Abstract
The microbial communities that inhabit the intestinal tract are essential for mammalian health. Communication between the microbiota and the host establishes and maintains immune homeostasis, enabling protective immune responses against pathogens while preventing adverse inflammatory responses to harmless commensal microbes. Specific bacteria, such as segmented filamentous bacteria, Clostridium species, and Bacteroides fragilis, are key contributors to immune homeostasis in the gut. The cellular and molecular interactions between intestinal microbes and the immune system are rapidly being elucidated. Here, we review advances in our understanding of the microbial populations that shape the mucosal immune system and create a protective defense that prevents infection while tolerating friendly commensals.
Introduction
The mammalian intestinal tract is colonized by an estimated 1013–1014 bacteria. These microbes aid the host by breaking down food into absorbable products in exchange for an environment with a constant influx of nutrients necessary for their survival. Thus, the microbial communities that inhabit the mammalian intestine, termed the intestinal microbiota, maintain a symbiotic relationship with their host. Because the gut is open to the environment, the risk of infection with exogenous pathogenic organisms is real. The immune system must be both tolerant to the microbial communities it contains and able to efficiently respond to infection. The microbiota itself plays a critical role in preventing the outgrowth of pathogenic organisms. This “colonization resistance” can be disrupted by changes in the complexity and density of the microbiota. Germ-free (GF) mice or mice carrying a low complexity microbiota, in addition to being highly susceptible to a variety of intestinal pathogens, also have altered mucosal immune responses [1,2].
The scale of current efforts to understand the composition and dynamics of the microbiota reflects the growing understanding of its critical role in whole organism homeostasis. It is now recognized that changes in the composition and density of the gut flora can have profound effects on the development of not only inflammatory bowel disease, but also autoimmunity in tissues that are not in direct contact with the microbiota. This review will address the role of the intestinal microbiota in host defense and in shaping immune compartments that contribute to inflammatory responses and autoimmunity.
The complex microbiota protects against infection
The intestinal tract of mammals harbors many bacterial species that have not been cultured in the laboratory. This has limited our understanding of the complexity of the intestinal microbiota until recent years, when cultivation-independent 16S rRNA gene sequencing techniques began to be employed more widely for this purpose. We now know that following antibiotic treatment, the density and composition of the intestinal microbiota is severely affected [3–5]. Further, lasting changes in the composition of microbial communities after cessation of antibiotic treatment permit colonization by organisms that pose a threat, such as vancomycin-resistant Enterococcus (VRE), even though bacterial density has been restored [3]. A recent study suggests that the ability of a pathogen to colonize the intestinal tract and cause disease is positively correlated with the abundance of closely related species [1], perhaps reflecting the development of niches that exclude unrelated but tolerate related classes of microbes. These findings suggest that the presence or absence of specific bacterial species and the changes these may effect on surrounding microbial communities and the host’s immune defenses contribute to the establishment of colonization resistance.
In antibiotic treated mice, colonization resistance and pathogen clearance can be re-established by reintroducing normal microbiota, either by adoptive transfer or by co-housing with untreated mice [2,3]. At least two mechanisms that are not mutually exclusive and are supported by recent evidence may mediate pathogen clearance following recovery of the normal microbiota (Fig. 1). First, commensals may directly inhibit the growth of specific pathogens, and second, stimulation of immune effector functions by the microbiota, by restoring immune tone in the intestine, may clear the invading pathogens. Salmonella typhimurium is an enteric pathogen that triggers intestinal inflammation and exploits it to colonize the gut [6,7]. During primary S. typhimurium infection of antibiotic-treated mice, reconstitution of the conventional flora by adoptive transfer leads to pathogen clearance [2]. This process occurs efficiently in the absence of antibody responses that clear the pathogen during secondary infection [2], suggesting that clearance is a direct result of pressure of commensals on the pathogen. It remains unclear, however, whether other components of the immune response are induced by recovery of the microbiota following primary S. typhimurium infection. The mechanism by which the normal microbiota directly limits the growth of pathogens is not fully elucidated and it may differ between infections, given the complexity of the microbiota and its effects on innate and adaptive immune responses.
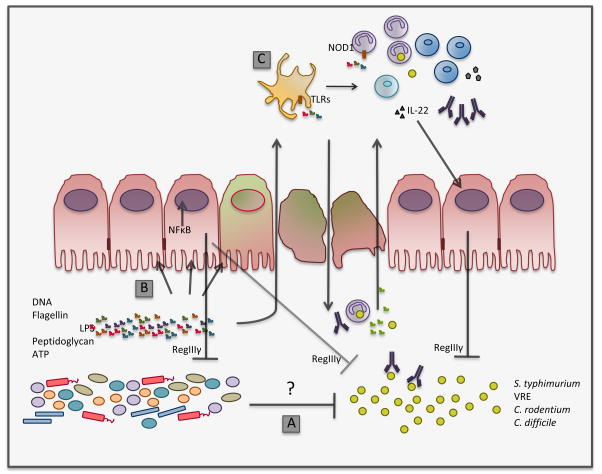
Figure 1.
Major mechanisms that may contribute to the restoration of colonization resistance. Antibiotic treatment results in depletion of the normal microbiota, rendering the host susceptible to colonization by potentially pathogenic microbes. Recovery of a commensal microbiota by adoptive transfer or cohousing with normal mice restores colonization resistance, leading to clearance of dangerous bacteria such as S. typhimurium and VRE. Two major mechanisms may contribute to pathogen clearance: the direct effect of the commensal flora on the growth of the pathogen (A) and restoration of immune tone induced by stimulation of immune receptors, preventing expansion of the pathogen (B and C). The mechanisms underlying direct pathogen inhibition by the microbiota remain ill-defined, but may include competition for nutritional resources and production of growth inhibitory molecules. As depicted in B, bacterial products stimulate innate immune receptors expressed on epithelial cells to restore immune homeostasis in the intestine. The production of RegIIIγ, an antimicrobicidal lectin that targets Gram-positive bacteria, by intestinal epithelial cells, helps maintain homeostasis of intestinal microbial communities and contributes to clearance of invading species such as VRE. RegIIIγ is elicited by direct sensing of the microbiota by intestinal epithelial cells (B) or, when bacterial products are found systemically, by sensing of bacterial products by cells of hematopoietic origin that express IL-22 (C). Following disruption of the epithelium, bacteria and their products reach the lamina propria and stimulate innate immune receptors, which recruits and activates neutrophils and monocytes, and stimulates IgA responses (C). Immune cells of the lamina propria are activated to generate an antimicrobial program that clears the pathogen and restores health to the epithelial barrier.
Immune activation elicits host defense
The importance of the microbiota in driving protective immune responses during intestinal infection is best illustrated by the finding that restoring signaling through innate immune receptors in antibiotic-treated mice can protect from intestinal infections. Many TLRs are expressed in the murine and human intestine, but their expression by the specific cell lineages of the gut is not fully elucidated. Toll-like receptor (TLR) 9 stimulation of antibiotic-treated mice by DNA derived from gut flora induces protective immune responses to the intracellular parasite Encephalitozoon cuniculi [8]. Engagement of the innate immune receptor NOD1 (nucleotide-binding, oligomerization domain-containing protein-1) by microbiota-derived peptidoglycan restores neutrophil bacterial killing capacity in antibiotic-treated mice, helping prevent sepsis following Streptococcus pneumoniae infection [9]. Antibiotic treated mice also have impaired capacity to mount protective cytokine responses during Toxoplasma gondii infection, supporting a defensive role for the microbiota during intestinal immune responses [10].
Restoring TLR4 or TLR5 signaling in antibiotic-treated mice by exogenous administration of LPS or flagellin, respectively, upregulates expression of the bactericidal lectin RegIIIγ and decreases colonization of the intestine with VRE (Fig. 1) [11,12]. RegIIIγ kills Gram-positive bacteria by binding carbohydrate motifs on the peptidoglycan bacterial wall, [13,14] and MyD88-mediated RegIIIγ [15] expression can be directly upregulated through TLR-mediated recognition of the microbiota by intestinal Paneth cells [16]. In contrast, following systemic flagellin administration, RegIIIγ expression in the epithelium is dependent on cells of hematopoietic origin and the expression of IL-22 [12,17]. IL-22 is a critical mediator of immune defense in the gut, and it is required to mount an effective response against the murine intestinal pathogen Citrobacter rodentium [18]. In addition to RegIIIγ upregulation, TLR5 stimulation prevents apoptosis and stimulates proliferation of intestinal epithelial cells, protecting mice from the damaging effects of radiation on gut tissues [19]. Interestingly, TLR5-deficient mice develop a microbiota that promotes the development of metabolic syndrome when transferred to TLR5-sufficient mice [20], suggesting that altered innate immune tone in the gut can result in changes to the microflora.
Colonization of the gut with segmented filamentous bacteria (SFB) increases mucosal defenses that protect against C. rodentium [21]. SFB are commensal organisms that adhere tightly to the small intestinal epithelium and elicit IL-17 and IL-22-producing Th17 cells in the lamina propria [21,22]. While GF and antibiotic-treated mice have markedly reduced numbers of Th17 cells in the lamina propria, colonization with SFB-containing microbiota results in increased Th17 cell and decreased Treg percentages within 2 weeks [22,23].
Microbiota and B cell responses
Given the important role of innate immune receptors in intestinal immune homeostasis and host defense, it is interesting to note that MyD88-deficient mice keep the microbiota at bay and prevent bacteremia. In a recent study, Slack and colleagues demonstrated the critical role of T-cell dependent IgG production in the context of severe innate immune deficiency [24]. Mice lacking the signaling mediators MyD88 and TRIF have elevated serum levels of IgG specific for microbiota components, preventing systemic bacterial infection [24]. Within the intestinal tract, mucosal IgA plays a critical role in shaping microbial populations and mediating pathogen clearance. Mice deficient in IgA production show expansion of SFB and other Clostridium-related anaerobes in the upper small intestine [25]. During Shigella infection, antigen-specific IgA ameliorates disease by immune exclusion, i.e., binding of bacteria at the mucosal layer to allow clearance and avoid contact with the epithelium [26]. In addition, IgA-bacterial complexes can translocate to Peyer’s patches (PP), where they inhibit production of inflammatory mediators that contribute to tissue damage [26].
Unlike IgG production, specific IgA responses lack typical memory characteristics but retain plasticity, enabling changes in the specificity of IgA as shifts in the dominant commensal species occur [27]. A robust IgA response specific for flagellin, a common antigen expressed by commensal bacterial species in the gut, prevents CD4+ T cell responses that could result in inflammation and tissue damage [28]. Treg provide help to IgA-producing B cells, and their depletion in vivo decreases intestinal IgA responses to flagellin and allows the expansion of flagellin-specific CD4+ T cells [28]. Conversely, transfer of Foxp3+ T cells to T cell-deficient mice restores formation of germinal centers in PP and intestinal IgA production [28,29]. Interestingly, Treg down-regulate Foxp3 expression in germinal centers and differentiate into follicular B helper T cells, which help sustain IgA production in PPs [29]. Follicular dendritic cells respond to direct TLR and retinoic acid receptor signaling by stimulating the proliferation, recruitment, and differentiation of lymphocytes in PP’s germinal centers [30]. Follicular dendritic cells in the PP are a major source of TGFβ, which is critical for promoting IgA class switching [30]. TGFβ also plays a role in T cell-independent IgA class-switching in isolated lymphoid follicles, whereas neutralization of TGFβ inhibits IgA production [31]. T cell-independent differentiation of IgA+ plasma cells can also be mediated by TLR5-expressing lamina propria DC following flagellin stimulation [32].
Role of the microbiota in directing T cell responses
Th17 cells are important mediators of immune defenses in the gut and are induced by the microbiota through several mechanisms (Fig. 2). Induction of Th17 cells in the small intestinal lamina propria by SFB appears to be mediated in part by the production of serum amyloid A, which is upregulated upon SFB colonization and can induce Th17 cell differentiation in vitro [21]. Phagocytosis of infected apoptotic cells by DC induces Th17 cells via production of IL-6 and TGFβ following recognition of microbial molecules [33]. Direct flagellin-mediated TLR5 stimulation of CD11bhiCD11chi lamina propria DC supports intestinal Th17 induction [32] through MyD88-dependent IL-6 production, a critical mediator of T cell homeostasis in the gut [32–34]. Lamina propria DC can also produce IL-6 and induce Th17 cells in GF mice following ATP administration; however, this occurs through a MyD88 and TRIF-independent mechanism [35]. The expansion of Th17 cells can also be negatively regulated by intestinal epithelial cells, which produce IL-25 following microbial recognition [36]. IL-25 inhibits the production of IL-23 in the lamina propria, which is required for T cell proliferation in the colon [37], thus limiting the Th17 program [36].
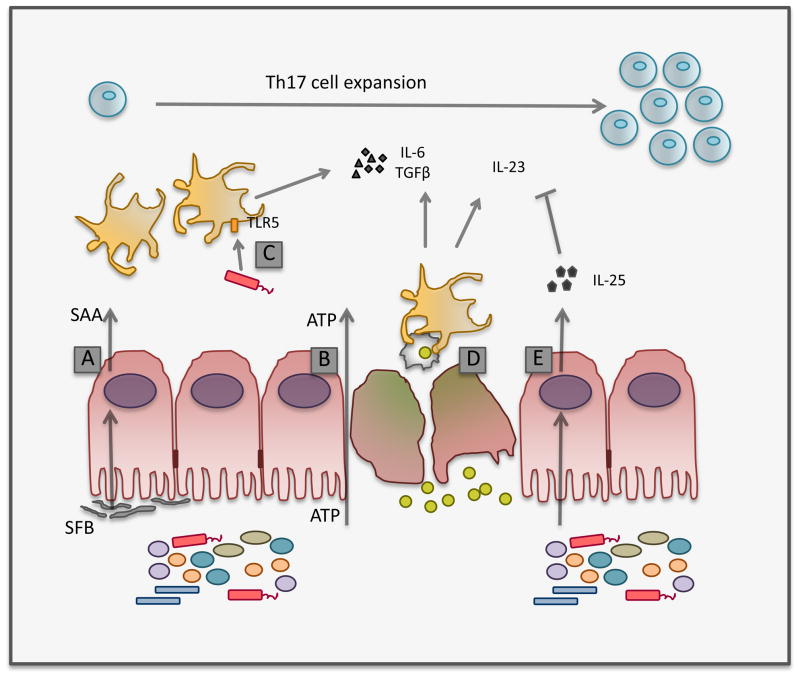
Figure 2.
The microbiota induces Th17 cell expansion through multiple mechanisms. SFB induces expression of serum amyloid A (SAA) in the small intestine, which leads to DC-mediated Th17 differentiation (A). ATP (adenosine 5′ triphosphate), which can be produced by the microbiota, contributes to Th17 differentiation by activating a subset of DC via a MyD88- and TRIF-independent mechanism (B). Flagellin-mediated stimulation of TLR5 expressed on CD103+ lamina propria DC induces IL-6 expression, contributing to the Th17 cell program (C). Phagocytosis of infected apoptotic cells by dendritic cells contributes to the production of Th17-inducing cytokines (D). Several DC subsets are present in the intestinal lamina propria, which may respond distinctly to these signals, contributing to Th17 cell expansion. The frequency of Th17 cells in the intestine is negatively regulated by microbiota-induced production of IL-25 by intestinal epithelial cells, which limits IL-23 production (E).
Activated γδ T cells in the lamina propria are also an important source of IL-17 and are regulated by the commensal microbiota, as reflected by the fact that GF and antibiotic-treated mice have lower percentages of activated γδ T cells [38]. IL-17 expression by γδ T cells is activated in vitro and in vivo by IL-23 and IL-1 [38,39]. Commensal bacteria also direct the function of γδ intraepithelial lymphocytes, which are critical in limiting bacterial penetration during intestinal injury [40].
Intestinal epithelial cells, which are in close contact with the microbiota, are critical for maintenance of innate and adaptive immune homeostasis in the gut. In the colon, Clostridium species signal through intestinal epithelial cells to mediate induction of IL-10-producing Treg in the colonic lamina propria [41]. IKKβ-dependent NF-κB signaling in intestinal epithelial cells regulates helper T cell responses that mediate clearance of the intestinal parasite Trichuris muris [42]. Further, NF-κB signaling in intestinal epithelial cells prevents inflammation by decreasing epithelial cell death. IKKβ- and MyD88-dependent signaling have a critical role in the regulation of colitis-associated cancer [43,44].
Inflammation and the microbiota
While microbiota-induced Th17 cytokines in the lamina propria can be crucial for protection against intestinal pathogens [18,21], they can also contribute to inflammation in the colon [35], and IL-23-responsive innate lymphoid cells in the lamina propria contribute to colitis in Rag−/− mice by producing IL-17 and IFNγ [45]. RORγt-deficient mice lack Th17 cells and lymphoid tissue inducer cells, resulting in an absence of lymph nodes, Peyer’s patches, and isolated lymphoid follicles. RORγt−/− mice are able to contain the microbiota by the formation of B cell-mediated tertiary lymphoid tissue, which supports production of protective IgG [46]. However, the increase in tertiary lymphoid tissue can also lead to exacerbated immune responses, aggravating chemically-induced colitis [46]. These findings illustrate the fine balance between immunity and inflammation in the gut.
T-bet deficiency in Rag2−/− mice (TRUC mice) leads to inflammation that closely resembles human ulcerative colitis, including the development of colitis-associated colorectal cancer [47,48]. Colitis in TRUC mice is driven by increased production of TNF-α by colonic DC, which is negatively regulated by T-bet. Antibiotic treatment can cure the colitis, and transfer of the microbiota from TRUC mice into wildtype recipients transmits colitis [47], indicating that this can be a microbiota-driven disease. Two bacterial species, Proteus mirabilis and Klebsiella pneumoniae, can elicit TNF-α production from T-bet−/− Rag−/− MyD88−/− bone marrow-derived DC [49]. These bacteria can drive TRUC colitis in specific-pathogen free wildtype mice but not in GF mice, indicating that there are other as yet unidentified bacterial species that contribute to colitis in this model [49].
Perhaps not surprisingly, a counterpart to these colitogenic species exists that ameliorates colitis; mice fed a fermented product containing Bifidobacterium animalis subsp. lactis have lower numbers of P. mirabilis and K. pneumoniae, and improved colitis scores [50]. In the skin, lipoteichoic acid, a TLR2 agonist derived from commensal Staphylococcus species inhibits TLR3-mediated inflammation following cutaneous injury [51]. Bacteroides fragilis, an intestinal commensal microbe belonging to the Bacteroidetes family, supports the differentiation of Foxp3+ T cells that express IL-10, thus protecting from colitis by decreasing detrimental inflammatory responses during Helicobacter hepaticus infection [52]. H. hepaticus is a Gram-negative commensal bacterium that reduces inflammatory responses in the intestine through its type VI secretion system, limiting intestinal inflammation and maintaining a symbiotic relationship with the host [53]. However, H. hepaticus can cause colitis in immunocompromised animals or following alterations to the microbiota, through mechanisms that are not entirely understood. B. fragilis-induced amelioration of colitis is mediated by a single B. fragilis-derived capsular component, polysaccharide A (PSA) [54], which, as we describe below, also plays a role in regulation of autoimmune processes in the central nervous system [55]. Expression of a single bacterial capsular polysaccharide, however, is insufficient for competitive intestinal colonization by B. fragilis [56]. Bacterial metabolites in the intestine, such as short-chain fatty acids, also limit inflammatory responses, in this case signaling through the receptor GPR43, and downregulating the expression of pro-inflammatory molecules on neutrophils [57]. These findings highlight the dual role of the microbiota, whose components can both cause as well as prevent inflammation.
Autoimmunity
While shifts in microbiota composition and density affect immune responses locally, it is now recognized that changes in bacterial species in the gut can also result in altered immunity in organs that are not in direct contact with the intestinal microbiota. Mice lacking an intestinal microbiota develop attenuated disease in models of autoimmune arthritis and autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. Colonization with SFB promotes progression to autoimmune arthritis through the induction of antigen-specific Th17 cells, which mediate the expansion of B cells in germinal centers and the production of autoantibodies involved in development of disease [58]. Similarly, GF mice develop markedly attenuated EAE, concomitant with decreased infiltration of the spinal cord by Th17 cells; and disease susceptibility is restored upon colonization with SFB [59]. Interestingly, colonization of GF mice with B. fragilis results in the expansion of IL-10-producing Tregs that limit pro-inflammatory responses and ameliorate EAE [54,55]. While the specific mechanism of Treg induction by B. fragilis-derived PSA is unclear, it is known to require TLR2 signaling [54]. While PSA-induced Tregs are effective suppressors of effector T cells, at least in part through IL-10 production, it remains unclear whether they can control specific inflammatory processes or if they have general suppressive potential that may be exploited more broadly to limit inflammation [54]. These findings support the concept of intestinal homeostasis maintained by a complex microbiota, whose individual components can contribute to immune activation or tolerance, together maintaining immune homeostasis in the gut and beyond.
In nonobese diabetic mice (NOD), which spontaneously develop autoimmune diabetes, MyD88 deficiency results in lower numbers of autoreactive CD8+ T cells in the pancreatic lymph nodes and autoreactive CD4+ T cells have lowered proliferative capacity, protecting mice from diabetes development [60]. Notably, however, the lack of a microbiota in GF NOD mice does not ameliorate disease [60], and the mechanism by which MyD88 signaling contributes to autoimmunity in these mice is not clear.
While Th17 cells are decreased in frequency in GF mice, a recent study demonstrates that Treg from GF mice do not have decreased suppressive capacity, and depletion of Treg in GF mice results in autoimmunity, similar to mice harboring conventional flora [61]. This finding suggests that autoimmune processes resulting from Treg deficiencies are not dependent on the presence of the microbiota.
Concluding remarks
Our appreciation for the role of the microbiota in shaping host innate and adaptive immunity has increased greatly in the last decade. These insights have established that changes in the density and composition of the intestinal microbiota should be considered when inflammatory processes are under investigation, including metabolic syndrome and chronic inflammatory responses. This is highlighted by the fact that C57BL/6 mice from different providers harbor distinct microbiotas that shape the cellular composition of the lamina propria [21]. Further, inbreeding of mouse colonies for extended periods of time may result in mouse strains harboring distinct gut floras, even within one animal facility. As discussed, this can have profound effects in studies of immune responses in the gut and elsewhere and should be taken into account. To equilibrate the microbiota of mouse strains, one may consider co-housing of animals or breeding strategies that result in littermate controls.
The identification of microbial species that affect the immune response and the mechanisms they employ, will continue to aid in our understanding of the way in which the microbiota communicates with the host to maintain immune homeostasis. These findings will continue to inform the development of microbial therapies that may alter immune responses in patients with autoimmunity, inflammatory bowel disease, and microbial infections.
Acknowledgments
Supported by NIH grants R01AI042135 and R37AI039031 to E.G. Pamer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stecher B, Chaffron S, Kappeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, von Mering C, et al. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 2010;6:e1000711. doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ, Heikenwalder M, et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010;6:e1001097. doi: 10.1371/journal.ppat.1001097. This study shows that during primary Salmonella infection, production of IgA and T cell responses are dispensable for pathogen clearance, suggesting that the normal microbiota enhances clearance of Salmonella from the intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. This work demonstrates that antibiotic-induced changes in the microbiota are long lasting and provide an environment in which vancomycin-resistant Enterococcus can colonize and dominate the microbiota. In humans, domination of VRE over the microbiota precedes bacteremia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–158. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. This article shows that tetrathionate, which is generated during inflammation, can be used by Salmonella as a respiratory electron acceptor, giving this pathogen a growth advantage over the microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. Microbiota-derived peptidoglycan is detectable in the systemic circulation and stimulates the NOD1 innate immune receptor to enhance neutrophil function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson A, Pifer R, Behrendt CL, Hooper LV, Yarovinsky F. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe. 2009;6:187–196. doi: 10.1016/j.chom.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010;201:534–543. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehotzky RE, Partch CL, Mukherjee S, Cash HL, Goldman WE, Gardner KH, Hooper LV. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proc Natl Acad Sci U S A. 2010;107:7722–7727. doi: 10.1073/pnas.0909449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandl K, Plitas G, Schnabl B, Dematteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII{gamma} and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Maele L, Carnoy C, Cayet D, Songhet P, Dumoutier L, Ferrero I, Janot L, Erard F, Bertout J, Leger H, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J Immunol. 2010;185:1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 19.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. This work, along with Ref. [22], identified SFB as a commensal species that induces Th17 cells in the intestine. This study demonstrates that SFB colonization can promote a protective response against C. rodentium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. This article, along with Ref. [21], identified SFB as the commensal species that induces Th17 cells in the intestine. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620. doi: 10.1126/science.1172747. This article demonstrates that innate immune deficient mice restrain the microbiota by increasing production of IgG specific for commensal microbes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthesy B, Phalipon A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol. 2009;183:5879–5885. doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- 27.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. This article demonstrates that Treg-mediated IgA responses directed towards commensals can specifically abrograte T cell responses in the gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 30•.Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. Follicular dendritic cells in Peyer’s patches receive signals through innate immune receptors, stimulating their production of factors that support immunoglobulin responses in the gut. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 33••.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. Phagocytosis of infected cells by dendritic cells triggers the production of cytokines that support Th17 differentiation and expansion, which contributes to mucosal immunity during infection. [DOI] [PubMed] [Google Scholar]
- 34.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 36•.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. Intestinal epithelial cells, in response to the microbiota, produce IL-25. Inhibition of IL-23 production by IL-25 prevents Th17 cell expansion in the colon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe. 2010;7:140–150. doi: 10.1016/j.chom.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182:3047–3054. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science. 2010 doi: 10.1126/science.1198469. This study demonstrates that spore-forming, Gram-positive Clostridia species induce Treg differentiation in the colon, but not the small bowel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 43.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 45.Buonocore S, Ahern PP, Uhlig HH, Ivanov, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, Eberl G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of ROR{gamma}t and LTi cells. J Exp Med. 2010 doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garrett WS, Punit S, Gallini CA, Michaud M, Zhang D, Sigrist KS, Lord GM, Glickman JN, Glimcher LH. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell. 2009;16:208–219. doi: 10.1016/j.ccr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. Two bacterial species are identified as contributors to colitis in TRUC mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veiga P, Gallini CA, Beal C, Michaud M, Delaney ML, DuBois A, Khlebnikov A, van Hylckama Vlieg JE, Punit S, Glickman JN, et al. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc Natl Acad Sci U S A. 2010;107:18132–18137. doi: 10.1073/pnas.1011737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, von Aulock S, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 53.Chow J, Mazmanian SK. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe. 2010;7:265–276. doi: 10.1016/j.chom.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 56.Liu CH, Lee SM, Vanlare JM, Kasper DL, Mazmanian SK. Regulation of surface architecture by symbiotic bacteria mediates host colonization. Proc Natl Acad Sci U S A. 2008;105:3951–3956. doi: 10.1073/pnas.0709266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Wu HJ, Ivanov, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. Colonization of GF mice with SFB restores susceptibility to autoimmune arthritis by stimulating Th17 cell expansion, which enhances autoimmune B cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Microbes and Health Sackler Colloquium: Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Chinen T, Volchkov PY, Chervonsky AV, Rudensky AY. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J Exp Med. 2010;207:2323–2330. doi: 10.1084/jem.20101235. Depletion of Treg in GF mice results in autoimmunity, establishing that the microbiota is dispensable for inflammatory responses in the absence of Treg. [DOI] [PMC free article] [PubMed] [Google Scholar]