Abstract
3, 5, 7-Trihydroxy-4'-methoxy-8-(3-hydroxy-3- methylbutyl)–flavone (ICT) is a novel derivative of Icariin (ICA), the major active ingredient of Herba Epimedii, a herb used in traditional Chinese and alternative medicine. We previously demonstrated its anti-inflammatory effect in murine innate immune cells and activated human PBMCs. We report herein that ICA or ICT treatment reduces the expression of MRP8/MRP14 and toll-like receptor 4 (TLR4) on human PBMCs. Administration of ICA or ICT inhibited tumor growth in 4T1-Neu tumor-bearing mice and considerably decreased MDSC numbers in the spleen of these mice. Further, we saw a restoration of IFN-γ production by CD8+ T cells in tumor bearing mice when treated with ICA or ICT. ICA and ICT significantly decreased the amounts of nitric oxide and reactive oxygen species in MDSC in vivo. When MDSC were treated in vitro with ICT, we saw a significant reduction in the percent of these cells with concomitant differentiation into dendritic cells and macrophages. Concomitant with this cell type conversion was a down-regulation of IL-10, IL-6 and TNF-α production. Decreased expression of S100A8/9 and inhibition of activation of STAT3 and AKT may in part be responsible for the observed results. In conclusion, our results showed that ICA, and more robustly, ICT, directly modulate MDSC signaling and therefore altered the phenotype and function of these cells, in vitro and in vivo.
Keywords: MDSC, ICA, ICT, Inflammation, TLR4, MRP8/14
1. Introduction
The delicate balance between inflammation and immune suppression is the basis of many diseases, including autoimmunity and cancer. In both cases, the presence of danger associated molecular patterns (DAMPs) provides a signal that perpetuates the immune response [1–3]. Myeloid-related protein-8 (MRP8) and -14 (MRP14), have recently been identified as important endogenous DAMPs and are capable of modulating innate immunity. S100A8 (also termed MRP8) and S100A9 (MRP14) exist mainly as a S100A8/A9 heterodimers (termed calprotectin) and are expressed by granulocytes, monocytes, and early differentiation of macrophages [4]. These DAMPs strongly correlate with an inflammatory immune response and become up-regulated in both autoimmunity and cancer [4, 5]. Calprotectin, which is recognized by TLR4, has been shown to induce reactive CD8+ T cells but more importantly, calprotectin has tumor-promoting properties that correlate with the recruitment of MDSC to the tumor site [6–9].
MDSCs are a heterogeneous group of myeloid cells comprised of hematopoietic progenitor cells and precursors of macrophages, granulocytes, dendritic cells (DCs) [10, 11]. These cells are a major component of the immune suppressive network responsible for T cell defects in cancer [12, 13]. S100A8 and S100A9 heterodimers are proinflammatory mediators that hamper cancer immunotherapy strategies by promoting the accumulation of MDSC that block T cell-mediated tumor immunity [8]. A previous study showed that preventing S100A8/A9 interaction with its receptor on MDSC using an anti-carboxylated glycan-specific antibody reduced MDSC levels in the blood and secondary lymphoid organs in tumor-bearing mice [8].
There is a renewed interest in the study of complementary and alternative remedies (CAM) for the treatment of many diseases, including cancer, since many of them have proven clinical efficacy. Herba Epimedii is the common name for the dried aerial parts of the epimidium plants E. breVicornum, E. sagittatum Maxim or E. koreanum Nakai, also commonly known as rowdy lamb herb, barrenwort, bishop’s hat, fairywings, horny goat weed or ying yang huo [14, 15]. Several of its components have been studied, but icariin (ICA) was identified as the major active ingredient of Herba Epimedii that mediates anti-inflammatory functions, which include down-regulation of TNF-α, PGE(2) and nitric oxide (NO), as well as inhibition of NF-κB p65 activation. Recently, we described a novel derivative of icariin named, 3, 5, 7-Trihydroxy-4'-methoxy-8-(3-hydroxy-3- methylbutyl)–flavone (ICT), which has shown similar anti-inflammatory effects in macrophage cell lines, human myeloid cells, and a mouse model of inflammation [16]. Here we studied the anti-inflammatory potential of ICA and ICT in human innate immune cells and further investigated the role of ICA and ICT on the TLR4 signaling pathway. We discovered that these compounds reduce the basal expression of TLR4 in unstimulated human innate immune cells. Furthermore, we uncovered a correlation between MRP8/14 suppression, which are known markers of tumor progression, and prevention of tumor growth in tumor bearing mice by reducing MDSC accumulation and activation restoration of the functionality of effector CD8+ T cells.
2. Materials and methods
2.1. Reagents and antibodies
Icariin (ICA) and Icaritin (ICT) were purchased from Shanghai Ronghe Co (Shanghai, China). Dichlorodihydrofluorescein diacetate (DCFDA) was purchased from Molecular Probes (Eugene, O`R). The following antibodies were purchased from eBiosciences: anti-mouse-Ly6G and Ly6C PE, anti-mouse-CD11b APC, anti-mouse-CD8 PerCP-Cy5.5, anti-mouse CD11c APC, anti-mouse I-Ad FITC, anti-mouse F4/80 FITC, anti-mouse CD86 PE, anti-human-TLR4 APC, and all isotype control antibodies. Cytometric Bead Array (CBA) Mouse Inflammation Kit, anti-mouse-IFNγ APC, anti-mouse-I-Ad FITC, anti-Ly6G and Ly6C biotynlated antibodies were obtained from BD eBiosciences and streptavidin microbeads were purchased from Miltenyi Biotech. Anti-human-MRP8, anti-human-MRP14, anti-mouse S100A8 and anti-mouse S100A9 antibodies were purchased from Santa Cruz. Antibodies against total and phosphorylated forms of AKTSer473, STAT1, STAT3 and ERK were purchased from Cell Signaling Technology, Inc.
2.2. Mice, tumor establishment and ICT administration
Female BALB/c mice at 6 to 8 weeks of age were purchased from the National Cancer Institute (Frederick, MD). All mice were kept in pathogen-free conditions in the animal facility of Moffitt Cancer Center. All experiments were performed in accordance with pre-approved institutional protocols within the guidelines of the Animal Care and Use Committee. The mouse tumor cell line 4T1-Neu was grown in RPMI 1640 with 10% heat-inactivated fetal bovine serum with 100 units/ml penicillin, 100 µg/ml streptomycin, and 2 mM glutamine. BALB/c mice were inoculated subcutaneously (s.c.) in the flank with 5×105 4T1 mammary carcinoma cells. Tumor growth was monitored with bidirectional tumor measurements using calipers every 2–3 days and tumor volume was calculated using the formula V = 0.4ab2 with "a" as the larger diameter and "b" as the smaller diameter. ICT or ICA were given at a dose of 100 mg/kg via intraperitoneal (i.p.) injection three times a week starting on day 7 after tumor inoculation until completion of the experiment. The control group was given an equivalent amount of vehicle solvent.
2.3. Isolation of Gr-1+ splenocytes
Spleens were harvested under sterile conditions. Splenocytes pooled from 5 mice per group were prepared by lysing red blood cells using ACK lysing buffer. 1 × 108 splenocytes were resuspended in 0.9 ml of cold MACS buffer (0.5% BSA in PBS with 2 mM EDTA), incubated with 100 µl of biotinylated anti Ly-6C and Ly-6G (Gr-1) antibodies (Miltenyi Biotec) for 20 min at 4°C. Cells were washed with cold MACS buffer to remove unbound antibodies, and then incubated with 100 µl of streptavidin microbeads for 15 min at 4°C. The Gr-1+ cell population was isolated on a MACS column according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). The purity of the Gr-1+ cell population was evaluated by flow cytometry and exceeded 90%.
2.4. Western Blot Analysis
Cells were harvested, washed with ice-cold PBS, and lysed in 1% NP40, 10 mM Tris, 140 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride, 10 mM iodoacetamide, 50 mM NaF, 1 mM EDTA, 1 mM sodium orthovanadate, 0.25% sodium deoxycholate, 100 µl 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), leupeptin, aprotonin (ALA), and 100 µl of phosphatase inhibitor cocktails I and II (Sigma). Cell lysates were centrifuged at 12,000 × g for 15 min to remove nuclei and cell debris. The protein concentration of the soluble extracts was determined by using the Bio-Rad (Bradford) protein assay. For Western blots, 30–50 µg of protein was separated by 10% SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and probed with primary antibodies as indicated and then secondary antibodies conjugated with horseradish peroxidase. The specific proteins were detected by the enhanced chemiluminescence detection system (ECL, Amersham). The equal loading of protein sample was verified with an actin-specific antibody (Sigma, St Louis, MO, USA).
2.5. Flow cytometry
One million cells were incubated for 30 min on ice in the dark in staining media (0.5% BSA in PBS) with the relevant antibodies for surface antigens, and then washed with PBS. For intracellular staining of IFN-γ, cells were labeled with anti-CD8-PerCP-Cy5.5, fixed, permeabilized in Cytofix/Cytoperm buffer (BD Biosciences) for 20 min at 4°C, and washed with a 1X Perm/Wash solution (BD Biosciences). The cells were incubated with anti-IFN-γ-APC antibody for 30 min on ice. After washing, the samples were analyzed using a LSRII (BD Pharmingen), and the results were analyzed using Flowjo software (TreeStar).
2.6. Quantitative real-time PCR
Total RNA was extracted from cells using Trizol-Reagent according to the manufacturer's instructions (Life Technologies, Bethesda, MD). Reverse-transcription (RT) reactions were performed using iScriptTM cDNA Synthesis kit (Bio-Rad, Hercules, CA). Oligonucleotide primers for amplifying MRP8 were MRP8-F (5'- GGGATGACCTGAAGAAATTGCTA-3') and MRP8-R (5'- TGTTGATATCCAACTCTTTGAACCA -3'). Primers for amplifying MRP14 were MRP14-F (5'- GTGCGAAAAGATCTGCAAAATTT -3') and MRP14-R (5'-GGTCCTCCATGATGTGTTCTATGA -3'). Primers for amplifying TLR4 were TLR4-F (5'-ATTCCCGGTGTGGCCATT -3') and TLR4-R (5'-ACACCACAACAATCACCTTTCG -3'). Quantitative RT–polymerase chain reaction (qRT-PCR) reactions were performed by iQ SYBR Green Supermix of Bio-Rad. A negative control without cDNA template was run with every assay. Transcript copy number per subject was calculated by normalization to GAPDH expression.
2.7. ROS production
The oxidation-sensitive dye DCFDA (Molecular Probes/Invitrogen) was used to measure ROS production by MDSC. Cells were incubated at room temperature in RPMI in the presence of 3 µM DCFDA with or without 300nM phorbol 12-myristate 13-acetate (PMA) for 30 min, washed with PBS, and then labeled with anti–CD11b-APC and anti–Gr-1-PE antibodies. After incubation on ice for 20 min, cells were washed with PBS and analyzed using flow cytometry.
2.8. Arginase activity
Arginase activity was measured in cell lysates, as Kusmartsev and Gabrilovich described. In brief, cells were lysed for 30 min with 100 µl of 0.1% Triton X-100. Subsequently, 100 µl of 25 mM Tris-HCl and 10 µl of 10 mM MnCl2 were added, and the enzyme was activated by heating for 10 min at 56°C. Arginine hydrolysis was conducted by incubating the lysate with 100 µl of 0.5 M L-arginine (pH 9.7) at 37°C for 120 min. The reaction was stopped with 900 µl of H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v). Urea concentration was measured at 540 nm after addition of 40 µl of α-isonitrosopropiophenone (dissolved in 100% ethanol), followed by heating at 95°C for 30 min.
2.9. NO production
Equal volumes of culture supernatants (100 µl) were mixed with Greiss reagent solution (1% sulfanilamide in 5% phosphoric acid and 0.1% N-1-naphthylethylenediamine dihydrochloride) in double-distilled water and incubated for 10 min at room temperature. Absorbance of the mixture was measured at 550 nm using a microplate plate reader (Bio-Rad). Nitrite concentrations were determined by comparing the absorbance values for the test samples to a standard curve generated by serial dilution of 0.25 mM sodium nitrite.
2.10. CBA assay
Cultured supernatants from ICA-treated, ICT-treated or control DMSO-treated MDSCs were collected at 24 h. The concentrations of IL-10, TNF-α and IL-6 in the culture supernatants were measured by commercially available CBA Mouse Inflammation kit (BD Biosciences). Briefly, 50 µl of mixed capture beads were incubated with 50 µl of supernatant and 50 µl of PE detection reagent for 2 h at room temperature. Then, the immunocomplexes were washed and analyzed using FACSCalibur affixed with a 488-nm laser, according to the manufacturer's protocol. The data was processed with the accompanying FACSDiva and BD CBA software.
2.11. Statistical analysis
The statistical significance between values was determined by Student’s t test. All data were expressed as the mean ± SD. Values of P < 0.05 were considered statistically significant. For all experiments, the graphs represent the mean of three separate experiments and the error bars represent the standard error.
3. Results
3.1. ICA and ICT downregulates surface expression of TLR4 on PBMCs from healthy donors
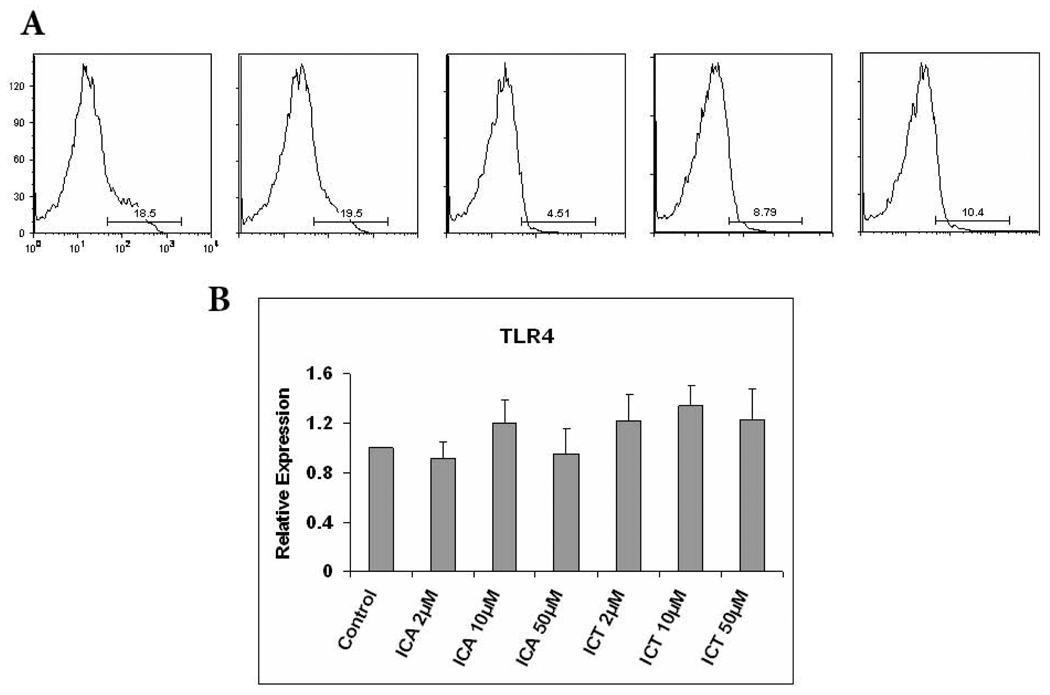
As described in the introduction, we have previously demonstrated in both human and mice, that ICA and ICT are capable of decreasing the expression of TLR4 on LPS-stimulated myeloid cells. To explore whether ICA or ICT are able to modulate the expression of TLR4 in unstimulated cells, PBMCs from healthy donors were incubated with different concentrations of ICA, ICT or vehicle for 24 h and then evaluated for their expression of TLR4. As shown in Fig. 1A, we found that treatment of PBMCs with 50 µM of ICA or treatment with 10 µM or 50 µM of ICT resulted in substantial loss of TLR4 surface expression, while 10 µM of ICA had no effect on TLR4 surface expression. Since these were unstimulated cells, we determined whether ICA or ICT exposure influenced TLR4 downregulation at the mRNA level. As shown in Fig. 1B, ICA and ICT treatment had no effect on TLR4 mRNA indicating that their action is probably mediated by the modulation of protein without affecting transcription.
Fig. 1.
TLR4 expression was decreased by ICA and ICT in unstimulated PBMC. A) PBMCs were treated with ICA, ICT or vehicle control for 24 h, flow cytometric analysis of TLR4 expression on the cell surface in PMBCs. B) PBMCs from healthy donor were incubated with ICA, ICT or vehicle for 24 h, RNA was extracted and expression of TLR4 was evaluated by quantitative real-time PCR as described in Materials and Methods.
3.2. MRP8/MRP14 expression by PBMCs is reduced upon treatment with ICA or ICT
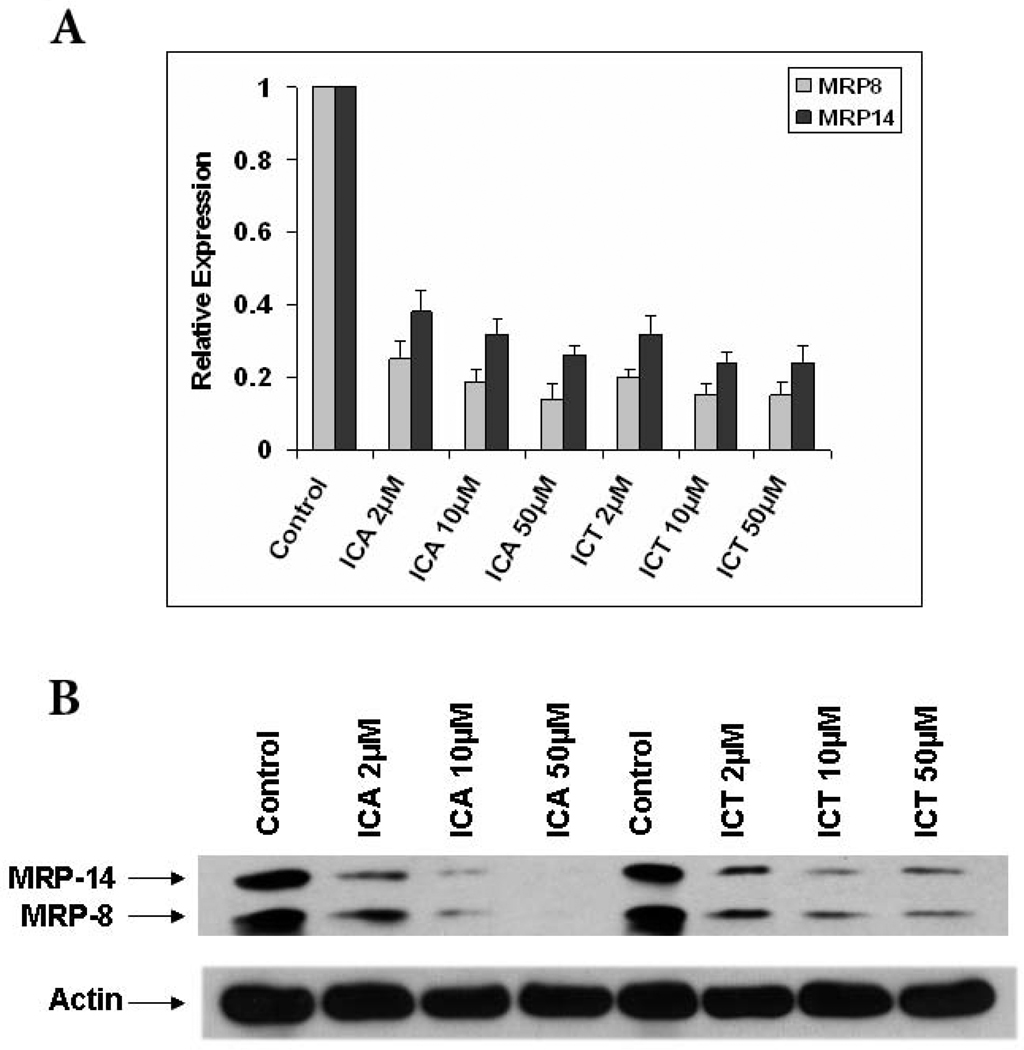
MRP8 and MRP14 are endogenous activators of TLR4 and therefore considered important pro-inflammatory mediators in acute and chronic inflammation [6, 17]. Since these TLR4 DAMPs are capable of modulating inflammation, we investigated whether ICA and ICT could affect their expression in human PBMCs. We incubated PBMCs from healthy donors with different concentrations of either compound and observed that the expression of MRP8 and MRP14 was dramatically decreased at the mRNA and protein levels (Fig. 2A and B).
Fig. 2.
ICA and ICT down-regulates the expression of MRP8/14 in PMBCs. A) PBMCs from healthy donor were treated with ICA, ICT or vehicle (DMSO) for 48 h, RNA was extracted and expression of MRP8/14 was evaluated by quantitative real-time PCR as described in Materials and Methods. B) PBMCs were treated with ICA, ICT or vehicle for 48 h and then lysed, western blots were performed with antibodies specific for MRP8/14 or Actin.
3.3. ICA and ICT displays strong anti-tumor properties and decreases the percent of MDSCs in 4T1-Neu mammary tumor bearing mice
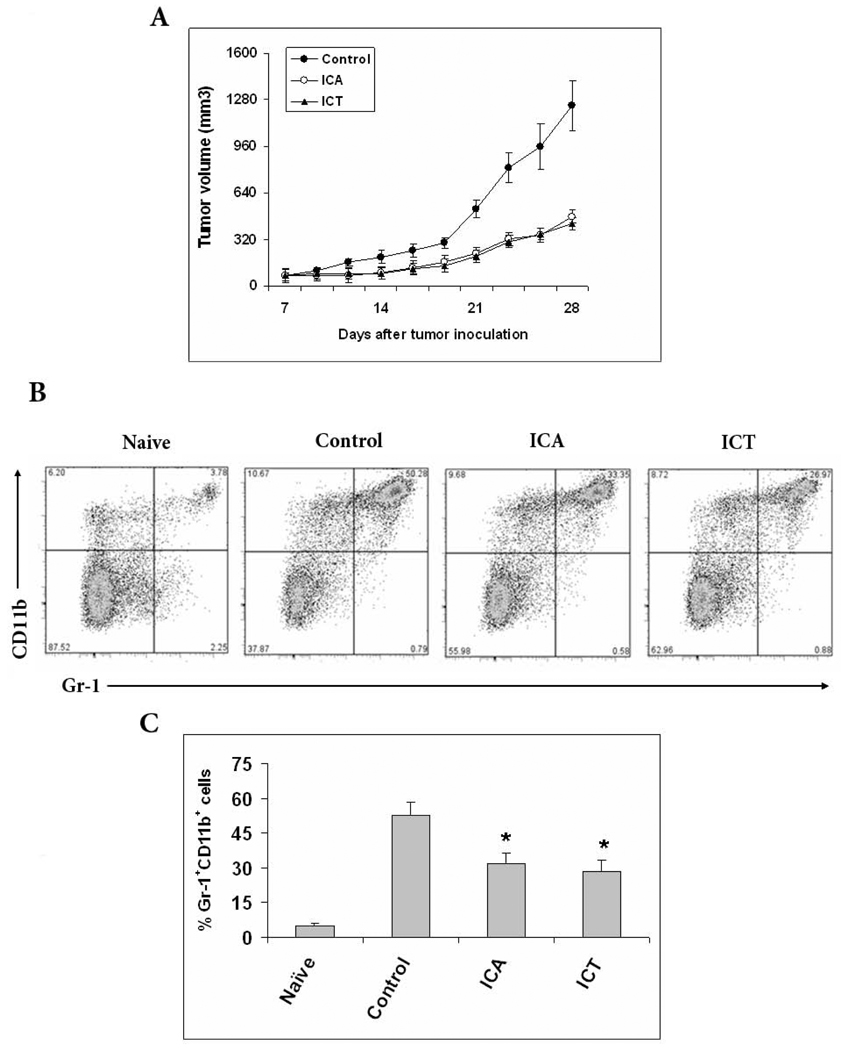
MRP8/14 has recently gained attention as a molecular marker up-regulated in various human cancers, hence their regulation by ICA and ICT could prove to have anti-tumor effects [18]. To examine the in vivo antitumor effect of ICA and ICT, 4T1 mammary carcinoma cells (5×105) were injected s.c. in the right suprascapular area of BALB/c mice on day 0. Treatment was administered as indicated in “material and methods” (section 2.2). Each group, consisting of five mice, was treated with i.p. injection of 100 mg/kg of ICA, 100 mg/kg of ICT, or vehicle. We observed that ICA and ICT significantly delayed tumor progression as compared to untreated tumor-bearing mice (Figure 3A) out to 28 days post tumor inoculation. The vehicle-treated group had tumors that were 1243.7 ±173.4 mm3 compared with 479.22 ± 40.7 mm3 in the ICA-treated group and 427.57 ±53.3 mm3 in the ICT-treated group. The study was terminated at this point because the tumors of the vehicle treated mice were exceeding IACUC humane standards. This indicates that apart from their anti-inflammatory properties, ICA and ICT have potent anti-tumor effects against 4T1-neu tumors.
Fig. 3.
ICA and ICT treatment retarded tumor growth and reduced Gr-1+CD11b+ MDSCs in the spleens of mice bearing tumors. A) 4T1 tumors were established in BALB/c mice, When tumor reached 1 cm in diameter (about 7 days post injection), mice were treated with 100mg/kg of ICA, 100mg/kg of ICT or vehicle (i.p.) three times weekly until completion. Tumor size (mean and SD) was monitored every 2 to 3 days. Each group includes five mice. B) Spleen cells from naïve or tumor bearers were stained with PE-conjugated anti–Gr-1 and APC-conjugated anti-CD11b antibodies. The percentages of double-positive Gr-1+CD11b+ cells are shown for spleens of five mice per group. C) Same as B were columns represent the mean of three separate experiments; bars, SE.*, statistically significant differences compared with controls (P < 0.05).
MRP8/MRP14 is highly expressed by activated myeloid cells and our research has effectively demonstrated that ICA and ICT can modulate the expression of pro-inflammatory molecules. Furthermore, immature myeloid subsets with suppressive properties have gained strong recognition due to their link to cancer [7]. To assess the effect of ICA and ICT on these suppressive cells, collectively called MDSC, we analyzed the percentage of MDSC in the spleens of 4T1-Neu tumor-bearing mice with or without treatment of ICA or ICT. Cells expressing Gr-1+CD11b+ on their surface were considered to be MDSC. 28 days post tumor inoculation, the spleens of vehicle treated tumor-bearing mice had 52.3±5.9% of MDSCs, whereas the ICA and ICT treatment significantly reduced the proportion of Gr-1+CD11b+ cells to 31.8±4.6% and 28.2±5.1%, respectively. This data is shown as a representative experiment (Fig. 3B) or as the mean of the percentage ± SE of three identically conducted experiments, both reflecting the same trend (Fig. 3C).
3.4. ICA and ICT restores the functional activity of CD8+ T cells
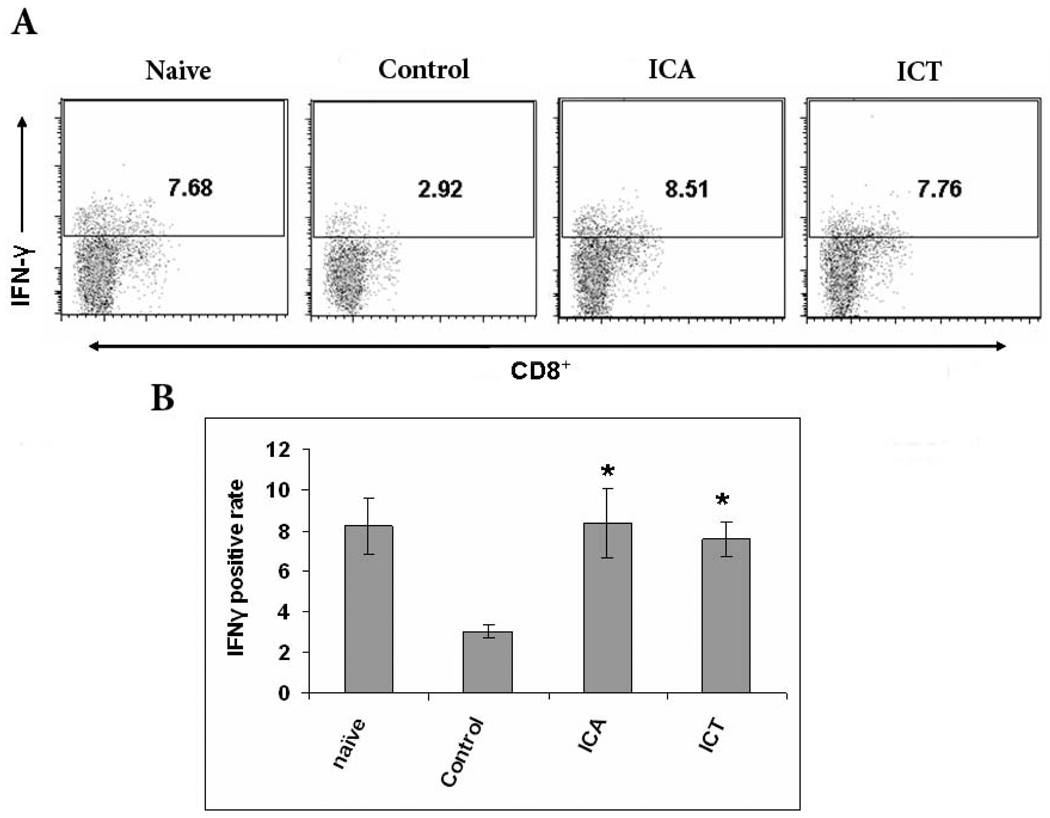
Inhibition of antigen-specific CD8+ T cells is one of the main suppressive characteristics of MDSCs. If ICA and ICT can reduce MDSCs numbers, we reasoned then that T-cell function should correspondingly recover in ICA and ICT-treated mice. To test this hypothesis, we analyzed IFN-γ production as a measure of T-cell function. Spleens of tumor-bearing mice treated with ICA, ICT, or vehicle were harvested and stained for intracellular IFN-γ and surface CD8. We found that splenocytes from tumor bearing mice treated with ICA or ICT had a significantly higher percent of IFN-γ producing CD8+ T cells compared to vehicle control-treated mice (Fig. 4A and B). Thus, these results indicate that ICA or ICT treatment can restore the functional capacity of CD8+ T cells within the spleens of tumor-bearing mice.
Fig. 4.
ICA and ICT treatment of tumor-bearing mice generates significant IFN-γ production in CD8+ T cells. A) Flow cytometric analysis of cells isolated from spleens of naive mice, vehicle-treated tumor bearers, ICA-treated tumor bearers, and ICT-treated tumor bearers. Splenocytes were labeled with anti-CD8 and anti-IFN-γ antibodies. CD8+ cells were gated and the proportion of IFN-γ-positive cells was calculated. B) Same as A where columns represent the mean cell number of IFN-γ–producing cells (n = 5); bars, SE. *, statistically significant differences compared with vehicle (P < 0.05).
3.5. Regulation of MDSC function by ICA and ICT in vivo
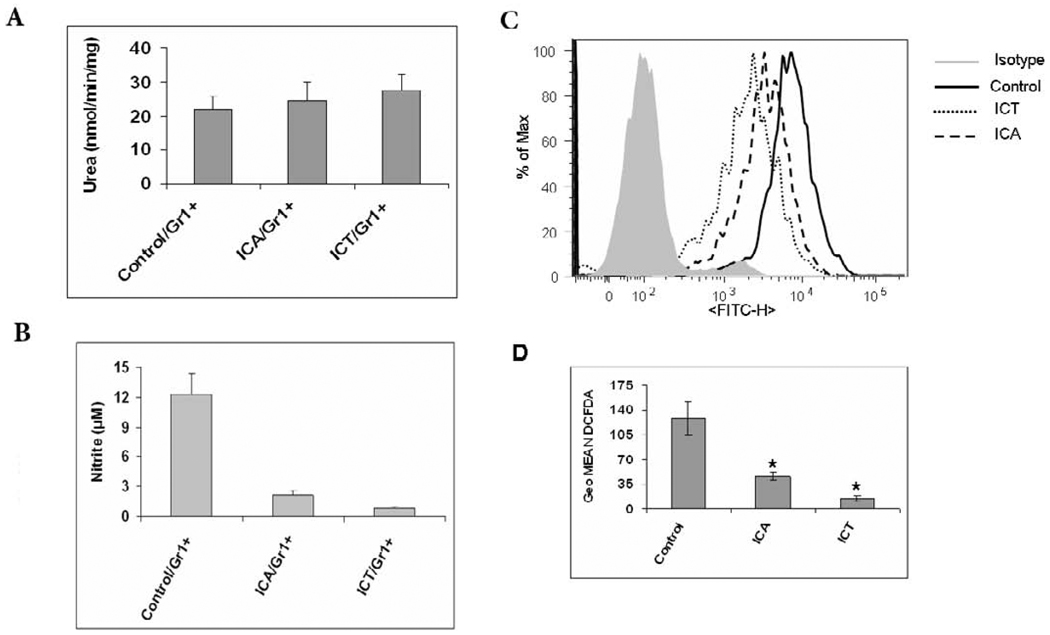
Several major factors contribute to MDSC-mediated immune suppression. These include the production of arginase, NO, and reactive oxygen species (ROS). We next investigated the suppressive mechanisms that may be affected by ICA and ICT treatment in vivo. We observed that ICA and ICT had no affect on arginase activity (Fig. 5A,), but NO and ROS production by MDSC from tumor-bearing mice were significantly decreased upon treatment with both compounds (5B, 5C and 5D). These data further define the function of ICT and ICA in MDSC in vivo as we show that not only do these compounds reduce the percent of MDSC in the spleens of tumor bearing mice, but also both inhibit suppressive functions of MDSC.
Fig. 5.
ICA and ICT significantly reduced the level of NO and ROS, whereas had no effect on arginase activity in MDSC. MDSCs were isolated from spleens of 4T1 tumor-bearing mice, cultured and suppressive function was assayed by A) Arginase activity, B) NO activity and C) the level of ROS in cells measured using DCFDA staining and flow cytometry (representative histogram). Splenocytes from mice were incubated at room temperature in the presence of 3 µM DCFDA with or without 300 nM PMA for 30 min, washed with PBS, and then stained with antibodies against CD11b-APC and Gr1-PE, and then was analyzed using flow cytometry. D) Same as C where columns represent the mean of three separate experiments. * Statistically significant differences compared with vehicle (P < 0.05).
3.6. Regulation of MDSC maturation and cytokine secretion by ICA and ICT in vitro
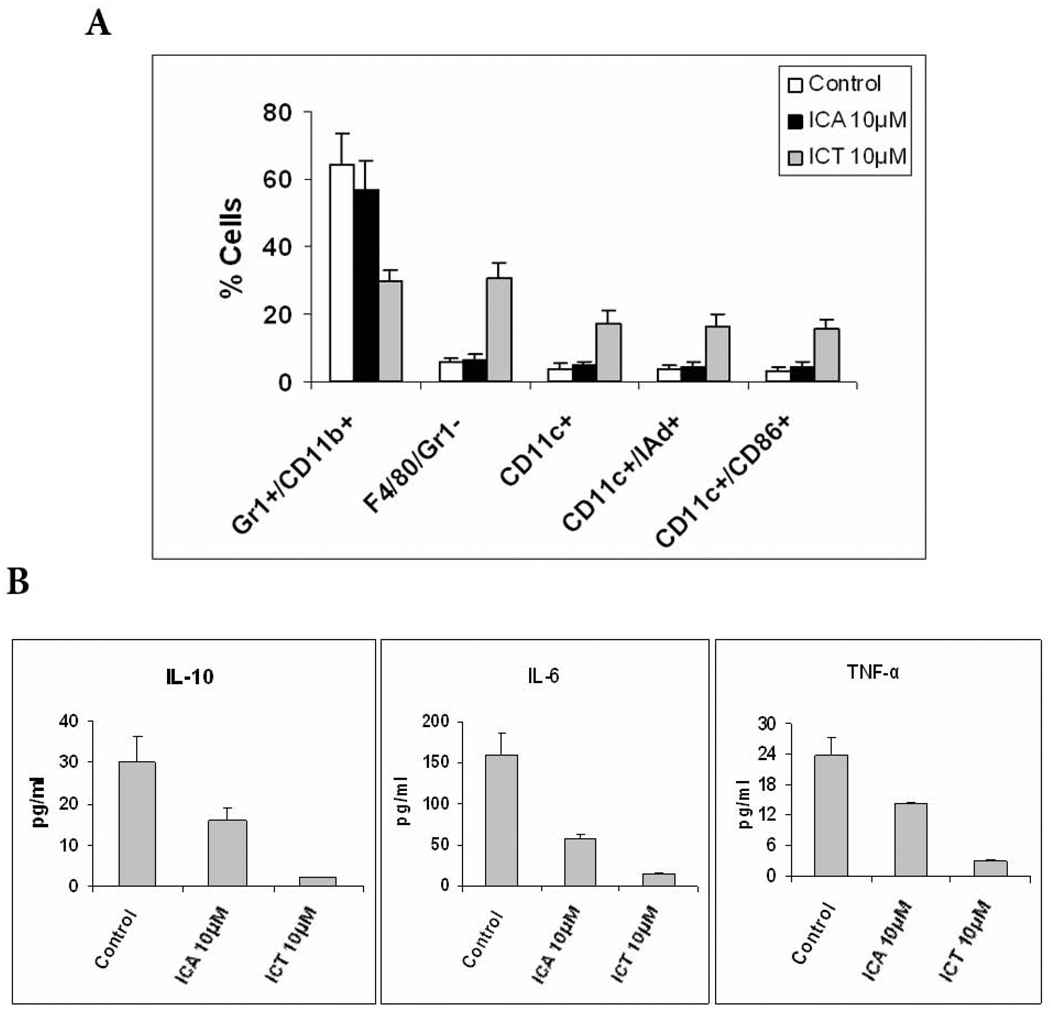
To evaluate the effect of ICA and ICT on MDSC differentiation in vitro, MDSCs were isolated from the spleens of 4T1 tumor-bearing mice and cultured with 4T1 tumor cell conditioned medium (TCCM) and GM-CSF for 5 days. To generate TCCM, cells were kept for 48 h in medium with reduced (3%) fetal bovine serum (FBS) concentration. During the first 24 h of culture, cells were treated with ICA, ICT, or vehicle. MDSCs were then stained for F4/80, CD11C, I-Ad, and CD86 to analyze if ICA or ICT can induce MDSC maturation and differentiation into macrophages and/or dendritic cells (DCs). Our data show that ICT substantially reduces the proportion of MDSCs and induces their differentiation into macrophages and DCs. However, vehicle or ICA had no effect on the proportion of MDSCs, F4/80+ macrophages or DCs (Fig. 6A).
Fig. 6.
Effects of ICA or ICT on differentiation of MDSC. A) MDSC were isolated from spleens of 4T1 tumor-bearing mice, they were cultured with GM-CSF and TCCM for 5 d in the presence of ICA or ICT. Cell phenotype was evaluated by flow cytometry. *, P<0.05, versus cells treated with vehicle alone. B) Cytokine analysis of supernants of MDSCs cultured in the presence of vehicle, ICA or ICT for 24h.
To further characterize the effect of ICA and ICT in modulating the MDSC population, we analyzed cytokine secretion by MDSCs that have been cultured with or without ICA or ICT. We discovered that both ICA and ICT reduced the amounts of IL-10, IL-6 and TNF-α in the supernatant of these cell cultures (P < 0.05) (Fig. 6B).
3.7. Mechanism of ICT effect on MDSC in vitro
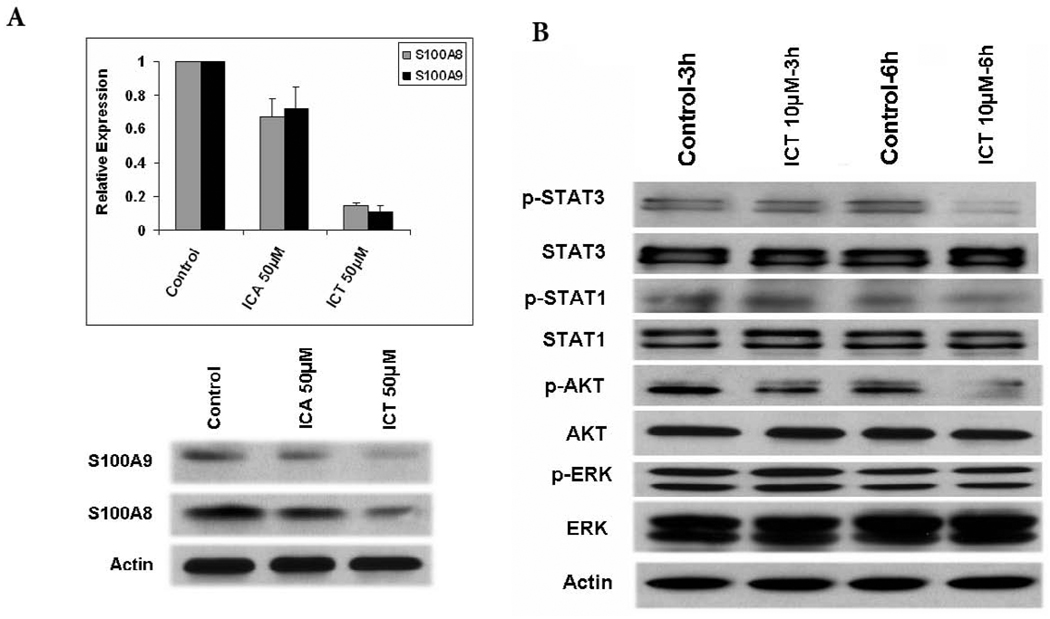
Our study showed that ICA and ICT inhibited the expression of MRP8/14 in human PBMCs. Since S100A8/A9 (MRP8/14) complexes play a central role in the induction and maintenance of MDSC in tumor-bearing mice, we next analyzed whether ICA and ICT could downregulate the expression of S100A8/A9 in MDSCs from tumor bearing mice. As shown in Fig. 7A, MDSCs treated with ICT showed a significant downregulation of S100A8/A9, whereas ICA treatment had minimal effect on S100A8/A9 expression by MDSCs.
Fig. 7.
ICT selectively inhibits MDSCs via AKT and STAT3 signaling. A) MDSC were isolated from spleens of 4T1 tumor-bearing mice and treated with different concentrations (10 µM or 50 µM) of ICA or ICT for 48 h. RNA was extracted from MDSC, and the expression of MRP8/14 was measured by quantitative real-time PCR and western blot. B) Western blot analysis of different proteins in MDSC after treatment with 10 µM ICT or vehicle DMSO for 3 and 6 h.
Collectively, our results suggest that ICT is more suppressive of MDSCs from tumor-bearing mice than ICA. We then decided to focus our efforts on understanding the functional mechanisms of ICT in MDSC. Stat3 activation is known to play a critical role in accumulation of MDSC. Further, Akt and ERK1/2 are commonly hyperactivated in pathological conditions, such as cancer. Therefore we examined the expression of STAT3, STAT1, AKT, and ERK, and their phosphorylated counterparts in MDSCs isolated from the spleens of tumor-bearing mice cultured in the presence of 10µM of ICT or DMSO for 3h and 6h. Western blot analysis revealed that ICT had no effect on total STAT3 and total AKT protein levels in MDSCs, but clearly inhibited the phosphorylated of STAT3 and AKT (pSTAT3 and pAKT) as early as 6 hours after treatment as determined by western blot (Fig. 7B). However, ICT did not have any notable inhibitory effects on pSTAT1 or pERK (Fig. 7B).
4. Discussion
The immune response is a multisided dynamic equilibrium that must be tightly regulated to prevent diseases or cure them. Our quest to understand the properties of ICA and ICT has provided us with further evidence that regulating this equilibrium will generate tools for future use against cancer as well as many other diseases. We investigated the role of these compounds in modulation of inflammatory responses by demonstrating their ability to downregulate basal levels of TLR4 and MRP8/14. Moreover, these compounds were able to prevent tumor growth in vivo by inhibiting suppressive immune mechanisms.
Our combined observations from this study, as well as previous ones, have provided further insight into the mechanisms of these compounds. Based on our previous findings, LPS-stimulated murine and human myeloid cells (monocytes and macrophages), exhibited lower levels of TNF-α, TLR4, and CD14 after treatment with ICA and ICT [16, 19, 20]. These changes correlate with studies in other inflammatory disease model [20–23], although all of which studied the effects of ICA and ICT in LPS stimulated systems. Here we wanted to investigate the role of ICT and ICA on cells that are unstimulated, or rather, a more physiological model. Interestingly, we observed that TLR4 is reduced at the surface protein level without stimulation, but mRNA levels remain unchanged. The exact mechanism for this disparity in TLR4 expression is unknown at this time. It is possible that TLR4 is being directly interacted with ICT or ICA and being subsequently endocytosed.
While bacterial lipopolysaccharide (LPS) is the canonical activator of TLR4, it has recently been shown that MRP8/MRP14 is an endogenous ligand for TLR4 [6, 24–27]. Considering that the expression of MRP8/14 may have an impact on the expression of TLR4, we were prompted to look at the levels of MRP8/14 upon treatment with ICT and ICA. We found that both mRNA and protein levels of MRP8/MRP14 were down-regulated in the presence of ICA and ICT. The down-regulation of MRP8/MRP14 may account for some of the anti-inflammatory properties of ICA and ICT; however, it is unlikely to be the only factor. Exactly how ICA and ICT lead to the decreased expression of TLR4 and its ligand, MRP8/14 remains unknown and warrants further investigation.
Chronic inflammation has been shown to promote tumor progression by blocking adaptive immunity through the induction of MDSC, which prevent the activation of T cells [28]. If inflammation facilitates tumor progression through the induction of more suppressive MDSC by signaling through the TLR4 pathway, then it is possible that a decreased pro-inflammatory microenvironment may reduce the potency of MDSC. Along with previous studies, here we have confirmed that ICA and ICT inhibit inflammatory responses by down-regulating TLR4 expression. Furthermore, the importance of TLR4 and MRP8/14 has been highlighted in many diseases, including cancer [4, 17, 29, 30]. Specifically, expression of both TLR4 and MRP8/MRP14 is increased in various tumors implicating their function in the pathogenesis or development of these diseases [18, 31–33]. Hence, while our original intention to study changes in MRP8/MRP14 was based on its strong relationship with TLR4, seeing that this DAMP, normally present in cells and only released upon stimulation, was down-regulated at both the protein and the mRNA levels lead us to hypothesize that ICA and ICT could have a role in preventing carcinogenesis [4]. Based on that premise, we shifted our studies to a murine tumor model where we could observe a direct impact on tumor progression. Indeed we observed strong tumor suppressive function with both compounds. Furthermore, our data demonstrate that treatment of tumor-bearing mice with ICA or ICT correlated with a major decrease in the number of splenic MDSC.
MDSC are one of the major factors mediating tumor-associated immune suppression, and their removal results in improvement of immune response in cancer, which could be potentially beneficial for various immunotherapeutic strategies. Moreover, we see that elimination of MDSC in ICA or ICT-treated animals results in increased IFN-γ production by CD8+ T cells. Similar results were shown in MRP8/MRP9 knockout mice in which the reduction of MDSCs correlated with tumor shrinkage and greater anti-tumor CD8+ effector cells [7, 34, 35]. We therefore investigated the possible mechanism of ICA and ICT in the regulation of MDSC function. Arginase, NO, and ROS are all implicated in MDSC–mediated T-cell suppression [10, 36]. Moreover, ROS production has been shown to be directly involved in MDSC-mediated T-cell suppression, and also acts as one of the major factors for preventing DC differentiation in tumor-bearing mice. Interestingly, the blockade of ROS production by MDSC promoted their differentiation into dendritic cells [37, 38]. Our in vivo experiments showed that ICA and ICT downregulates the level of NO and ROS in MDSC, but has no effect on the level of arginase. Additionally, both compounds similarly mediated an antitumor effect and decreased the proportion of MDSC in tumor-bearing mice. ICT, however, uniquely decreased the proportion of MDSC and induced MDSC to differentiate into mature DCs in vitro. It is known that the tumor burden directly affects the expansion of MDSC [39]. It is possible that ICT reduces the numbers of MDSC by direct inhibition of tumor growth; however, it is also likely that ICT treatment reduces the numbers of MDSC via an indirect immunomodulatory mechanism. Our results show that ICA and ICT may modulate the tumor microenvironment by decreasing MDSC numbers, but these compounds also downregulate cytokines, IL-10, IL-6 and TNF-α, molecules known to have a role in inflammation and MDSC modulation.
Observations on mouse cancer models have suggested that tumor-derived factors induce the expansion of MDSCs and inhibit their differentiation into DCs by activation of JAK2–STAT3 pathways in MDSCs [10, 40]. Since STAT3 is the main transcription factor that regulates the expansion of MDSCs, we evaluated the effect of ICT on STAT3 and other signal transduction pathways in MDSC. ICT treatment diminished pSTAT3 and pAKT in MDSCs; however, ICT did not have any notable inhibitory effects on ERK or STAT1 activation. S100A8/A9 proteins have a crucial role in regulating MDSC expansion, and provide a link between inflammation and immune suppression in cancer [10]. Recent findings suggest that S100A8/A9 proteins inhibit the differentiation of MDSCs into DCs and macrophages through a STAT3-dependent mechanism [10]. Our data show that ICT significantly down-regulates S100A8/9 expression by MDSC in vitro, which suggests a major immunomodulatory effect of ICT is by downregulation of S100A8/9, STAT3, and AKT in MDSCs, leading to their differentiation into macrophages and DCs. Overall, these studies show ICT has direct effects on MDSC signaling, phenotype and function, in vitro and in vivo. Our study demonstrates the potent immune and anti-tumor effects elicited by ICA and its derivative ICT, which warrants their future exploration pre-clinically as adjuvants or perhaps even stand-alone chemotherapeutic agents in cancer.
Acknowledgements
This project was supported by National Institutes of Health grant AI056213 and James and Esther King Program Team Science Project Grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ' DAMP' view of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3:382–390. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 4.Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 5.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 6.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 7.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JJ, Li SP, Wang YT. Optimization for quantitative determination of four flavonoidsin Epimedium by capillary zone electrophoresis coupled with diode array detection using central composite design. J Chromatogr A. 2006;1103:344–349. doi: 10.1016/j.chroma.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Mun YJ, Im SJ, Han JH, Lee HS, Woo WH. Effects of the aqueous extract of epimedii herba on the antibody responses in mice. Int Immunopharmacol. 2001;1:935–944. doi: 10.1016/s1567-5769(01)00030-3. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Du J, Xu C, Le J, Liu B, Xu Y, et al. In vivo and in vitro anti-inflammatory effects of a novel derivative of icariin. Immunopharmacol Immunotoxicol. 2010 Mar 25; doi: 10.3109/08923971003725144. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+T cells. Nat Med. 16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 18.Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622–1631. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao YX, et al. Icariin attenuates LPS-induced acute inflammatory responses: involvement of PI3K/Akt and NF-kappaB signaling pathway. Eur J Pharmacol. 642:146–153. doi: 10.1016/j.ejphar.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Wu JF, Dong JC, Xu CQ. Effects of icariin on inflammation model stimulated by lipopolysaccharide in vitro and in vivo. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:330–334. [PubMed] [Google Scholar]
- 21.Guo J, Li F, Wu Q, Gong Q, Lu Y, Shi J. Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine. doi: 10.1016/j.phymed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Xie JY, Dong JC, Cui Y. Effect of epimedium herb on RANTES and monocyte chemotactic protein-3 expression in lung tissue of asthmatic rats. Zhongguo Zhong XiYi Jie He Za Zhi. 2008;28:238–241. [PubMed] [Google Scholar]
- 23.Johansen RF, Sorensen B, Ingerslev J. Acquired haemophilia: dynamic whole blood coagulation utilized to guide haemostatic therapy. Haemophilia. 2006;12:190–197. doi: 10.1111/j.1365-2516.2006.01166.x. [DOI] [PubMed] [Google Scholar]
- 24.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 26.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 27.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 28.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 29.Lin WJ, Yeh WC. Implication of Toll-like receptor and tumor necrosis factor alpha signaling in septic shock. Shock. 2005;24:206–209. doi: 10.1097/01.shk.0000180074.69143.77. [DOI] [PubMed] [Google Scholar]
- 30.Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm Des. 2006;12:4229–4245. doi: 10.2174/138161206778743501. [DOI] [PubMed] [Google Scholar]
- 31.Voelcker V, Gebhardt C, Averbeck M, Saalbach A, Wolf V, Weih F, et al. Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp Dermatol. 2008;17:100–107. doi: 10.1111/j.1600-0625.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 32.Arai K, Takano S, Teratani T, Ito Y, Yamada T, Nozawa R. S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Curr Cancer Drug Targets. 2008;8:243–252. doi: 10.2174/156800908784533445. [DOI] [PubMed] [Google Scholar]
- 33.Muller H, Haug U, Rothenbacher D, Stegmaier C, Brenner H. Evaluation of serum and urinary myeloid related protein-14 as a marker for early detection of prostate cancer. J Urol. 2008;180:1309–1312. doi: 10.1016/j.juro.2008.06.025. discussion 12-3. [DOI] [PubMed] [Google Scholar]
- 34.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 35.Varga G, Ehrchen J, Tsianakas A, Tenbrock K, Rattenholl A, Seeliger S, et al. Glucocorticoids induce an activated, anti-inflammatory monocyte subset in mice that resembles myeloid-derived suppressor cells. J Leukoc Biol. 2008;84:644–650. doi: 10.1189/jlb.1107768. [DOI] [PubMed] [Google Scholar]
- 36.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67:11021–11028. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 38.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 39.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 16:1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]