Abstract
Subthalamic nucleus deep brain stimulation (STN-DBS) is currently the treatment of choice for medication-resistant levodopa-related motor complications in patients with Parkinson’s disease (PD). While STN-DBS often results in meaningful motor improvements, consensus regarding long-term neuropsychological outcome continues to be debated. We assessed the cognitive outcomes of 19 STN-DBS patients compared to a group of 18 medically-managed PD patients on a comprehensive neuropsychological battery at baseline and two years post-surgery. Patients did not demonstrate changes in global cognitive functioning on screening measures. However, neuropsychological results revealed impairments in nonverbal recall, oral information processing speed, and lexical and semantic fluency in STN-DBS patients compared to PD controls 2 years post-surgery in these preliminary analyses. Additionally, reliable change indices revealed that approximately 50% of STN-DBS patients demonstrated significant declines in nonverbal memory and oral information processing speed compared to 25% to 30% of PD controls, and 26% of STN-DBS patients declined on lexical fluency compared to 11% of PD patients. Approximately 30% of both groups declined on semantic fluency. The number of STN-DBS patients who converted to dementia 2 years following surgery was not significantly different from the PD participants (32% versus 16%, respectively). Our results suggest that neuropsychological evaluations may identify possible mild cognitive changes following surgery.
Keywords: Parkinson’s Disease, deep brain stimulation, neuropsychology, cognition, reliable change
INTRODUCTION
Bilateral subthalamic nucleus deep brain stimulation (STN-DBS) is currently the treatment of choice for treatment resistant tremor and motor complications in patients treated with levodopa for Parkinson’s disease (PD).[1, 2] This procedure has been successful in ameliorating dyskinesias, decreasing levodopa medications, and increasing “on” medication time and health-related quality of life (QOL).[3, 4]
However, less is known regarding the cognitive outcome following STN-DBS. Initial research suggested that STN-DBS did not lead to significant changes in short-term global cognitive skills when assessed with brief screening measures.[5–7] Conversely, studies utilizing more comprehensive neuropsychological batteries revealed short-term, mild impairments in verbal fluency, memory and executive function.[8–13] Despite evidence of mild short-term cognitive impairments, long-term cognitive effects of STN-DBS have not been as thoroughly assessed. While some studies have not found cognitive changes following DBS[6] other investigations have documented declines in verbal fluency, verbal memory, information processing speed and executive function.[10–12] A meta-analysis on STN-DBS cognitive outcome research found that 41% of patients had evidence of cognitive impairments in verbal memory and fluency, executive functioning, attention, working memory, mental speed, and response inhibition an average of 13 months following surgery.[13] These studies suggest that the cognitive effects of STN-DBS remain underestimated and highlight the need to perform comprehensive neuropsychological evaluations to better capture the full effects of STN-DBS.
The purpose of the present study was to investigate the cognitive effects of bilateral simultaneous STN-DBS using a comprehensive neuropsychological assessment and a matched medically-managed PD control group two years following surgery. Moreover, we present reliable change indices (RCIs) to compare statistically reliable cognitive changes between the groups for each neuropsychological measure over time. Additionally, dementia caseness analyses using both DSM-IV-TR[14] and Emre[15] criteria were performed.
METHODS
Participants
Nineteen PD patients who had bilateral simultaneous STN-DBS were compared to 18 non-surgical PD patients using comprehensive neuropsychological assessment at baseline and at a 2 year follow-up evaluation. The STN-DBS patients were consecutive patients who underwent STN-DBS for the treatment of PD from the Baylor College of Medicine Parkinson’s Disease and Movement Disorders Center (BCM-PDMDC). The non-surgical PD patients were a convenience sample of PD patients from the PDMDC who were medically managed, who chose not to undergo surgery, or who were recruited from PD community support groups. The PD patients were matched to the STN-DBS group at the time of recruitment on an on-going basis. However, as this sample constitutes a sub-sample of patients whose 6 month cognitive outcome was previously reported[16], the patient groups were then matched post-hoc for these analyses based on the availability of 2 year data. All PD and STN DBS patients underwent 3 neuropsychological evaluations: Baseline, 6 months, and 2 year follow-up.
Inclusion criteria included a diagnosis and clinical findings of moderately advanced (“off” medication Hoehn and Yahr score of 3 or 4) idiopathic PD with response to levodopa, complicated by disabling motor fluctuations and dyskinesias. Patients with Mini-Mental Status Examination (MMSE) scores ≤23 and psychiatric complications that could interfere with compliance were excluded. All procedures were approved by the BCM Institutional Review Board.
Procedures
Neurosurgical procedures
One neurosurgeon (RKS) completed all operative procedures according to previously described methods.[17]
Neurological evaluations
PD patients were evaluated in the “on” motor state at baseline and at 2 years; STN-DBS patients were evaluated in both the “on” and the “off” states for the same time periods. All patients were evaluated by a movement disorder neurologist (JJ) certified in the use of the UPDRS.
Neuropsychological evaluations
Neuropsychological tests administered included (18): Mental Status: MMSE, Dementia Rating Scale (DRS); Memory: Rey Auditory Verbal Learning Test Total Trials 1–5 (RAVLT-total), short-term recall (RAVLT-stm), and long-term recall (RAVLT-ltm), and the Brief Visual Memory Test-Revised total trials 1–3 (BVMT-R-total) and delayed recall (BVMT-R delay); Information Processing: Symbol Digit Modalities Test oral administration (SDMT), Trail Making Test Part A (Trails A), Digit Span from the Wechsler Adult Intelligence Scale, 3rd edition; Stroop Color-Word Test score (Stroop Word); Language: Controlled Oral Word Association Test (VF) and semantic fluency (SF), and Boston Naming Test (BNT); Executive Functioning: Trail Making Test Part B (Trails B), Wisconsin Card Sorting Test perseverative errors (WCST), and Stroop Color-Word Test (Stroop Color-Word); and Visuospatial Functioning: Clock Drawing. Three alternate forms were counterbalanced across participants for the RAVLT and the BVMT-R for each of the three evaluations that the patients underwent.
Data Analyses
Repeated measures ANOVAs and ANCOVAs were conducted with group (STN-DBS vs. PD) as the between subjects variable and neuropsychological outcomes as the within subjects variable. Standard scores were used for all outcomes except for MMSE and Clock Drawing. Partial eta-squared (η2) was used as the effect size for F tests. RCIs were computed to assess for statistically reliable decline, increase, or no change in a neuropsychological score from baseline to 2 years while taking into account the reliability of the test, indicating a significant change in scores not attributable to measurement variability.[19] Due to the exploratory nature of identifying the cognitive side effect profile of the surgery, the p-value was set at p ≤ 0.05 for all analyses. With the large number of comparisons being proposed in this exploratory research, we acknowledge that there is an inflated risk of Type I error. A Bonferroni correction would adequately address this concern by reducing Type I error. However, particularly when a Bonferroni correction is applied to a small data set, Type II error increases, hence a potential cognitive side effect of STN-DBS may not be identified. Consequently, we have chosen not to employ a Bonferroni correction for this preliminary investigation. We have included effect sizes, or the degree of experimental effects, for each of the findings along with the exact p-value observed. These effects sizes are comparable across studies even with different sample sizes (20); thus allowing for the evaluation of the statistical significance of these preliminary results. However, caution should be exercised when interpreting our findings and we acknowledge that this data analysis is exploratory in nature.
In addition, dementia caseness analyses were conducted using the criteria established by Emre et al[15] and DSM-IV-TR[14] independently to identify participants who met criteria for PD dementia (PDD). A patient was designated as having PDD according to Emre’s criteria [15] with a reliable decline based on the RCI score and an impairment of at least 2 standard deviations below the mean on standardized scores in at least 2 cognitive domains. Patients were categorized as meeting PDD DSM-IV-TR criteria with a reliable decline based on the RCI scores and impairment (2 standard deviations below mean) in memory and at least one other cognitive domain To further evaluate the progression of cognitive changes, a caseness analysis of PD-Mild Cognitive Impairment (MCI) was also conducted using the criteria adopted by Caviness and colleagues [21]. A patient was designated as having PD-MCI with cognitive performance at least 2 standard deviations below the age corrected mean score in one cognitive domain (as established by Emre’s [15] criteria).
RESULTS
Subjects
Table 1 presents baseline demographic information for STN-DBS patients and PD controls. Groups were matched post-hoc on age, gender, baseline Hoehn and Yahr staging, duration of PD, and baseline MMSE scores. However, the STN-DBS group had significantly less education and higher baseline dopaminergic medication usage. Education correlated significantly (ps < 0.05) with Digit Span, BVMT-R Total Recall, and BNT and thus was entered as a covariate. To control for changes in dopaminergic medications over time, a change score was computed by subtracting dose at baseline from 2 years. Change in dopaminergic medication use correlated significantly and was used as a covariate for Stroop Word and Trails A.
Table 1.
Demographics for PD and STN-DBS groups
| PD (n=18) | STN-DBS (n=19) | p value | |
|---|---|---|---|
| Sex (%Male) | 83% | 53% | 0.08 |
| Age (y) | 66.6 (9.0) | 62.1 (10.3) | 0.16 |
| Education (y) | 16.6 (1.20) | 13.6 (1.71) | 0.001 |
| Duration (y) | 7.50 (4.22) | 10.1 (6.24) | 0.15 |
| MMSE | 28.7 (1.19) | 27.9 (3.41) | 0.37 |
| Dopaminergic medication (mg) | 468.4 (293.0) | 1017.6 (411.2) | 0.001 |
| Hoehn and Yahr Staging | 1.0–3.0 | 1.5–3.0 | 0.85 |
Attrition Rates for STN-DBS
The neuropsychological performance 6 months following surgery was previously reported by York et al.[16] for 23 STN-DBS patients. Four of these patients did not return for their 2 year follow-up evaluation (17% attrition from 6 months to 2 years). Of these four patients, two patients died between their 6 month and 2 year evaluation, one patient moved out of state and could not be contacted, and one patient refused to complete the evaluation due to an increase in psychological distress.
Outcomes
Motor Outcome
The UPDRS motor “on” scores revealed that the STN-DBS patients demonstrated improved motor functioning at 2 years as compared to the PD control group (F(1,27)=4.28, p=0.04, η2=0.13). STN-DBS patients also demonstrated a significant decrease in their levodopa medication usage over time [F(1,23)=10.0, p=0.004, η2=0.30]. PD patients demonstrated an increase in dopaminergic medication usage from baseline (M=468.4 mg, SD=293.0) to 2 years (M=1040.0 mg, SD=131.4) compared to a decline for the STN-DBS patients from baseline (M=1017.6 mg, SD=411.2) to 2 years (M=599.3 mg, SD=460.1).
Neuropsychological Outcome
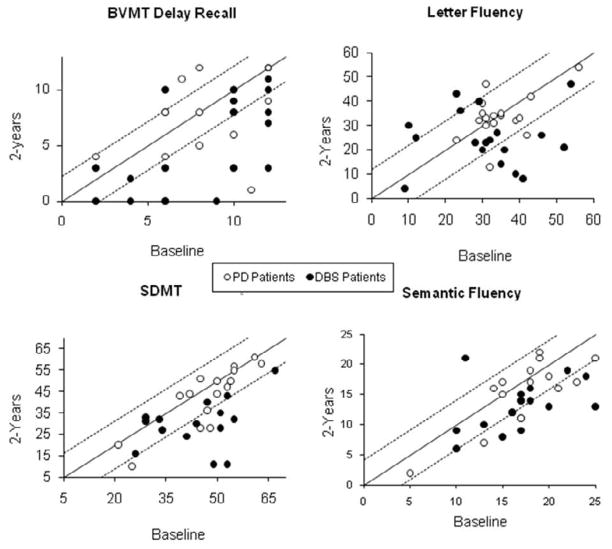
Table 2 provides raw score means and standard deviations for neuropsychological outcomes by group over time. Table 3 provides 95% RCI criterion for each measure and the percentage of PD and STN-DBS patients with a reliable change in scores from baseline to 2 years. RCI data for significant outcomes are illustrated in Figure 1.
Table 2.
Cognitive outcome for the DBS and PD control groups
| Baseline M(SD) | 2-years M(SD) | Interaction | |||
|---|---|---|---|---|---|
| Domain/test | PD | DBS | PD | DBS | p value |
| General ability | |||||
| MMSE | 28.7 (1.19) | 27.9 (3.41) | 27.7 (2.17) | 27.5 (2.84) | 0.62 |
| DRS | 138.1 (5.41) | 134.3 (9.46) | 134.9 (10.1) | 132.8 (9.53) | 0.49 |
| Memory | |||||
| RAVLT Total | 41.7 (12.8) | 37.8 (11.5) | 38.6 (15.9) | 33.2 (11.2) | 0.18 |
| RAVLT STM | 7.89 (3.82) | 7.53 (3.53) | 7.11 (3.68) | 6.22 (3.89) | 0.55 |
| RAVLT Delay Recall | 7.39 (4.00) | 7.84 (3.82) | 7.11 (4.03) | 5.94 (4.14) | 0.11 |
| BVMT Total Recall* | 20.1 (7.30) | 18.6 (7.33) | 20.6 (8.62) | 12.5 (7.78) | 0.17 |
| BVMT Delay Recall | 8.33 (3.50) | 7.89 (3.54)a | 8.25 (3.49) | 4.84 (4.07)a | 0.03 |
| Information Processing | |||||
| Symbol Digit | 45.2 (12.5)a | 40.9 (13.5)b | 40.6 (16.4)a | 29.2 (12.2)b | 0.03 |
| Trails A^ | 41.3 (30.6) | 49.3 (19.1) | 55.2 (54.7) | 57.4 (23.5) | 0.29 |
| Digit Span* | 17.4 (3.60) | 16.1 (3.51) | 16.6 (4.00) | 13.6 (3.31) | 0.08 |
| Stroop Word^ | 86.3 (18.9) | 78.4 (12.2) | 81.2 (19.0) | 75.6 (19.5) | 0.46 |
| Language | |||||
| Letter Fluency | 36.2 (9.06) | 31.4 (12.5)a | 35.6 (11.6) | 24.5 (11.4)a | 0.01 |
| Semantic Fluency | 17.3 (4.41) | 16.8 (4.37)a | 14.7 (5.55) | 13.6 (5.23)a | 0.04 |
| Boston Naming* | 57.0 (2.10) | 53.4 (7.37) | 57.2 (2.60) | 54.6 (4.26) | 0.44 |
| Executive Function | |||||
| Trails B | 103.0 (61.2) | 148.4 (68.6) | 123.5 (78.7) | 189.3 (103.0) | 0.35 |
| Stroop Color-Word | 30.1 (9.10) | 32.7 (10.4) | 29.7 (13.1)) | 30.4 (14.4) | 0.07 |
| WCST Pers. Errors | 9.53 (5.95)) | 13.7 (7.28) | 11.1 (12.6) | 19.2 (16.4) | 0.12 |
| Visuospatial | |||||
| Clock Command | 9.50 (0.92)a | 8.58 (1.64) | 8.76 (1.60)a | 8.84 (1.46) | 0.01 |
Raw score means and standard deviations for neuropsychological outcomes. Means within the same row that share a letter are significantly different ( p <0.05).
Test was significantly related to education level. Means and standard deviations for these outcomes are raw scores, and the p value is based on analyses conducted with education corrected standard scores.
Test was significantly correlated with changes in dopaminergic medication levels. For these analyses, the p value is based on analyses conducted with the medication change score included as a covariate. M=mean, SD=standard deviation, PD=parkinson’s disease, DBS=deep brain stimulation
Table 3.
Reliable change indices (percentages) for cognitive scores for the STN-DBS and PD groups
| PD |
STN-DBS |
||||||
|---|---|---|---|---|---|---|---|
| Domain/test | Decline % | No change % | Improve % | Decline % | No change % | Improve % | 95% RCI criterion |
| General ability | |||||||
| MMSE | 44.4 | 38.9 | 16.7 | 38.9 | 50.0 | 11.1 | 1.30 |
| DRS | 33.3 | 61.1 | 5.60 | 31.6 | 52.6 | 15.8 | 3.40 |
| Memory | |||||||
| RAVLT Total | 22.2 | 66.7 | 11.1 | 55.6 | 38.9 | 5.60 | 8.80 |
| RAVLT STM | 27.8 | 61.1 | 11.1 | 38.9 | 44.4 | 16.7 | 3.00 |
| RAVLT Delay Recall | 5.60 | 88.9 | 5.6 | 22.2 | 72.2 | 5.60 | 4.50 |
| BVMT-R Total | 18.8 | 56.3 | 25.0 | 42.1 | 52.6 | 5.30 | 5.60 |
| BVMT-R Delay | |||||||
| Recall | 25.0 | 62.5 | 12.5 | 47.4 | 47.4 | 5.3 | 2.20 |
| Information Processing | |||||||
| SDMT | 29.4 | 70.6 | 0.00 | 52.9 | 41.2 | 5.90 | 11.0 |
| Trails A | 33.3 | 61.1 | 5.60 | 38.8 | 44.4 | 16.7 | 9.30 |
| Digit Span | 47.1 | 23.5 | 29.4 | 50.0 | 38.9 | 11.0 | 1.90 |
| Stroop Word | 12.5 | 87.5 | 0.00 | 37.5 | 37.5 | 25.0 | 13.8 |
| Language | |||||||
| Letter Fluency | 11.1 | 83.3 | 5.6 | 26.3 | 68.4 | 5.3 | 12.0 |
| Semantic Fluency | 29.4 | 70.6 | 0.00 | 29.4 | 64.7 | 5.90 | 4.10 |
| Boston Naming | 5.90 | 88.2 | 5.90 | 37.5 | 56.3 | 6.30 | 2.70 |
| Executive Function | |||||||
| Trails B | 35.3 | 64.7 | 0.00 | 52.9 | 23.5 | 23.5 | 20.9 |
| Stroop Color-Word | 17.6 | 64.7 | 17.6 | 42.9 | 21.4 | 35.7 | 7.50 |
| WCST Pers. Errors | 15.4 | 69.2 | 15.4 | 26.7 | 66.7 | 6.70 | 8.30 |
| Visuospatial | |||||||
| Clock Command | 47.1 | 47.1 | 5.9 | 15.8 | 42.1 | 42.1 | 0.80 |
Percentage of PD and DBS participants meeting 95% RCI criterion for reliable decline or increase or no change over time on neuropsychological measures. For some variables there was missing data; for those variables corrected percentages are provided.
Figure 1.
Reliable Change Indices for outcomes with significant interaction effects. Points represent individual raw scores. Solid line represents zero change. Values above the top dotted line represent a reliable increase in scores from baseline to 2 years. Values below the lower dotted line represent a reliable decrease in scores from baseline to 2 years. Values between the dotted lines represent no reliable change.
General Ability & Mental Status
The interaction effect for MMSE and DRS was non-significant (p=0.62, η2=0.007; p=0.49, η2=0.01, respectively).
Memory
There was not a significant Group X Time interaction effect for RAVLT total, short-term or long-term recall scores [p=0.18, η2=0.05; p=0.55, η2=0.01; p=0.11, η2=0.07]. While no significant difference was found in BVMT-R total recall scores (p=0.17, η2=0.06), BVMT-R delayed recall scores differed significantly between the groups after 2 years (p=0.03, η2=0.13). The STN-DBS patients demonstrated a greater decline on nonverbal recall 2 years after surgery [t(1,18)=4.00, p=0.001] as compared to medically managed PD patients [t(1,15)=0.54, p=0.60]. RCI indicated that 47% of STN-DBS patients had a reliable decline in BVMT-R-delayed recall scores compared to 25% of PD patients.
Information Processing
For SDMT, there was a significant Group X Time interaction effect [F(1,32)=5.19, p=0.03, η2=0.14]. STN-DBS patients demonstrated a larger significant decline at 2 years compared to baseline [t(1,16)=4.35, p=0.001] as compared to the PD patients [t(1,16)=2.29, p=0.04]. RCI indicated that 53% of STN-DBS patients had a reliable decline in SDMT compared to 29% of PD patients. Interaction effects were non-significant for Trails A, Digit Span, and Stroop Word (p=0.29, η2=0.05; p=0.08, η2=0.09; p=0.46, η2=0.03, respectively).
Language
For VF, there was a significant Group X Time interaction effect [F(1,34)=6.65, p=0.01, η2=0.16]. STN-DBS patients had a significant decline in VF scores [t(1,18)=3.31, p=0.004], with no change in scores for PD patients [t(1,16)=−0.12, p=0.91]. RCI scores indicated that 26% of STN-DBS patients had a reliable decline in VF scores compared to 11% of the PD patients. A significant interaction effect was also found for SF [F(1,32)=4.20, p=0.04, η2=0.12], with STN-DBS patients demonstrating a significant decline in scores at the 2 year evaluation compared to baseline [t(1,16)=3.08, p=0.007], with no change in the PD groups’ scores [t(1,16)=1.67, p=0.11]. RCI scores indicated that both STN-DBS and PD patients had a 29% reliable decline in SF scores. No significant Group X Time interaction effect was found for BNT (p=0.44, η2=0.20].
Executive Function
Stroop Color-Word scores demonstrated a trend toward significance [F(1,29)=3.44, p=0.07, η2=0.11], with STN-DBS patients demonstrating a greater decline in scores at 2 years compared to baseline [t(1,16)=1.86, p=0.08], with no change for the PD patients [t(1,16)=−0.25, p=0.80]. RCI indicated that 43% of STN-DBS patients had a reliable decline in Stroop Color Word scores compared to 18% of the PD patients. Interaction effects were non-significant for Trails B and WCST Perseverative errors (p=0.35, η2=0.03; p=0.12, η2=0.09, respectively).
Visuospatial Function
For Clock Command, there was a significant interaction effect [F(1,34)=6.07, p=0.01, η2=0.15]. PD patients tended to be more impaired at the 2 year evaluation as compared to baseline [t(1,16)=2.30, p=0.04], versus the STN-DBS patients whose scores remained stable over this time [t(1,18)=−1.05, p=0.31]. It should be noted that although the PD patients demonstrated a slight decline in their clock drawing scores over time, they remained within the intact range and this decline is not considered a clinically significant change in performance. RCI indicated that 47% of PD patients had a reliable decline in Clock Command scores compared to 16% of the STN-DBS patients.
Dementia Caseness Analysis
Patients were classified as meeting PDD criteria based independently upon Emre’s[15] and DSM-IV-TR[16] criteria using the RCI indices as a measure of reliable decline in combination with a deficit of at least 2 standard deviations. Emre and DSM-IV-TR criteria [14, 15] resulted in identical dementia designations. No PD or STN-DBS participants met criteria for PDD at baseline. At 2 years, 6 of the DBS patients (32%) met criteria for PDD compared to 3 PD patients (16%), which was not significantly different from each other based on frequencies (p=0.21).
Mild Cognitive Impairment Caseness Analysis
Patients were classified as meeting PD-MCI criteria with a deficit of at least 2 standard deviations below the age corrected mean in one of the four cognitive domains identified by Emre et al.’s [17] criteria. Five of the STN-DBS participants (26%) and 3 of the PD participants (17%) met criteria for PD-MCI at baseline (p=0.12). Of the 5 STN-DBS patients who met criteria for PD-MCI at baseline, 3 STN-DBS patients converted to PDD and the remaining 2 patients continued to meet criteria for PD-MCI at 2 years. The 3 PD patients who met criteria for PD-MCI at baseline all converted to PDD by the 2 year evaluation. At 2 years, an additional 4 STN-DBS patients (21%) converted to PD-MCI compared to 3 additional PD patients (17%). In total, at the 2 year evaluation 6 of the STN-DBS patients (32%) met criteria for PD-MCI compared to 3 PD patients (16%) (p=0.21). When PD-MCI and PDD diagnoses were combined, the STN-DBS patients revealed a trend toward demonstrating increased cognitive impairment following surgery, with 12/19 STN-DBS patients (63%) demonstrating at least PD-MCI 2 years following surgery as compared to 6/18 PD patients (33%) (p = 0.06).
DISCUSSION
Using a comprehensive neuropsychological assessment, we examined the cognitive effects of bilateral simultaneous STN-DBS 2 years after surgery compared to a matched PD medically-managed group and present RCIs for each of the cognitive measures. These exploratory findings suggest that STN-DBS patients 2 years following surgery demonstrated impairments in nonverbal memory (BVMT-R-delay), oral information processing speed (SDMT), and language (VF & SF). These findings were further emphasized with RCIs indicating that approximately 50% of the STN-DBS patients demonstrated reliable impairments on nonverbal memory and oral information processing speed compared to approximately 30% of PD participants. Additionally, of the STN-DBS patients, almost 30% declined on verbal fluency abilities compared to 11% of the PD patients. In both groups, a 29% reliable decline was found for semantic fluency, although the STN-DBS group demonstrated a larger decline on this measure. These results are consistent with recent reports on STN-DBS, which have found sustained deficits in multiple cognitive domains, including memory, attention, and frontostriatal functioning post-operatively when comprehensive neuropsychological measures were administered.[5, 8, 13]
The contradictory literature regarding the neurocognitive abilities following STN-DBS may be explained in part by methodological factors. Many early studies that failed to find cognitive changes following surgery utilized screening measures, which are not designed to identify subtle changes in cognitive domains associated with PD, such as language and executive functioning.[6, 7] In a large randomized controlled study investigating DBS versus medical management for the treatment of PD which employed a comprehensive neuropsychological evaluation, Weaver and colleagues [22] reported that there were statistically significant treatment differences 6 months after surgery in working memory, processing speed, verbal fluency, and delayed visual recall. Furthermore, Williams and colleagues [23] also reported that a cognitive screening measure was not sufficiently sensitive to detect the postoperative cognitive changes that were revealed on a more detailed neuropsychological battery, including verbal fluency and vocabulary. These findings taken together with the current results suggest comprehensive neuropsychological assessments are preferable to detect the cognitive impairments evident after STN-DBS and that reliance on cognitive screening measures may have previously underestimated post-operative cognitive changes.
In addition to examining neuropsychological impairment, it is beneficial to investigate how these impairments manifest in the emergence of PDD in individual patients. Dementia caseness analyses did not reveal differences in the number of STN-DBS and PD patients who developed cognitive impairments significant enough to meet stringent criteria for PDD 2 years after surgery (32% versus 16%, respectively). All of the patients categorized as PDD met criteria proposed by both Emre [15] and DSM-IV TR [14]. Emre et al. [15] criteria differs from the DSM-IV-TR[14] requirements in that PDD is diagnosed by impairment in any two cognitive domains; thus, memory impairment is not a required deficit as it is in the DSM-IV-TR [14]. The present data suggest that over the course of 2 years, dementia occurs in relatively equal numbers between PD patients treated with STN-DBS and medically managed PD patients, regardless of criteria used to determine dementia status.
To further characterize the cognitive changes over time, PD-MCI caseness analyses were also conducted. These analyses revealed a similar profile of conversion to PD-MCI over the two years for the STN-DBS and PD groups, with 21% of the STN-DBS patients and 17% of the PD patients converting to PD-MCI 2-years following the surgery. The previously reported short-term cognitive outcome of this sample reported that one STN-DBS patient met criteria for PDD at 6 months according to the DSM-IV TR criteria [14]. Using Emre’s PDD criteria [15], an additional STN-DBS patient would have met criteria for PDD at 6 months. Although none of the STN-DBS patients were demented at baseline, 26% met criteria for PD-MCI prior to surgery, 42% were PD-MCI and 11% PDD at 6 months and 32% were PD-MCI and 32% were PDD at 2 years.
Investigating the baseline, 6 months and 2 year cognitive profiles of the 6 STN-DBS who were diagnosed as PDD revealed a gradual progression in cognitive impairments that occurred over time. The 2 STN-DBS patients who converted to dementia 6 months following the surgery demonstrated a continued worsening of their cognitive performance over the intervening 18 months revealing impairments in verbal and nonverbal short-term memory, verbal fluency, and executive functioning at two years. Three of the 6 PDD patients met criteria for PD-MCI at 6 months and revealed declines in verbal fluency and short-term verbal recall at the 2 year evaluation. The final PDD patient did not meet criteria for PD-MCI at 6 months but showed a significant decline in his cognitive performance between the 6 month and 2 year evaluation. In comparison, the 3 medically managed PD patients who were demented at the two year evaluation met criteria for PD-MCI at the baseline and the 6 month evaluations, demonstrating a mild decline in cognitive performance over time. The additional 3 PD patients who met criteria for PD-MCI at the 2 year evaluation had not met criteria previously, and they did not demonstrate a change in their performance between their baseline and 6 month evaluations. Overall, the STN-DBS patients who met criteria for PD-MCI at 6 months were more likely to either dement or demonstrate a decline in cognitive performance at two years. This finding suggests that for a subset of patients the surgery may result in a deterioration of cognitive functioning that may then continue to progress to PDD. Further research with more closely spaced time periods for each evaluation is needed to more definitively comment on the progression of the cognitive symptoms following STN-DBS.
The impact of disease progression on the post-surgical outcome continues to be debated, thus underscoring the importance of a suitable control group when examining the cognitive outcome following STN-DBS. It is still not known whether post-operative cognitive impairments are solely attributable to PD progression, the effects of the surgery, or both. Factors that indicate greater disease progression (e.g., age, duration of illness) were either matched between groups or statistically controlled for in our analyses and the groups did not differ on the number of patients with mild cognitive impairment at baseline. Consequently, similar cognitive impairments should be present in the PD and STN-DBS patients at 2 years. Overall, the STN-DBS patients showed a non-significant trend toward more cognitive impairment 2 years following surgery as compared to the PD patients, with an overall gradual decline in cognition over the two years. However, it is possible that the STN-DBS patients may have been at increased risk for cognitive decline due to more severe disease at baseline.
Reliable change indices (RCI) are the preferred statistic used to examine the influence of disease progression over time by controlling for test-retest reliability.[9, 16, 24] RCIs supplement traditional statistical analyses by providing an observation of performance across time accounting for changes due to factors not related to cognitive impairments. Additionally, comparing reliable change in STN-DBS patients to PD patients, allows for conclusions to be drawn about the effect of STN-DBS on cognition above and beyond the impairment associated with PD progression. RCI data in the present study indicated significant impairments in nonverbal memory, oral information processing speed, and verbal fluency beyond that of the PD comparison group. However, RCI analyses do not account for baseline differences between PD and STN-DBS patients, warranting some caution when interpreting these findings.
Understanding of neural circuitry in PD and the pathophysiology of neuropsychological changes following STN-DBS can lead to therapeutic improvements. Due to the STN’s rich connections with the dorsolateral prefrontal cortex and limbic structures,[25, 26] stimulation of this area could alter cognition. Poor performance on measures of VF (related to mental flexibility in the frontal-striatal circuitry) is related to high frequency stimulation, with a beneficial effect during low frequency stimulation.[27] Hence, high frequency STN-DBS stimulation could lead to declines over time in VF as seen in the present sample. Reductions in dopaminergic medication after STN-DBS may also contribute to cognitive impairments following surgery.[28–30] However, in the current study, changes in dopaminergic medication were statistically controlled for suggesting that medication changes are unlikely to account completely for the cognitive impairments demonstrated.
Several limitations of this study should be acknowledged and addressed in future research. First, due to the proven efficacy of the surgery for the motor symptoms of PD, a long-term randomized investigation of STN-DBS was not performed. We instead used a convenience sample of medically managed PD patients recruited from the PDMDC and community resources. Second, due to the inability to randomize patients, we matched the STN-DBS and PD patients on relevant demographic and medical variables at baseline or statistically controlled for variables to help account for between group differences (education and levodopa medication change). It remains uncertain whether these factors could be fully accounted for through our statistical corrections. It is possible that the STN-DBS patients, who were slightly younger in age (p>0.05) with longer disease duration (p>0.05) and were taking more levodopa medication at baseline (p<0.05) as compared to the PD sample, may have been at increased risk for cognitive decline presurgically; thus biasing our findings. Additional research is needed to better ascertain the variables contributing to cognitive impairments following STN-DBS and to more clearly delineate the progression of these cognitive changes over time. Third, our sample size was relatively small; therefore, we were unable to analyze predictors of decline. Fourth, due to the preliminary nature of this data and the small sample size, we chose not to use a Bonferroni correction to reduce the risk of Type II error. We acknowledge that our findings may be affected by the Type 1 error rate inflation. Fifth, we did not address the influence of stimulation alone on cognition. Future studies should investigate long-term cognitive performance in both “on” and “off” stimulation states.
In summary, the present study investigated cognitive outcome two-years after STN-DBS compared to a medically-managed PD group on a comprehensive neuropsychological evaluation and reported RCIs for each of the neuropsychological measures. STN-DBS patients did not demonstrate changes in global cognitive functioning on screening measures. However, in our small exploratory sample, neuropsychological evaluation revealed declines for STN-DBS patients in nonverbal memory, oral information processing speed, and language functioning compared to the PD patients. Our results suggest that neuropsychological evaluations may be useful in delineating cognitive changes that may potentially occur following STN-DBS.
Acknowledgments
This material is the result of work funded by a NIH/NINDS K23 grant (PI: M.K. York and supported with resources from the Department of Veterans Affairs, Michael E. DeBakey Veteran’s Affairs Hospital, Houston, Texas.
The authors wish to thank the National Parkinson’s Foundation and the Parkinson’s disease patients who willingly gave of their time to participate in this research project.
Footnotes
Author Contributions
Michele K. York – execution of project and review and critique of statistics and manuscript
Amy E. Williams - execution of project including statistical analyses
Gladys Marina Arzola – execution of project and manuscript
Adriana M. Strutt - Patient assessment and review and critique of statistics and manuscript
Richard Simpson - Surgical procedures and review and critique of manuscript
Joseph Jankovic - Treating neurologist and review and critique of manuscript
Competing Interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s Disease. N Engl J Med. 2006 August 31;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner-Fisman G, Herzog J, Fisman D, Tamma F, Lyons K, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006 Jun;21(S14):S290–304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 3.Siderowf A, Jaggi J, Xie S, Loveland-Jones C, Leng L, Hurtig H, et al. Long-term effects of bilateral subthalamic nucleus stimulation on health-related quality of life in advanced Parkinson’s disease. Mov Disord. 2006 Jun;21(6):746–53. doi: 10.1002/mds.20786. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara J, Diamond A, Hunter C, Davidson A, Almaguer M, Jankovic J. Impact of STN- DBS on life and health satisfaction in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010 Mar;81(3):315–9. doi: 10.1136/jnnp.2009.184127. [DOI] [PubMed] [Google Scholar]
- 5.Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004 Jun;75(6):834–9. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schupbach M, Gargiulo M, Welter ML, Mallet L, Behar C, Houeto JL, et al. Neurosurgery in Parkinson disease: a distressed mind in a repaired body? Neurology. 2006;66(12):1811–6. doi: 10.1212/01.wnl.0000234880.51322.16. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Oroz M, Obeso J, Lang A, Houeto J, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005 Oct;128(10):2240–9. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 8.Contarino MF, Daniele A, Sibilia AH, Romito LMA, Benlivoglio AR, Gainotti G, et al. Cognitive outcome 5 years after bilateral chronic stimulation of subthalamic nucleus in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(3):248–52. doi: 10.1136/jnnp.2005.086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahodne LB, Okun MS, Foote KD, Fernandez HH, Rodriguez RL, Kirsch-Darrow L, et al. Cognitive declines one year after unilateral deep brain stimulation surgery in parkinson’s disease: A controlled study using reliable change. Clin Neuropsychol. 2008;23(3):385–405. doi: 10.1080/13854040802360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schupbach WMM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet AM, et al. Stimulation of the subthalamic nucleus in Parkinson’s disease: A 5 year follow up. J Neurol Neurosurg Psychiatry. 2005;76(12):1640–4. doi: 10.1136/jnnp.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krack P, Batir A, Van Blercom Ng, Chabardes S, Fraix Vr, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349(20):1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 12.Zangaglia R, Pacchetti C, Pasotti C, Mancini F, Servello D, Sinforiani E, et al. Deep brain stimulation and cognitive functions in Parkinson’s disease: A three-year controlled study. Mov Disord. 2009 Aug 15;24(11):1621–8. doi: 10.1002/mds.22603. [DOI] [PubMed] [Google Scholar]
- 13.Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism Relat Disord. 2006 Jun;12(5):265–72. doi: 10.1016/j.parkreldis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition - text revision (DSMIV-TR) Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 15.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 16.York MK, Dulay M, Macias A, Levin HS, Grossman R, Simpson R, et al. Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79(7):789–95. doi: 10.1136/jnnp.2007.118786. [DOI] [PubMed] [Google Scholar]
- 17.Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A, et al. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg. 2007;106(4):621–5. doi: 10.3171/jns.2007.106.4.621. [DOI] [PubMed] [Google Scholar]
- 18.Lezak M, Howieson D, Loring D. Neuropsychological Assessment. 4. Oxford; New York: [Google Scholar]
- 19.Jacobson NS, Turax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Cons Clin Psychol. 1991;59:12–9. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum Associates; Hillsdale, New Jersey: [Google Scholar]
- 21.Caviness J, Driver-Dunckley E, Connor D, Sabbagh M, Hentz J, Noble B, Evidente V, Shill H, Adler C. Defining mild cognitive impairment in Parkinson’s disease. Movement Disorders. 2007;22(9):1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 22.Weaver F, Follett K, Stern M, et al. Bilateral Deep Brain Stimulation vs Best Medical Therapy for Patients With Advanced Parkinson Disease: A Randomized Controlled Trial. JAMA. 2009;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams A, Gill S, Varma T, et al. on behalf of the PD SURG collaborative group. Deep brain stimulation surgery plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trail): a randomised, open label trial. Lancet Neurology. 2010 doi: 10.1016/S1474-4422(10)70093-4. online ahead of pub April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurology. 2008;7(7):605–14. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- 25.Temel Y, Blokland A, Steinbusch H, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005 Aug;76(6):393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Haegelen C, Verin M, Broche BA, Prigent F, Jannin P, Gibaud B, et al. Does subthalamic nucleus stimulation affect the frontal limbic areas? A single-photon emiision computed tomography study using a manual anatomical segmentation method. Surg Radiol Anat. 2005;27:389–413. doi: 10.1007/s00276-005-0021-8. [DOI] [PubMed] [Google Scholar]
- 27.Wojtecki L, Timmermann L, Jorgens S, Sudmeyer M, Maarouf M, Treuer H, et al. Frequency-dependent reciprocal modulation of verbal fluency and motor functions in subthalamic deep brain stimulation. Arch Neurol. 2006;63:1273–6. doi: 10.1001/archneur.63.9.1273. [DOI] [PubMed] [Google Scholar]
- 28.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–43. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 29.Mattay Vs, Tessitore A, Callicott JH, bertolino A, Goldberg TE, Chase TN, et al. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann Neurol. 2002;51:156–64. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- 30.Kulisevsky J, Garcia-Sanchez C, Berthier ML, Barbanoj M, Pascual-Sedano B, Gironell A, et al. Chronic effects of dopaminergic replacement on cognitive function in Parkinson’s disease: A two-year follow-up study of previously untreated patients. Mov Disord. 2000;15:613–26. doi: 10.1002/1531-8257(200007)15:4<613::aid-mds1005>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]