatively differ from the main analyses based on 111 postmenopausal women. When FSH and bio E2 were removed from the models, the associations for weight and race reported above remained statistically significant.
Discussion
This cross-sectional study of postmenopausal women aged 50–64 years recruited from primary care practices or the community found insufficient evidence to support an association between FSH or bio E2 and bone density measured by DXA or by distal radial pQCT after adjustment for patient-related factors and other hormonal variables. The correlational analysis showed that FSH concentrations were higher and bio E2 concentrations were lower on average in thin, white women compared with heavier nonwhite women, and the former group had lower bone density. However, the multivariable analysis showed that weight and race were independently associated with areal BMD, while FSH and bio E2 were not significant determinants. This suggests that the inverse relationship between FSH and BMD was mostly due to confounding variables. Race was the only factor showing a statistically significant association with a pQCT outcome (cortical area at 33% distal radius). This indicates a less important role of weight in determining pQCT outcomes at a non-weight-bearing site compared to areal BMD at weight-bearing sites.
FSH would warrant consideration as a screening biomarker if high concentrations were consistently associated with lower bone strength, or if it was found to be a more robust marker than easily measurable patient-related factors such as weight. Past longitudinal studies have shown associations between FSH and bone density loss in pre- and postmenopausal study populations. Riggs et al. found that in postmenopausal women, several significant correlations existed between bio E2, bio T, and FSH vs. cortical and trabecular bone loss in the distal radius, distal tibia, and lumbar spine [5]. While their analysis also included IGF, C-telopeptide, and propeptide of type I collagen, it did not account for body weight. Sowers et al., following pre- and early perimenopausal women into the menopausal transition, found that lumbar spine and hip BMD losses were most strongly related to the interaction between initial FSH concentration and longitudinal FSH change and not to sex steroid concentrations, after adjustment for BMI [4]. We chose to include weight rather than BMI in our models because Robbins et al. found weight alone to be a much better predictor of BMD than BMI [17]. We acknowledge that a longitudinal study might show a different relationship between FSH and bone loss. Also, because FSH concentrations rise significantly in the perimenopausal period then plateau after menopause [18], associations between FSH and low bone density may be different in perimenopausal women compared to our postmenopausal study population.
Our data suggest that loading was more important than hormonal effects at weight-bearing sites in younger postmenopausal women. Weight is known to be a key predictor of osteoporosis [19–22] and bone loss [23–25] in postmenopausal women. In their meta-analysis of the WHI, EPIDOS, and CHS databases, Robbins et al. speculated that increased weight promoted load induced changes in the skeleton [17]. Increased mechanical loading of bone is associated with increased bone strength [26]. Accordingly, Beck et al. found femur BMD and geometric strength to be greater in overweight and obese postmenopausal women aged 50–79 compared with normal weight and underweight in a cross-sectional analysis, and the heavier patient group reported a history of fewer fractures at the hip and other central body sites, but not in the extremities [27]. Increased weight is also associated with increased estrogen production by aromatization in adipose tissue [28] and lower concentrations of SHBG [29], both of which increase the bioavailable fraction of estrogen. In our study, weight did not predict cortical or trabecular bone volumetric mass in the radius, a site where body weight should contribute less to the loading environment. The lean mass and fat mass components of weight may be relevant factors, but they could not be included in our analysis because of collinearity.
Race was significantly associated with areal BMD at most weight bearing sites, and with cortical area at the 33% radius site. Mean bone density is greater in black women than in white women in the US general population [30]. The nonwhite group in our study population comprised of 27 black women and four women of other races, meaning that the race comparison was mostly black vs. white in our analysis. As such, our results were consistent with population norms for bone density by race which suggest that genetic factors underlie cortical size.
Our study has several limitations. As in all cross-sectional analyses, we could only show associations between hormonal measures and bone density; the results do not demonstrate cause–effect relationships. Importantly, the results do not apply to the rate of bone loss, which could cause marked changes in bone density in subsequent decades after this early stage of the postmenopausal period. Our study population was small, but comparable in size to past cross-sectional studies of associations between hormonal factors and pQCT measures of bone. We used a Bonferroni adjustment to adjust for multiple hypothesis testing. Because this approach is conservative and may increase type II error (actual association is not detected) [15, 31], the associations we found with near statistical significance might become significant in a larger study. Factors such as inhibins and serotonin were not included in our analysis, and might have altered our results.
In conclusion, our multivariable analysis did not find a significant association between FSH or bio E2 and bone density measures by DXA or pQCT in postmenopausal women aged 50–64 years. Instead, the easily obtainable measures of weight and race were more useful than hormonal factors as clinical indicators of bone density and should be included in analyses of potential bone biomarkers.
Acknowledgments
We acknowledge Brent Henderson for his assistance with the data collection and database management and Dan Schiferl of Bone Diagnostic Inc. for his training and supervision of the pQCT analysis. The project described was funded by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R03AR055734) and National Center for Research Resources (K23RR024685, M01RR00046, and UL1RR025747). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the funding agencies.
Footnotes
Conflicts of interest None.
Contributor Information
M. L. Gourlay, Email: margaret_gourlay@med.unc.edu, Department of Family Medicine, University of North Carolina, Chapel Hill, NC, USA; University of North Carolina, Aycock Building, Manning Drive, CB #7595, Chapel Hill, NC 27599-7595, USA.
J. S. Preisser, Department of Biostatistics, University of North Carolina, Chapel Hill, NC, USA
C. A. Hammett-Stabler, Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, NC, USA
J. B. Renner, Department of Radiology, University of North Carolina, Chapel Hill, NC, USA
J. Rubin, Department of Medicine, University of North Carolina, Chapel Hill, NC, USA; Department of Pharmacology, University of North Carolina, Chapel Hill, NC, USA
References
- 1.Sun L, Peng Y, Sharrow A, Iqbal J, Zhang Z, Papachristou D, Zaidi S, Zhu LL, Yaroslavskiy B, Zhou H, Zallone A, Sairam M, Kumar T, Bo W, Braun J, Cardoso-Landa L, Schaffler M, Moonga B, Blair H, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Cheng Y, Xu W, Bian Z. Protective effects of follicle-stimulating hormone inhibitor on alveolar bone loss resulting from experimental periapical lesions in ovariectomized rats. J Endod. 2010;36(4):658–663. doi: 10.1016/j.joen.2010.01.011. doi:S0099-2399(10)00048-8[pii]10.1016/j.joen.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Rendina D, Gianfrancesco F, De Filippo G, Merlotti D, Esposito T, Mingione A, Nuti R, Strazzullo P, Mossetti G, Gennari L. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur J Endocrinol. 2010;163(1):165–172. doi: 10.1530/EJE-10-0043. doi:EJE-10-0043[pii]10.1016/j.joen.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Sowers M, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 5.Riggs BL, Melton LJ, 3rd, Robb R, Camp J, Atkinson EJ, McDaniel L, Amin S, Rouleau P, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu ZR, Wang AH, Wu XP, Zhang H, Sheng ZF, Wu XY, Xie H, Luo XH, Liao EY. Relationship of age-related concentrations of serum FSH and LH with bone mineral density, prevalence of osteoporosis in native Chinese women. Clin Chim Acta. 2009;400(1–2):8–13. doi: 10.1016/j.cca.2008.09.027. doi:S0009-8981(08)00480-4[pii]10.1016/j.cca.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Gao J, Tiwari-Pandey R, Samadfam R, Yang Y, Miao D, Karaplis A, Sairam M, Goltzman D. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone. Endocrinology. 2007;148:2613–2621. doi: 10.1210/en.2006-1404. [DOI] [PubMed] [Google Scholar]
- 8.Drake M, McCready L, Hoey K, Atkinson E, Khosla S. Effects of suppression of follicle stimulating hormone secretion on bone resorption markers in postmenopausal women. J Clin Endocrinol Metab. 2010;95(11):5063–5068. doi: 10.1210/jc.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devleta B, Adem B, Senada S. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. J Bone Miner Metab. 2004;22:360–364. doi: 10.1007/s00774-004-0495-1. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Study Group . WHO technical report series, no 843. Geneva: 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis; p. 129. [PubMed] [Google Scholar]
- 11.Engelke K, Adams J, Armbrecht G, Augat P, Bogado C, Bouxsein M, Felsenberg D, Ito M, Prevrhal S, Hans D, Lewiecki E. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–162. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Augat P, Fuerst T, Genant H. Quantitative bone mineral assessment at the forearm: a review. Osteoporos Int. 1998;8:299–310. doi: 10.1007/s001980050068. [DOI] [PubMed] [Google Scholar]
- 13.Khosla S, Melton L, Atkinson E, O'Fallon W, Klee G, Riggs B. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 14.StataCorp . Stata Statistical Software: Release SE 9.2. College Stations: 2005. [Google Scholar]
- 15.Bland J, Altman D. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khosla S, Riggs B, Robb R, Camp J, Achenbach S, Oberg A, Rouleau P, Lr M. Relationship of volumetric bone density and structural parameters at different skeletal sites to sex steroid levels in women. J Clin Endocrinol Metab. 2005;90:5096–5103. doi: 10.1210/jc.2005-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins J, Schott AM, Azari R, Kronmal R. Body mass index is not a good predictor of bone density: results from WHI, CHS, and EPIDOS. J Clin Densitom. 2006;9:329–334. doi: 10.1016/j.jocd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Gracia C, Sammel M, Freeman E, Lin H, Langan E, Kapoor S, Nelson D. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12:128–135. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- 19.Wildner M, Peters A, Raghuvanshi V, Hohnloser J, Siebert U. Superiority of age and weight as variables in predicting osteoporosis in postmenopausal white women. Osteoporos Int. 2003;14:950–956. doi: 10.1007/s00198-003-1487-z. [DOI] [PubMed] [Google Scholar]
- 20.Zhai G, Hart D, Valdes A, Kato B, Richards J, Hakim A, Spector T. Natural history and risk factors for bone loss in postmenopausal Caucasian women: a 15-year follow-up population-based study. Osteoporos Int. 2008;19:1211–1217. doi: 10.1007/s00198-008-0562-x. [DOI] [PubMed] [Google Scholar]
- 21.Koh L, Ben Sedrine W, Torralba T, Kung A, Fujiwara S, Chan S, Huang Q, Rajatanavin R, Tsai K, Park H, Reginster JY. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12:699–705. doi: 10.1007/s001980170070. [DOI] [PubMed] [Google Scholar]
- 22.Waugh E, Lam M, Hawker G, McGowan J, Papaioannou A, Cheung A, Hodsman A, Leslie W, Simonoski K, Jamal S. Risk factors for low bone mass in healthy 40–60 year old women: a systematic review of the literature. Osteoporos Int. 2009;10:1–21. doi: 10.1007/s00198-008-0643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macdonald H, New S, Campbell M, Reid D. Influence of weight and weight change on bone loss in perimenopausal and early postmenopausal Scottish women. Osteoporos Int. 2005;16:163–171. doi: 10.1007/s00198-004-1657-7. [DOI] [PubMed] [Google Scholar]
- 24.May H, Murphy S, Khaw KT. Age-associated bone loss in men and women and its relationship to weight. Age Aging. 1994;23:235–240. doi: 10.1093/ageing/23.3.235. [DOI] [PubMed] [Google Scholar]
- 25.Harris S, Dallal G, Dawson-Hughes B. Influence of body weight on rates of change in bone density of the spine, hip, and radius in postmenopausal women. Calcif Tissue Int. 1992;50:19–23. doi: 10.1007/BF00297292. [DOI] [PubMed] [Google Scholar]
- 26.Forwood M. Mechanical effects on the skeleton: are there clinical implications? Osteoporos Int. 2001;12:77–83. doi: 10.1007/s001980170161. [DOI] [PubMed] [Google Scholar]
- 27.Beck T, Petit M, Wu G, LeBoff M, Cauley J, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the Women's Health Initiative-Observational Study. J Bone Miner Res. 2009;24:1369–1379. doi: 10.1359/JBMR.090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grodin J, Siiteri P, MacDonald P. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973;36:207–214. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- 29.Wu F, Ames R, Evans M, France J, Reid D. Determinants of sex hormone-binding globulin in normal postmenopausal women. Clin Endocrinol (Oxf) 2001;54:81–87. doi: 10.1046/j.1365-2265.2001.01183.x. [DOI] [PubMed] [Google Scholar]
- 30.Looker A, Wahner H, Dunn W, Calvo M, Harris T, Heyse S, Johnston C, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 31.Perneger T. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]