Summary
Morbidity, the state of being diseased, is an important aspect of pathogenesis that has gone relatively unstudied in fruit flies. Our interest is in characterizing how bacterial pathogenesis affects various physiologies of the fly. We chose to examine the fly ovary because we found bacterial infection had a striking effect on fly reproduction. We observed decreased egg laying after bacterial infection that correlated with increased bacterial virulence. We also found that bacteria colonized the ovary in a previously undescribed manner; bacteria were found in the posterior of the ovary, adjacent to the lateral oviduct. This local infection in the ovary resulted in melanization and activation of the cellular immune response at the site of infection.
Introduction
Fruit flies have served as a trusted model for innate immunity. Work in the fly revealed the importance of the Toll signaling pathway as a central regulator of innate immune responses [1]. This field has gone on to describe the fly’s transcriptional response to a variety of immune elicitors and has taken a systematic look at the requirements for phagocytosis [2, 3]. Recently, microbiologists have taken an interest in using the fly to identify virulence factors of pathogens that affect fly survival [4–15]. One interaction between host and pathogen that has gone relatively unstudied in flies is sickness. To date, most fly immunity work has focused on the mechanistic aspects of the immediate innate immune response. This work has concentrated on the production of antimicrobial peptide transcripts while other physiological effects of infection have been ignored; yet morbidity, the state of being diseased, is important. To put this in perspective, malaria kills 1,000,000 people per year but it causes 500,000,000 cases of disease (WHO Roll Back Malaria Report 2006); the impact of non-lethal infection is enormous and the biology behind this is important. In addition to being a model for innate immunity, the fly can also serve as a model for the physiological changes triggered by the host’s response to infection that cause what we call “disease”. We address what it means to be a sick fly by challenging flies with microbes that cause disease in wild type flies. We use microbes that ultimately kill the fly because we found that these microbes also cause profound pathological effects and we are interested in defining the effects of bacterial pathogenesis on the various physiologies of the fly. We chose to examine the ovary because we found bacterial infection had a striking effect on fly reproduction.
Insect disease can be reflected in reproductive fitness [16]. Insects generally invest their resources heavily in reproduction and resource competition can occur when the insect is parasitized. When parasite biomass is relatively large, direct competition for nutrients between insect and parasite reduces host reproduction. This is the case for fruit flies infested with mites and for mosquitoes and bark beetles infected with nematodes. When parasite biomass is relatively small, such as in a malaria-infected mosquito, the insect host may divert resources from reproduction and invest in tissue repair and the production of antimicrobial compounds.
Fertility and immunity are linked. Schwartz and Koella (2004), and Ahmed and Hurd (2006), injected mosquitoes with negatively charged beads or lipopolysaccharide to elicit an immune response and saw a decrease in egg production while sham injection of saline had no effect [17, 18]. Zerofsky et al. (2005) established that jabbing fruit flies with a mixture of heat-killed Micrococcus luteus and Escherichia coli also reduced egg production compared to wounding alone [19]. Immune responses can clearly reduce fertility but the mechanisms underlying this have not been identified.
The existing data suggest any immune response can trigger changes in fertility in insects. To study the role microbial virulence plays in this process, we injected three lethal gram-negative bacterial species (Salmonella typhimurium, Serratia marcescens and Burkholderia cenocepacia) into female Drosophila melanogaster and observed a dramatic decrease in egg production in comparison to flies injected with non-pathogenic gram-negative bacteria or medium alone. Using a less virulent S. typhimurium mutant, we showed that decreased egg laying was correlated with increased bacterial virulence.
We found these pathogens infected the ovary in a previously undescribed manner; bacteria were found in the posterior of the ovary, adjacent to the lateral oviduct in an area we describe as the “debris zone”. This local infection in the ovary resulted in melanization and activation of the cellular immune response at the site of infection.
Results and Discussion
Persistent infections reduce lifespan and reproduction
To examine the effects of infection on D. melanogaster reproduction, we injected four gram-negative bacterial species into fruit flies. We chose Salmonella typhimurium, Burkholderia cenocepacia and Serratia marcescens because each caused a lethal infection in wild type flies with comparable killing kinetics (Figure 1a) and persistent bacterial loads (Figure 1b). We compared egg laying during these lethal infections to injection with gram-negative Escherichia coli, which raises an innate immune response but is cleared and does not kill a wild type fly faster than wounding alone (Figures 1a and 1b).
Figure 1. Persistent bacterial infections reduce lifespan and reproduction.
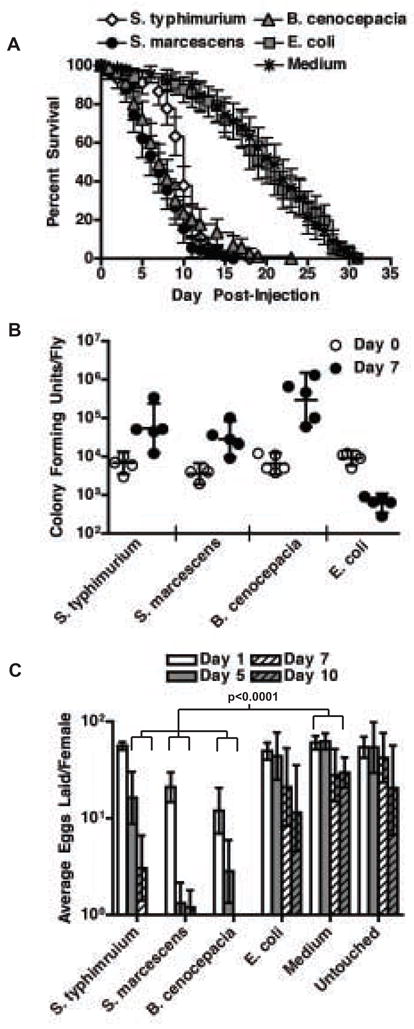
(a) Survival of female flies injected with bacteria or medium. n=90 flies. Symbols indicate mean. Bars indicate 95% confidence intervals. p<0.0001 when comparing S. typhimurium- or S. marcescens- or B. cenocepacia-injected flies to E. coli- or medium-injected flies by logrank test.
(b) Bacterial growth in individual female flies. n=5 flies. The geometric mean and 95% confidence intervals are indicated.
(c) Average eggs laid per female fly injected with bacteria or medium or left untouched. n=18. The geometric mean and 95% confidence intervals are indicated. Significance was determined by two-tailed unpaired t-test. p=.043 for E. coli- vs. medium-injected on day 10. See Supplemental Figure 1 for raw data and full time course. Note: There were no living flies on day 10 post-injection with B. cenocepacia or S. marcescens.
We measured egg laying by counting the number of eggs laid per day. We found average daily egg production in females injected with a lethal dose of bacteria was significantly decreased in comparison to flies injected with non-lethal bacteria (E. coli) or medium alone (Figure 1c and Supplemental Figure 1). The reduction in egg laying was most dramatic in the 2–3 days before death when most infected females ceased laying eggs. We found no significant difference between medium-injected flies and unmolested flies demonstrating that a wounding response does not affect egg laying. Extracellular (S. marcescens, B. cenocepacia) as well as a facultative intracellular pathogen (S. typhimurium) were able to cause a reduction in egg laying. We found no significant difference between E. coli-injected and medium-injected flies until the last day of the time course, corroborating published reports that a transient immune response in response to a non-lethal microbe can reduce egg laying, albeit to a much lesser degree than what we observe with a lethal microbe.
Virulence correlates with reduced egg production
To determine whether egg laying correlated with bacterial virulence we injected flies with mutant S. typhimurium that cannot produce either of their two type III secretion systems (SPI1-/SPI2-). These bacteria are unable to inject their virulence proteins into cells [20]. These are well-characterized virulence factors that have been assayed for their ability to allow entry into cells as well as intracellular replication in the mouse macrophages. SPI1-/SPI2- (SPI) S. typhimurium showed greatly reduced lethality in the fly (Figure 2a) yet bacteria were maintained at levels higher than wild type as we had published previously (Figure 2b) [4].. Flies injected with wild type S. typhimurium significantly reduced egg production with respect to E. coli infected flies while egg laying in flies injected with SPI typhimurium were statistically indistinguishable from flies injected either with E. coli or wild type S. typhimurium using ANCOVA (Figure 2C and Supplemental Figure 2). We take this to mean that the SPI mutant bacteria produced an intermediate phenotype and thus bacterial virulence correlates with reduced egg production. Since bacterial biomass was higher in SPI S. typhimurium injected flies than in wild type S. typhimurium injected flies, reduced egg production does not likely result from direct nutrient competition between the bacteria and the fly.
Figure 2. Bacterial virulence correlates with reduced egg production.
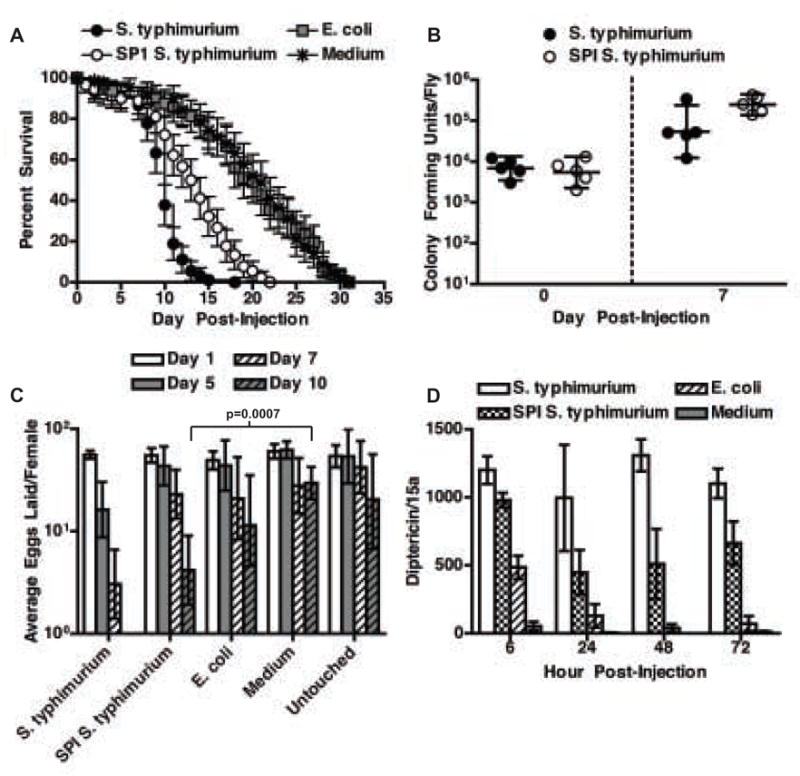
(a) Survival of female flies injected with bacteria or medium. n=90 flies. Symbols indicate mean. Bars indicate 95% confidence intervals. p<0.0001 when comparing S. typhimurium- to SPI S. typhimurium-injected flies by logrank test.
(b) Bacterial growth in individual female flies. n=5 flies. The geometric mean and 95% confidence intervals are indicated.
(c) Average eggs laid per female fly injected with bacteria or medium, or left untouched. n=18 flies. The geometric mean and 95% confidence intervals are indicated. Significance was determined by two-tailed unpaired t-test. See Supplemental Figure 2 for raw data and full time course.
(d) Diptericin RNA transcript levels in female flies injected with bacteria or medium. Values are normalized to day 0. n=3 RNA samples from 5 flies each. The mean and standard deviation are indicated. p<0.05 for wild type vs. SPI S. typhimurium at all time points as determined by two-tailed unpaired t-test.
To determine whether the strength of the immune response correlated with reduced egg production we measured induction of the antimicrobial peptide diptericin over the course of SPI and wild type S. typhimurium infections as a proxy for the intensity of the immune response (Figure 2d). This antimicrobial peptide is induced largely by the Imd pathway in response to gram-negative bacterial infections and has been used previously as a marker of the intensity of an immune response in genetic screens [21]. Following injection with E. coli, flies transiently upregulated diptericin in comparison to medium injection alone. In contrast, flies injected with S. typhimurium persistently upregulated diptericin. Expression in flies injected with SPI S. typhimurium was on average half that found in flies injected with wild type S. typhimurium. SPI S. typhimurium elicited a smaller immune response even though flies contained more bacteria. These results support the hypothesis that the fly diverts resources away from the reproductive system in proportion to the size of the immune response.
Ovaries degenerate during infection
Ovaries in infected flies withered (Figure 3a). By one week post-injection, ovaries from infected flies contained few to no post-vitellogenic egg chambers. Because it was reported previously that malaria infection induces apoptosis of egg follicles in mosquitoes [22], we measured the effect of lethal bacterial infection on the death of fly ovarian egg chambers. We counted the ratio of degenerate egg chambers to total egg chambers in ovaries from S. typhimurium-injected versus medium-injected flies. Degenerate egg chambers were identified by staining with acridine orange, a nucleic acid dye that stains dying egg chambers in a bright punctate pattern. Whereas the ratio of acridine positive egg chambers to total egg chambers in control flies remained constant during the weeklong experiment, the ratio increased dramatically in ovaries of S. typhimurium-injected flies (Figure 3b) suggesting that pathogenesis increased cell death in fly ovaries.
Figure 3. Ovaries degenerate during infection.
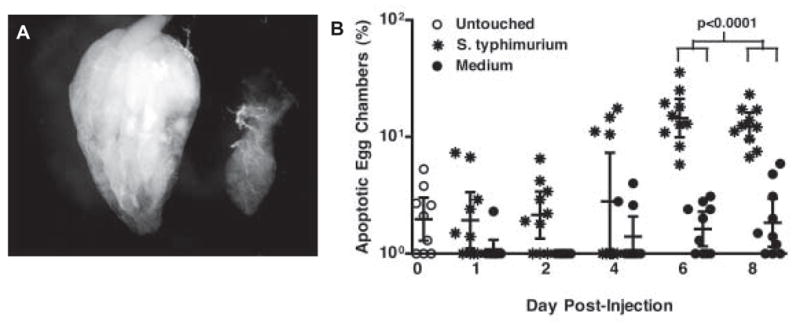
(a) DIC image of a single ovary dissected 7 days post-injection from a medium-injected female fly (left) and a S. typhimurium-injected female fly (right). All ovaries are shown posterior end (oviduct) up.
(b) Percentage of dying egg chambers per ovary pair from female flies injected with S. typhimurium or medium. n=10 ovaries. The geometric mean and 95% confidence intervals are indicated. Significance was determined by two-tailed unpaired t-test.
Bacteria colonize the fly ovary
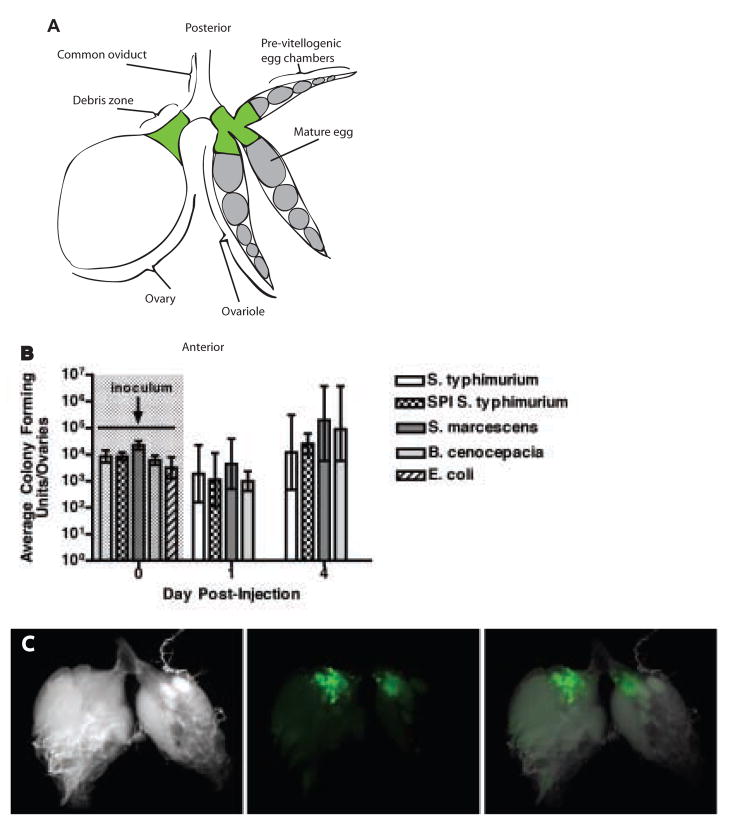
To determine whether bacteria were infecting the ovary we measured the bacterial load in ovaries isolated from infected flies. We found each pathogenic bacterial species (S. typhimurium, B. cenocepacia, and S. marcescens) colonized the ovary (Figure 4b and Supplemental Figure 3) whereas E. coli did not.
Figure 4. Bacteria colonize the fly ovary.
(a) Diagram describing the anatomy of the ovary. Each fly has two ovaries. The one on the left is shown whole while the one on the right is shown in an exploded view revealing three of the approximately 8 ovarioles that make up the ovary. Development proceeds from the anterior to the posterior end of the ovary, where eggs are laid through the common oviduct. The “debris zone” where bacteria and hemocytes are found is marked in green.
(b) Bacterial growth in ovaries of female flies injected with bacteria. n=5 ovary pairs. The geometric mean and 95% confidence intervals are indicated. See Supplemental Figure 3 for raw data and full time course.
(c) Ovaries dissected from a female fly 2 days post-injection with S. typhimurium pmig-1. DIC image (left), localization of green bacteria in the debris zone (center), overlay (right).
We were then curious to determine the location of bacteria within the ovary. To do this we used S. typhimurium that expressed green fluorescent protein. During the first week post-injection, S. typhimurium colonized the ovary adjacent to the lateral oviducts at the opening to the ovarioles (Figure 4c). This is the same region where apoptotic cellular material accumulated during infection (data not shown). For this reason, we call the area of bacterial colonization within the ovary the “debris zone”.
Replication of bacteria within the debris zone of the ovary has not been reported previously. This site of replication is not unique to S. typhimurium as we also observed Listeria monocytogenes within the debris zone (data not shown). It is unclear why bacteria preferentially colonize this area. This region may provide a nutrient rich environment or provide protection from the humoral immune response of the fly. Alternatively, if the fly ovary is capable of directly responding to the presence of pathogen, then colonization of the debris zone may have evolved as a method of pathogen sampling in order to shut off reproduction and reallocate resources rapidly. Because bacteria can actually colonize the ovary, it is difficult to separate the factors that might cause reduced egg laying; the ovary might be responding to the presence of pathogen, tissue damage caused by the bacteria or systemic cues produced by a sick fly.
Hemocytes respond to ovary infection
Because phagocytic cells have been reported in fly oviducts [23], we were curious to determine if they respond to infection in the ovary. Using transgenic flies (hemolectin delta-Gal4, UAS-GFP flies) that express GFP specifically within hemocytes (circulating phagocytes) [24], we observed hemocytes attached to the common and lateral oviducts (Figure 5a). We challenged hemolectin delta-Gal4, UAS-GFP flies with S. typhimurium. Upon infection of the ovary, oviduct hemocytes became enlarged and elongated (Figure 5e and 5f). Over a period of days, hemocytes accumulated adjacent to the site of infection (Figures 5b). The hemocytes became squamous and formed sheets surrounding the infection (Figure 5c). Ten days post-injection, the infected portions of the ovary appeared to be faintly melanized (Figure 5d). Similar results were observed in ovaries dissected from flies challenged with SPI S. typhimurium, but not in E. coli-challenged flies (data not shown).
Figure 5. Fly hemocytes respond to ovary infection.
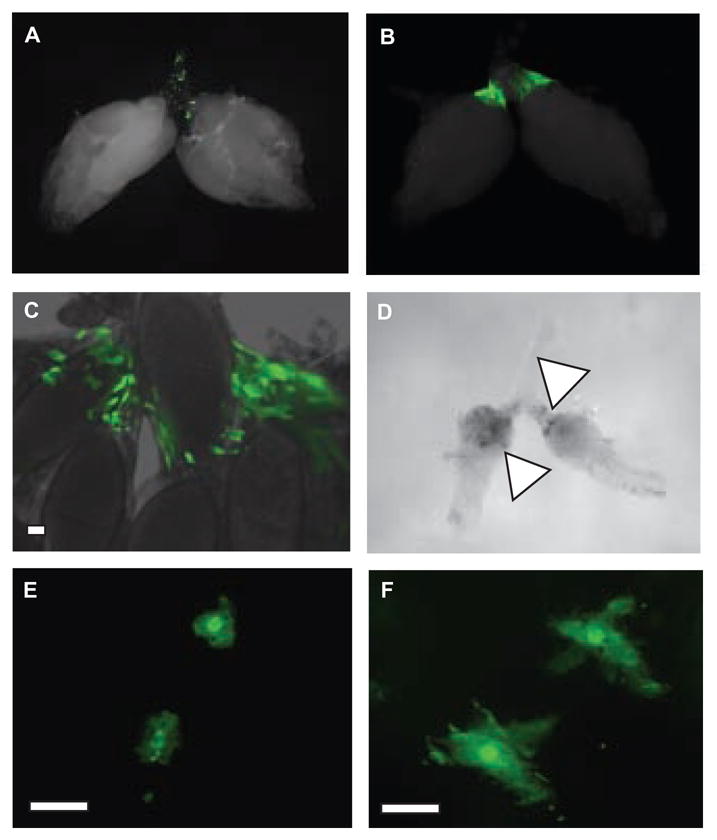
(a) Ovary dissected from a hemolectin delta-Gal4, UAS-GFP female fly 7 days post-injection with medium. Note the green fluorescent hemocytes in the debris zone.
(b–c) Ovaries dissected from hemolectin delta-Gal4, UAS-GFP female flies 7 days post-injection with S. typhimurium. The morphology of these cells differs from that seen in uninfected ovaries in (a) The white bar indicates 10 μm.
(d) DIC image of ovaries from a female fly 10 days-post injection with S. typhimurium. Note the dark regions (marked with a triangle) that appear melanized.
(e–f) Oviducts dissected from hemolectin delta-Gal4, UAS-GFP female flies 7 days post-injection with medium(e) or S. typhimurium(f). Note the diffence in the morphology of the labeled cells in uninfected versus infected ovaries. The white bar indicates 10 μm.
How hemocyte activation and accumulation at a site of bacterial infection within a tissue contributes to pathogenesis has not been studied in the adult fly. Infiltration of phagocytic cells into a site of infection in mammals contributes to localized inflammation and tissue damage [25]. Likewise, accumulation of activated hemocytes at sites of infection may contribute to morbidity in the fly, and therefore, the cellular response in the ovary should be considered when analyzing reproductive fitness during infection.
Concluding Remarks
In conclusion, bacterial infection of the fly is not merely defined by immune activation and survival, but also by tissue-specific physiological changes. As illustrated by the fly ovary, pathogenesis is complicated; disease correlates with bacterial virulence and may involve both local and systemic host factors. Tissue-specific pathogenesis beyond the ovary should be explored because failure of multiple organs defines disease and is what ultimately leads to fly death.
Experimental Procedures
Fly stocks and bacterial strains
Flies were maintained at 25°C on dextrose food supplemented with dry yeast. All experiments were performed with one-week-old female Oregon R flies unless otherwise specified. Hemocyte behavior was monitored using hemolectin delta-Gal 4, UAS-GFP flies [24]. The following bacterial strains were used in this study: Salmonella typhimurium strain SL1344 [26], Salmonella typhimurium strain SL1344 orgA::Tn5lacZY ssrA::miniTn5 (SPI), Serratia marcescens strain DB1140 [27], Salmonella typhimurium strain SL1344 pmig-1 [28], Burkholderia cenocepacia strain K56-2 [29], and Escherichia coli strain DH5α [30]. The SPIS. typhimurium strain was generated by P22 phage transduction from S. typhimurium strain P3F4 into S. typhimurium strain BJ66, selecting for colonies resistant to both kanamycin (30 μg/ml) and tetracycline (5 μg/ml) [31, 32]. All bacteria were grown at 37°C in LB with appropriate antibiotics. S. typhimurium strains were grown standing. All other bacteria were grown shaking.
Infections and survival curves
Bacteria (OD600=0.1; approximately 10,000 cfu) and LB medium were injected into the anterior abdomen on the ventrolateral surface of the fly using a pulled glass needle. A 50 nl sample was injected into each fly using a Picospritzer III injector (Parker Hannifin, Rohnert Park, California, United States). One-week-old female flies were used for all experiments. After injection, flies were kept at 29°C. All survival experiments were performed in triplicate with 30 flies per replicate. Experiments were repeated at least three times. Survival curves were plotted using the Kaplan-Meier method. Significance was determined by comparing survival curves of bacteria-injected flies to medium-injected flies using the logrank test.
Quantifying bacterial proliferation
Five flies per time point were homogenized in LB medium using a small pestle in a 1.5 ml centrifuge tube. Diluted homogenates were plated on LB agar. Colonies were counted after overnight incubation at 37°C. To assess bacterial proliferation in the ovary, ovaries were dissected from female flies in PBS and then washed four times in 5 ml PBS before being homogenized and plated as above. A 100 μl aliquot of the last wash was always plated to ensure that it did not contain bacteria. Five pairs of ovaries were analyzed per time point.
Quantifying egg production
Individual one-week-old non-virgin female flies were placed with a single male fly in a compartment of a lab-made plastic fly condominium. The fly condominium was placed on an apple juice egg laying plate supplemented with yeast paste [33]. Egg laying plates were replaced daily. Egg production was assessed at 29°C by counting the total number of eggs laid per female. Eighteen females, the maximum occupancy of the fly condominium, were studied per condition per experiment. Dead flies don’t lay eggs and were thus excluded from the egg counting rates.
Quantifying antimicrobial peptide response
Total RNA was extracted from five flies per sample using a Qiagen RNeasy Kit (Qiagen, Valencia, California, United States). Quantitative real-time RT-PCR was performed using rTth polymerase (Applied Biosystems, Foster City, California, United States). Diptericin induction was measured as previously described [34]. Diptericin values were normalized to the amount of D. melanogaster ribosomal protein 15a transcript in each sample. All time points were performed in triplicate.
Quantifying dying egg chambers
Each day five pairs of ovaries were dissected in PBS, stained in 10 μg/ml acridine orange (Sigma-Aldrich, St. Louis, Missouri, United States) for 1 minute and then washed 4 times in 1 ml PBS. Individual ovarioles were separated using a glass needle. The ovaries were placed on a slide and covered with a coverslip. The acridine orange staining was observed under a Leica MZ3 fluorescent dissecting microscope (Leica, Wetzlar, Germany) using GFP epifluorescence optics. For each ovary, the percentage of dying egg chambers was determined by dividing the number of acridine orange positive egg chambers by the total number of egg chambers.
Statistical Analysis
Most statistical analyses were performed using the program GraphPad Prism with the exception of the ANCOVA in supplemental figure 2, which was performed using the program R.
Supplementary Material
Supplemental Figure 1. Persistent bacterial infections reduce reproduction (raw data)
Total eggs laid per individual female fly injected with bacteria or medium, or left untouched. n=18 flies. The geometric mean and 95% confidence intervals are indicated.
Supplemental Figure 2. Bacterial virulence correlates with reduced egg production (raw data)
Total eggs laid per female fly injected with bacteria or medium, or left untouched. n=18 flies. The geometric mean and 95% confidence intervals are indicated. The table shows p-values, computed by ANCOVA, for the differences in the changes in egg-laying rates in different infection states. Sinificant p values are shown in bold. These data support the conclusion that the egg laying rates of flies injected with wild type Salmonella (SL1344) differ from that of flies injected with E. coli, LB or left untouched as p<0.05. The egg laying rates of the flies injected with the SPI1/2 mutant lies between that of wild type Salmonella (SL1344) and other treatments because it is not significantly different from either wild type Salmonella or E. coli and LB injection but is significantly different from uninjected flies.
Supplemental Figure 3. Bacteria colonize the fly ovary (raw data)
Bacterial growth in ovaries of female flies injected with bacteria. n=5 ovary pairs. The geometric mean and 95% confidence intervals are indicated.
Acknowledgments
We thank the Falkow, Tan and LiPuma labs for bacterial reagents. We thank Janelle Ayres, Linh Pham and Mimi Shirasu-Hiza for comments on the manuscript. We thank Marc Dionne for performing ANCOVA. This work was supported by NIH RO1 AI053080 and an NSF Graduate Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 2.Ayres JS, Schneider DS. Genomic dissection of microbial pathogenesis in cultured Drosophila cells. Trends Microbiol. 2006;14(3):101–4. doi: 10.1016/j.tim.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Brennan CA, Anderson KV. Drosophila: the genetics of innate immune recognition and response. Annu Rev Immunol. 2004;22:457–83. doi: 10.1146/annurev.immunol.22.012703.104626. [DOI] [PubMed] [Google Scholar]
- 4.Brandt SM, Dionne MS, Khush RS, Pham LN, Vigdal TJ, Schneider DS. Secreted Bacterial Effectors and Host-Produced Eiger/TNF Drive Death in a Salmonella-Infected Fruit Fly. PLoS Biol. 2004;2(12):e418. doi: 10.1371/journal.pbio.0020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guichard A, Park JM, Cruz-Moreno B, Karin M, Bier E. Anthrax lethal factor and edema factor act on conserved targets in Drosophila. Proc Natl Acad Sci U S A. 2006;103(9):3244–9. doi: 10.1073/pnas.0510748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson DL, Lines JL, Pesci EC, Venturi V, Storey DG. Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect Immun. 2004;72(10):5638–45. doi: 10.1128/IAI.72.10.5638-5645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng LW, Portnoy DA. Drosophila S2 cells: an alternative infection model for Listeria monocytogenes. Cell Microbiol. 2003;5(12):875–85. doi: 10.1046/j.1462-5822.2003.00327.x. [DOI] [PubMed] [Google Scholar]
- 8.Basset A, Tzou P, Lemaitre B, Boccard F. A single gene that promotes interaction of a phytopathogenic bacterium with its insect vector, Drosophila melanogaster. EMBO Rep. 2003;4(2):205–9. doi: 10.1038/sj.embor.embor730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Lara J, Needham AJ, Foster SJ. Invertebrates as animal models for Staphylococcus aureus pathogenesis: a window into host-pathogen interaction. FEMS Immunol Med Microbiol. 2005;43(3):311–23. doi: 10.1016/j.femsim.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Needham AJ, Kibart M, Crossley H, Ingham PW, Foster SJ. Drosophila melanogaster as a model host for Staphylococcus aureus infection. Microbiology. 2004;150(Pt 7):2347–55. doi: 10.1099/mic.0.27116-0. [DOI] [PubMed] [Google Scholar]
- 11.Fauvarque MO, Bergeret E, Chabert J, Dacheux D, Satre M, Attree I. Role and activation of type III secretion system genes in Pseudomonas aeruginosa-induced Drosophila killing. Microb Pathog. 2002;32(6):287–95. doi: 10.1006/mpat.2002.0504. [DOI] [PubMed] [Google Scholar]
- 12.Chamilos G, Lionakis MS, Lewis RE, Lopez-Ribot JL, Saville SP, Albert ND, Halder G, Kontoyiannis DP. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis. 2006;193(7):1014–22. doi: 10.1086/500950. [DOI] [PubMed] [Google Scholar]
- 13.D’Argenio DA, Gallagher LA, Berg CA, Manoil C. Drosophila as a model host for Pseudomonas aeruginosa infection. J Bacteriol. 2001;183(4):1466–71. doi: 10.1128/JB.183.4.1466-1471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apidianakis Y, Rahme LG, Heitman J, Ausubel FM, Calderwood SB, Mylonakis E. Challenge of Drosophila melanogaster with Cryptococcus neoformans and role of the innate immune response. Eukaryot Cell. 2004;3(2):413–9. doi: 10.1128/EC.3.2.413-419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau GW, Goumnerov BC, Walendziewicz CL, Hewitson J, Xiao W, Mahajan-Miklos S, Tompkins RG, Perkins LA, Rahme LG. The Drosophila melanogaster toll pathway participates in resistance to infection by the gram-negative human pathogen Pseudomonas aeruginosa. Infect Immun. 2003;71(7):4059–66. doi: 10.1128/IAI.71.7.4059-4066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurd H. Host fecundity reduction: a strategy for damage limitation? Trends Parasitol. 2001;17(8):363–8. doi: 10.1016/s1471-4922(01)01927-4. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz A, Koella JC. The cost of immunity in the yellow fever mosquito, Aedes aegypti depends on immune activation. J Evol Biol. 2004;17(4):834–40. doi: 10.1111/j.1420-9101.2004.00720.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed AM, Hurd H. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect. 2006;8(2):308–15. doi: 10.1016/j.micinf.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Zerofsky M, Harel E, Silverman N, Tatar M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell. 2005;4(2):103–8. doi: 10.1111/j.1474-9728.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 20.Zaharik ML, Gruenheid S, Perrin AJ, Finlay BB. Delivery of dangerous goods: type III secretion in enteric pathogens. Int J Med Microbiol. 2002;291(8):593–603. doi: 10.1078/1438-4221-00179. [DOI] [PubMed] [Google Scholar]
- 21.Khush RS, Lemaitre B. Genes that fight infection: what the Drosophila genome says about animal immunity. Trends Genet. 2000;16(10):442–9. doi: 10.1016/s0168-9525(00)02095-3. [DOI] [PubMed] [Google Scholar]
- 22.Hopwood JA, Ahmed AM, Polwart A, Williams GT, Hurd H. Malaria-induced apoptosis in mosquito ovaries: a mechanism to control vector egg production. J Exp Biol. 2001;204(Pt 16):2773–80. doi: 10.1242/jeb.204.16.2773. [DOI] [PubMed] [Google Scholar]
- 23.Nezis IP, Stravopodis DJ, Papassideri I, Robert-Nicoud M, Margaritis LH. Dynamics of apoptosis in the ovarian follicle cells during the late stages of Drosophila oogenesis. Cell Tissue Res. 2002;307(3):401–9. doi: 10.1007/s00441-001-0498-3. [DOI] [PubMed] [Google Scholar]
- 24.Goto A, Kadowaki T, Kitagawa Y. Drosophila hemolectin gene is expressed in embryonic and larval hemocytes and its knock down causes bleeding defects. Dev Biol. 2003;264(2):582–91. doi: 10.1016/j.ydbio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Kuby J. Immunology. 3. xxiv. New York: W.H. Freeman; 1997. p. 664. [Google Scholar]
- 26.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291(5812):238–9. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 27.Flyg C, Kenne K, Boman HG. Insect pathogenic properties of Serratia marcescens: phage-resistant mutants with a decreased resistance to Cecropia immunity and a decreased virulence to Drosophila. J Gen Microbiol. 1980;120(1):173–81. doi: 10.1099/00221287-120-1-173. [DOI] [PubMed] [Google Scholar]
- 28.Valdivia RH, Falkow S. Fluorescence-based isolation of bacterial genes expressed within host cells. Science. 1997;277(5334):2007–11. doi: 10.1126/science.277.5334.2007. [DOI] [PubMed] [Google Scholar]
- 29.Darling P, Chan M, Cox AD, Sokol PA. Siderophore production by cystic fibrosis isolates of Burkholderia cepacia. Infect Immun. 1998;66(2):874–7. doi: 10.1128/iai.66.2.874-877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Vol. 3 Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 31.Jones BD, Falkow S. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect Immun. 1994;62(9):3745–52. doi: 10.1128/iai.62.9.3745-3752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman JA, Rappl C, Kuhle V, Hensel M, Miller SI. SpiC is required for translocation of Salmonella pathogenicity island 2 effectors and secretion of translocon proteins SseB and SseC. J Bacteriol. 2002;184(18):4971–80. doi: 10.1128/JB.184.18.4971-4980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashburner M. Drosophila. Vol. 2 Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 34.Schneider D, Shahabuddin M. Malaria parasite development in a Drosophila model. Science. 2000;288(5475):2376–9. doi: 10.1126/science.288.5475.2376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Persistent bacterial infections reduce reproduction (raw data)
Total eggs laid per individual female fly injected with bacteria or medium, or left untouched. n=18 flies. The geometric mean and 95% confidence intervals are indicated.
Supplemental Figure 2. Bacterial virulence correlates with reduced egg production (raw data)
Total eggs laid per female fly injected with bacteria or medium, or left untouched. n=18 flies. The geometric mean and 95% confidence intervals are indicated. The table shows p-values, computed by ANCOVA, for the differences in the changes in egg-laying rates in different infection states. Sinificant p values are shown in bold. These data support the conclusion that the egg laying rates of flies injected with wild type Salmonella (SL1344) differ from that of flies injected with E. coli, LB or left untouched as p<0.05. The egg laying rates of the flies injected with the SPI1/2 mutant lies between that of wild type Salmonella (SL1344) and other treatments because it is not significantly different from either wild type Salmonella or E. coli and LB injection but is significantly different from uninjected flies.
Supplemental Figure 3. Bacteria colonize the fly ovary (raw data)
Bacterial growth in ovaries of female flies injected with bacteria. n=5 ovary pairs. The geometric mean and 95% confidence intervals are indicated.