Summary
A number of noteworthy technology advances in DNA vaccines research and development over the past few years have led to the resurgence of this field as a viable vaccine modality. Notably, these include - optimization of DNA constructs; development of new DNA manufacturing processes and formulations; augmentation of immune responses with novel encoded molecular adjuvants; and the improvement in new in vivo delivery strategies including electroporation (EP). Of these, EP mediated delivery has generated considerable enthusiasm and appears to have had a great impact in vaccine immunegenicity and efficacy by increasing antigen delivery upto a 1000 fold over naked DNA delivery alone. This increased delivery has resulted in an improved in vivo immune response magnitude as well as response rates relative to DNA delivery by direct injection alone. Indeed the immune responses and protection from pathogen challenge observed following DNA administration via EP in many cases are comparable or superior to other well studied vaccine platforms including viral vectors and live/attenuated/inactivated virus vaccines. Significantly, the early promise of EP delivery shown in numerous pre-clinical animal models of many different infectious diseases and cancer are now translating into equally enhanced immune responses in human clinical trials making the prospects for this vaccine approach to impact diverse disease targets tangible.
Introduction: The Promise of DNA Vaccines
The concept of using DNA to immunize people was first advanced in the early 1990s and immediately gained widespread recognition due to its apparent simplicity and elegance [1–3]. What could be simpler than simply injecting a DNA plasmid encoding the antigen of interest into host cells and letting the host-cellular machinery carry out the tasks of protein translation and antigen processing and presentation in vivo? Indeed the simplicity of this concept for eliciting meaningful immune responses was exemplified by the rapid translation of molecular biology constructs in vivo into immune responses and protection in some challenge models in small animals – notably mice [4,5].
Over the years other advantages of DNA vaccination came to the fore. DNA remains the only vectored platform that does not induce anti-vector immunity making it suitable for vaccine regimens that include both priming as well as boosts. Additionally, manufacturing of plasmid DNA is considerably faster and easier than most other vaccine platforms and relies primarily on bacterial hosts for production. Indeed manufacture of small-scale non-GMP research grade plasmid material has become a commodity business and the difficulties associated with manufacturing and handling live/attenuated viral vaccines as well as large variability in potency from lot-to-lot are largely not an issue with DNA. Furthermore, DNA is relatively stable at room temperature making the requirement for maintaining the vaccine cold-chain less critical compared to other vaccine platforms. In addition, manufacturing of DNA can be done extremely safely especially as compared to killed pathogenic vaccine platforms.
From the vaccinologists’ perspective, DNA, due to its ability to combine the power of genomics with in vivo antigen expression, provides a tantalizing opportunity to easily customize vaccines through the use of molecular biology. Indeed it can be said that DNA vaccines bring to fore the strengths of molecular biology and genetic engineering to harness the potential of the immune system. The ability to easily combine multiple plasmids or disparate gene products into a single formulation without apparent loss of potency allows the possibility to formulate multi-component vaccines targeting multiple antigens or even multiple pathogens simultaneously [6,7]. Similarly, a seasonal flu vaccine combining DNA plasmids targeting influenza A/H1N1, H3N2, and influenza B strains can be readily contemplated and coupled for in vivo delivery with an A/H5N1 vaccine thus allowing for the simultaneous targeting of both seasonal and pandemic strains [8]. Just as important, such vaccine can be designed to increase the breadth of the immune responses and potentially increase pathogen coverage. Thus approaches such as the use of synthetic consensus immunogens and mosaics – both approaches available simply in a DNA based platform - are expanding the notion of vaccine design to focus on developing “universal” vaccines to simultaneously target multiple divergent but related strains of given pathogens [9–13].
And yet for all the promise, the early DNA vaccine human clinical trials failed to meet immunogenicity end points. The translation of results from preclinical models to humans was largely ineffective bringing into question the scalability of induction of immune responses from small animals to humans. Was this inability due to limitations of vaccine dose (delivery on a weight by weight basis)? Or vaccine potency? Or due to differences in the immune systems of animals versus humans to recognize DNA based antigens differently? Or possibly a combination of these factors?
Research in these areas led to important discoveries on the role of DNA to activate innate immunity and the identification of potential receptors and/or intracellular sensors for double stranded DNA including TLR9 [14,15], DAI (DNA-dependent activator of interferon regulatory factors)[16] [17], AIM2 [18,19], and HMGB (high mobility group box) proteins[20]. Similarly, research into improving the potency of DNA vaccines through the use of conjugates to recruit T-cell help [21,22] or inducing effector T-cells [21,23] or through the use of molecular adjuvants [24–27] has resulted in a better understanding of the immune system and its response to DNA based vaccines. These approaches are very interesting and have shown recent early promise in small clinical trials [28,29].
Electroporation Enhanced DNA Vaccine Delivery
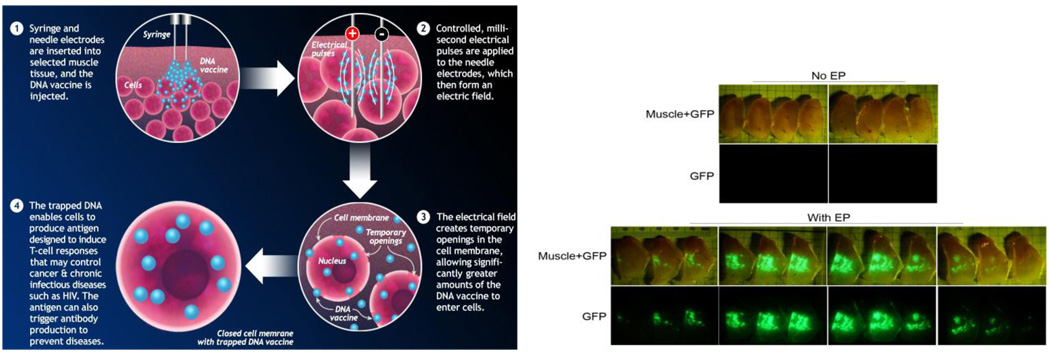
Electroporation (EP) is a method to introduce macromolecules such as nucleic acids into cells, either in vivo or in vitro, via the application of brief electric pulses to induce transient and reversible permeabilization of the cell membrane (Figure 1). Over the last decade the technique has evolved from an experimental technique to now being used in several clinical trials to deliver nucleic acids as well as drugs to a variety of target tissues [30]. A number of mechanistic studies have helped develop the hypothesis that during EP, transient pores are formed as a function of the transmembrane voltage [31,32]. During the period of membrane destabilization (on the nano to milli second timescale), macromolecules present in the extracellular medium surrounding the target cells gain access to the intracellular milieu [33]. After the EP pulses, a slow resealing of the membrane occurs on the second to minutes time scale. Although the exact mechanism of translocation of DNA across the membrane pores is debated (electrophoretic facilitation versus passive diffusion) the end result of the process is that upwards of 100–1000 fold enhancement of plasmid delivery and gene expression can be achieved relative to delivery of DNA alone without electroporation (see references below).
Figure 1.
(a) Schematic depicting the EP process. (b) Enhancement of gene expression following DNA delivery with EP. GFP plasmid was delivered to rabbit muscle via IM injection without EP (top panels) or IM injection with EP (bottom panels). The injected muscle was harvested and then sectioned into 1 mm thick sections to visualize GFP expression either under white light (Muscle + GFP) or under a UV lamp (GFP). The highly fluorescent GFP expression is observed only when the DNA is delivered via EP – representing a 100–1000 fold enhancement in gene delivery to the target tissue (Unpublished GFP images courtesy of Inovio Pharmaceuticals).
Translating In vivo Expression to Immunogenicity and Efficacy
The observation that in vivo electroporation can dramatically improve gene delivery has led to a great deal of interest to assess the consequences of this delivery to enhancing immunogenicity and effectiveness of DNA vaccines in specific model systems. While it has been difficult to quantitatively measure directly the enhancement in plasmid delivery and its translation to increased transgene expression, and consequent induction of immune responses in the same animals, a number of experiments have measured this correlation indirectly. Increased expression following DNA delivery via EP has been measured quantitatively by assessing reporter gene products (GFP and SEAP) at the injection site or their circulating levels in the sera [53]. In parallel, experiments with vaccine antigens comparing delivery with or without EP have shown increased immune responses (cellular and humoral) at significantly lower doses (dose-sparing). Indeed data from several vaccine candidates spanning cancer and infectious disease published in the last two years has demonstrated that a 10–100 fold enhancement of immune responses as well as protection from pathogenic challenge is routinely achievable in various animal models of disease including SIV/HIV[34–39], malaria [40,41], HCV[42], HBV [43–46], Botulinum toxins A, B, E [47], HPV[48,49], Anthrax [50], influenza [51,52].
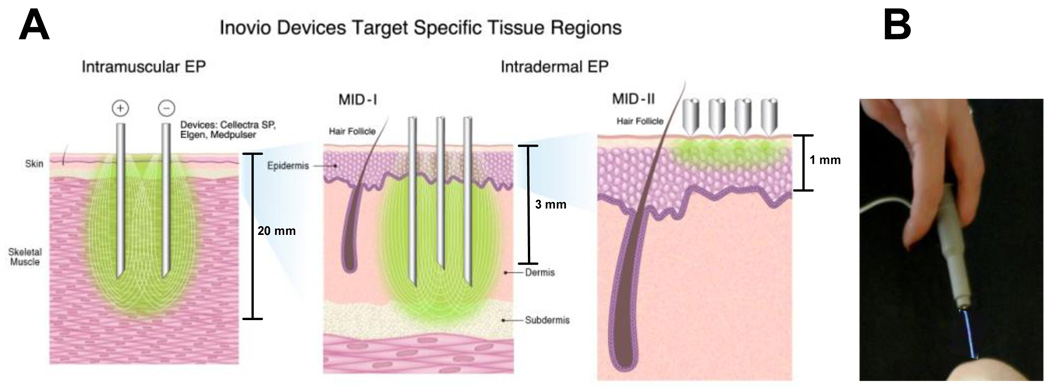
Much of the early work for EP mediated delivery of DNA vaccines was conducted using intramuscular (IM) delivery. Recently however, there has been an increasing shift towards developing intradermal (ID) delivery systems to complement delivery to the muscle [6,53–55]. The skin is the most accessible organ of the human body, is easily monitored as well as being a highly immunocompetent target organ [56,57]. A variety of experimental EP devices have been reported in the literature to target the skin. Some use plates or webs which deliver the electrical charge to the skin. In addition devices which target through the skin and access deeper dermal layers have also been studied. The immune responses with many of these have been variable, however the ability to transfect in small animals has been reported. We recently reported the development of a minimally invasive device that merely scratches the surface of the skin [54]. Indeed studies from our group have shown that electrodes that target different depths and varying degrees of skin/muscle invasion induce unique immune phenotypes by contacting different and unique immune compartments of the skin/muscle. These range from non-contact (piezoelectric assisted delivery[58]) to minimally invasive (ID/SQ delivery) to IM delivery (Figure 2) [53,54,59]. Studies in this area illustrate a superior level of immune control that can be demonstrated by specific immune compartment targeting in vivo.
Figure 2.
Electroporation devices developed to target the different depths of the skin/muscle. IM devices include Cellectra®-5P, ELGEN™, Medpulser. The minimally invasive devices (MID) target the dermis/sub-cutaneous layers (MID-I)[53] or the epidermis/stratum corneum (MID-II)[54]. Also shown is a non-contact device where EP is facilitated by piezoelectric discharge (PID)[58].
Prospects for Safety and Tolerability
For the vaccine developer, a consistent story emerging from over 15+ years of clinical development with plasmid based DNA vaccines collected across 100s of human clinical trials covering 1000s of healthy and diseased subjects spanning studies in infectious diseases, cancer, and gene therapy delivery is that of an excellent and consistently unremarkable safety profile. These safety trends have continued to be observed in studies where the DNA has been delivered via EP. Indeed published toxicology studies in animal models have largely yielded no adverse findings and published human clinical studies have not noted vaccine associated serious adverse events when DNA was administered as the drug substance either with or without EP [29,55,60,61]. Tissue biodistribution studies in animals have noted that when found at all, DNA is present only at the injection site (usually skin and muscle). There is a rapid decay in plasmid copy numbers over time [60,61] and early concerns surrounding plasmid integration[62] into the host genome remain unsubstantiated.
EP studies in cancer and HIV amongst others have had good patient recruitment and trial retention, suggesting that this platform can be associated with good patient compliance in diverse clinical protocols including cancer immune therapy [29], HCV therapy [63], HIV prophylaxis [55], and influenza prophylaxis (trials ongoing). The predominant adverse findings (grade 1/2) associated with the IMEP procedure is transient pain that rapidly decays to background within 25 – 30 minutes [64,65]. The transient pain associated with the IMEP procedure was noted to be further decreased substantially in the case of ID EP applicators [60,66], and thus the rationale for the increasing trend towards IDEP. The decreased invasiveness of DNA delivery, shallower depth of penetration and lower current (Amps) parameters to effect optimal delivery all translate into a more tolerable procedure for the subject supporting the observation that IDEP vaccination procedures appear comparable to routine ID/IM vaccination with a needle and syringe.
EP Enhanced Clinical Immunogenicity and Efficacy of DNA Vaccines
The last two years have also seen the completion of the first DNA EP clinical trials and publication of Phase I/II immunogenicity and efficacy data. The exciting findings are that similar to the pre-clinical animal model data, EP appears to enhance the immunogenicity of DNA vaccines relative to DNA alone in the human clinical setting as well. Specifially, Ottensmeier et al reported results from a prostate cancer DNA vaccine study where they detected IFN-γ producing CD8+ T-cells against the target PSMA peptide in approximately 60% of the cases and that EP delivery of DNA stimulated T-cell responses more quickly and with a greater magnitude compared to the cohort that received the DNA vaccine without EP[67]. This group also reported induction of strong humoral responses to the fragment C domain (DOM) of tetanus toxin that was conjugated to the PSMA peptide to facilitate CD4+ T-cell help. The patients receiving the DNA vaccine via EP induced stronger antibody titers (over 14 fold) to DOM relative to those receiving the vaccine without EP[29]. Similarly, Sällberg and colleagues recently reported data from their HCV therapy trial with an NS3/4a based DNA vaccine delivered via EP. The authors noted significant induction of antigen specific IFN-γ producing T-cells in the HCV infected subjects receiving the vaccine via EP. The authors also noted transient reduction in viral load (0.6 log10 to 2.4 log10) in 5/12 vaccinated subjects [63].
Beyond the therapeutic vaccination regimens, EP delivery of DNA has made inroads into prophylactic vaccine regimens as well. Vasan and colleagues reported the induction of antigen specific IFN-γ producing T-cells (response rates and magnitude) when a DNA based HIV-1 candidate vaccine expressing Clade C/B env, gag, pol, nef, and tat genes (ADVAX) was delivered using EP [68] compared to an earlier study where ADVAX was administered via IM injection alone [69]. While overall response rates were only 13–33% in the IM DNA study [68,69] and consistent with the poor immunogenicity reported in other DNA vaccine studies, the application of EP increased the response rates to over 75% in the mid and high dose cohorts and magnitude by upto 70 fold over the same dose delivered IM [68].
We recently reported preliminary safety and immunogenicity data from a HPV-16/18 E6 and E7 DNA based candidate vaccine delivered via EP [70]. The study was conducted as a dose escalation study in three cohorts (0.3, 1, and 3 mg of each of two DNA plasmids in the vaccine formulation) and data from the first two dose cohorts was presented at the 50th ICAAC meeting in Boston. No SAEs or vaccine-related Grade 3 or 4 AEs were reported. The antigen specific antibodies and T-Cell ELISpots observed were higher than previous reports from prior studies of HPV poxviral, peptide or DNA vaccines [71,72] and these were observed even at low DNA doses.
Other currently ongoing prophylactic vaccine clinical trials with the DNA EP platform include HIV (two trials sponsored by NIH/DAIDS and the HVTN (HVTN-080) or the USMHRP (RV-262)), avian influenza (Inovio), malaria (NIAID/Ichor), and HIV (Karolinska institute/Cytopulse).
Prospects for Clinical Efficacy and Product Development Success
As exemplified above, the early clinical trial data has largely concurred with the larger animal model data in supporting the hypothesis that EP delivery of DNA vaccines enhances immunogenicity of the DNA delivered vaccines. The early clinical trials have focused on development of vaccines for hard to treat targets with a clear unmet need (HIV, HCV and cancer), or for diseases where a cellular immune response was considered important (Immune therapeutic vaccine regimens for HIV, HCV, cancer) – largely as a result of dogma arising from the early days of DNA vaccination.
The key prevailing misconceptions those early studies being:
As a platform DNA was good solely at eliciting cellular immune responses but not humoral immune responses
DNA vaccination led to a predominantly CD4+ biased response with minimal induction of CD8+ T-cells
The cost of DNA vaccine manufacturing (and EP delivery) would make this vaccine platform suitable only for therapeutic vaccination scenarios and out of reach of routine prophylactic vaccination.
Due to low potency, DNA is primarily useful as a priming modality in a prime-boost setting.
The application of EP delivery to DNA vaccination has arguably changed our view of all of the conventional wisdom and the weight of recent evidence suggests a much broader potential of DNA vaccination. Indeed, the approach has proved promising in eliciting both cellular and humoral immune responses in animal models and humans. Induction of strong CD8+ T-cells in addition to CD4+ T-cells in primates and humans has been another hallmark of EP delivery and different from what has been observed with DNA alone [34,36,53]. Importantly, although the current costs of cGMP DNA manufacturing at small scale remain relatively high (on the order of $50–100/mg at a 1–10g scale of manufacturing using 500L fermenter process trains), the relative ease of plasmid DNA manufacturing and scale up makes it likely that in the future manufacturers will find ways to lower manufacturing costs by 2–3 log10 at commercial scale (upwards of 10Kg using 3,000 – 30,000L fermenter process trains) [73]. Regardless, while commercial scale up with DNA has not been attempted largely due to the lack of relevant late stage vaccine products, the projected costs of manufacturing DNA at scale appear to be competitive with other licensed or other evaluated vaccine platforms – live/attenuated virus, inactivated virus, VLP, or viral vectored (Ad5, MVA). Furthermore, unlike the other platforms that often require mammalian cell culture DNA does not face the same risks with carryover of adventitious viruses [74,75], or large variability in lot-to-lot potency, or the safety of a live vialed final product.
As the ultimate goal of DNA vaccine research should be to develop life saving products in the face of other alternative vaccine development choices and not just find ways to improve immunogenicity of DNA at any cost, a fair question to ask is: How does the DNA EP technology stack up to other licensed vaccines or developmental vaccine platforms in terms of immunogenicity and efficacy?
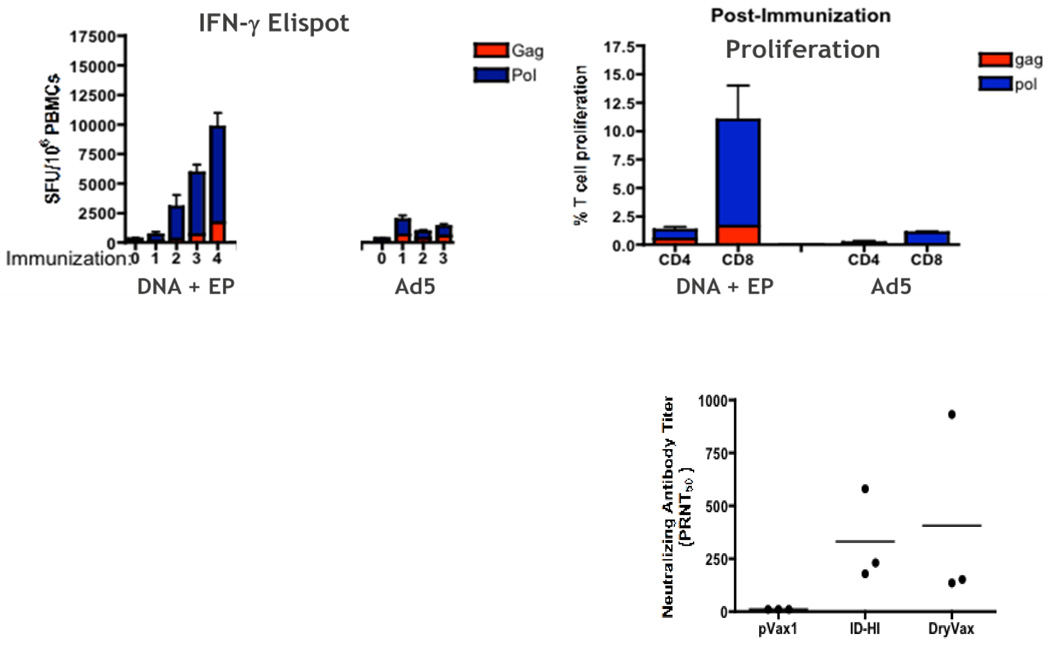
Several recent publications have attempted to answer this question directly in relevant nonhuman primate (NHP) models. Hirao et al evaluated the Merck Ad5 SIV vaccine – an important benchmark for new T-cell based vaccine development – against an optimized SIV DNA vaccine delivered via the CELLECTRA® EP device and noted significant differences in the quantity of IFN-γ responses by ELISpot, greater proliferative capacity of CD8+ T-cells, and increased polyfunctionality of both CD4+ and CD8+ T-cells in the DNA vaccinated group compared to the Ad5 group [39]. Importantly Ad5 immunizations failed to boost following the first vaccination, while the DNA induced responses were continually boosted with three subsequent immunizations (Figure 3a).
Figure 3.
(a, top) Comparison of cellular immune responses elicited by an optimized SIV DNA vaccine delivered via EP versus an optimized Ad5 SIV vaccine. The DNA EP vaccine yielded stronger and continuously boostable ELISpot responses relative to the Ad5 vaccine. The DNA EP vaccine also led to better proliferative capacity of both CD4+ and CD8+ T-cells and improved polyfunctionality (Figure adapted from [39]). (b, right) Comparison of NAb responses induced by a DNA EP smallpox vaccine to those induced by the licensed Dryvax® live attenuated vaccine in a NHP model (Figure adapted from [7]).
In another NHP study, Livingston and coworkers compared the efficacy of an Anthrax DNA vaccine delivered with or without EP and compared the efficacy in a challenge model to that achieved with a licensed anthrax vaccine [50]. The authors report a 100 fold enhanced immune response when the DNA vaccine was delivered via EP compared to standard IM injection. The DNA EP vaccine conferred protection to the animals in a subsequent lethal Bacillus anthracis spore challenge comparable to that achieved with the licensed attenuated anthrax vaccine.
We recently reported on a NHP challenge study with a multicomponent DNA vaccine for smallpox [7]. This vaccine consisted of an 8-plasmid formulation and was delivered in microvolumes via IDEP delivery. We observed high titer antibody responses against all 8 DNA encoded antigens and the vaccinated animals were protected against a lethal monkey pox challenge. The neutralizing antibody titers measured in the vaccinated animals were comparable to those seen with the FDA licensed live attenuated Dryvax® vaccine (Figure 3b). The study also underscored a powerful feature of DNA vaccination – that of being able to develop multi-component vaccine formulations to mimic immune responses from live viral infections and added a new dimension to our ability to design vaccines against complex human and animal pathogens.
In summary, while the lead DNA-EP vaccine programs are still only in the Phase I/II stage, the weight of the available data suggests that many of the desired goals for this platform are within reach and that the approach is likely to have a very bright future.
Cautionary Factors
While the field of DNA vaccines is entering its third decade, in vivo EP in the clinical setting is still in its relative infancy. There is much to learn about the clinical effects of transient electric fields on tissues. The results to date with DNA vaccines and EP have created a highly favorable safety profile but, while the safety database of DNA vaccines (with or without EP) is now at several thousand individuals (across 100s of DNA vaccine trials) and growing rapidly, it still has not reached the maturity levels observed with the licensed vaccines. Thus potential concerns around long term persistence and potential for integration will have to be addressed on a case by case basis.
Another cautionary aspect of the emerging literature is that while EP delivery may generally improve DNA immunogenicity relative to other methods, there are significant differences in the immune responses elicited depending on the pulse patterns, voltage-current and field strengths, electrode configurations, and impedence of target tissues. Similarly electrode shape, size, and DNA vaccine formulations (optimized sequence, dose, concentration, buffers) also play a critical role in the induction of immune responses and may need to be optimized depending on the particular vaccine target specifications for immunogenicity and efficacy. Based on these considerations, EP delivery of DNA should be viewed in terms of a combination product during its development and that importantly, not all EP device – DNA vaccine combinations are likely to lead to the same outcome based on different design and delivery parameters. This aspect is particularly critical to acknowledge for a newly emergent field in terms of rationalizing some of the variability of immune responses reported in the literature across DNA constructs and across EP devices. From the standpoint of vaccine product development, the apparent lack of standardization may well prove to be challenging, if every vaccine-device combination needed to be ultimately optimized in humans. In that regards the DNA vaccine-EP delivery combination is no different from conventional vaccine approaches (live/attenuated/VLP/recombinant) where considerations such as route of delivery (IM, ID, IV, oral, nasal), dose, and choice of adjuvants/formulations/excipients also need to be optimized in pre-clinical and early Phase I/II studies in response to safety, immunogenicity, stability, and market considerations. However, we remain optimistic that common themes will emerge to further simplify the development paradigms as the first DNA vaccines progress through Phase II – III clinical development and are licensed.
Concluding Remarks
The combination of highly optimized DNA delivered by advanced EP is clearly an important and exciting area of investigation. The numbers of positive outcome studies of DNA-EP in the clinic are steadily increasing and support the notion that this is a vaccine product platform with broad applicability. Excitingly, the immune responses seen to date mimic those seen with viral infections in terms of the induction of both cellular and humoral responses and the magnitude and breadth of the responses. The ability to break tolerance in the cancer setting is also a new and encouraging observation in the few clinical studies reported to date. It is also encouraging that the scalability and the economics of manufacturing at scale allow the DNA vaccine platform to be competitive to other vaccine strategies for either therapeutic or prophylactic vaccine scenarios as well as their deployment in developed or resource poor settings. If these important developments continue to mount and additional successes are reported in research laboratories and in the clinic, we may well look back on this decade as the “DNA vaccine decade”.
Acknowledgements
We thank Drs. Kate Broderick, Feng Lin, Jian Yan and Amir Khan for critical comments and editorial help with the manuscript. NYS acknowledges grant/contract support from the DAIDS/NIAID/NIH (HHSN272200800063C) and MVI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The DBW laboratory notes possible commercial conflicts associated with this work from consulting fees, stock ownership, Advisory Board or Review Board Service, speaking support among others which may include the following companies: Pfizer, Inovio, BMS, VGXI, Virxsys, Ichor, Merck, Althea, Aldevron, Novartis, and possibly others.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* Of special interest
** Of outstanding interest
- 1.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 2.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Ugen KE, Srikantan V, Agadjanyan MG, Dang K, Refaeli Y, Sato AI, Boyer J, Williams WV, Weiner DB. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fattori E, La Monica N, Ciliberto G, Toniatti C. Electro-gene-transfer: a new approach for muscle gene delivery. Somat Cell Mol Genet. 2002;27:75–83. doi: 10.1023/a:1022927822244. [DOI] [PubMed] [Google Scholar]
- 5.Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006;12:216–222. doi: 10.1016/j.molmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirao LA, Draghia-Akli R, Prigge JT, Yang M, Satishchandran A, Wu L, Hammarlund E, Khan AS, Babas T, Rhodes L, et al. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J Infect Dis. 2010;203:95–102. doi: 10.1093/infdis/jiq017. This paper highlights in a significant non-human primate vaccine challenge model many of the key aspects of DNA vaccines that make the platform attractive for vaccine development: Formulation and delivery of 8 plasmids at high concentrations and microvolumes; DNA delivery comparing intradermal and intramuscular electroporation both of which were found to be effective in controlling viremia and protecting the animals from lethality; and induction of strong humoral and celular immune responses to smallpox antigens. The immune response induced protected the animals from a lethal monkeypox challenge and were found to be comparable to those achieved by a licensed vaccine.
- 8.Choo AY, Broderick KE, Kim JJ, Sardesai NY. DNA-based influenza vaccines: evaluating their potential to provide universal protection. IDrugs. 2010;13:707–712. [PubMed] [Google Scholar]
- 9.Laddy DJ, Yan J, Corbitt N, Kobasa D, Kobinger GP, Weiner DB. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine. 2007;25:2984–2989. doi: 10.1016/j.vaccine.2007.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan J, Yoon H, Kumar S, Ramanathan MP, Corbitt N, Kutzler M, Dai A, Boyer JD, Weiner DB. Enhanced cellular immune responses elicited by an engineered HIV-1 subtype B consensus-based envelope DNA vaccine. Mol Ther. 2007;15:411–421. doi: 10.1038/sj.mt.6300036. [DOI] [PubMed] [Google Scholar]
- 11.Santra S, Liao HX, Zhang R, Muldoon M, Watson S, Fischer W, Theiler J, Szinger J, Balachandran H, Buzby A, et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med. 2010;16:324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang KA, Yan J, Draghia-Akli R, Khan A, Weiner DB. Strong HCV NS3- and NS4A-specific cellular immune responses induced in mice and Rhesus macaques by a novel HCV genotype 1a/1b consensus DNA vaccine. Vaccine. 2008;26:6225–6231. doi: 10.1016/j.vaccine.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramanathan MP, Kuo YC, Selling BH, Li Q, Sardesai NY, Kim JJ, Weiner DB. Development of a novel DNA SynCon tetravalent dengue vaccine that elicits immune responses against four serotypes. Vaccine. 2009;27:6444–6453. doi: 10.1016/j.vaccine.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 15.Spies B, Hochrein H, Vabulas M, Huster K, Busch DH, Schmitz F, Heit A, Wagner H. Vaccination with plasmid DNA activates dendritic cells via Toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J Immunol. 2003;171:5908–5912. doi: 10.4049/jimmunol.171.11.5908. [DOI] [PubMed] [Google Scholar]
- 16.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Choi MK, Ban T, Yanai H, Negishi H, Lu Y, Tamura T, Takaoka A, Nishikura K, Taniguchi T. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci U S A. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 21.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 22.Hung CF, Hsu KF, Cheng WF, Chai CY, He L, Ling M, Wu TC. Enhancement of DNA vaccine potency by linkage of antigen gene to a gene encoding the extracellular domain of Fms-like tyrosine kinase 3-ligand. Cancer Res. 2001;61:1080–1088. [PubMed] [Google Scholar]
- 23.Zanetti M. T for two: when helpers need help. Autoimmun Rev. 2005;4:571–578. doi: 10.1016/j.autrev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K, Wang B, Boyer JD, Weiner DB. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- 25.Schadeck EB, Sidhu M, Egan MA, Chong SY, Piacente P, Masood A, Garcia-Hand D, Cappello S, Roopchand V, Megati S, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine. 2006;24:4677–4687. doi: 10.1016/j.vaccine.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 26.Arcuri M, Cappelletti M, Zampaglione I, Aurisicchio L, Nicosia A, Ciliberto G, Fattori E. Synergistic effect of gene-electro transfer and adjuvant cytokines in increasing the potency of hepatitis C virus genetic vaccination. J Gene Med. 2008;10:1048–1054. doi: 10.1002/jgm.1217. [DOI] [PubMed] [Google Scholar]
- 27.Morrow MP, Yan J, Pankhong P, Ferraro B, Lewis MG, Khan AS, Sardesai NY, Weiner DB. Unique Th1/Th2 phenotypes induced during priming and memory phases by use of interleukin-12 (IL-12) or IL-28B vaccine adjuvants in rhesus macaques. Clin Vaccine Immunol. 2010;17:1493–1493. doi: 10.1128/CVI.00181-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson FK, Ottensmeier CH, Rice J. DNA vaccines against cancer come of age. Curr Opin Immunol. 2010;22:264–270. doi: 10.1016/j.coi.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 29. Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, Stevenson F, Ottensmeier CH. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009;20:1269–1278. doi: 10.1089/hum.2009.067. The authors demonstrate the induction of humoral responses in humans with a DNA vaccine delivered with electroporation. The data suggests the possibility of expanding the scope of the DNA-EP approach beyond cellular immune targets to potentially accessing prophylactic vaccine targets that require induction of humoral immune responses.
- 30. Draghia-Akli R, Khan A. Nancy Templeton Smith CRC Press, editor. Gene and cell therapy: Therapeutic mechanisms and strategies. 2009:363–371. The authors have provided a comprehensive overview of the early development of in vivo electroporation and proposed mechanism of action and discuss the clinical translation of the DNA-EP approaches in different disease areas.
- 31.Cukjati D, Batiuskaite D, Andre F, Miklavcic D, Mir LM. Real time electroporation control for accurate and safe in vivo non-viral gene therapy. Bioelectrochemistry. 2007;70:501–507. doi: 10.1016/j.bioelechem.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Trollet C, Bloquel C, Scherman D, Bigey P. Electrotransfer into skeletal muscle for protein expression. Curr Gene Ther. 2006;6:561–578. doi: 10.2174/156652306778520656. [DOI] [PubMed] [Google Scholar]
- 33.Becker SM, Kuznetsov AV. Local temperature rises influence in vivo electroporation pore development: a numerical stratum corneum lipid phase transition model. J Biomech Eng. 2007;129:712–721. doi: 10.1115/1.2768380. [DOI] [PubMed] [Google Scholar]
- 34.Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, Garcia-Hand D, Abdullah R, Braun R, Montefiori DC, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007;81:5257–5269. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Ewald BA, Lynch DM, Denholtz M, Abbink P, Lemckert AA, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008;82:4844–4852. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, Betts MR, Draghia-Akli R, Weiner DB. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26:3112–3120. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, Alicea C, Weiss D, Treece J, Pal R, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26:5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon AJ, Casimiro DR, Finnefrock AC, Davies ME, Tang A, Chen M, Chastain M, Kath GS, Chen L, Shiver JW. Enhanced in vivo transgene expression and immunogenicity from plasmid vectors following electrostimulation in rodents and primates. Vaccine. 2008;26:5202–5209. doi: 10.1016/j.vaccine.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 39. Hirao LA, Wu L, Satishchandran A, Khan AS, Draghia-Akli R, Finnefrock AC, Bett AJ, Betts MR, Casimiro DR, Sardesai NY, et al. Comparative analysis of immune responses induced by vaccination with SIV antigens by recombinant Ad5 vector or plasmid DNA in rhesus macaques. Mol Ther. 2010;18:1568–1576. doi: 10.1038/mt.2010.112. The adenovirus 5 vectored platform has been considered as the most potent platform for the induction of cellular immune responses. The authors compared the cellular immune responses induced by an optimized SIV DNA vaccine to those from an optimized SIV Ad5 vaccine in a non-human primate model. The DNA-EP responses were found to be superior to those from the Ad5 vaccine in magnitude, ability to continually boost beyond the initial prime, increased breadth of immune response and increased proliferative capacity of the antigen specific T-cells (CD4+ and CD8+).
- 40.Dobano C, Widera G, Rabussay D, Doolan DL. Enhancement of antibody and cellular immune responses to malaria DNA vaccines by in vivo electroporation. Vaccine. 2007;25:6635–6645. doi: 10.1016/j.vaccine.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 41.LeBlanc R, Vasquez Y, Hannaman D, Kumar N. Markedly enhanced immunogenicity of a Pfs25 DNA-based malaria transmission-blocking vaccine by in vivo electroporation. Vaccine. 2008;26:185–192. doi: 10.1016/j.vaccine.2007.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahlen G, Soderholm J, Tjelle T, Kjeken R, Frelin L, Hoglund U, Blomberg P, Fons M, Mathiesen I, Sallberg M. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J Immunol. 2007;179:4741–4753. doi: 10.4049/jimmunol.179.7.4741. [DOI] [PubMed] [Google Scholar]
- 43.Luxembourg A, Hannaman D, Wills K, Bernard R, Tennant BC, Menne S, Cote PJ. Immunogenicity in mice and rabbits of DNA vaccines expressing woodchuck hepatitis virus antigens. Vaccine. 2008;26:4025–4033. doi: 10.1016/j.vaccine.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 44.van Drunen Littel-van den Hurk S, Luxembourg A, Ellefsen B, Wilson D, Ubach A, Hannaman D, van den Hurk JV. Electroporation-based DNA transfer enhances gene expression and immune responses to DNA vaccines in cattle. Vaccine. 2008;26:5503–5509. doi: 10.1016/j.vaccine.2008.07.093. [DOI] [PubMed] [Google Scholar]
- 45.Kim CY, Kang ES, Kim SB, Kim HE, Choi JH, Lee DS, Im SJ, Yang SH, Sung YC, Kim BM, et al. Increased in vivo immunological potency of HB-110, a novel therapeutic HBV DNA vaccine, by electroporation. Exp Mol Med. 2008;40:669–676. doi: 10.3858/emm.2008.40.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nystrom J, Chen A, Frelin L, Ahlen G, Koh S, Brass A, Peterson DL, Fons M, Milich DR, Hultgren C, et al. Improving on the ability of endogenous hepatitis B core antigen to prime cytotoxic T lymphocytes. J Infect Dis. 2010;201:1867–1879. doi: 10.1086/652808. [DOI] [PubMed] [Google Scholar]
- 47.Trollet C, Pereira Y, Burgain A, Litzler E, Mezrahi M, Seguin J, Manich M, Popoff MR, Scherman D, Bigey P. Generation of high-titer neutralizing antibodies against botulinum toxins A, B, and E by DNA electrotransfer. Infect Immun. 2009;77:2221–2229. doi: 10.1128/IAI.01269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Best SR, Peng S, Juang CM, Hung CF, Hannaman D, Saunders JR, Wu TC, Pai SI. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27:5450–5459. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seo SH, Jin HT, Park SH, Youn JI, Sung YC. Optimal induction of HPV DNA vaccine-induced CD8+ T cell responses and therapeutic antitumor effect by antigen engineering and electroporation. Vaccine. 2009;27:5906–5912. doi: 10.1016/j.vaccine.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 50. Livingston BD, Little SF, Luxembourg A, Ellefsen B, Hannaman D. Comparative performance of a licensed anthrax vaccine versus electroporation based delivery of a PA encoding DNA vaccine in rhesus macaques. Vaccine. 2010;28:1056–1061. doi: 10.1016/j.vaccine.2009.10.111. The authors provide another example of a direct comparison of the immunogenicity and efficacy in a non-human primate model of a DNA vaccine delivered via electroporation to that induced by a licensed vaccine and demonstrate that the DNA-EP platform has the potential to yield comparable immune responses and protection from challenge to vaccines that have been licensed for human use.
- 51.Laddy DJ, Yan J, Khan AS, Andersen H, Cohn A, Greenhouse J, Lewis M, Manischewitz J, King LR, Golding H, et al. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J Virol. 2009;83:4624–4630. doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laddy DJ, Yan J, Kutzler M, Kobasa D, Kobinger GP, Khan AS, Greenhouse J, Sardesai NY, Draghia-Akli R, Weiner DB. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS One. 2008;3:e2517. doi: 10.1371/journal.pone.0002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008;26:440–448. doi: 10.1016/j.vaccine.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 54.Broderick KE, Shen X, Soderholm J, Lin F, McCoy J, Khan AS, Yan J, Morrow MP, Patel A, Kobinger GP, et al. Prototype development and preclinical immunogenicity analysis of a novel minimally invasive electroporation device. Gene Ther. 2010 Oct 21; doi: 10.1038/gt.2010.137. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Dolter KE, Evans CF, Ellefsen B, Song J, Boente-Carrera M, Vittorino R, Rosenberg TJ, Hannaman D, Vasan S. Immunogenicity, safety, biodistribution and persistence of ADVAX, a prophylactic DNA vaccine for HIV-1, delivered by in vivo electroporation. Vaccine. 2010;29:795–803. doi: 10.1016/j.vaccine.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 56.Tobin DJ. Biochemistry of human skin--our brain on the outside. Chem Soc Rev. 2006;35:52–67. doi: 10.1039/b505793k. [DOI] [PubMed] [Google Scholar]
- 57.Martinon F, Kaldma K, Sikut R, Culina S, Romain G, Tuomela M, Adojaan M, Mannik A, Toots U, Kivisild T, et al. Persistent immune responses induced by a human immunodeficiency virus DNA vaccine delivered in association with electroporation in the skin of nonhuman primates. Hum Gene Ther. 2009;20:1291–1307. doi: 10.1089/hum.2009.044. [DOI] [PubMed] [Google Scholar]
- 58.Broderick K, Kardos T, McCoy JR, Fons MP, Kemmerrer S, Sardesai NY. Piezoelectric permeabilization of mammalian dermal tissue for in vivo DNA delivery leads to enhanced protein expression and increased immunogenicity. Human Vaccines. 2011 doi: 10.4161/hv.7.0.14559. in press. [DOI] [PubMed] [Google Scholar]
- 59.Lin F, Shen X, McCoy JB, Mendoza JM, Yan J, Kemmerrer S, Khan AS, Weiner DB, Broderick KE, Sardesai NY. A novel prototype device for electroporation-enhanced DNA vaccine delivery simultaneously to both skin and muscle. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.12.057. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 60.Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, Brave A, Wahren B, Pisa P. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One. 2009;4:e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brave A, Johansson U, Hallengard D, Heidari S, Gullberg H, Wahren B, Hinkula J, Spetz AL. Induction of HIV-1-specific cellular and humoral immune responses following immunization with HIV-DNA adjuvanted with activated apoptotic lymphocytes. Vaccine. 2010;28:2080–2087. doi: 10.1016/j.vaccine.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 62.Ledwith BJ, Manam S, Troilo PJ, Barnum AB, Pauley CJ, Griffiths TG, 2nd, Harper LB, Beare CM, Bagdon WJ, Nichols WW. Plasmid DNA vaccines: investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology. 2000;43:258–272. doi: 10.1159/000053993. [DOI] [PubMed] [Google Scholar]
- 63.Sallberg M, Frelin L, Diepolder HM, Jung MC, Mathiesen I, Fons M, Ahlen G, Chen M, Weiland O. Therapeutic DNA vaccination followed by standard-of-care therapy in patients with chronic hepatitis C: a rapid clearance of viremia. Molecular Therapy. 2010;18:S110. doi: 10.1038/mt.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallace M, Evans B, Woods S, Mogg R, Zhang L, Finnefrock AC, Rabussay D, Fons M, Mallee J, Mehrotra D, et al. Tolerability of two sequential electroporation treatments using MedPulser DNA delivery system (DDS) in healthy adults. Mol Ther. 2009;17:922–928. doi: 10.1038/mt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan J, Pankhong P, Shen X, Giffear M, Lee J, Harris D, Diaz D, Khan A, Bagarazzi M, Boyer J, Weiner DB, Sardesai NY. Phase I safety and immunogenicity of HPV 16 and 18 DNA vaccines delivered via electroporation. Mol Ther. 2010;18:S184. [Google Scholar]
- 66.Lee J, Khan A, Giffear M, Diep-Lam P, Zifchak L, Maslow J, Tebas P, Sardesai NY. Tolerability of EP by Cellectra device following intradermal administration of saline in healthy volunteers. ASGCT Conference poster. 2010 [Google Scholar]
- 67.Ottensmeier C, Low L, Mander A, Tjelle T, Campos-perez J, Williams T, Heath C, Dearnaley D, Mathiesen I, Stevenson F. DNA fusion gene vaccination, delivered with or without in vivo electroporation: a potent and safe strategy for inducing antitumor immune response in prostate cancer. AACR Meeting Abstracts. 2008:2843. [Google Scholar]
- 68.Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, Boente-Carrera MM, Vittorino RM, Caskey M, Andersen J, et al. In vivo electroporation enhances the immunogenicity of ADVAX, a DNA-based HIV-1 vaccine candidate, in healthy volunteers. AIDS Vaccine Conference Abstract. 2009:55. doi: 10.1371/journal.pone.0019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasan S, Schlesinger SJ, Huang Y, Hurley A, Lombardo A, Chen Z, Than S, Adesanya P, Bunce C, Boaz M, et al. Phase 1 safety and immunogenicity evaluation of ADVAX, a multigenic, DNA-based clade C/B' HIV-1 candidate vaccine. PLoS One. 2010;5:e8617. doi: 10.1371/journal.pone.0008617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bagarazzi M, Yan J, Shen X, Giffear M, Lee J, Khan A, Harris D, Pankhong P, Shedlock D, Boyer J, Weiner D, Sardesai N. Immunotherapy of post-LEEP CIN2/3 with HPV 16 and 18 E6/E7 DNA vaccines/electroporation. 50th ICAAC Boston, poster G1-202. 2010 Sept Sept 12–15; [Google Scholar]
- 71.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, Pardoll D, Wu TC. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15:361–367. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 73.Listner K, Bentley L, Okonkowski J, Kistler C, Wnek R, Caparoni A, Junker B, Robinson D, Salmon P, Chartrain M. Development of a highly productive and scalable plasmid DNA production platform. Biotechnol Prog. 2006;22:1335–1345. doi: 10.1021/bp060046h. [DOI] [PubMed] [Google Scholar]
- 74.Victoria JG, Wang C, Jones MS, Jaing C, McLoughlin K, Gardner S, Delwart EL. Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. J Virol. 2010;84:6033–6040. doi: 10.1128/JVI.02690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Onions D, Kolman J. Massively parallel sequencing, a new method for detecting adventitious agents. Biologicals. 2010;38:377–380. doi: 10.1016/j.biologicals.2010.01.003. [DOI] [PubMed] [Google Scholar]