Abstract
Parkinson's disease (PD) is a neurodegenerative disorder marked by the selective loss of dopaminergic neurons, leading to a decrease of the neurotransmitter dopamine (DA). DA is metabolized by monoamine oxidase to 3,4-dihydroxyphenyacetaldehyde (DOPAL). While the mechanism of pathogenesis of PD is unknown, DOPAL has demonstrated the ability to covalently modify proteins and cause cell death at concentrations elevated from physiologic levels. Currently, the identities of protein targets of the aldehyde are unknown, but previous studies have demonstrated the ability of catechols and other DA-catabolism products to interact with and inhibit tyrosine hydroxylase (TH). Given that DOPAL is structurally related to DA and is a highly reactive electrophile, it was hypothesized to modify and inhibit TH.
The data presented in this study positively identified TH as a protein target of DOPAL modification and inhibition. Furthermore, western blot analysis demonstrated a concentration-dependent decrease in antibody recognition of TH. DOPAL in cell lysate significantly inhibited TH activity as measured by decreased L-DOPA production. Inhibition of TH was semi-reversible, with the recovery of activity being time and concentration-dependent upon removal of DOPAL. These data indicate DOPAL to be a reactive DA-metabolite with the capability of modifying and inhibiting an enzyme important to DA synthesis.
Keywords: Tyrosine hydroxylase, Parkinson's disease, enzyme inhibition, dopamine metabolite
1. INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disorder marked by the selective loss of dopaminergic neurons in the substantia nigra. Such a condition leads to a decrease in the important neurotransmitter dopamine (DA), causing a variety of symptoms including motor impairment. Currently, the mechanism of pathogenesis is unknown; however, studies have revealed a link to both environmental causes, such as pesticides, as well as endogenously produced oxidative stress (Andersen 2004, Jenner 2003, Fleming et al. 1994).
DA is metabolized by monoamine oxidase (MAO) to the reactive intermediate 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is then further oxidized by mitochondrial aldehyde dehydrogenase 2 (ALDH2) to 3,4-dihydroxyphenylacetic acid (DOPAC), and in a lesser pathway, by cytosolic aldehyde reductase (ALR) to 3,4-dihydroxyphenylethanol (DOPET) (Burke et al. 2004). While previous work has established DA to be an endogenous neurotoxin, capable of auto-oxidation leading to protein modification (Graham 1978, Graham et al. 1978, Stokes et al. 1999), the metabolite DOPAL was found to be several orders of magnitude more toxic than DA (Burke 2003, Burke et al. 2003). Physiological concentrations of DOPAL were measured to be ~2–3 μM, and it was shown that when levels of DOPAL are slightly elevated (6.6 μM), there is a decrease in TH-positive cells, indicating dopaminergic cell death (Kristal et al. 2001, Mattammal et al. 1995, Burke et al. 2003, Burke 2003). Furthermore, DOPAL has been implicated in protein modification (Rees et al. 2007, Helander & Tottmar 1989, Ungar et al. 1973, Nilsson & Tottmar 1987, LaVoie et al. 2005). Studies have demonstrated the ability of DOPAL to covalently modify Lys residues via the aldehyde (Rees et al. 2009), forming a Schiff-base structure predicted to interfere with normal protein function. Currently, specific protein targets of DOPAL are unknown, but previous studies have revealed that catechols and other DA-metabolism products interact with and inhibit tyrosine hydroxylase (TH), the rate-limiting step in DA synthesis (Laschinski et al. 1986, Xu et al. 1998).
Tyrosine hydroxylase (EC 1.14.13.41) catalyzes the oxidation of L-tyrosine to L-DOPA, and L-amino acid decarboxylase converts L-DOPA to DA (Nagatsu et al. 1964, Elsworth & Roth 1997). Due to the importance TH plays in the synthesis of DA, a decrease or inhibition of this enzyme is predicted to yield a decrease in DA. Furthermore, studies have shown L-DOPA to have trophic properties, leading to an increase in TH+ neurons in both male and female rats with 6-hydroxydopamine lesions (Datla et al. 2001, Murer et al. 1998). Therefore, inhibition of TH activity may also lead to a decrease in cell function and viability.
It is hypothesized that DOPAL is an endogenously produced neurotoxin that modifies and inhibits enzymes important to DA biosynthesis, such as TH, leading to a decrease in DA production. The work presented here confirms DOPAL toxicity in dopaminergic PC6-3 cells, demonstrates inhibition of TH by DOPAL, and for the first time positively identifies TH as a protein target of DOPAL modification.
2. Materials and Methods
2.1. Materials
DOPAL was biosynthesized as previously described using enzyme-catalyzed conversion of DA to DOPAL by rat liver MAO (Nilsson & Tottmar 1987), and the concentration was determined using an ALDH assay with NAD (Ungar et al. 1973) and HPLC analysis as described below. 3,4-dihydroxyphenylethanol (DOPET) was obtained via reduction of DOPAL using a 10-fold excess of sodium borohydride. Tyrosine, L-DOPA, DA, DOPAC, and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
2.2. Cell culture
PC6-3 cells were cultured in RPMI 1640 medium supplemented with heat-inactived 10% horse serum, 5% fetal bovine serum, penicillin (10 IU/mL) and streptomycin (10 mg/mL). PC6-3 cells are a subline of the pheochromocytoma (rat adrenal medulla) PC-12 cell line. They were chosen due to a decreased tendency from the parent line to aggregate, as well as decreased background cell death, and they are a homogeneous population that can be easily maintained (Strack 2002, Pittman et al. 1993). PC-12 are also are a widely accepted model for DA synthesis and metabolism (Kitazawa et al. 2001, Hirata et al. 1998). Furthermore, PC12 cells assume a neuronal cell phenotype in the presence of nerve growth factor, and have important neurochemical processes similar to dopaminergic neurons (Shafer & Atchison 1991, Seegal et al. 1989). Cells were grown in a 10 cm tissue culture dish at 37 °C in 5% CO2 for 3 days. Cells were then seeded into six-well plates (1 × 105) and were incubated at 37 °C in 5% CO2 for 3 days prior to the addition of nerve growth factor (NGF) (50 ng/mL) to stimulate cell differentiation. PC6-3 cells were then kept in the same conditions for 4 days prior to use. For experiments involving treatment with DOPAL, cellular media was removed in order to eliminate DOPAL interaction with serum proteins and replaced with HEPES-buffered media containing 115 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5.5 mM glucose, 1 mM NaH2PO4, and 15 mM HEPES (pH 7.4) to which DOPAL (5, 10, 25, 50 μM) was added. Cells were then incubated for 2 hr at 37 °C in 5% CO2.
2.3. Aminophenylboronic acid (APBA) protein separation
PC6-3 cells were cultured and incubated with DOPAL as previously described. Control (i.e. no DOPAL) and 50 μM DOPAL conditions were used in APBA protein separation. After 2 h, extracellular media was removed and 300 μL lysis buffer (0.1% Triton-X in 10 mM KH2PO4) was added and plates were placed on dry ice 5 min to rupture cell membranes. Lysate was then collected and supernatant was used for APBA isolation of DOPAL-bound proteins as previously described with some modification (LaVoie et al. 2005). The boronic acid component of the APBA resin binds covalently to vicinal diols (i.e. catechols) under alkaline conditions, and the diol or catechol can be subsequently released via acidic conditions as described below. DOPAL has been shown to modify proteins amines (e.g., Lys) via the aldehyde to yield a Schiff-base, leaving the catechol on DOPAL available for reaction with the APBA resin (Rees et al. 2009). Briefly, 100 μL of resin was washed and equilibrated 3 times in 50 mM sodium phosphate buffer (pH 7.4) and centrifuged at 10,000 × g for 3 min. After removal of the final wash, 100 μL of sample (either control or 50 μM DOPAL lysate) and 100 μL of buffer were added to the resin and allowed to incubate for 4 h at room temperature on a shaker table. The resin was subsequently washed with 300 μL of 1:1 acetonitrile:50 mM sodium phosphate buffer 3 times, and 300 μL of distilled water 3 times, centrifuging after each wash fraction (10,000 × g, 3 min). In order to elute protein bound to the APBA resin, the resin was washed with 100 μL of 1% trifluoroacetic acid (TFA) 2 times, 100 μL of 1:1 acetonitrile:1% TFA once, and 100 μL of 1:1 acetonitrile:H2O once, centrifuging after each release fraction (10,000 × g, 3 min). The pH of the each release fraction was immediately neutralized using 5 μL of 1 M sodium phosphate buffer (pH 7.4). A 10% gel was used for SDS-PAGE gel electrophoresis and Coomassie blue staining was used for protein separation and detection.
2.4. Proteomic analysis
Bands of interest from the APBA resin-bound fractions were excised and digested using trypsin as previously described (Jimenez 1998). Samples were then subjected to LC-ESI-MS/MS analysis using a Thermo LTQ-XL linear ion trap mass spectrometer. TH, with a molecular weight of approximately 60 kDa, was identified as a protein of interest using the TrEMBL database for rat. Scaffold (version Scaffold_3_00_05, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm (Keller et al. 2002). Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (Nesvizhskii et al. 2003). Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
2.5. Western blot analysis
PC6-3 cell lysate was collected as described below. Lysate was incubated with DOPAL (10, 50, 100 μM) for 4 h at 37 °C. Sodium cyanoborohydride (1 mM) was added to help stabilize Schiff-base adducts formed. 1 μg of protein was loaded and a 7.5% acrylamide gel was used to separate via SDS-PAGE. After separation, protein was transferred to nitrocellulose membrane for 1.25 h at 20 V, and blocked over night at 4 °C in 3% BLOTTO. Primary rabbit anti-TH antibody was used at a dilution of 1:1000 for 2 h at room temperature, and horseradish-peroxidase-conjugated secondary goat-anti-rabbit antibody was used at a dilution of 1:10000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1.5 h at room temperature. Between blocking and antibody incubations, membranes were washed 3 times using 0.05 M Tris, 0.9% NaCl containing 0.05% Tween-20 (TBS-T) for 3 minutes at room temperature. Staining for actin was used as a loading control. Primary and secondary antibody dilutions were 1:500 and 1:10000, respectively (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Signal was detected using an Amersham ECL-plus Western Blotting Detection Kit according to manufacturer instructions. Band density was measured using ImageJ and compared to control lanes.
2.6. Tyrosine hydroxylase isolation and activity assay
PC6-3 cells were grown as previously described (1 × 105) in 10 cm tissue culture dishes as used as a source of TH. Undifferentiated cells, which contain levels of TH several fold higher than NGF-differentiated cells, (Shafer & Atchison 1991) were then washed 3 times with cold 10 mM sodium phosphate buffer (pH 6.4) (Hegstrand et al. 1979). Cells were then scraped and collected in 500 μL of buffer. They were then sonicated at 3 second intervals 10 times, and the lysate was centrifuged using a Sorvall Discovery SE Ultraspeed centrifuge at 100,000 × g for 1 h at 4 °C. The cytosolic fraction (i.e. supernatant) was collected to be used in TH activity assays. Protein concentration was determined using a Thermo Scientific Pierce BCA Protein Assay. Using a Molecular Devices Spectra-Max plate reader, the absorbance was measured at 562 nm and a standard curve was used to determine the protein concentration in samples. Lysate was stored at −80 °C until assays were performed. Cell lysate was utilized for a variety of reasons, including, TH activity has been successfully measured previously in lysate with results exhibiting similar L-DOPA production (Cartier et al., Laschinski et al. 1986, Laschinski et al. 1984). Furthermore, TH activity is stable up to a year when stored in these conditions (Laschinski et al. 1986), and lysate provides conditions for the enzyme similar to those in a whole cell model. Preliminary data revealed the exclusion of catalase and dithiothreitol in activity assays did not adversely affect the activity of TH (data not shown). Activity was assessed using HPLC to monitor L-DOPA production. Briefly, control groups contained tyrosine (100 μM) and tetrahydrobiopterin (0.5 mM), which were added to lysate (0.2 mg/mL); time points were taken at 0, 30, 60, 90, and 120 minutes and the reaction was stopped by the addition of 5% (v/v) perchloric acid. In inhibition assays, varying concentrations of DOPAL (0.1, 0.5, 1.0, 2.5, 5, and 10 μM) was also added and time points were taken as previously described. In reversibility assays, DOPAL (5, 10, or 20 μM), tyrosine (100 μM), and tetrahydrobiopterin (0.5 mM) were added to lysate (0.2 mg/mL) and preincubated (30, 60, or 90 min). After preincubation, all reactions were subjected to a Micro Bio-Spin 6 column twice (Bio-Rad, Hercules, CA) in order to entirely remove excess DOPAL (use of column once removed ~90% of DOPAL, data not shown). Control groups (i.e. no DOPAL) were also subjected to the spin columns. Tyrosine and tetrahydrobiopterin were then reintroduced to the system (100 μM and 0.5 mM, respectively) and time points were taken as previously described. In reversibility assays, the % recovery is defined as the production of L-DOPA in experimental conditions (preincubation with DOPAL) as compared to the controls where no DOPAL was present. Results were subtracted from 100 in order calculate the % recovery of activity after the removal of DOPAL. All assays were performed in 10 mM sodium phosphate buffer (pH 6.4).
2.7. HPLC analysis of L-DOPA production
An Agilent 1200 Series Capillary HPLC system with a photodiode array detector set to absorbance at 202 and 280 nm was used. 15 μL of sample was injected, and separation was achieved using a Phenomenex Luna C18 column (1 × 150 mm, 100 Å) and a mobile phase consisting of 97% 0.1% trifluoroacetic acid (v/v) in HPLC-grade water, and 3% acetonitrile (v/v). The flow rate was 50 μL/min and retention times were determined for DA, L-DOPA, tyrosine, and DOPAL to be 6.8, 7.9, 10.5, and 11.2 minutes, respectively, using standard samples. Conversion of area to concentration units was achieved using a calibration curve of standards.
2.8. Cytotoxicity assay
To determine the cytotoxicity of DOPAL in PC6-3 cell cultures, cell viability was measured using the 3-(4,5-dimetylthiazol-2-yl)-2,3-diphenyltetrazolium bromide (MTT) reduction assay. In this assay, the conversion of yellow tetrazolium salt MTT to purple formazan is achieved by active mitochondrial reductases found in functional cells. In brief, NGF-differentiated cells were treated with varying concentrations of DOPAL (0, 5, 10, 25, 50 μM) for 2 h at 37 °C. Following this, yellow MTT (0.5 mg/mL) was added to each well, and further incubated for 2 h at 37 °C. The medium was then removed, and the purple formazan crystals were dissolved in DMSO. The formazan product absorbs at 570 nm, and a Molecular Devices Spectra-Max plate reader was used to measure the absorbance in each well. Absorbance values for the experimental wells were compared to controls (no DOPAL). As a second method of determining cell viability, the trypan blue method was used as previously described (Zhang et al. 2010).
2.9. Statistical analysis
All linear regression and statistical analysis were performed using the software GraphPad Prism 5.0 (Graph Pad Software, San Diego, CA) Data for cells treated with DOPAL were compared to the controls and significant differences (p < 0.05) were determined using an ANOVA with a Tukey post-test (this was also used to analyze % recovery data for reversibility studies). Data for TH activity and L-DOPA formation was determined using a linear regression.
3. RESULTS
3.1. Tyrosine hydroxylase identified as a protein target of DOPAL
PC6-3 cells were incubated with DOPAL (0, 50 μM) as previously described in the methods section. Lysate was subjected to APBA resin, and DOPAL-modified proteins were separated. SDS-PAGE electrophoresis was used to further separate proteins. The most prevalent band at approximately 60 kDa was excised and an in-gel digestion was performed using trypsin. Using LC-ESI-MS/MS and the TrEMBL database, 4 peptides were matched to identify tyrosine hydroxylase as the band of interest with a 95% confidence interval. Based on mascot ion scores which ranged from 47.2–141.2, these results positively identify tyrosine hydroxylase as a protein target of DOPAL modification.
3.2. DOPAL interferes with antibody recognition of tyrosine hydroxylase
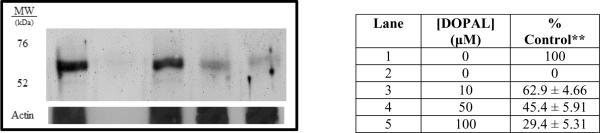
PC6-3 cell lysate containing tyrosine hydroxylase was incubated with DOPAL for 4 hours and western blot analysis was performed to investigate the effect on antibody recognition of the enzyme. Figure 1 shows the concentration-dependent decrease in antibody recognition, with almost complete loss of staining when 100 μM DOPAL was present. The % of TH staining in lane 5 as compared to the control (lane 1) is significantly decreased. These results suggest that DOPAL modifies tyrosine hydroxylase and interferes with the ability of the antibody to recognize the protein.
Fig. 1.
DOPAL interferes with antibody recognition of TH in a concentration-dependent manner. PC6-3 cell lysate as positive control (Lane 1), Bovine serum albumin (BSA) as a negative control (Lane 2), Lysate treated with 10 μM DOPAL (Lane 3), 50 μM DOPAL (Lane 4), and 100 μM DOPAL (Lane 5) % Control refers to integrated staining density for tyrosine hydroxylase (60 kDa). ** Control values represent the mean ± SEMs (n = 3), figure represents an example of gel analysis.
3.3. Tyrosine hydroxylase is inhibited by DOPAL
As shown in Figure 2A and B, treatment of PC6-3 cell lysate with tyrosine, tetrahydrobiopterin, and varying concentrations of DOPAL (0, 0.1, 0.5, 1.0, 2.5, 5.0, and 10 μM) for 2 h resulted in a concentration-dependent inhibition of enzyme activity. HPLC analysis was used to quantify the production of L-DOPA.
Fig. 2.
Treatment of PC6-3 cell lysate with varying concentrations of DOPAL leads to TH inhibition and a decrease in L-DOPA production. (A) L-DOPA production over 2 h shows significant inhibition by DOPAL. (B) Enhancement of 2A DOPAL treatment experiments. (C) Comparison of initial linear slopes shows the concentration-dependent decrease in L-DOPA production. Values shown represent the mean ± SEMs (n = 4). **Significantly different from the control (p < 0.05). In all graphs: (●) control, (--) 0.1 μM DOPAL, (□) 0.5 μM DOPAL, (▲) 1.0 μM DOPAL, (◇) 2.5 μM DOPAL, (◆) 5.0 μM DOPAL, (○) 10.0 μM DOPAL.
All concentrations of DOPAL tested yielded inhibition of TH. Controls (i.e. no DOPAL present) showed a time-dependent increase in L-DOPA production, while the presence of DOPAL illustrated significant inhibition of tyrosine hydroxylase activity (Figure 2B shows an enlargement of the L-DOPA production in the presence of DOPAL). It is important to note that TH activity in PC6-3 control cell lysate is similar as compared to activity demonstrated in previous studies using PC-12 cells (Laschinski et al. 1986, Shafer & Atchison 1991).
Analysis of linear slopes indicated over 80% inhibition of the enzyme at the physiologically relevant concentration of 0.5 μM DOPAL. As the concentration of DOPAL increased to 10 μM greater than 95% inhibition of TH activity was exhibited (Figure 2C). Furthermore, initial studies found that 30 μM DOPAL resulted in complete enzyme inhibition, with the initial linear slope not being significantly different from zero (data not shown).
3.4. DOPAL inhibition of tyrosine hydroxylase is semi-reversible
As previously described in the methods section, reversibility of DOPAL-mediated inhibition of TH was studied using PC6-3 cell lysate. Briefly, preincubation of DOPAL (5, 10, 20 μM) was carried out for 30, 60, or 90 minutes. Excess DOPAL was then removed and substrate and cofactor (i.e. tyrosine and tetrahydrobiopterin, respectively) were re-introduced. It is important to note that control groups (i.e. no DOPAL) were submitted to the same conditions as DOPAL groups, and no effect on TH activity can be seen (i.e. L-DOPA production was not significantly changed).
As Figure 3A, B, and C demonstrate, recovery of TH activity displays both time and concentration-dependent elements. Furthermore, figure 3D shows a linear decrease in TH recovery as both preincubation time and concentration of DOPAL increases. These results indicate the semi-reversibility of TH inhibition by DOPAL is dependent upon both concentration and exposure time, and may have implications for the onset and progression of PD.
Fig. 3.
TH displays semi-reversible inhibition after PC6-3 cell lysate was incubated with 5, 10, or 20 μM DOPAL for 30, 60, or 90 min and excess DOPAL removed. (A), (B), and (C) demonstrate recovery of activity which displays time and concentration-dependent elements and 30, 60 and 90 min, respectively. (D) indicates a linear decrease in recovery of TH as preincubation time and DOPAL concentration increase as determined by HPLC analysis. (n = 3). In all graphs: (●) control, (*) 5 μM DOPAL, (▲) 10 μM DOPAL, (■) 20 μM DOPAL. *Significantly different from time zero (p < 0.05).
3.5. DOPAL is cytotoxic in PC6-3 cell models
To determine the toxicity of DOPAL, varying concentrations of DOPAL (i.e. 5, 10, 25, and 50 μM) were applied to NGF-differentiated PC6-3 cells for 2 h. Mitochondrial dysfunction was measured using the MTT assay as described in the methods. As Figure 4 reveals, there is a concentration-dependent increase in mitochondrial dysfunction. Furthermore, concentrations of DOPAL as low as 10 μM applied for only 2 h show significant dysfunction as compared to controls (p < 0.05). As a second method of detecting viable cells and confirming MTT results, the trypan blue method was used. Cell viability was significantly different from control at 2 h for 10 (60.9 ± 9.7%), 25 (55.1 ± 5.3%), and 50 μM (41.0 ± 9.1%) DOPAL incubations based on one-way ANOVA with Tukey post-test (p < 0.05) for n = 3. Treatment with 5 uM DOPAL did not yield a significant change in cell viability (85.9 ± 14.9%). These data illustrate even slight elevations from normal DOPAL concentrations are detrimental to cell viability.
Fig. 4.
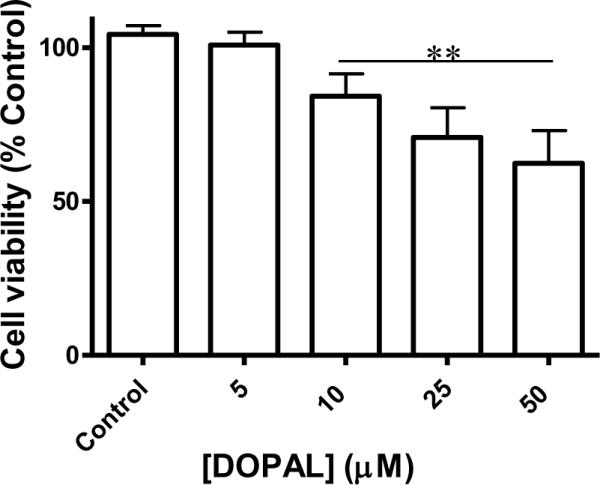
DOPAL-induced cytotoxicity in PC6-3 cells. Treatment with DOPAL (0, 5, 10, 25, and 50 μM) for 2 h lead to significant mitochondrial dysfunction as compared to controls when DOPAL levels were as low as 10 μM. Values represent means ± SEMs (n = 4). **Significantly different from control values (p < 0.05)
4. DISCUSSION
DOPAL, an intermediate of DA catabolism, is an endogenous neurotoxin known to covalently modify proteins (Rees et al. 2009, Burke et al. 2003, Rees et al. 2007) and hypothesized to be a factor in the pathogenesis of PD (Kristal et al. 2001, Burke et al. 2004). While brain levels of DOPAL were measured to be between 2–3 μM under normal conditions, studies have shown that slight elevations (i.e., 6.6 μM) can lead to cell death in dopaminergic cells and a decrease in TH-positive immunostaining (Burke 2003).
Earlier work demonstrated DOPAL to be an electrophile capable of protein modification (Lamensdorf et al. 2000, Kristal et al. 2001). The current study positively identifies TH as a protein target of the DA metabolite. Cells were incubated with DOPAL, and after lysis, proteins were separated via APBA. Proteomic analysis of the cell lysate revealed the presence of TH in the fraction bound to the APBA resin, indicating the enzyme to be covalently modified by DOPAL. Such a result is not unexpected given TH has been shown to be targeted by DA-related quinones. In previous work, DA-quinones and other catecholamine quinones were found to covalently modify the enzyme and inhibit activity (Xu et al. 1998, Kuhn et al. 1999). Furthermore, results of previous studies demonstrated the ability of other DA catabolism products, such as DOPAC, to inhibit TH as well (Laschinski et al. 1986). Overall, these results establish TH to be susceptible to protein modification via DA-related electrophiles and indicate that a mechanism of toxicity for DA neurons may involve TH. While the mechanism behind toxicity and cell death in the presence of DOPAL is currently unknown, it is hypothesized that toxicity and inhibition of TH are distinct processes which ultimately lead to dopaminergic cell death. Previous data reveal DA has the potential to modify other important proteins such as Parkin and α-synuclein leading to aggregation and overwhelming the capacity of proteasome clearance (Lees et al. 2009, LaVoie et al. 2005, Conway et al. 2001) It is therefore possible that DOPAL modifies several other important proteins found in neurons, leading to similar results that may decrease neuronal function in general. As discussed before, L-DOPA has demonstrated trophic properties; therefore, a decrease in L-DOPA production may lead to a decrease in cell viability (Datla et al. 2001, Murer et al. 1998). The link between DOPAL toxicity and inhibition of TH is currently being investigated in a whole cell model.
In previous studies, the APBA resin, which is reactive towards vicinal diols such as those found in catechols, was used to isolate DA-modified proteins (LaVoie et al. 2005). The current study employed the use of APBA to obtain proteins covalently modified by DOPAL. While it might be argued the TH modification is due to oxidized DA or DOPAC, most likely the enzyme adduct is the result of a covalent reaction with DOPAL. DOPAL is significantly more reactive towards proteins than either DOPAC or DA (Burke et al. 2003). While DA is reactive towards proteins, it requires oxidation to the quinone, which then rapidly rearranges to form dopaminochrome in the absence of thiols (Graham 1978, Graham et al. 1978, Stokes et al. 1999, Hastings et al. 1996). In addition, in the current studies, cells were loaded with DOPAL (50 μM) prior to lysis and separation by APBA, ensuring DOPAL uptake and elevated concentrations within the cells. Such results indicate TH modification within the cell is a result of DOPAL interaction with the enzyme.
Further support for DOPAL modification of TH can be seen in Figure 1, which indicates the interference of antibody recognition of TH due to DOPAL adducts. Previous studies established that DA-quinones react with proteins through Cys residues (Kuhn et al. 1999). In contrast, DOPAL is known to modify proteins via Lys residues (Rees et al. 2009). Adduction of Lys or Cys residues on TH may lead to changes in the enzyme structure and conformation, which could negatively impact the activity of the enzyme. It is conceivable inhibition of TH presented in Figure 2 is a result of DOPAL modification to the substrate (i.e. tyrosine) binding site. There are several Lys residues in the catalytic domain of TH, including a critical Lys within one of the 4 α-helices that line the active site cleft (Goodwill et al. 1998). Modification may change the conformation of the active-site cleft, leading to a decrease in TH function. As shown in Figure 3 though, the majority of inhibition is reversible, with almost 90% activity recovery demonstrated after removal of DOPAL. Due to the fact that DOPAL shares many structural characteristics with DA, it is also possible DOPAL may compete for the pterin binding site in a fashion similar to DA, leading to feedback inhibition (Nakashima et al. 2009). It is also known that the structural basis of catechols binding to TH involves the chelation of iron, and in the case of DA, this leads to inhibition of catalytic activity of TH (Kuhn et al. 1999). While there is the possibility that DOPAL may cause TH degradation, it is important to note that the time frame of these experiments is fairly short (2 or 4 hr) as compared to studies done by others in the field (Burke et al. 2003). There is also no evidence of higher or lower molecular weight bands in western blot analysis. Furthermore, TH activity is recovered in preincubation experiments, indicating DOPAL-modification inhibits the enzyme instead of causing protein degradation. Although the structural basis of DOPAL's interaction with TH is currently unknown, studies are in progress to elucidate the mechanism of TH inhibition by DOPAL. In both cases there would be a decrease in the activity of the enzyme, leading to lower levels of both L-DOPA and DA.
In cell lysate experiments involving TH inhibition and reversibility, DOPAL stability was investigated as a control. Preparation of cell lysate for experiments appears to remove cytosolic ALDH and ALR, and HPLC analysis revealed that DOPAL does not exhibit metabolism over 2 h, and no DA, DOPAC, or DOPET was detected in these control experiments (data not shown). Overall, these results indicate that TH inhibition in the cell lysate model is due to the presence of DOPAL.
It is important to note the potency of DOPAL as an inhibitor of TH activity. Several studies have investigated the effect of catechols on TH activity, including L-DOPA, norepinephrine, DOPAC, and DA. Several demonstrated the ability to inhibit TH, with IC50 values ranging from 2.3 μM for norepinephrine, to 35 μM for L-DOPA (Laschinski et al. 1986, Udenfriend et al. 1965). It is clear from the work presented here that DOPAL is a significantly more potent inhibitor than DA or other DA-metabolites. Furthermore, the results displayed in Figure 3 demonstrate a link between recovery of enzyme activity and the length of exposure to DOPAL, specifically, time-dependent inactivation. These data may be important in the onset of PD. There is evidence suggesting the aging process leads to an increase in both oxidative stress as well as MAO activity, and a slight decrease in ALDH metabolism of aldehydes (Andersen 2004, Oreland & Gottfries 1986, Meyer et al. 2004). Such a case may lead to an age-dependent increase in DOPAL levels within a cell, yielding chronic exposure of TH to elevated DOPAL concentration. The data presented here predict such an event would cause inhibition of TH activity, as well as an inability of the enzyme to recover even if DOPAL levels are later reduced.
This work demonstrates the significant DOPAL-mediated inhibition of TH activity which leads to a decrease in L-DOPA production. Previous results have reported cellular DOPAL levels to be between 2–3 μM and based on these findings, such concentrations are predicted to inhibit TH activity. However, it is important to note that DOPAL production and metabolism occur in the vicinity of the mitochondria while TH activity is contained mostly in the cytosol (McGeer et al. 1971, Tank et al. 1981). DOPAL is quickly metabolized by ALDH and ALR at or near the mitochondria, and is not a long-lived species capable of accumulating in the cytosolic fraction.
Elevations in DOPAL concentration may occur with age, when oxidative stress and lipid peroxidation products increase (Andersen 2004). Studies have shown that products of lipid peroxidation (i.e. 4-hydroxy-2-nonenal and malondialdehyde) are capable of inhibiting both ALDH and ALR in cells. This leads to a decrease in aldehyde metabolism by these enzymes and an accumulation of DOPAL (Jinsmaa et al. 2009). Such a case may yield DOPAL-mediated inhibition of TH. Further investigation of the effect of elevated DOPAL in cell models is planned to more fully answer this question.
Overall, these results establish DOPAL to be toxic to PC6–3 cells and an inhibitor of TH activity, leading to a decrease in the production of L-DOPA. Furthermore, TH was positively identified as a protein target of DOPAL and preincubation of TH with DOPAL reveals a time and concentration-dependent decrease in the ability of the enzyme to recover activity. Work is in progress to study the effect of DOPAL on TH activity within a cell and to better understand the link between DOPAL toxicity and inhibition of TH. Furthermore, studies are planned to identify sites of DOPAL-mediated covalent modification to investigate the irreversible modification by DOPAL of TH. These data may prove useful in elucidating the mechanism of inhibition of TH by DOPAL and will contribute to the understanding of the pathogenesis of PD.
Acknowledgements
This work was supported by NIH R01 ES15507 (J.A.D.). We thank the Proteomics Facility at The University of Iowa for their work in identification of protein bands of interest and helpful discussion regarding the results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest. The authors declare that there are no conflicts of interest.
References
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Burke WJ. 3,4-dihydroxyphenylacetaldehyde: a potential target for neuroprotective therapy in Parkinson's disease. Curr Drug Targets CNS Neurol Disord. 2003;2:143–148. doi: 10.2174/1568007033482913. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Chung HD, et al. Neurotoxicity of MAO Metabolites of Catecholamine Neurotransmitters: Role in Neurodegenerative Diseases. Neurotoxicology. 2004;25:101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson's disease pathogenesis. Brain Res. 2003;989:205–213. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- Cartier EA, Parra LA, Baust TB, Quiroz M, Salazar G, Faundez V, Egana L, Torres GE. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J Biol Chem. 285:1957–1966. doi: 10.1074/jbc.M109.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Datla KP, Blunt SB, Dexter DT. Chronic L-DOPA administration is not toxic to the remaining dopaminergic nigrostriatal neurons, but instead may promote their functional recovery, in rats with partial 6-OHDA or FeCl(3) nigrostriatal lesions. Mov Disord. 2001;16:424–434. doi: 10.1002/mds.1091. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Roth RH. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson's disease. Exp Neurol. 1997;144:4–9. doi: 10.1006/exnr.1996.6379. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson's disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- Goodwill KE, Sabatier C, Stevens RC. Crystal structure of tyrosine hydroxylase with bound cofactor analogue and iron at 2.3 A resolution: self-hydroxylation of Phe300 and the pterin-binding site. Biochemistry. 1998;37:13437–13445. doi: 10.1021/bi981462g. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Graham DG, Tiffany SM, Bell WR, Jr., Gutknecht WF. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol. 1978;14:644–653. [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Reactive dopamine metabolites and neurotoxicity: implications for Parkinson's disease. Adv Exp Med Biol. 1996;387:97–106. doi: 10.1007/978-1-4757-9480-9_13. [DOI] [PubMed] [Google Scholar]
- Hegstrand LR, Simon JR, Roth RH. Tyrosine hydroxylase:--examination of conditions influencing activity in pheochromocytoma, adrenal medulla and striatum. Biochem Pharmacol. 1979;28:519–523. doi: 10.1016/0006-2952(79)90245-4. [DOI] [PubMed] [Google Scholar]
- Helander A, Tottmar O. Reactions of biogenic aldehydes with hemoglobin. Alcohol. 1989;6:71–75. doi: 10.1016/0741-8329(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Adachi K, Kiuchi K. Activation of JNK pathway and induction of apoptosis by manganese in PC12 cells. J Neurochem. 1998;71:1607–1615. doi: 10.1046/j.1471-4159.1998.71041607.x. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36-28. [DOI] [PubMed] [Google Scholar]
- Jimenez CRH,L, Qui Y, Burlingame AL. Current Protocols in Protein Science. John Wiley and Sons; New York: 1998. [Google Scholar]
- Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem Res Toxicol. 2009;22:835–841. doi: 10.1021/tx800405v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, Burke WJ. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic Biol Med. 2001;30:924–931. doi: 10.1016/s0891-5849(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE, Jr., Thomas DM, Elferink LA. Tyrosine hydroxylase is inactivated by catechol-quinones and converted to a redox-cycling quinoprotein: possible relevance to Parkinson's disease. J Neurochem. 1999;73:1309–1317. doi: 10.1046/j.1471-4159.1999.0731309.x. [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Eisenhofer G, Harvey-White J, Hayakawa Y, Kirk K, Kopin IJ. Metabolic stress in PC12 cells induces the formation of the endogenous dopaminergic neurotoxin, 3,4-dihydroxyphenylacetaldehyde. J Neurosci Res. 2000;60:552–558. doi: 10.1002/(SICI)1097-4547(20000515)60:4<552::AID-JNR14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Laschinski G, Kittner B, Brautigam M. Inhibition of striatal tyrosine hydroxylase by low concentrations of apomorphine. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:114–118. doi: 10.1007/BF00500904. [DOI] [PubMed] [Google Scholar]
- Laschinski G, Kittner B, Brautigam M. Direct inhibition of tyrosine hydroxylase from PC-12 cells by catechol derivatives. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:346–350. doi: 10.1007/BF00500085. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Mattammal MB, Haring JH, Chung HD, Raghu G, Strong R. An endogenous dopaminergic neurotoxin: implication for Parkinson's disease. Neurodegeneration. 1995;4:271–281. doi: 10.1016/1055-8330(95)90016-0. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL, Wada JA. Distribution of tyrosine hydroxylase in human and animal brain. J Neurochem. 1971;18:1647–1658. doi: 10.1111/j.1471-4159.1971.tb03738.x. [DOI] [PubMed] [Google Scholar]
- Meyer MJ, Mosely DE, Amarnath V, Picklo MJ., Sr. Metabolism of 4-hydroxy-trans-2-nonenal by central nervous system mitochondria is dependent on age and NAD+ availability. Chem Res Toxicol. 2004;17:1272–1279. doi: 10.1021/tx049843k. [DOI] [PubMed] [Google Scholar]
- Murer MG, Dziewczapolski G, Menalled LB, Garcia MC, Agid Y, Gershanik O, Raisman-Vozari R. Chronic levodopa is not toxic for remaining dopamine neurons, but instead promotes their recovery, in rats with moderate nigrostriatal lesions. Ann Neurol. 1998;43:561–575. doi: 10.1002/ana.410430504. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Levitt M, Udenfriend S. Tyrosine Hydroxylase. The Initial Step in Norepinephrine Biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- Nakashima A, Hayashi N, Kaneko YS, Mori K, Sabban EL, Nagatsu T, Ota A. Role of N-terminus of tyrosine hydroxylase in the biosynthesis of catecholamines. J Neural Transm. 2009;116:1355–1362. doi: 10.1007/s00702-009-0227-8. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Tottmar O. Biogenic aldehydes in brain: on their preparation and reactions with rat brain tissue. J Neurochem. 1987;48:1566–1572. doi: 10.1111/j.1471-4159.1987.tb05702.x. [DOI] [PubMed] [Google Scholar]
- Oreland L, Gottfries CG. Brain and brain monoamine oxidase in aging and in dementia of Alzheimer's type. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:533–540. doi: 10.1016/0278-5846(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Pittman RN, Wang S, DiBenedetto AJ, Mills JC. A system for characterizing cellular and molecular events in programmed neuronal cell death. J Neurosci. 1993;13:3669–3680. doi: 10.1523/JNEUROSCI.13-09-03669.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JN, Florang VR, Anderson DG, Doorn JA. Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate. Chem Res Toxicol. 2007;20:1536–1542. doi: 10.1021/tx700248y. [DOI] [PubMed] [Google Scholar]
- Rees JN, Florang VR, Eckert LL, Doorn JA. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem Res Toxicol. 2009;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Brosch K, Bush B, Ritz M, Shain W. Effects of Aroclor 1254 on dopamine and norepinephrine concentrations in pheochromocytoma (PC-12) cells. Neurotoxicology. 1989;10:757–764. [PubMed] [Google Scholar]
- Shafer TJ, Atchison WD. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12:473–492. [PubMed] [Google Scholar]
- Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Strack S. Overexpression of the protein phosphatase 2A regulatory subunit Bgamma promotes neuronal differentiation by activating the MAP kinase (MAPK) cascade. J Biol Chem. 2002;277:41525–41532. doi: 10.1074/jbc.M203767200. [DOI] [PubMed] [Google Scholar]
- Tank AW, Weiner H, Thurman JA. Enzymology and subcellular localization of aldehyde oxidation in rat liver. Oxidation of 3,4-dihydroxyphenylacetaldehyde derived from dopamine to 3,4-dihydroxyphenylacetic acid. Biochem Pharmacol. 1981;30:3265–3275. doi: 10.1016/0006-2952(81)90598-0. [DOI] [PubMed] [Google Scholar]
- Udenfriend S, Zaltzman-Nirenberg P, Nagatsu T. Inhibitors of purified beef adrenal tyrosine hydroxylase. Biochem Pharmacol. 1965;14:837–845. doi: 10.1016/0006-2952(65)90103-6. [DOI] [PubMed] [Google Scholar]
- Ungar F, Tabakoff B, Alivisatos SG. Inhibition of binding of aldehydes of biogenic amines in tissues. Biochem Pharmacol. 1973;22:1905–1913. doi: 10.1016/0006-2952(73)90050-6. [DOI] [PubMed] [Google Scholar]
- Xu Y, Stokes AH, Roskoski R, Jr., Vrana KE. Dopamine, in the presence of tyrosinase, covalently modifies and inactivates tyrosine hydroxylase. J Neurosci Res. 1998;54:691–697. doi: 10.1002/(SICI)1097-4547(19981201)54:5<691::AID-JNR14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G, Wang Z. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int. 2010;57:547–555. doi: 10.1016/j.neuint.2010.06.021. [DOI] [PubMed] [Google Scholar]