Abstract
Post-weaning social isolation of rats is utilized as a model of early life stress. We have previously demonstrated that rats exposed to post-weaning social isolation exhibit greater anxiety-like behaviors as adults. Furthermore, these rats exhibit greater density of corticotropin-releasing factor (CRF) type 2 receptors in the dorsal raphe nucleus. Therefore, we examined whether antagonism of CRF2 receptors in the dorsal raphe nucleus reverses the effects of post-weaning social isolation on anxiety states. Male rats were reared in isolation or in groups from day of weaning (postnatal day [PND] 21) to mid-adolescence (PND42) and then allowed to develop to early adulthood housed in groups. At PND62, rats were either infused with vehicle, the CRF1 receptor antagonist antalarmin (0.25-0.5 μg) or the CRF2 receptor antagonist antisauvagine-30 (2 μg) into the dorsal raphe nucleus, 20 minutes prior to being introduced to the elevated plus maze. Isolation-reared rats showed reduced open arm behavior compared to group-reared rats, confirming the anxiogenic effects of post-weaning social isolation. Infusion of the CRF2 receptor antagonist, but not the CRF1 receptor antagonist, into the dorsal raphe nucleus of isolation-reared rats increased open arm behavior when compared to that of group-reared rats. Overall, the findings suggest that CRF2 receptors within the dorsal raphe nucleus mediate anxiety-like states following post-weaning social isolation, and CRF2 receptors may represent an important target for the treatment of anxiety disorders following early life stressors.
Keywords: Social isolation, corticotropin-releasing factor, anxiety, antisauvagine-30, antalarmin, elevated plus maze
1. Introduction
Early-life stress and neglect are associated with the later-life development of psychiatric disorders, such as anxiety and depression [2,26]. Post-weaning social isolation is an effective rat model used to study the neural mechanisms underlying the detrimental effects of early-life stress [14,24]. Post-weaning social isolation of male rats increases anxiety-like behaviors in adulthood within a variety of paradigms [3,22-24,27,35-36]. Furthermore, adult anxiety-like behaviors resulting from post-weaning social isolation restricted to early-mid adolescence do persist through to adulthood even if the rat is re-housed into groups after the isolation period [3-4,22-24,36].
However, there are some inconsistencies among studies as to whether social isolation increases adult anxiety-like behavior in the elevated plus maze (EPM) [24]. For example, social isolation of rats from early pre-adolescence to the beginning of mid-adolescence (PND22 to 35) did not alter anxiety-like behavior on the EPM tested during early adulthood [33]. Similarly, rats isolated from the end of pre-adolescence to early adulthood (PND28-62) did not show altered anxiety-like behaviors on the EPM [7]. However, combined findings from numerous other studies using the EPM as an end measure suggest that anxiety-like behaviors are detected in early adulthood if isolation occurs in early pre-adolescence (PND21-22) and persists until at the middle period of mid-adolescence (PND42) [22-24,27,35-36]. This critical period appears to coincide with the development of social behaviors and neural systems underlying anxiety and motivated behaviors [2,24,30]. The first goal of the current study was to directly test whether social isolation of rats restricted to the suggested critical period (from PND21-42), which produces robust anxiety states in adulthood when tested in a social interaction paradigm [22-24], also produces heightened anxiety-like behavior in adult rats tested in the EPM.
Recent reports have implicated altered corticotropin-releasing factor (CRF) mediation of serotonin (5-HT) release as underlying heightened anxiety states in adult rats exposed to early-life social isolation [21-24]. For example, post-weaning social isolation prolongs CRF-induced serotonin release in the nucleus accumbens of adult rats, an effect thought to be mediated by increased expression of CRF2 receptors in the brainstem serotonergic cell body region, the dorsal raphe nucleus (dRN) [21]. Furthermore, infusion of d-Phe-CRF, an antagonist of both CRF1 and CRF2 receptors, into the dRN reduced heightened social anxiety-like behaviors of adult rats exposed to post-weaning social isolation [23]. However, whether the heightened anxiety states following post-weaning social isolation are mediated by CRF1 or CRF2 receptors in the dRN has not been tested to date.
Pharmacological studies indicate a role for both CRF1 and CRF2 receptors in mediating anxiety-like behaviors in animal models [31]. Most often, peripheral or central administration of CRF1 receptor antagonists decrease anxiety behavior [13,25,31]. Similarly, CRF2 receptor antagonists administered centrally or specifically in to the dRN reduce fear and anxiety states in animal models [15,17,32,34]. However, adult rats exposed to early-life social isolation show increased levels of CRF2 receptors in the dRN but no change in expression of CRF1 receptors [21]. Therefore, the current study tested the hypothesis that increased CRF2 receptors in the dRN mediate heightened anxiety states following post-weaning social isolation.
2. Methods and Materials
2.1 Animals and Social Isolation Procedures
Male Sprague-Dawley rats were bred by the Animal Resource Center at the University of South Dakota. Rats were obtained from 4 separate litters for Experiment 1, and 14 different litters for Experiment 2. Within each litter, equal numbers of rats were randomly assigned to either group or isolation conditions at weaning. On PND21, rats were weaned and housed in groups of three or in isolation for three weeks. At PND42 (mid-adolescence) [2,24,30], rats were weighed and re-housed into groups of three within the same rearing condition groups until testing. Group-reared rats were also re-housed into different groups at this stage to control for the social disruption [21-23]. This social isolation paradigm results in increased CRF2 receptors in the dRN and heightened social anxiety states in adult rats [21-24]. Rats were housed under a 12-hour reverse light cycle, with lights off at 10 am, and all testing was conducted in the dark phase of the light cycle. Animals had continuous access to food and water, and cages were changed once per week to ensure minimal handling. The University of South Dakota Animal Care and Use Committee approved all animal procedures.
2.2 Experiment 1: Effects of Post-Weaning Social Isolation on Behaviors in the Elevated Plus Maze
Rats (12 isolates and 12 reared in groups) were tested on the EPM at PND56, corresponding to early adulthood [2,24,30]. The EPM (Noldus Information Technology, Leesburg, VA) was raised 1 m above the floor, with four arms 50 cm in length and 11 cm wide. Two arms were surrounded by walls, enclosing those arms of the maze. Testing was performed in a dark room under red lighting. Each rat was placed into the center portion of the maze facing an open arm and was allowed to explore the maze for 5 minutes. The total distance moved, total time spent in the open arms, and number of open arm entries were measured by Ethovision XT 5.1 (Noldus Information Technology).
2.3. Experiment 2: Effects of CRF1 and CRF2 Receptor Antagonism in the dRN on Anxiety-Like Behaviors
2.3.1. Surgery
Sixty-eight rats (not used in experiment 1) underwent stereotaxic surgery on PND56 to implant a drug infusion cannula above the dRN. Rats were anesthetized with ketamine/xylazine ip. (ketamine 80 mg/kg, Met-Vet, Libertyville, IL; xylazine 16 mg/kg, RX Veterinary Products, Westlake, TX) and placed in a the stereotaxic apparatus (David Kopf Institute, CA, USA) on a heating pad. The nose bar was set at -3.7 mm to ensure the skull was flat. Under aseptic conditions, a 22 gauge guide cannula (5 mm long; Plastics One, Roanoke, VA) was inserted into the brain (-7.8 mm posterior and 2.7 mm lateral to bregma according to Paxinos and Watson [28]), 2 mm above the dRN at a 23-degree angle to avoid the cerebral aqueduct [23]. Three screws were placed into the skull to serve as anchors, and dental acrylic was applied to secure the guide cannula. The analgesic ketoprofen (5 mg/kg, im; Med-Vet) was administered upon completion of the surgery. Rats were allowed to recover for 2-3 days prior to acclimation procedures.
2.3.2. Acclimation and Infusion Procedure
Rats were acclimated to handling and infusion procedures for three consecutive days [23,34]. Rats were gently held in cloth, a 30 gauge cannula (2 mm longer than the guide) was inserted through the guide cannula and 0.5 μl of artificial cerebrospinal fluid (aCSF) was infused at a rate of 0.5 μl/min using a microinfusion pump (Stoelting, Wood Dale, IL). The cannula remained in situ for one minute after the infusion to allow diffusion away from the tip.
2.3.3. Drug Infusion and Elevated Plus Maze Testing
Following three days of acclimation, rats were taken into the dark testing room and an infusion of vehicle (0.5 μl, 2%EtOH/2% Camphor in aCSF) or CRF2 receptor antagonist antisauvagine-30 (ASV-30; 2 μg/0.5 μl, Sigma-Aldrich, St. Louis, MO) [10,20,34] or CRF1 receptor antagonist antalarmin (0.25 μg/0.50 μl or 0.5 μg/0.5 μl, Tocris Bioscience, Ellisville, MO) [20]was made into the dRN as described in section 2.3.2. The 2 μg concentration of ASV and the 0.25 μg concentration of antalarmin have been shown sufficient to block the effects of CRF1 or CRF2 stimulation without affecting the other receptor type [20]. The 0.5 μg concentration of antalarmin was also used since the 0.25 μg concentration produced mixed behavioral results. The 0.5 μl infusion has been shown to be sufficient to distribute bilaterally through the dRN, but not to effectively diffuse into surrounding brain regions [9,20,23]. Twenty minutes following infusion [23,34], the rat was placed in the center of the EPM and testing proceeded as described in section 2.2.
2.3.4. Histology
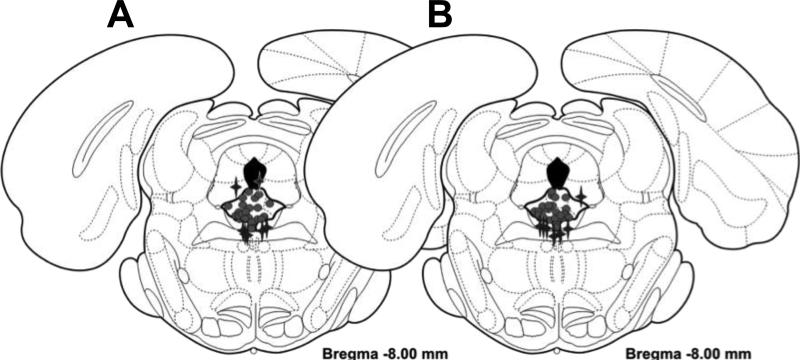
After testing, the rats were euthanized with 1 ml Fatal Plus (390 mg/ml, Vortech Pharmaceuticals, Dearborn, MI), brains removed and fixed in 10% formalin (Fischer, Fair Lawn, NJ) for a minimum of three days. Brains were sliced frozen at 60 μm on a microtome through the dRN, mounted and stained with cresyl violet. Cannula placements were confirmed by at least two experimenters blind to treatment and results. Only data from animals with placements within the dRN were used in the following analysis. However, there were sufficient numbers of ASV-treated rats that received an infusion of ASV outside but adjacent (within 0.5 mm) of the dRN for analysis to examine the specificity of the infusion (Figure 2; Table 1).
Figure 2.
Cannula placements within and surrounding the dorsal raphe nucleus (dRN) for (A) group-reared and (B) isolation-reared rats. Circles indicate placements within the dRN and stars indicate placements within 0.5 mm of the dRN. Figures adapted from [28].
Table 1.
Effects of antisauvagine-30 infusions adjacent to the dRN (within 0.5 mm) on behavior within the elevated plus maze of young adult rats reared in isolation or group conditions.
| Behavior | Group-reared | Isolation-reared |
|---|---|---|
| Number of open arm entries | 13.71 ± 01.48 | 9.14 ± 00.99* |
| Time spent in open arms (s) | 105.31 ± 16.90 | 46.54 ± 08.13* |
| Total distance moved (cm) | 2794.57 ± 68.68 | 2665.61 ± 76.31 |
P < 0.05. N = 7 per group.
2.4. Statistical Analysis
Following behavioral testing for Experiment 1, group and isolation-reared rats were compared using Mann-Whitney Rank Sum Tests since the data was not normally distributed. For Experiment 2, behavioral data were analyzed with separate 2-way ANOVAs for each behavioral measure. When a significant effect of social treatment (P ≤ 0.05) was observed, it was further assessed with a Student-Newman-Keuls (SNK) post hoc test for multiple pair wise comparisons between isolation and group-reared rats within the same drug treatment. When a significant effect of drug treatment was observed, data were analyzed within a rearing condition (group or isolation) with separate 1-way ANOVAs, and significant data (p ≤ 0.05) were further assessed with a Holm-Sidak (H-S) post hoc test for multiple comparisons against the control (vehicle-treated) groups. To examine whether the anxiolytic effects of CRF2 receptor antagonism in the dRN could be related to diffusion into the cerebral aqueduct, duration of time spent in open arms and frequency of entries to open arms in the isolation group were regressed against the distance of the infusion cannula tip from the aqueduct using linear regression ANOVA. All analyses were performed using Sigma Stat 3.5 (Systat Software Inc., Point Richmond, CA).
3. Results
3.1. Experiment 1: Effects of Post-Weaning Social Isolation on Behaviors in the Elevated Plus Maze
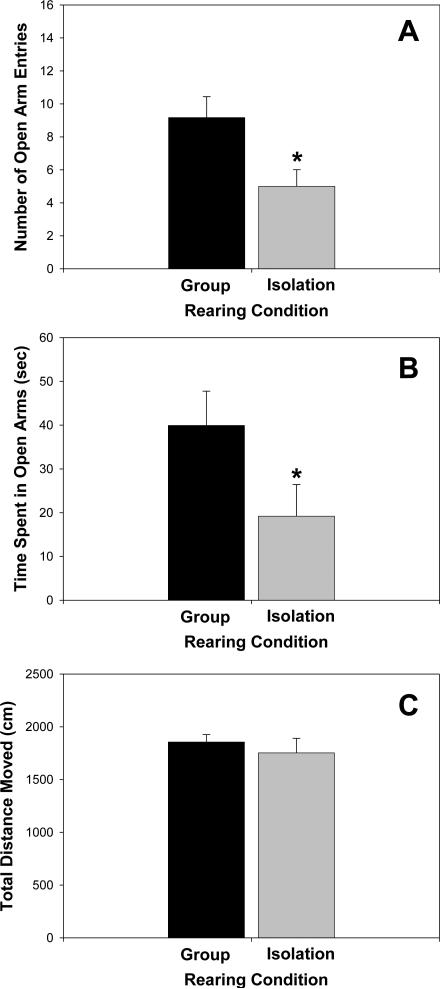
Early-life social isolation (PND21-42) increased anxiety-like behaviors when compared with group-reared rats, as tested on the EPM. Rats reared in social isolation from PND21-42 showed a significant decrease in the number of open arm entries (T(22) = 158.00, P = 0.039; Figure 1A) and in the amount of time spent in the open arms (T(22) = 134.0, P = 0.031; Figure 1B) when compared to group-reared rats. There was no statistical difference between isolation and group-reared rats in distance moved within the entire maze (T(22) = 162.0, P = 0.507; Figure 1C).
Figure 1.
Behavior on the elevated plus maze (EPM) of young adult rats reared in isolation or group conditions. Isolation-reared rats showed (A) reduced number of open arm entries and (B) reduced time spent in open arms of the EPM, indicative of increased anxiety-like behavior. (C) Total distance moved in the EPM did not differ between rearing conditions. *P < 0.05. N = 12 per group.
3.2. Experiment 2: Effects of CRF1 and CRF2 Receptor Antagonism in the dRN on Anxiety-Like Behaviors
3.2.1. Cannula Placements
The majority of dRN cannula placements were in the dorsal, ventral, and ventrolateral portions of the dRN (Figure 2). Anterior-posterior measurements ranged from -7.3 to -8.3 mm from bregma, with the majority being between -7.8 and -8.3 mm from bregma. There were sufficient numbers of ASV-treated rats that received an injection of ASV outside but adjacent (within 0.5 mm) of the dRN for analysis of the specificity of the dRN infusion effects (Figure 2). These placements were in the medial longitudinal fasciculus, decussation of the superior cerebral peduncle, cerebral aqueduct, or in the central grey dorsal to the dRN. The dRN and adjacent cannula placements did not differ between social isolation and group-reared rats (Figures 2A-B).
3.2.2. Effects of CRF Receptor Antagonists in the dRN on Anxiety-Like Behavior
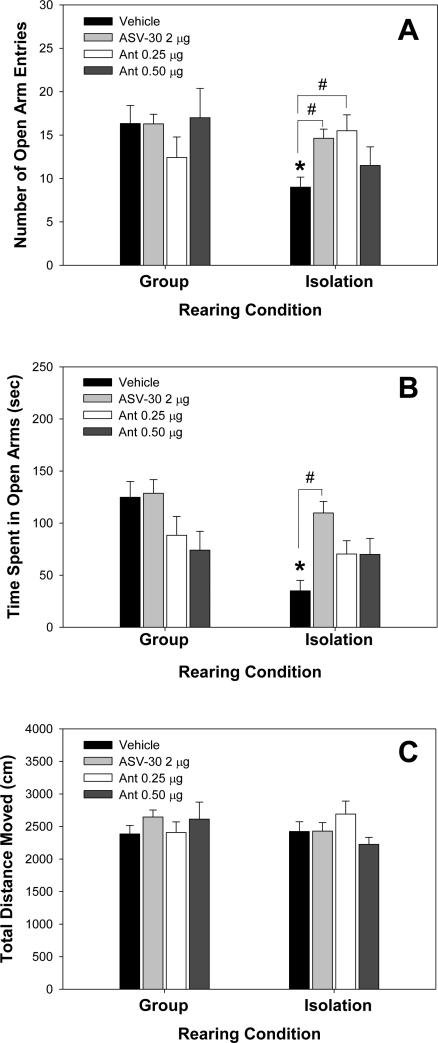
Rats reared in groups or social isolation (PND21-42) received infusions of either the CRF2 receptor antagonist ASV-30 (2 μg); the CRF1 receptor antagonist antalarmin (0.25 or 0.5 μg) or vehicle 20 minutes prior to testing on the EPM. For number of entries into the open arms (Figure 3A), a significant effect of social treatment was present (F(1,45) = 4.083, P = 0.049), but there was no significant effect of dRN treatment (F(3,45) = 0.662, P = 0.580), and a trend was found between social treatment and drug treatment (F(3,45) = 2.731, P = 0.055). One-way ANOVA analysis within group-reared animals showed there was no significant effect of dRN infusion on open arm entries (F(3,22) = 0.870, P = 0.472, Figure 3A). However, a one-way ANOVA within isolation-reared animals showed a significant effect of dRN treatment on open arm entries (F(3,21) = 3.278, P = 0.041, Figure 3B). A post hoc analysis within isolation treatment showed that rats that received an ASV-30 infusion entered the open arms more times than those receiving the vehicle treatment (H-S, P = 0.028; Figure 3A). Similarly, within isolation treatment, animals receiving 0.25 μg antalarmin infusion entered the open arms more times than those receiving vehicle treatment (H-S, P = 0.012, Figure3A). However, within isolation treatment, there was no difference in the number of entries into the open arms between the 0.50 μg antalarmin treatment compared to vehicle treatment (H-S, P = 0.538; Figure 3A). Post hoc analyses between social treatments within a drug treatment showed that within vehicle treatment, isolation-reared animals exhibited a decrease in the number of entries into open arms compared to group-reared animals (SNK, P = 0.020, Figure 3A). However, within ASV-30 treatment, isolation-reared rats showed no difference in the number of open arm entries compared to group-reared rats (SNK, P = 0.526, Figure 3A). Within 0.25 μg antalarmin treatment, isolation-reared animals did not differ in the number of open arm entries compared to group-reared animals (SNK, P = 0.243, Figure 3A). Finally, within 0.50 μg antalarmin treatment, isolation-reared animals did not differ from group-reared rats in the number of entries into the open arms (SNK, P=0.077, Figure 3A).
Figure 3.
Effects of corticotropin-releasing factor (CRF) receptor antagonists infused into the dorsal raphe nucleus (dRN) on behavior in the elevated plus maze (EPM) of young adult rats reared in isolation or group conditions. (A) Isolation-reared rats showed reduced number of open arm entries that was reversed by dRN infusion of either the CRF2 receptor antagonist antisauvagine-30 (ASV-30) or 0.25 μg of the CRF1 receptor antagonist antalarmin (Ant). (B) Isolation-reared rats showed reduced time spent in open arms of the EPM, which was reversed by dRN infusion of the CRF2 receptor antagonist ASV-30. (C) Total distance moved in the EPM did not differ between rearing conditions or dRN infusion treatment groups. *Significantly different from vehicle-treated group-reared rats; #significantly different from vehicle-treated isolation-reared rats; P < 0.05. N = 6-8 per group.
For the amount of time spent in open arms, there was a significant effect of social treatment (F(1,45) = 9.895, P=0.003), dRN treatment (F(3,45) = 4.605, P = 0.007), and a significant interaction between social treatment and drug treatment (F(3,45) = 3.039, P = 0.039, Figure 3B). One-way ANOVA within the group-housed animals showed no significant effect of dRN treatment (F(3,22) = 2.729, P = 0.068), but there was a significant effect of dRN treatment within the isolation-housed animals (F(3,21) = 5.281, P = 0.007, Figure 3B). Post hoc analysis showed that within the group subject to isolation rearing, animals that received ASV-30 infusion spent more time in the open arms (Figure 3B) compared to those receiving vehicle treatment (H-S, P < 0.001). Within isolation rearing, no significant difference in time spent in open arms was seen between vehicle and 0.25 μg antalarmin treatments (H-S, P = 0.079), or between vehicle and 0.50 μg antalarmin treatments (H-S, P = 0.131). Post hoc analyses between social treatments within a drug treatment showed that within vehicle treatment, isolation-reared animals spent less time in the open arms when compared to group-reared animals (SNK, P < 0.001; Figure 3B), but no significant difference was found between isolation- and group-reared rats infused with ASV-30 (SNK, P = 0.334), infused with 0.25 μg antalarmin (SNK, P = 0.357), or infused with 0.50 μg antalarmin (SNK, P = 0.850, Figure 3B).
Neither rearing condition nor dRN treatment had any effect on locomotor activity within the EPM (Figure 3C). For total distance moved in the EPM, no significant difference was found between social treatments (F(1,45) = 0.363, P = 0.550), between dRN treatments (F(3,45) = 0.430, P = 0.733), nor was there a significant interaction between social treatment and dRN treatment (F(3,45) = 1.608, P = 0.201, Figure 3C).
3.2.3. Regional Specificity of the Effects of ASV-30 on Anxiety-Like Behavior
There was no significant relationship between distance of dRN infusion from the aqueduct with duration of time spent in open arms (r2 = 0.04, P = 0.652), or with the frequency of entries into open arms (r2 = 0.05, P = 0.604) in isolates, suggesting that diffusion into the ventricular system could not explain the anxiolytic effects of ASV-30 in this group. Furthermore, Infusion of ASV-30 within 0.5 mm of the dRN failed to reduce anxiety-like behaviors of isolation-reared rats (Table 1). Animals reared in isolation entered the open arms significantly fewer times than group-reared animals (T(12) = 69.00, P = 0.038; Table 1). Similarly animals reared in social isolation spent significantly less time in the open arms compared to group-reared animals (T(12) = 71.00, P = 0.017, Table 1). However, there were no significant differences between isolation- and group-reared animals in in the total distance moved within the maze (T(12) = 64.00, P = 0.165, Table 1).
4. Discussion
4.1. Effects of Post-Weaning Social Isolation on Behaviors in the Elevated Plus Maze
Rats reared in isolation from PND21 to PND42 entered the open arms fewer times and spent less time in open arms of the EPM when tested in early adulthood, as compared to group-reared controls. Furthermore, post-weaning social isolation did not have an effect on the total distance moved in the EPM, suggesting that reduced entries and time spent in open arms exhibited by isolation-reared rats were not due to deficits in exploration or locomotion. Together, these measures are indicative of increased anxiety-like behavior of isolation-reared rats [8,16,34]. This is consistent with earlier work suggesting that post-weaning social isolation results in anxiety-like behavior in early adulthood within multiple tests of anxiety states [14,24,35-36].
Furthermore, the current findings support the idea that isolation initiated early in pre-adolescence results in adult anxiety-like behavior detectable on the EPM [24,35-36], since others have not detected heightened anxiety states on the EPM when rats are socially isolated from the beginning of adolescence (PND28) [7]. Combined, present and past findings [7,24,33,35-36] suggest that isolation must not only begin in pre-adolescence, but it must last well into mid-adolescence to detect heightened anxiety states in early adulthood on the EPM.
While isolation-reared rats from both experiments of the current study showed significantly enhanced anxiety-like behaviors in the EPM (in the absence of CRF receptor antagonist infusion), the absolute number of entries and time spent in opens arms for all groups were much lower in Experiment 1 as opposed to Experiment 2. This has been observed previously [34], where rats that did not receive surgery, pre-handling acclimation, or infusions spent less time in open arms compared to rats that undergo these procedures, as was the case for Experiment 2. Once possibility is that the pre-handling may reduce anxiety states and increase activity within the EPM. However, the effect of infusion, either due to the damage caused by cannula insertion or the infusion of an ethanol-based vehicle, may also have produced this anxiolytic effect. These possibilities could be differentiated by future experimentation.
4.2. Effects of CRF Receptor Antagonists in the dRN on Anxiety-Like Behavior Following Post-weaning Social Isolation
The current study demonstrated that pre-infusion of the dRN with the CRF2 receptor antagonist ASV-30, at a concentration known to block CRF-mediation of 5-HT release [10,20], reversed anxiety-like behavior of adult rats exposed to post-weaning social isolation. Furthermore, this effect was specific to ASV-30 infusion into the dRN since infusions of this antagonist adjacent (within 0.5 mm) to the dRN did not reverse the anxiety-like behavior of isolation-reared rats. Interestingly, ASV-30 infused into the dRN had no effect on group-reared rats, suggesting that CRF2 receptor-mediation of behavior on the EPM may be dependent on pre-exposure to stressors. Overall, findings suggest that CRF2 receptors in the dRN mediate anxiety-like behavior of rats exposed to social isolation during a critical period of development.
It is known that CRF stimulation of adenylate cyclase (as is observed with CRF2 receptor stimulation) in whole brain homogenates undergoes developmental reduction from PND 20 to PND28 [18]. Thus, social isolation initiated at PND21 may reduce the developmental pruning of CRF2 receptors in the dRN to result in enhanced CRF2 receptor levels and function as measured in early adulthood [21,24]. However, there is no information currently available specifically regarding the developmental trajectory of the expression and function CRF2 receptors in the dRN, and how this may be affected by stress during PND21-42. Thus, this is an important area for future research to explore.
While the role of CRF2 receptors in mediating anxiety-states following social isolation have not been previously explored, a recent report shows CRF2 receptor knockout mice show hyper-locomotion following social isolation that is not apparent in wildtype mice [11]. This finding suggests that the effects of social isolation on locomotion are ameliorated by CRF2 receptor function [11]. Thus, the specific role of CRF2 receptors in mediating the behavioral effects of social isolation may depend on the end behavioral measure.
The ability of the CRF1 receptor antagonist antalarmin to alter anxiety-like behaviors of isolation-reared animals was not robust. To illustrate, 0.25 μg, but not 0.5 μg, antalarmin infused into the dRN increased entries the open arms of isolation-reared rats. However, neither concentration altered time in the open arms for isolation-reared rats. The 0.25 μg concentration of antalarmin into the dRN is sufficient to completely block CRF1 receptor-mediated serotonin release [20]. Overall, CRF1 receptors in the dRN appear to have a less pronounced effect in mediating anxiety-like behaviors following post-weaning social isolation as compared to CRF2 receptors, in line with the findings that post-weaning social isolation increased CRF2 receptor levels in the dRN but had no effect on CRF1 receptors [21].
Although no previous studies have reported the effects of CRF1 receptor antagonism in the dRN on anxiety-like behavior, many have found that systemic or central administration of CRF1 receptor antagonists reduce anxiety-like behavior in a variety of animal models (as reviewed by [31]). Specifically, Lundkvist et al. [25] demonstrated that adult male Sprague-Dawley rats exhibited decreased anxiety-like behavior on the EPM following systemic administration of CP 154,526, a CRF1 receptor antagonist. Interestingly, higher doses of the antagonist had no effect on anxiety behavior when compared to vehicle injection [25], similar to the lack of response with the higher concentration of antalarmin in the current study. The current findings tentatively imply that CRF1 receptors in the dRN may not be an important locus of the anxiolytic effects of centrally-administered CRF1 receptor antagonists. However, a role for CRF1 receptors in the dRN for mediating anxiety states following post-weaning social isolation should not be ruled out until further testing is conducted.
An increasing body of literature, including the current study, provides support for CRF2 receptors in mediating anxiety states. For example, the CRF2 receptor agonist urocortin II (UcnII) infused into the septum increases anxiety-like behavior of mice, which is blocked by infusion of astressin-2B, a CRF2 receptor antagonist [17]. Takahashi et al. [32] show that an infusion of ASV-30 into the lateral ventricle reduces anxiety behaviors in several tests of anxiety, including the EPM. With regards to CRF2 receptors in the dRN, Vuong et al [34] demonstrated that ASV infused into the dRN at the same concentration as used in the current study, reversed anxiety-like behaviors of adult male rats during amphetamine withdrawal. These findings stand in contrast to those obtained from CRF2 receptor knockout mice that exhibit increased anxiety-like behaviors, implying that activity of CRF2 receptors is anxiolytic [5-6]. However, it has been suggested that the increased concentrations of CRF mRNA in the amygdala of CRF2 knockout mice [5] could enhance anxiety-states in this model [32], perhaps by acting on CRF1 receptors throughout the brain.
Stimulation of CRF2 receptors increases 5-HT neural activity in the dRN and increases the release of 5-HT in the limbic system [1,10,20,29], and 5-HT has been shown to be associated with fear and anxiety behavior, especially 5-HT released from the dRN [9,19]. Therefore, CRF2 receptor-mediation of anxiety states within the dRN of isolation-reared rats implies that enhanced 5-HT release within regions of the limbic system may underlie increased anxiety following early-life stress.
4.3. Conclusions
Post-weaning isolation restricted to PND21-42 results in increased anxiety-like behaviors in the EPM, which are mediated by CRF2 receptors in the dRN. A role for CRF1 receptors in the dRN in mediating heightened anxiety states following post-weaning social isolation cannot be excluded, but is not indicated by the current study. Given the lack of ASV-30 effect on control rats or on general locomotor activity, the CRF2 receptor may be a potential selective pharmacological target for anxiety disorders resulting from early-life stress.
Acknowledgements
We would like to thank Dr. Michael Watt for his assistance, Dr. Jodi Lukkes for helpful advice and Drs. Kenneth Renner and Cliff Summers for comments on earlier versions of this manuscript. This work was funded by grants NIH R01 DA019921 and NIH P20 RR015567. AB was supported by an UDiscover fellowship from The University of South Dakota.
Abbreviations
- 5-HT
Serotonin
- aCSF
Artificial cerebrospinal fluid
- Ant
Antalarmin
- ASV-30
Antisauvagine-30
- CRF
Corticotropin-releasing factor
- CRF1
Corticotropin-releasing factor type 1 receptors
- CRF2
Corticotropin-releasing factor type 2 receptors
- dRN
Dorsal raphe nucleus
- EPM
Elevated plus maze
- H-S
Holm-Sidak
- PND
Postnatal day
- SNK
Student-Newman-Keuls
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphé nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 3.Arakawa H. Interaction between isolation rearing and social development on exploratory behavior in male rats. Behav. Processes. 2005;70:223–234. doi: 10.1016/j.beproc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Arakawa H. Ontogenetic interaction between social relationships and defensive burying behavior in the rat. Physiol. Behav. 2007;90:751–759. doi: 10.1016/j.physbeh.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GE, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 6.Bale TL. Sensitivity to stress: Dysregulation of CRF pathways and disease development. Horm. Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Brenes JC, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav. Brain Res. 2009;197:125–137. doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Dawson GR, Tricklebank MD. Use of the elevated plus maze in search for novel anxiolytic agents. Trends Pharacol. Sci. 1995;16:33–36. doi: 10.1016/s0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- 9.Forster GL, Feng N, Watt MJ, Korzan WJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphé nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphé nucleus activity. Eur. J. Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gresack J, Powell S, Geyr M, Poore MS, Coste S, Risbrough V. CRF2 null mutation increases sensitivity to isolation rearing effects on locomotor activity in mice. Neuropeptides. 2010;44:349–53. doi: 10.1016/j.npep.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol. Behav. 2003;79:471–478. doi: 10.1016/s0031-9384(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 13.Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc. Natl. Acad. Sci. USA. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical behavioral consequences. Crit. Rev. Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 15.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J. Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handley SL, McBlane JW. An assessment of the elevated x-maze for studying anxiety and anxiety-modulating drugs. J. Pharmacol. Toxicol. Methods. 1993;29:129–138. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- 17.Henry B, Vale W, Markou A. The effect of lateral septum cortictropin-releasing factor 2 activation on anxiety is modulated by stress. J. Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J. Neurosci. 1988;8:4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- 20.Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphé differentially affect serotonin release in the nucleus accumbens. Eur. J. Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009;158:845–855. doi: 10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm. Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Lukkes JL, Vuong SM, Scholl JL, Oliver H, Forster GL. Corticotropin-releasing factor receptor antagonism within the dorsal raphé reduces social anxiety following early-life social isolation. J. Neurosci. 2009;32:9955–9960. doi: 10.1523/JNEUROSCI.0854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci. 2009d;3(1):1–12. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundkvist J, Chai Z, Teheranian R, Hasanvan H, Bartfai T, Jenk F, Widmer U, Moreau J. A non peptidic corticotropin releasing factor receptor antagonist attenuates fever and exhibits anxiolytic-like activity. Eur. J. Pharmacol. 1996;309:195–200. doi: 10.1016/0014-2999(96)00337-8. [DOI] [PubMed] [Google Scholar]
- 26.National Clearinghouse on Child Abuse and Neglect . Long-term consequences of child abuse and neglect. U.S. Department of Health and Human Services; 2005. [Google Scholar]
- 27.Parker V, Morinan A. The socially-isolated rat as a model for anxiety. Neuropharmacology. 1986;25:663–664. [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. third ed. Academic Press; New York: 1997. [Google Scholar]
- 29.Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors of neurochemically identified neurons in the rat dorsal raphé nucleus reveals dual actions. J. Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi LK. Role of CRF1 and CRF2 receptors in fear and anxiety. Neurosci. Biobehav. Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF2 receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902:135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 1999;34:129–138. [PubMed] [Google Scholar]
- 34.Vuong SM, Oliver HA, Scholl JL, Oliver KM, Forster GL. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav. Brain Res. 2010;208:278–281. doi: 10.1016/j.bbr.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol. Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]