Abstract
Combining antiangiogenic agents with traditional cytotoxic chemotherapy offers the potential to target both vascular and cellular components of a growing tumor mass. Here, we examined the antitumor activity of the vascular endothelial growth factor antibody, Bevacizumab (Avastin®) in combination with the topoisomerase I inhibitor, Irinotecan (CPT-11) against human head and neck squamous cell carcinoma (HNSCC) xenografts. Bevacizumab was administered daily (at 5 mg/kg or 20 mg/kg) to nude mice bearing FaDu HNSCC xenografts for 28 days with the first dose beginning seven days prior to Irinotecan (100 mg/kg, weekly × 4). Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and immunohistochemical (IHC) methods were employed to study the antiangiogenic effects of Bevacizumab in vivo. Kinetics of tumor response to treatment was studied by monitoring tumor volume over a 60-day period. DCE-MRI detected a significant reduction in vascular permeability following treatment with Bevacizumab (5 mg/kg) while high dose Bevacizumab (20 mg/kg) induced significant microvascular damage and tumor necrosis, confirmed by immunohistochemistry (IHC). Irinotecan alone resulted in complete tumor regression (cures) in ~40% of animals while Bevacizumab alone did not result in any cures. Treatment with Bevacizumab (5 mg/kg/day × 28 days) in combination with Irinotecan (100 mg/kg, weekly × 4) was highly effective in inhibiting FaDu tumor growth and resulted in complete tumor regression in 80% of animals. These results demonstrate that long term administration of Bevacizumab effectively modulates chemotherapeutic efficacy against HNSCC xenografts. Further investigation into the therapeutic potential of this combination strategy against HNSCC is warranted.
Keywords: Bevacizumab, Irinotecan, MRI, Head and neck cancer, human tumor xenografts, Combination therapy
INTRODUCTION
Head and neck squamous cell carcinomas (HNSCC) constitute a diverse group of neoplasms with varying biological behavior, metastatic potential and prognosis1. Management of patients with head and neck cancer is a complex process that often involves a combination of treatment modalities such as surgery, chemotherapy and radiation. Combinatorial approaches such as concurrent chemoradiotherapy have shown clinical benefit in patients with locally advanced disease2. However, the majority of patients with head and neck cancer experience severe treatment-induced toxicities and are at increased risk of disease recurrence and loco-regional metastasis. Clearly, there is a need to examine the potential of novel targeted combination strategies that are likely to improve the therapeutic efficacy against HNSCC with minimal or no toxicity.
It is now well-recognized that angiogenesis is an essential requirement for neoplastic growth and one of the hallmarks of cancer3,4. As a result, targeting tumor angiogenesis has received a lot of attention over the last decade and several agents are actively being pursued for their antiangiogenic and antitumor activity in preclinical models and/or in clinical trials5,6. In 2004, Bevacizumab (Avastin®), a humanized monoclonal antibody against the vascular endothelial growth factor (VEGF), was the first antiangiogenic agent to receive FDA-approval for clinical use in patients with metastatic colorectal cancer in combination with chemotherapy5–7. Since then, the agent has received additional clinical approval in advanced cancers of the lung, breast, kidneys and brain5–7. Given the strong angiogenic dependence of HNSCC, preclinical studies have investigated the potential of combining antiangiogenic agents and chemotherapy with encouraging results8,9. However, extensive investigation into the activity of Bevacizumab in combination with chemotherapy against HNSCC has not been carried out.
The camptothecin analogue, Irinotecan (CPT-11; Camptosar®) is an FDA-approved agent used for treatment of metastatic colorectal cancer alone or in combination with 5-fluorouracil and leucovorin10–12. Irinotecan is also clinically active against recurrent or metastatic head and neck cancer when used alone and in combination with chemotherapeutic agents such as cisplatin or docetaxel13–15. Therefore, in this study, we investigated the effects of Bevacizumab on the therapeutic efficacy of Irinotecan against FaDu HNSCC xenografts. First, the effects of Bevacizumab treatment on the vascular function of HNSCC xenografts were studied using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and immunohistochemistry (IHC). Subsequently, short-term and long-term outcome following Bevacizumab-Irinotecan combination therapy was determined by studying the kinetics of tumor response and relapse in nude mice bearing FaDu HNSCC.
MATERIALS AND METHODS
Tumor model system
Female athymic nude mice (nu/nu, body weight, 20–25 g), eight- to 12- weeks of age, were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and kept 5 mice per cage with water and food ad libitum. FaDu cells were maintained as a monolayer in RPMI 1640 supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA). Xenografts were initially established by subcutaneous implantation of 106 cells from culture and subsequently passaged in vivo by transplantation of ~ 50 mg non-necrotic tumor tissue over several generations as described previously16. All studies were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Drugs
Irinotecan (CPT-11) was purchased from Pfizer, Inc. (formerly Pharmacia & Upjohn Company Kalamazoo, MI) in a ready-to-use formulation at a concentration of 20 mg/ml. Bevacizumab (Avastin®) was purchased from Genentech (San Francisco, CA) in 100 mg vials. All drugs were diluted in sterile saline to obtain the desired final concentration for injection.
Study design
For all studies, Irinotecan was administrated intravenously (i.v.) at a dose of 100 mg/kg by tail-vein injection once a week for 4 weeks (weekly × 4). Bevacizumab monotherapy was evaluated at 5 mg/kg or 20 mg/kg by intraperitoneal (i.p.) injection daily for 14–28 days. For combination treatment, tumor-bearing mice were treated with Irinotecan (100 mg/kg, weekly × 4) and Bevacizumab at 5 mg/kg (Bevacizumab 5) or 20 mg/kg (Bevacizumab 20) daily for 28 days, with the first dose of Bevacizumab administered 7 days prior to Irinotecan treatment beginning on the same day of tumor implantation.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) experiments were performed in a 4.7T MR scanner (General Electric, Fremont, CA) with AVANCE digital electronics (Bruker Medical, Billerica, MA) dedicated for preclinical research. Anesthetized mice were placed on an MR-compatible sled equipped with temperature and respiratory sensors and positioned in the scanner. Preliminary scout images were acquired on sagittal and axial planes for slice positioning. T1-weighted dynamic contrast-enhanced MRI was performed using the intravascular contrast agent, albumin-gadopentetate dimeglumine (albumin-GdDTPA) according to previously described methods17–19. The change in T1-relaxation rate (ΔR1) was calculated for tumor and normalized to the vascular relaxation enhancement (ΔR1tumor/blood) to estimate changes in tumor vascular permeability following treatment17–19. All post processing and analysis were performed using Analyze™ (Analyze Direct, Overland Park, KS) and MATLAB (Version 7.0, Math works Inc., Natick, MA). T1-relaxation maps were calculated on a pixel-by-pixel basis.
Measurement of tumor response
Two axes of the tumor (L, longest axis; W, shortest axis) were measured with a Vernier caliper. Tumor volume (mm3) was calculated from the dimensions using the formula, V = ½(L × W2). Measurements were taken once a day during treatment and 2 to 3 times a week thereafter. Animals were randomized into one of 5 different treatment groups on day 7 after tumor transplantation (when the tumors reached approximately 200–250 mm3). Tumor response was expressed as a partial response (PR) when tumor volume was temporarily reduced by at least 50% of initial tumor size and as complete response (CR) when tumor was undetectable by palpation at the site of transplantation16,20. Animals with no visible/palpable tumor at the end of the 60-day period were considered to be cured.
Immunohistochemical detection of intratumoral microvessels
Whole tumor specimens were placed in zinc containing fixative overnight and processed to generate paraffin blocks. Haematoxylin-eosin stained slides were used for as a guide for general orientation. Deparaffinized sections were immunostained with mAb CD31 to visualize microvessels as described earlier18,19. All CD31 positive intratumoral microvessels were counted at 400× magnification in each individual microscopic field on the viable parts of the entire tumor without any selection criteria. Single CD31-positive endothelial cells without any visible lumen were not counted. The results were reported as the average microvessel density (MVD) per high power field. All histopathological and immunohistochemical analyses and counting of microvessels were performed by an experienced pathologist (K.T).
Statistical analysis
All statistical analyses were performed using GraphPad Prism Version 5.00 for Windows (GraphPad Software, San Diego, CA). Measured values are reported as the mean ± standard error of the mean and p-values <0.05 were considered statistically significant. MRI examinations were performed on a total of 16 tumors (Controls, n = 5; Bevacizumab 5 mg/kg, n = 6; Bevacizumab 20 mg/kg, n=5) implanted subcutaneously in the flanks of nude mice. Linear regression analysis of ΔR1 (tumor/blood) was performed to estimate differences in vascular permeability. Tumor growth measurements were performed on a total of 80 mice bearing FaDu tumors (Control group, n = 20; CPT-11 alone, n = 20; Bevacizumab 5 mg/kg, n=10, Bevacizumab 20 mg/kg, n=10, CPT 11 + Bevacizumab 5 mg/kg, n = 10; CPT-11 + Bevacizumab 20 mg/kg, n = 10). 1/10 animals in the CPT 11 + Bevacizumab 5 group accidentally died during the course of the experiment. One-way analysis of variance with Bonferroni’s multiple comparisons test was used to compare differences between all 4 groups. Two-tailed unpaired t test was used to compare differences between individual groups.
RESULTS
Antiangiogenic effects of Bevacizumab in HNSCC
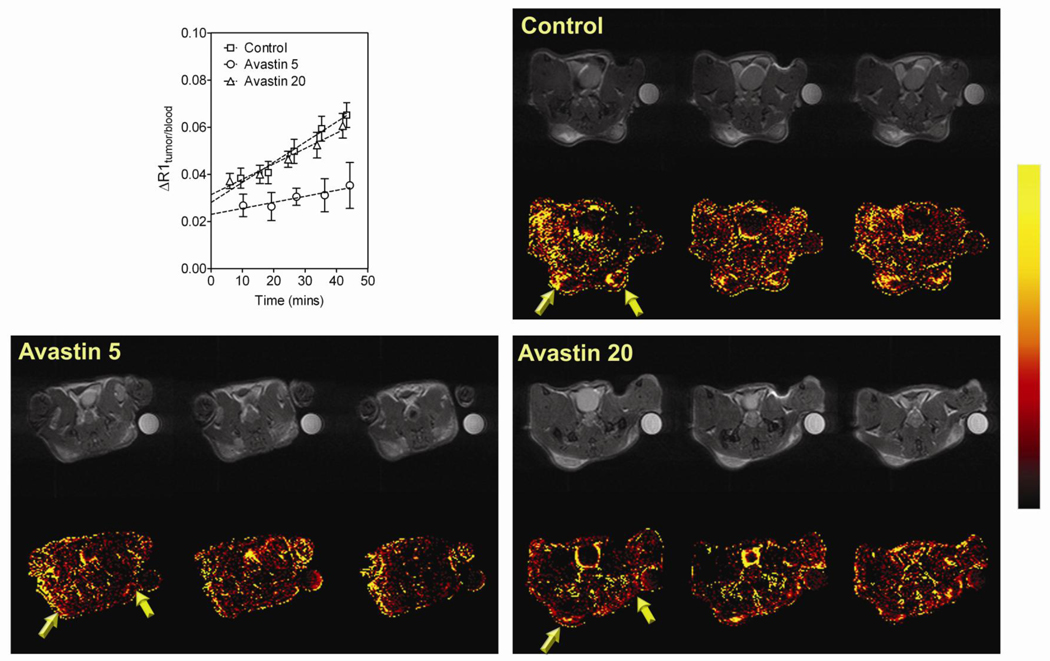
We first examined the antiangiogenic effects of Bevacizumab in FaDu HNSCC xenografts using DCE-MRI. Nude mice bearing FaDu tumors were treated with Bevacizumab at 5 mg/kg or 20 mg/kg daily for a period of 7 days beginning on the day of implantation. Animals were imaged on day 7 to examine changes in vascular function following treatment. Contrast agent concentration was estimated by calculating the change in T1-relaxation rate (ΔR1) of tumor and blood. Linear regression analysis of normalized ΔR1 (tumor/blood) was performed to compute relative vascular volume (y-intercept at t=0) and vascular permeability (slope) of tumors (Figure 1, upper left panel). Permeability maps were also calculated on a pixel-by-pixel basis to provide images of contrast agent accumulation within the tumor over ~45 minutes. Permeability maps of an individual mouse bearing FaDu xenograft (3 contiguous slices) from each of the 3 groups (Control, Bevacizumab 5 mg/kg and Bevacizumab 20 mg/kg) are shown in Figure 1 along with corresponding raw proton images. A color look-up table was applied to the calculated permeability maps to allow improved visualization of treatment-induced changes in vascular permeability. As shown in Figure 1 upper right panel, all three slices of the control FaDu tumor showed hyper-permeable regions within the tumor characterized by leakage of the contrast agent (arrows) and a steady increase in normalized ΔR1 values. In contrast, permeability maps of the tumor treated with 5 mg/kg of Bevacizumab showed minimal change in enhancement following contrast (lower left panel) and a significant reduction in vascular permeability of FaDu tumors compared to untreated controls (p<0.01 between slopes). Interestingly, permeability map and the ΔR1 profile of the mouse treated with Bevacizumab at 20 mg/kg/day for 7 days was similar to the controls (Figure 1, p=0.11 between slopes).
Figure 1. MRI-based assessment of antiangiogenic activity of Bevacizumab against HNSCC.
Plot shows the normalized change in T1-relaxation rate (ΔR1 tumor/blood) of control FaDu tumors (n=5) and Bevacizumab-treated FaDu tumors at 5 mg/kg (Bevacizumab 5, n=6) or 20 mg/kg (Bevacizumab 20, n=5) over ~45 minutes following administration of the MR contrast agent. Linear regression analysis of normalized ΔR1 (tumor/blood) was performed to estimate tumor vascular volume (y-intercept) and permeability (slope). Panel of images represent permeability maps of individual mice bearing FaDu tumors (3 contiguous slices) from each of the 3 groups (Control, Bevacizumab 5 mg/kg and Bevacizumab 20 mg/kg) along with corresponding raw proton images. A color look-up table was applied to the calculated permeability maps to allowed improved visualization of treatment-induced changes in vascular permeability.
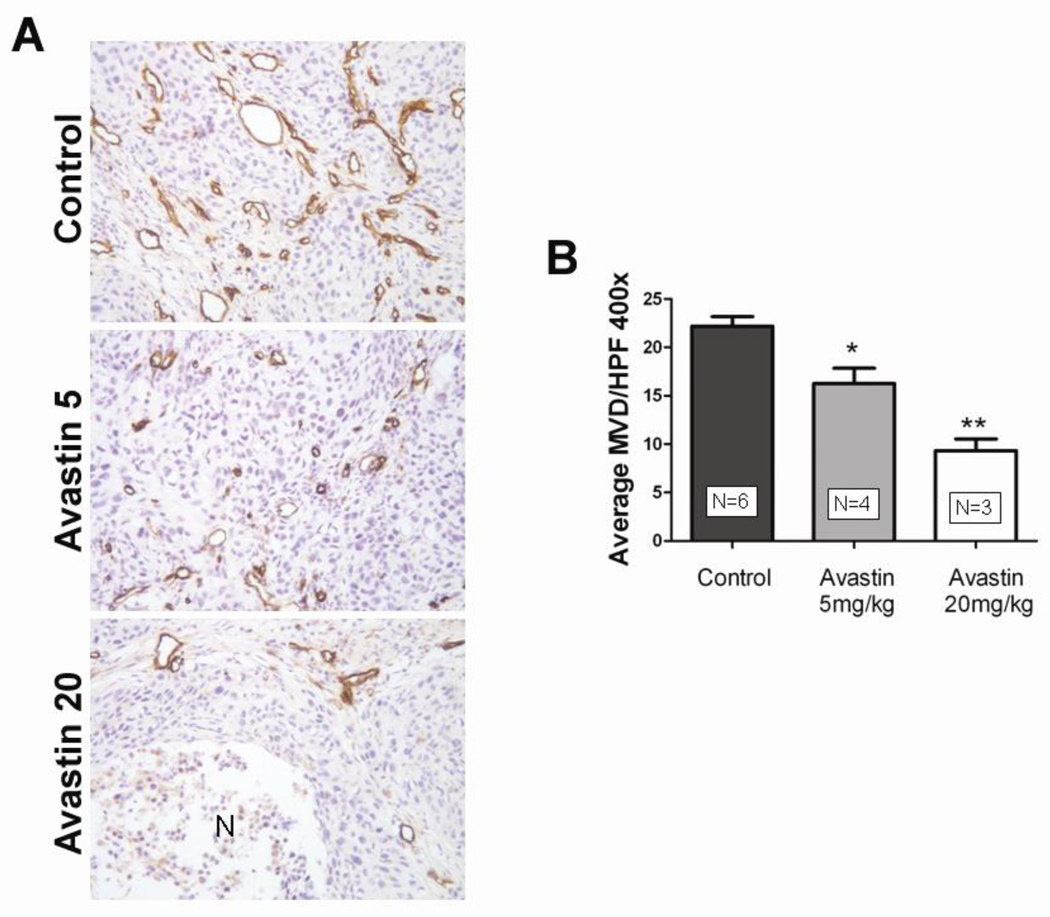
To further investigate the effects of Bevacizumab monotherapy on FaDu tumor vasculature, FaDu tumor-bearing mice were treated with Bevacizumab at 5 mg/kg or 20 mg/kg for a period of 14 days at the end of which the tumors were excised and immunostained for the endothelial cell adhesion molecule CD31. Figure 2A shows representative microphotographs of CD31-immunostained FaDu tumor sections obtained from mice treated with 5 mg/kg (middle panel) and 20 mg/kg of Bevacizumab (lower panel). Corresponding CD31-immunostained section of a control tumor is also shown. In comparison to the well-vascularized control tumor, treatment with 5 mg/kg of Bevacizumab resulted in a significant decrease in MVD (Fig 2B, p<0.01). FaDu tumor sections of animals treated with Bevacizumab at 20 mg/kg revealed in multiple focal areas of tumor necrosis devoid of any microvessels and even greater reduction in microvessel density (p<0.002).
Figure 2. Immunohistochemical assessment of FaDu vascular response to Bevacizumab treatment.
(A) Representative microphotographs of CD31-immunostained tumor sections obtained from a control animal and following treatment with 5 mg/kg or 20 mg/kg Bevacizumab. Multiple, focal necrosis (N) devoid of microvessels was seen with high dose Bevacizumab treatment. (B) Bar graph shows intratumoral microvessel density counts of control FaDu tumors and tumors obtained from animals treated with Bevacizumab. A significant dose dependent reduction in microvessel counts was observed with Bevacizumab treatment compared to untreated control tumors. Values are reported as average MVD counts per high power field.
Short-term response of HNSCC xenografts to Bevacizumab and Irinotecan
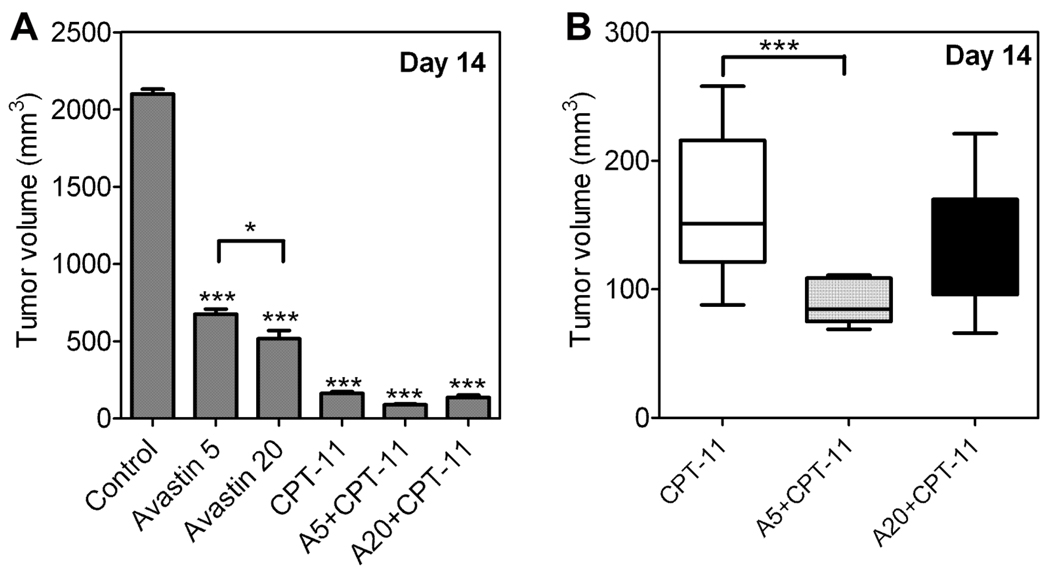
We then examined the short-term tumor response of HNSCC xenografts to Irinotecan alone and in combination with Bevacizumab at the two doses (5 and 20 mg/kg) studied using MRI. On day 14 when the mice were sacrificed to harvest the tumors for histopathological evaluation after MRI studies, the tumors were weighed for all animals in control and 5 treatment groups, Bevacizumab alone (5 and 20 mg/kg/day), Irinotecan alone (100 mg/kg), Bevacizumab 5 mg/kg + Irinotecan and Bevacizumab 20 mg/kg + Irinotecan and compared to untreated controls. The tumor volume (mean ± sem) of animals in the control group was 2100 ± 30.9 mm3 (n=20). Bevacizumab treatment (n=10 per group) resulted in significant tumor growth inhibition (p<0.001) at 5 mg/kg (675 ± 33.9 mm3) and 20 mg/kg (516 ± 53 mm3). Differences in tumor volume between the two Bevacizumab doses were also statistically significant (p<0.05). Tumor volumes of animals treated with Irinotecan alone, Bevacizumab 5 mg/kg + Irinotecan and Bevacizumab 20 mg/kg + Irinotecan, were 162 ± 11.7 mm3 (n=20), 89 ± 5.0 mm3 (n=10) and 136.2 ± 15.8 mm3 (n=10), respectively. All 5 treatment groups showed a statistically significant reduction in tumor volume on day 14 compared to untreated controls as shown in Figure 3A (one-way ANOVA, p<0.0001). However, when compared to Irinotecan monotherapy, only the combination of Bevacizumab 5 mg/kg + Irinotecan showed a statistically significant reduction in tumor volume on day 14 (Fig 3B, p<0.001).
Figure 3. Short-term response of HNSCC xenografts to Bevacizumab-Irinotecan combination treatment.
(A) Caliper-based tumor volume measurements for animals in the 5 treatment groups along with untreated controls on day 14 post implantation when the tumors were harvested and weighed for immunohistochemistry. All 5 treatment groups showed a statistically significant reduction in tumor volume on day 14 compared to untreated controls (p<0.0001). (B) Scatter plot of individual tumor volumes of animals treated with Irinotecan (CPT-11) alone, Bevacizumab 5 + CPT-11 and Bevacizumab 20 + CPT-11. Only animals treated with Bevacizumab 5 + CPT-11 showed a statistically significant reduction in tumor volume on day 14 (p<0.001) compared to treatment with CPT-11 alone. Asterisks above the error bar indicate statistical significance in comparison to controls.
Long-term Bevacizumab treatment enhances chemotherapeutic efficacy against HNSCC
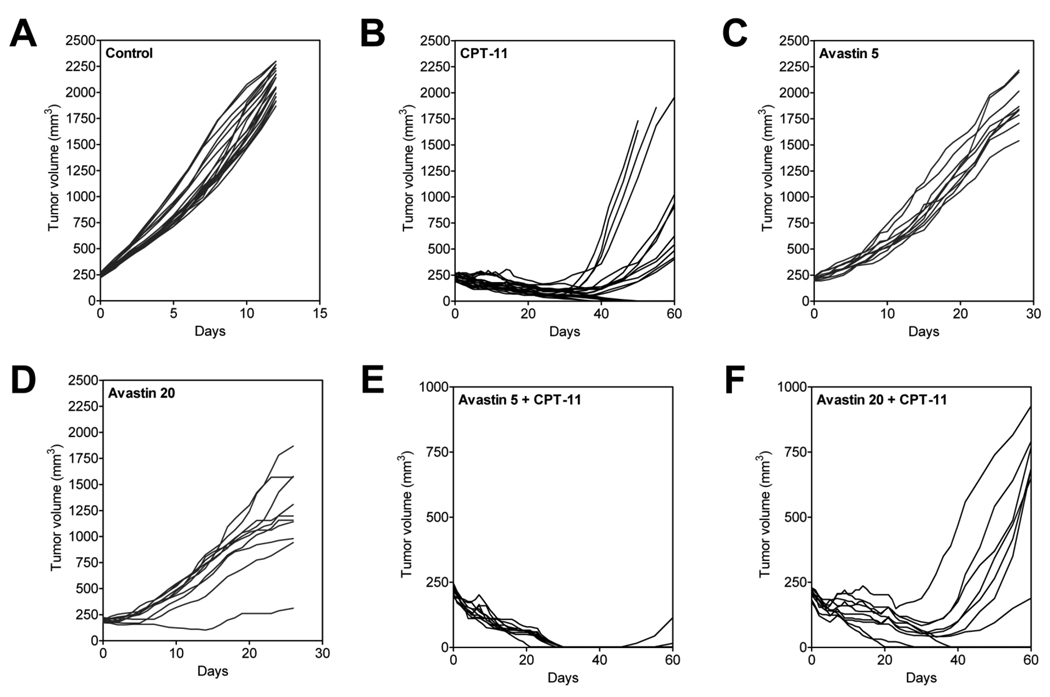
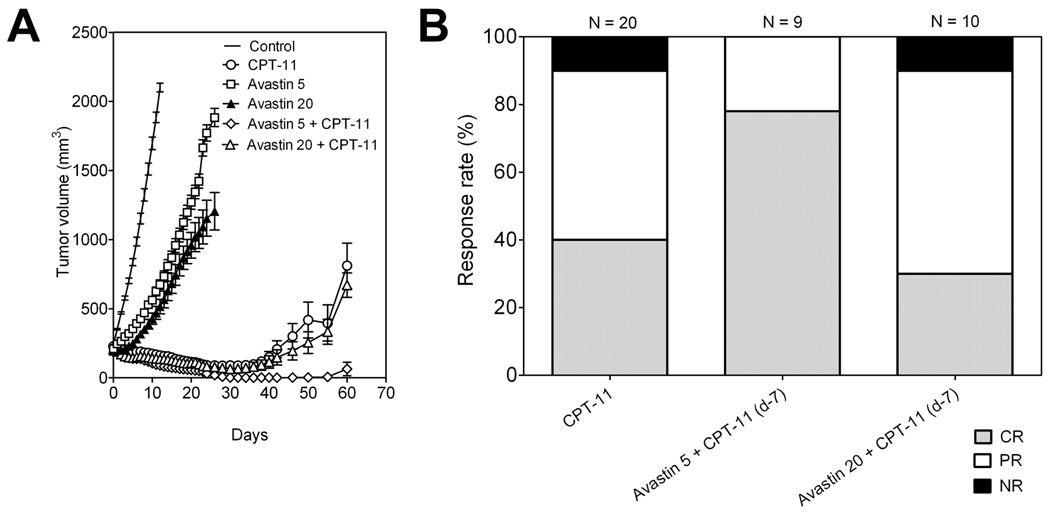
The long-term response of FaDu HNSCC xenografts to CPT-11 and Bevacizumab alone and in combination was studied by monitoring the change in tumor volume over a 60-day period. Individual tumor growth curves of FaDu xenografts in all 6 groups are shown in Figure 4. As mentioned above, control tumors reached ~2000 mm3 volume approximately 12–14 days post randomization (Fig 4A). CPT-11 treatment alone resulted in complete regression of tumors in 8/20 animals during a 50 day period with a considerable delay in tumor regrowth observed compared to controls (Fig 4B). Although significant tumor growth inhibition compared to controls was observed, treatment with Bevacizumab alone at 5 mg/kg or 20 mg/kg did not result in any tumor regression (Fig 4C and 4D). Combination treatment with Bevacizumab (5 mg/kg) and Irinotecan resulted in a marked tumor growth inhibition led to complete ‘cure’ of tumors in 7/9 animals within ~30 days (Fig 4E). Tumor response observed with the combination of Irinotecan with Bevacizumab at a dose of 20 mg/kg was comparable to the response observed with Irinotecan treatment alone, with complete tumor regression observed only in 3/10 animals (Fig 4F). The reduction in tumor growth at the end of 30 days with the combination of Bevacizumab 5 mg/kg with Irinotecan was significantly greater compared to CPT-11 alone (p<0.001) and the combination of Bevacizumab 20 mg/kg with Irinotecan (p<0.01). This trend persisted up to the end of the 60-day period of evaluation.
Figure 4. Long-term tumor response to Bevacizumab-Irinotecan combination treatment.
Individual tumor growth curves of FaDu xenografts in all experimental groups over a 60 day period are shown. All 3 treatment groups (B,E,F) resulted in a significant delay in tumor re-growth compared to untreated controls. Maximal therapeutic benefit was observed with the combination of Bevacizumab 5 mg/kg and CPT-11 (100 mg/kg).
Figure 5A shows the change in mean ± sem of FaDu tumor volume over the 60-day period. A summarized bar graph showing CR, PR and NR (% values) is shown in Figure 5B. Treatment with Irinotecan was associated with 40% CR and 50% PR in FaDu xenografts, respectively. The combination of Bevacizumab at 20 mg/kg with Irinotecan resulted in a similar profile with 30% CR and 60% PR in FaDu xenografts. In contrast, Bevacizumab 5 mg/kg with Irinotecan resulted in a higher and durable response of ~80% CR and 20% PR. Interestingly, while 10% of tumors (NR) failed to respond to Irinotecan alone and in combination with Bevacizumab at 20 mg/kg, all tumors showed a partial or complete response to the combination of Bevacizumab 5 mg/kg and Irinotecan.
Figure 5. Long-term low-dose Bevacizumab enhances long-term chemotherapeutic efficacy against HNSCC.
(A) Change in FaDu tumor volume of animals in control and 5 treatment groups over the 60-day period. Significant differences in tumor volume were observed between CPT-11 alone and Bevacizumab 5 + CPT-11 groups. (B) Summarized bar graph showing CR, PR and NR (% values) following treatment with CPT-11 alone and in combination with Bevacizumab. Treatment with CPT-11 was associated with CR and PR values of 40% and 50 % PR, respectively. The combination of Bevacizumab 20 + CPT-11 resulted in a similar profile with 30% CR and 60% PR in FaDu xenografts. In contrast, Bevacizumab 5 + CPT-11 resulted in a higher and durable response of ~80% CR and 20% PR.
DISCUSSION
The overall goal of this study was to investigate the therapeutic potential of the combining Bevacizumab with Irinotecan in a preclinical xenograft model of human head and neck cancer. The results of our study demonstrate that continuous administration of a biologically active dose of Bevacizumab significantly enhances the antitumor activity of Irinotecan against HNSCC. Bevacizumab (5 mg/kg) markedly potentiated the antitumor efficacy of Irinotecan resulting in a significant increase in CR from 40% with Irinotecan monotherapy to ~80% with the combination. This enhanced antitumor activity of the combination of Bevacizumab and Irinotecan was observed to be schedule-dependent. Even when Bevacizumab was used at 5 mg/kg, significant enhancement of therapeutic efficacy was seen only with sequential administration of Bevacizumab for seven days prior to Irinotecan treatment. Concurrent administration of Bevacizumab and Irinotecan on the same day did not exhibit any significant increase in CR compared to Irinotecan monotherapy (data not shown). At a higher dose Bevacizumab (20 mg/kg) decreased the microvessel density of the tumors (Fig. 2). However, combination treatment at this dose did not exhibit any potentiation of antitumor activity compared to Irinotecan alone (Fig. 3–5). One possible explanation for this observation is that the marked inhibition of angiogenesis by high dose (20 mg/kg) Bevacizumab treatment hindered intratumoral delivery of Irinotecan leading to poor treatment outcome.
In clinical studies, Bevacizumab has been administered at doses ranging from 5–10 mg/kg once every two weeks or 15 mg/kg once every 3 weeks21,22. Extensive preclinical pharmacology and pharmacodynamic evaluation of Bevacizumab has also been carried out in a number of tumor models23 [and references within]. The doses used in our study (5 mg/kg and 20 mg/kg (~100 ug and 400 ug per dose, respectively) are comparable to doses previously studied in human tumor xenograft models. In our experience, mice appear to tolerate higher doses of Bevacizumab since we did not observe any significant toxicity even at 20 mg/kg. Although Bevacizumab shares pharmacological properties its murine counterpart (A4.6.1, from which it was derived), it is generally believed to be specific for human VEGF and does not bind to murine VEGF23,24. Nevertheless, recent studies have demonstrated weak binding (≤0.1% of binding to human VEGF-A) of Bevacizumab to murine VEGF-A24,25. However, Bevacizumab fails to inhibit the activity of murine VEGF-A even at extremely high molar concentrations24. This lack of cross reactivity with murine VEGF is at least partly accountable for differences in efficacy and toxicity of Bevacizumab in combination with chemotherapy between mice and humans23,25.
The camptothecin analogue, Irinotecan (CPT-11; Camptosar®) is approved for clinical use in patients with advanced colorectal cancer10,11. The drug undergoes enzymatic breakdown to its active metabolite, SN-38, which stabilizes the complex formed between double-stranded DNA and the nuclear enzyme, topoisomerase-126. The formation of this complex induces replication fork arrest and chromatid breaks by preventing relaxation of DNA supercoiling during replication26. Since HNSCCs have been associated with high topoisomerase-1 activity27, we examined the antitumor activity of Irinotecan against FaDu HNSCC xenografts alone and in combination with Bevacizumab. While the activity of Irinotecan has been demonstrated against a variety of experimental tumors and human tumor xenografts, only a few reports have investigated its activity in head and neck cancer13,28. Yazici et al., have demonstrated enhanced therapeutic efficacy with the combination of VEGFR tyrosine kinases and Irinotecan28. Although Irinotecan does not have a standard role in HNC management, the agent has exhibited activity in combination with cisplatin and docetaxel in patients with recurrent and metastatic head and neck cancer14,15. Irinotecan has also shown modest activity as a single agent in chemonaive and previously treated head and neck cancer13,29. The combination of irinotecan and docetaxel with or with radiation showed promising results with a high CR rate achieved in patients with advanced head and neck cancer30.
Bevacizumab is a humanized monoclonal antibody against VEGF, a potent endothelial mitogen and a principal regulator of the angiogenic switch in most solid tumors including HNSCC5–7. Since 2004, the drug has received FDA-approval for clinical use in patients with advanced metastatic colorectal carcinoma, renal cell carcinoma, breast and non-small cell lung cancer. Preclinical and clinical studies have demonstrated the potential of Bevacizumab in the combination setting against head and neck cancers8,9,21,31. Using an orthotopic model of head and neck cancer, Bozec et al., have recently shown that the combination of the Bevacizumab, Erlotinib and radiation results in marked tumor growth inhibition compared to monotherapy8. Increased antitumor activity has also been observed with the combination of bevacizumab and paclitaxel against HNSCC31. A phase I/II study, the combination of Erlotinib and Bevacizumab was well tolerated and showed a sustained benefit and CR in some patients with recurrent or metastatic head and neck cancer21.
The biological response of tumors to angiogenic inhibition is distinct from the antitumor responses seen with cytotoxic agents. As a result, evaluation of antiangiogenic therapy demands estimation of multiple response variables reflective of the underlying changes in tumor physiology rather than a simple assessment of tumor morphology. Jain and colleagues have demonstrated that angiogenic inhibition with Bevacizumab leads to a transient change in tumor vascular phenotype to one that closely resembles normal tissue vasculature32. During this window of ‘normalization’, reduction in vascular permeability and consequently, lowering of the interstitial fluid pressure are observed32,33. These changes contribute to enhancement of the antitumor efficacy of chemo- and radiotherapy by improving drug delivery and tumor oxygenation, respectively32–34. Wildiers et al have previously demonstrated increased intratumoral availability of Irinotecan in colorectal cancers following treatment with an anti-VEGF antibody35. The antiangiogenic effects of Bevacizumab (decreased microvessel density, reduced permeability and interstitial fluid pressure) seen in human tumor xenografts is generally attributed to its interaction with only the human VEGF secreted by the xenograft and not by the host (mouse)23,24.
A majority of studies investigating the significance of angiogenesis or the potential of antiangiogenic agents in HNSCC have utilized immunohistochemical techniques for assessment of tumor vascularity. Immunohistochemical endpoints such as microvessel density (MVD) are important read-outs of vascular status, but analysis of tissue samples does not account for potential spatial or temporal heterogeneity within the tumor and the invasive nature of the methodology prevents longitudinal assessment within the same subject. Here, using a clinically-relevant imaging technique (MRI), we have demonstrated the effects of Bevacizumab on head and neck tumor angiogenesis. We have previously utilized contrast-enhanced MRI to study the vascular characteristics of head and neck tumor models and their response to antivascular strategies in vivo18,19. Recently, using MRI, we have demonstrated the presence of angiogenic heterogeneity between head and neck subsites using patient tumor-derived head and neck squamous cell carcinoma xenografts18. In the present study, using a similar method, we examined the vascular response of HNSCC xenografts to Bevacizumab treatment. Our results show that, Bevacizumab induced changes in tumor vascular permeability are dose dependent. Similar observations using MRI have been previously reported in the literature36,37. Raatschen et al., have previously reported reduction in vascular permeability detected by MRI as a useful biomarker of tumor response to Bevacizumab36. In addition to measurement of changes in vascular permeability and perfusion, MRI has also been recently used to estimate reduction in edema associated with gliomas following angiogenic inhibition37. With the increased development of molecular targeted therapies, non-invasive imaging techniques such as MRI will continue to play an important role for diagnostic and prognostic assessment in oncology.
In conclusion, the results of the present study demonstrate a favorable therapeutic interaction between Bevacizumab and Irinotecan. However, optimal therapeutic synergy appears to be dependent on the dose and schedule of Bevacizumab. While Bevacizumab-Irinotecan combination treatment is currently being evaluated in patients with gliomas in clinical trials, to the best of our knowledge, therapeutic activity of this combination has not been studied in head and neck tumor models. The positive results of the present study highlight the potential of this drug combination in head and neck cancer. Our MRI and immunohistochemistry results are consistent with previously observed effects of Bevacizumab on tumor vascular function and point towards vascular ‘normalization’ as the possible mechanism. However, it is likely that other molecular mechanisms contribute to the observed therapeutic benefit with combination treatment. Therefore, further investigation into the mechanisms involved in tumor cellular and vascular response to this combination is warranted.
Acknowledgments
FUNDING
This work was supported by grants from the National Cancer Institute (NCI; R21CA133688 (M.S) and the Alliance Foundation (M.S.) and utilized core resources supported by RPCI’s Cancer Center Support Grant from the NCI P30CA16056 (Trump, DL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
None declared.
REFERENCES
- 1.Forastiere AA, Trotti A, Pfister DG, Grandis JR. Head and neck cancer: recent advances and new standards of care. J Clin Oncol. 2006;24(17):2603–2605. doi: 10.1200/JCO.2006.07.1464. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis. Ann Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. Review. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6(9):507–518. doi: 10.1038/nrclinonc.2009.110. Review. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. Review. [DOI] [PubMed] [Google Scholar]
- 7.Midgley R, Kerr D. Bevacizumab--current status and future directions. Ann Oncol. 2005;16(7):999–1004. doi: 10.1093/annonc/mdi208. Review. [DOI] [PubMed] [Google Scholar]
- 8.Bozec A, Sudaka A, Fischel JL, Brunstein MC, Etienne-Grimaldi MC, Milano G. Combined effects of bevacizumab with erlotinib and irradiation: a preclinical study on a head and neck cancer orthotopic model. Br J Cancer. 2008;99(1):93–99. doi: 10.1038/sj.bjc.6604429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prichard CN, Kim S, Yazici YD, Doan DD, Jasser SA, Mandal M, et al. Concurrent cetuximab and bevacizumab therapy in a murine orthotopic model of anaplastic thyroid carcinoma. Laryngoscope. 2007;117(4):674–679. doi: 10.1097/MLG.0b013e318031055e. [DOI] [PubMed] [Google Scholar]
- 10.Saltz LB, Douillard JY, Pirotta N, Alakl M, Gruia G, Awad L, et al. Irinotecan plus fluorouracil/leucovorin for metastatic colorectal cancer: a new survival standard. Oncologist. 2001;6(1):81–91. doi: 10.1634/theoncologist.6-1-81. [DOI] [PubMed] [Google Scholar]
- 11.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 12.Vanhoefer U, Harstrick A, Achterrath W, Cao S, Seeber S, Rustum YM. Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol. 2001;19(5):1501–1518. doi: 10.1200/JCO.2001.19.5.1501. [DOI] [PubMed] [Google Scholar]
- 13.Murphy BA, Cmelak A, Burkey B, Netterville J, Shyr Y, Douglas S, et al. Topoisomerase I inhibitors in the treatment of head and neck cancer. Oncology. 2001;15(7):47–52. [PubMed] [Google Scholar]
- 14.Gilbert J, Cmelak A, Shyr Y, Netterville J, Burkey BB, Sinard RJ, et al. Phase II trial of irinotecan plus cisplatin in patients with recurrent or metastatic squamous carcinoma of the head and neck. Cancer. 2008;113(1):186–192. doi: 10.1002/cncr.23545. [DOI] [PubMed] [Google Scholar]
- 15.Argiris A, Buchanan A, Brockstein B, Kolesar J, Ghebremichael M, Pins M, et al. Docetaxel and irinotecan in recurrent or metastatic head and neck cancer. Cancer. 2009;115(19):4504–4513. doi: 10.1002/cncr.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao S, McGuire JJ, Rustum YM. Antitumor activity of ZD1694 (Tomudex) against human head and neck cancer in nude mouse models: role of doing schedule and plasma thymidine. Clin Cancer Res. 1999;5(7):1925–1934. [PubMed] [Google Scholar]
- 17.Demsar F, Roberts TP, Schwickert HC, Shames DM, van Dijke CF, Mann JS, et al. A MRI spatial mapping technique for microvascular permeability and tissue blood volume based on macromolecular contrast agent distribution. Magn Reson Med. 1997;37(2):236–242. doi: 10.1002/mrm.1910370216. [DOI] [PubMed] [Google Scholar]
- 18.Seshadri M, Merzianu M, Tang H, Rigual NR, Sullivan M, Loree TR, et al. Establishment and characterization of patient tumor-derived head and neck squamous cell carcinoma xenografts. Cancer Biol Ther. 2009;8(23):2275–2283. doi: 10.4161/cbt.8.23.10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seshadri M, Toth K. Acute vascular disruption by 5,6-dimethylxanthenone-4-acetic acid in an orthotopic model of human head and neck cancer. Transl Oncol. 2009;2(3):121–127. doi: 10.1593/tlo.09103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by seleninum containing compounds against human tumor xenografts. Clin. Cancer Res. 2004;10(7):2561–2569. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 21.Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattan S, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10(3):247–257. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 23.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65(3):671–680. [PubMed] [Google Scholar]
- 24.Yu L, Wu X, Cheng Z, Lee CV, LeCouter J, Campa C, Fuh G, Lowman H, Ferrara N. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci. 2008 Feb;49(2):522–527. doi: 10.1167/iovs.07-1175. [DOI] [PubMed] [Google Scholar]
- 25.Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M, Zahn G, Cursiefen C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007 Jun;48(6):2545–2552. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- 26.Pommier Y, Pourquier P, Fan Y, Strumberg D. Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400(1–3):83–106. doi: 10.1016/s0167-4781(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 27.Masin JS, Berger SJ, Setrakian S, Stepnick DW, Berger NA. Topoisomerase I activity in squamous cell carcinoma of the head and neck. Laryngoscope. 1995;105(11):1191–1196. doi: 10.1288/00005537-199511000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Yazici YD, Kim S, Jasser SA, Wang Z, Carter KB, Jr, Bucana CD, Myers JN. Antivascular therapy of oral tongue squamous cell carcinoma with PTK787. Laryngoscope. 2005 Dec;115(12):2249–2255. doi: 10.1097/01.mlg.0000183861.53765.77. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert J, Dang T, Cmelak A, Shyr Y, Netterville J, Burkey B, et al. Single agent irinotecan for the treatment of metastatic or recurrent squamous carcinoma of the head and neck (SCCHN) Clinical Medicine: Oncology. 2007;1(7):67–71. [Google Scholar]
- 30.Koukourakis MI, Bizakis JG, Skoulakis CE, Kymizakis D, giatromanolaki A, Papadakis CE, et al. Combined irinotecan, docetaxel and conventionally fractionated radiotherapy in locally advanced head and neck cancer. A phase I dose escalation study. Anticancer Res. 1999;19(3B):2305–2309. [PubMed] [Google Scholar]
- 31.Fujita K, Sano D, Kimura M, Yamashita Y, Kawakami M, Ishiguro Y, et al. Antitumor effects of bevacizumab in combination with paclitaxel on head and neck squamous cell carcinoma. Oncol Rep. 2007;18(1):47–51. [PubMed] [Google Scholar]
- 32.Jain RK. Normalizing tumor vasculature with antiangiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 33.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 34.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, et al. Blockade of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59(14):3374–3378. [PubMed] [Google Scholar]
- 35.Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88(12):1979–1986. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raatschen HJ, Simon GH, Fu Y, Sennino B, Shames DM, Wendland MF, et al. Vascular permeability during antiangiogenesis treatment: MR imaging assay results as biomarker for subsequent tumor growth in rats. Radiology. 2008;247(2):391–399. doi: 10.1148/radiol.2472070363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27(15):2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]