Abstract
Purpose
In this prospective multi-centre observational cohort study, we investigated the effect of an intensified multidisciplinary pharmaceutical care programme on the adherence of cancer patients treated with capecitabine, a prodrug of fluorouracil.
Patients and methods
Twenty-four colorectal and 24 breast cancer patients participated in this study. Patients of the control group (n = 24) received standard care, patients of the intervention group (n = 24) received intensified pharmaceutical care consisting of written and spoken information. Adherence to capecitabine chemotherapy was measured using an electronic medication event monitoring system (MEMS™).
Results
Patients in the intervention group exhibited an enhanced but not significantly different mean overall adherence compared to the control group (97.9% vs 90.5%, p = 0.069). Mean daily adherence was significantly higher in the intervention group (96.8% vs 87.2%, p = 0.029). Variability of both adherence parameters was considerably reduced when pharmaceutical care was provided. At the end of the observation period of 126 days, the probability of still being treated with capecitabine was found to be 48% in the control group and 83% in the intervention group (p = 0.019, log-rank test). The relative risk for a deviating drug intake interval, i.e. <10 or >14 instead of 12 h, in the intervention group was found to be 0.51 (95% CI, 0.46–0.56) compared with the control group (p < 0.05, Chi-square test).
Conclusions
The provision of intensified pharmaceutical care can enhance adherence to and prolong treatment with capecitabine. The results underline the importance of multidisciplinary care to assure the effectiveness of oral chemotherapy.
Keywords: Adherence, Capecitabine, Oral chemotherapy, Pharmaceutical care
Introduction
Over the past decade, oral administration of anticancer agents has gained increasing importance. Venepuncture and the risk of extravasation are avoided, and oral administration allows greater autonomy because patients can take their drugs wherever they like. Capecitabine, cyclophosphamide, methotrexate, temozolomide and the so called ‘targeted drugs’ such as imatinib, lapatinib, sorafenib and sunitinib are examples out of a long list of oral anticancer agents. Despite these advantages, oral administration is also accompanied by many challenges. The potential toxicity of anticancer agents, the recognition of adverse effects by the patient, the management of adverse effects and the importance of patient's adherence for treatment success are important issues that have to be addressed. Multidisciplinary patient care and a good patient education play a key role in a successful oral anticancer treatment [1–-4].
In this study, we focused on patients receiving chemotherapy with capecitabine, a prodrug of fluorouracil (5 FU) which is frequently used for the treatment of breast, colorectal and gastric cancer patients [5]. One capecitabine chemotherapy cycle consists of 2 weeks of twice daily drug intake followed by 7 days of break. Since it is an orally administered drug, patients take it usually at home. As for all oral anticancer agents, a high adherence to the prescribed dosage regimen is a major prerequisite for therapeutic efficacy.
Adherence to prescribed medication has been in the centre of interest in many chronic diseases. Several working groups have focussed on adherence to antihypertensive therapy or therapy of chronic heart failure and developed strategies for enhancing adherence [6–10]. Others have studied adherence in the elderly and patients with mental disorders [11, 12]. So far, there are only few data available which focus on adherence in cancer patients treated with an oral anticancer drug as summarised in a recent review [13]. Most data are available for tamoxifen. Waterhouse et al. used patient self-report, pill counts and microelectronic monitoring (Medication Event Monitoring System (MEMS)™) in 24 breast cancer patients to assess the adherence to oral tamoxifen. Adherence rates differed depending on the strategy of measurement: self-report resulted in an overall adherence of 98.6 ± 2.2%, pill counts of 92.1 ± 9.8% and microelectronic monitoring showed the lowest rate with 85.4 ± 17.2% [14]. Recently published results on the persistence to tamoxifen therapy in 462 breast cancer patients over 5 years revealed that 31% of patients who started tamoxifen failed to complete the recommended 5-year course [15]. Lebovits et al. concentrated on adherence to cyclophosphamide and prednisolone in 51 breast cancer patients, also assessed by patient self-report. Non-adherence was defined as ingesting 90% or less of the total prescribed dosage during 6 months of treatment. Criteria for non-adherence were met by 43% of the patients [16]. Adherence to oral etoposide measured in 12 patients with small cell lung cancer by using an electronically monitored tablet bottle adherence was reported to be 93.2 ± 12.0% [17].
First data on adherence to capecitabine using MEMS™ have recently been published. Only 76% of elderly breast cancer patients took 80% or more of the prescribed doses [18]. In contrast, in a younger breast cancer population median adherence was found to be 96% [19].
Although in general, adherence to oral anticancer therapy seems to be higher compared with other diseases, most of these studies have shown that there is always a certain number of cancer patients who might need specific interventions to assure adherence. In this study, we assessed the adherence of breast and colorectal cancer patients treated with capecitabine and the impact of a pharmaceutical care intervention on patient adherence. Patient education by pharmacists can improve patient outcome and adherence to oral drugs as shown in a Cochrane review for other diseases than cancer [20]. For oral chemotherapy, pharmacy services are probably underused even in cancer centres [5]. The pharmaceutical care intervention in this study was provided by a team of two registered pharmacists with extensive experience in this field and consisted of a combination of written and spoken information. Adherence was assessed by MEMS™ as this system provides precise and detailed information about patients' behaviour in taking their medication including dose timing and drug holidays. However, it does not prove that the patient really ingested the drug or took the correct number of pills. This indirect approach, however, currently provides the most reliable data on adherence [21].
Methods
Study design
The study was conducted as a prospective, multi-centred observational cohort study with control group. The control group receiving standard care was studied before the intervention group which received intensified pharmaceutical care. As the latter must be regarded as a complex intervention, some limitations with regard to the study design had to be accepted [22]. A non-randomized design was chosen for two reasons: Firstly, possible interactions between patients of the two study groups could have led to an undesired contamination with regard to the evaluation of adherence in the control group. Secondly, learning effects of participating physicians and nursing staff had to be avoided with regard to the pharmaceutical care intervention.
Patient recruitment and study population
The study was approved by the ethics committee of the University of Bonn, Germany. Patients were consecutively recruited at three hospital sites (two departments of internal medicine and one department of obstetrics and gynaecology) and three ambulatory oncology practices. The main inclusion criterion was that the patient started a chemotherapy regimen with capecitabine as a single agent or in combination with other agents for treatment of colorectal or breast cancer. Patients had to be included into the study within 2 weeks after the beginning of capecitabine therapy. The minimum age was defined as 18 years; patients had to be able to speak and read German language fluently and had to give written consent to participate. Exclusion criteria were any prior orally administrable chemotherapy and any disease compromising the patient to fully understand the purpose and course of the study (for example, Alzheimer's disease).
If an eligible patient was identified by one of the collaborating oncologists, the patient was contacted within 24 h to organize a first meeting to provide the patient with spoken and written information about the study. Patients were given at least 24 h to decide whether they would want to participate.
Sixty-nine ambulatory cancer patients were reported to the pharmaceutical care team by the participating oncologists. Fifty of those patients were included into the study, and data were evaluated. Of 19 patients not included, three patients did not give their consent to participate in the study and 11 patients did not meet at least one of the inclusion criteria. In five cases, other reasons prevented the inclusion into the study. One patient in each study group died during the observation time and the MEMS™ vials were not returned. Figure 1 provides an overview of the timeline of patient inclusion into the control and intervention group.
Fig. 1.
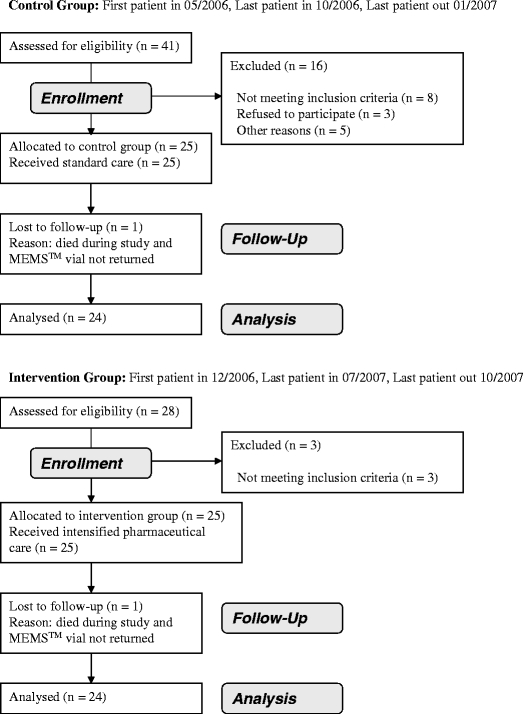
Flow diagram of patient recruitment
Pharmaceutical care intervention
The intensified pharmaceutical care service for patients of the intervention group consisted of a combination of written and spoken information provided by two registered pharmacists of the department of clinical pharmacy at the University of Bonn, Germany. The initial patient consultation included the general information about the study, the introduction to the study material and the course of the study. Upon inclusion into the study, the characteristics of the drug capecitabine, including mechanism of action, possible adverse events and their appropriate management, as well as the individual treatment regimen were explained in detail. Furthermore, patients were informed about the importance of a high adherence to this drug and the risks of inadequate compliant behaviour. Patients were also educated about any other additional medication they were taking. After conducting an interaction check and a change in medication in cooperation with the physician where necessary, patients were given a written dosing schedule by the pharmaceutical care team. This dosing schedule contained all current medications and special advice regarding the drug administration where necessary and was updated each cycle. Patients received a leaflet with information about the prevention and management of adverse effects of chemotherapy. They were contacted at least once during each cycle of capecitabine chemotherapy to inquire about any current therapy-related questions or problems and to reconfirm the ongoing individual therapeutic regimen. Issues discussed during these telephone consultations ranged from management of adverse effects, administration of chemotherapy and supportive therapy to questions regarding additional complementary treatment options. In some cases, the follow-up consultations took place on the hospital or oncology practice site.
The median time required for the initial patient consultation (including general information about the study etc.) was 75.0 min. During the following course of the study, a median of 2.2 consultations per cycle were held. The median duration of these consultations was 6.0 min.
Adherence measurement
Patients received an activated MEMS™ vial and were asked to use it to store their capecitabine medication for the duration of their participation in the study. As demanded by the responsible ethics committee for this study, they were informed that the lid of the vial contained a micro-chip that registered every opening of the container. They were instructed to take medication only from the container and not to open it for other reasons. Depending on the prescribed dosing regimen of capecitabine therapy (dosing in the observed patients ranged from two tablets twice daily to four tablets twice daily), the MEMS™ vial had to be refilled by the patient several times in the course of the study. Patients were therefore asked to schedule refills of the bottle at the same time as they were extracting tablets for regular drug intake to avoid extra openings. Additionally, patients received a documentation sheet to note any extraordinary opening of the vial. Openings noted by patients in this way were included into the adherence analysis. After completion of six cycles of capecitabine therapy or upon notification of an earlier stop of treatment through the participating oncologists, the MEMS™ containers were collected from patients.
Adherence and persistence analysis
Adherence of patients of both study groups was analysed using the data uploaded from the MEMS™ vials and patients' notes regarding extraordinary openings.
Overall adherence was calculated by dividing the number of actual openings recorded by the MEMS™ system by the number of expected openings. In addition, daily adherence was calculated by dividing the number of days with a correct number of openings of the MEMS™ vial by the number of days monitored. In the case of capecitabine therapy in this study, a day with a correct number of openings could either be a day with two openings (during the 2 weeks of therapy) or with no opening (during the 7 days of break). Therapy days with less or more than two openings or days with any number of openings during the scheduled therapy-free interval were counted as non-adherent days.
Furthermore, adherence data were evaluated with regard to persistence with therapy. For this purpose, duration of capecitabine treatment was compared between both study groups. Preplanned treatment discontinuations according to the respective regimen and those due to death of patients were censored.
Duration of drug intake intervals was assessed as time between two openings of the MEMS™ container. Intervals ≥24 h were excluded from this analysis. Intervals of 12 ± 2 h were defined as adherent. Relative risk was calculated for intervals outside this timeframe. Also, the ‘number needed to treat’ was calculated to prevent one non-adherent interval by the pharmaceutical care intervention.
Sample size calculation and statistical analysis
Daily adherence was selected as primary endpoint of this study; the other measured parameters were regarded as secondary endpoints. Since no prior data on daily adherence to capecitabine were available, we assumed a daily adherence of 79.1% for the control group according to a published meta-analysis [23]. Target daily adherence for the intervention group was 95%. Assuming an alpha error of 5% and an intra-patient correlation of 0.25 (estimated from the Pharmionic Knowledge Center database, Pharmionic Systems, Sion, Switzerland) resulted in a sample size of 17 patients for each study group (power of 80%). Considering a potential drop-out rate of 25% led to a final sample size of 22 patients for each group.
The statistical analysis in this study was performed using SPSS™ for Windows, Version 15.0 (SPSS Inc., USA) and Microsoft™ Excel 2000 (Microsoft Corporation, USA).
The non-parametric Mann–Whitney U test was used to compare adherence parameters in both patient groups. Duration of capecitabine treatment was compared by performing a Kaplan–Meier survival analysis and the log-rank test. Influence of the intervention on regularity of intake intervals was evaluated by 95% confidence intervals and the Chi-square test. The level of significance was set to p < 0.05.
Results
Patient characteristics
Adherence was assessed in 24 breast and 24 colorectal cancer patients. There was no statistically significant difference between the control and the intervention group with regard to age, gender and number of additional oral medications in use at the time of inclusion into the study. With regard to gender (p = 0.002, Mann–Whitney U test) and age (p = 0.001, Mann–Whitney U test), there was a statistically significant difference between the two diagnosis groups (breast and colorectal cancer) regardless of the study group affiliation. Table 1 summarises demographic characteristics of the study population and provides an overview of the observation period, the number of completed capecitabine cycles and the number of additional medications.
Table 1.
Demographic characteristics
| Sociodemographic characteristics | Control group (n = 24) | Intervention group (n = 24) |
|---|---|---|
| Mean (SD) age (years) | ||
| Breast cancer | 57.5 (11.6) | 55.9 (11.0) |
| Colorectal cancer | 69.8 (9.4) | 66.0 (12.0) |
| Sex | ||
| Female | 18 | 19 |
| Male | 6 | 5 |
| Diagnosis | ||
| Breast cancer | 12 | 12 |
| Colorectal cancer | 12 | 12 |
| Chemotherapy regimen at time of inclusion | ||
| Cap (monotherapy) | 11 | 12 |
| Cap Vin | 3 | 0 |
| Cap EC | 1 | 0 |
| Cap Pac | 1 | 2 |
| Cap Beva | 3 | 2 |
| Cap Ox | 3 | 4 |
| Cap Beva Ox | 2 | 0 |
| Cap Beva Iri | 0 | 1 |
| Cap Iri Cet | 0 | 1 |
| Cap Lap | 0 | 2 |
| Median (range) no. of additional medicationsa | ||
| Breast cancer | 4 (1–10) | 5 (2–15) |
| Colorectal cancer | 3.5 (0–8) | 7 (1–13) |
| Median (range) days monitored | ||
| Breast cancer | 64 (13–119) | 104 (9–119) |
| Colorectal cancer | 73 (21–128) | 118 (31–138) |
| Median (range) number of completed cycles | ||
| Breast cancer | 2.5 (0–6) | 4.0 (0–6) |
| Colorectal cancer | 2.5 (1–6) | 4.0 (1–5) |
Cap capecitabine, Vin vinorelbine, EC epirubicin + cyclophosphamide, Pac paclitaxel, Beva bevacizumab, Ox oxaliplatin, Iri irinotecan, Cet cetuximab, Lap lapatinib
aAt time of inclusion
Adherence analysis
The observation period in the control group ranged from 13 to 128 days and in the intervention group from 9 to 138 days. Tables 2 and 3 show the adherence results for each patient over the observation period expressed in overall and daily adherence. The intervention group showed an increased but not significantly different mean overall adherence of 97.9% (Median = 99.0%, p = 0.069, Mann–Whitney U test) compared with a mean overall adherence of 90.5% in the control group (Median = 96.2%). Mean daily adherence was significantly (p = 0.029) improved from 87.2% in the control group (Median = 93.8%) to 96.8% in the intervention group (Median = 98.5%). Figures 2 and 3 illustrate the differences in overall and daily adherence between the two study groups. Moreover, the intervention led to a decrease of variability with regard to the adherence parameters in the intervention group.
Table 2.
Adherence data of the control group
| Patient | No of openings (expected) | No of openings (actual) | Overall adherence (%) | Days with correct drug intake | Days monitored | Daily adherence (%) |
|---|---|---|---|---|---|---|
| B 01 | 31 | 29 | 93.5 | 21.5 | 23.0 | 93.5 |
| B 02 | 27 | 17 | 63.0 | 13.5 | 21.0 | 64.3 |
| B 03 | 148 | 142 | 95.9 | 108.0 | 116.0 | 93.1 |
| B 04 | 11 | 11 | 100.0 | 13.0 | 14.0 | 92.9 |
| B 05 | 166 | 161 | 97.0 | 113.0 | 118.0 | 95.8 |
| B 06 | 168 | 167 | 99.4 | 118.0 | 119.0 | 99.2 |
| B 07 | 73 | 70 | 95.9 | 48.0 | 51.0 | 94.1 |
| B 08 | 166 | 166 | 100.0 | 118.0 | 118.0 | 100.0 |
| B 09 | 108 | 128 | 118.5 | 67.0 | 92.0 | 72.8 |
| B 10 | 71 | 71 | 100.0 | 56.5 | 56.5 | 100.0 |
| B 11 | 26 | 26 | 100.0 | 13.0 | 13.0 | 100.0 |
| B 12 | 100 | 95 | 95.0 | 66.0 | 71.0 | 93.0 |
| C 01 | 106 | 75 | 70.8 | 51.0 | 76.0 | 67.1 |
| C 02 | 102 | 97 | 95.1 | 86.0 | 90.0 | 95.6 |
| C 03 | 152 | 124 | 81.6 | 95.0 | 118.0 | 80.5 |
| C 04 | 96 | 71 | 74.0 | 47.0 | 69.0 | 68.1 |
| C 05 | 40 | 37 | 92.5 | 25.5 | 28.0 | 91.1 |
| C 06 | 168 | 162 | 96.4 | 122.0 | 128.0 | 95.3 |
| C 07 | 52 | 26 | 50.0 | 13.0 | 36.0 | 36.1 |
| C 08 | 56 | 56 | 100.0 | 35.0 | 35.0 | 100.0 |
| C 09 | 28 | 28 | 100.0 | 21.0 | 21.0 | 100.0 |
| C 10 | 29 | 14 | 48.3 | 14.5 | 22.0 | 65.9 |
| C 11 | 56 | 60 | 107.1 | 107.0 | 112.0 | 95.5 |
| C 12 | 164 | 162 | 98.8 | 115.0 | 117.0 | 98.3 |
| Mean | 89.3 | 83.1 | 90.5 | 62.0 | 69.4 | 87.2 |
| Median | 84.5 | 71.0 | 96.2 | 53.8 | 70.0 | 93.8 |
| Range | 11–168 | 11–167 | 48.3–118.5 | 13.0–122.0 | 13.0–128.0 | 36.1–100.0 |
B breast cancer patient, C colorectal cancer patient
Table 3.
Adherence data of the intervention group
| Patient | No of openings (expected) | No of openings (actual) | Overall adherence (%) | Days with correct drug intake | Days monitored | Daily adherence (%) |
|---|---|---|---|---|---|---|
| B I01 | 165 | 165 | 100.0 | 117.5 | 117.5 | 100.0 |
| B I02 | 165 | 159 | 96.4 | 112.5 | 117.5 | 95.7 |
| B I03 | 163 | 166 | 101.8 | 111.5 | 116.5 | 95.7 |
| B I04 | 136 | 136 | 100.0 | 96.0 | 96.0 | 100.0 |
| B I05 | 31 | 29 | 93.5 | 21.0 | 22.5 | 93.3 |
| B I06 | 124 | 126 | 101.6 | 109.0 | 111.0 | 98.2 |
| B I07 | 20 | 21 | 105.0 | 9.5 | 10.5 | 90.5 |
| B I08 | 163 | 163 | 100.0 | 116.5 | 116.5 | 100.0 |
| B I09 | 112 | 113 | 100.9 | 76.0 | 77.0 | 98.7 |
| B I10 | 18 | 15 | 83.3 | 8.5 | 9.0 | 94.4 |
| B I11 | 18 | 17 | 94.4 | 8.0 | 9.0 | 88.9 |
| B I12 | 168 | 168 | 100.0 | 119.0 | 119.0 | 100.0 |
| C I01 | 112 | 109 | 97.3 | 112.0 | 119.0 | 94.1 |
| C I02 | 111 | 114 | 102.7 | 135.5 | 137.5 | 98.5 |
| C I03 | 138 | 138 | 100.0 | 117.0 | 118.0 | 99.2 |
| C I04 | 155 | 151 | 97.4 | 111.5 | 112.0 | 99.6 |
| C I05 | 111 | 109 | 98.2 | 75.5 | 76.5 | 98.7 |
| C I06 | 165 | 166 | 100.6 | 116.5 | 117.5 | 99.1 |
| C I07 | 160 | 155 | 96.9 | 125.5 | 128.5 | 97.7 |
| C I08 | 131 | 130 | 99.2 | 114.0 | 115.0 | 99.1 |
| C I09 | 167 | 165 | 98.8 | 118.5 | 118.5 | 100.0 |
| C I10 | 51 | 45 | 88.2 | 28.5 | 33.0 | 86.4 |
| C I11 | 45 | 43 | 95.6 | 30.0 | 30.5 | 98.4 |
| C I12 | 145 | 141 | 97.2 | 122.0 | 125.0 | 97.6 |
| Mean | 115.6 | 114.3 | 97.9 | 88.0 | 89.7 | 96.8 |
| Median | 133.5 | 133.0 | 99.0 | 111.8 | 115.8 | 98.5 |
| Range | 18–168 | 15–168 | 83.3–105.0 | 8.0–135.5 | 9.0–137.5 | 86.4–100.0 |
B breast cancer patient, C colorectal cancer patient
Fig. 2.
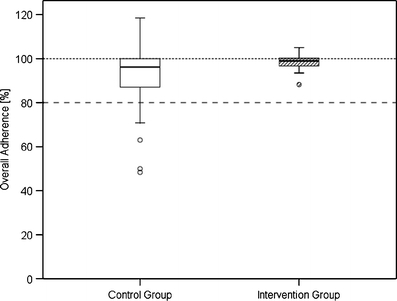
Boxplot of overall adherence in the control and intervention group
Fig. 3.
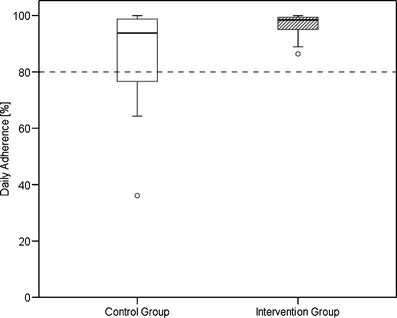
Boxplot of daily adherence in the control and intervention group
Table 4 presents an overview of the total and relative number of patients in both groups with adherence parameters below 90% and 80%. These adherence limits had been defined in the study protocol on the basis of empirical considerations. None of the patients having received the pharmaceutical care intervention showed an overall or daily adherence below 80%; whereas in the control group, five (21%) patients showed an overall and six (25%) a daily adherence below 80%.
Table 4.
Number of non-adherent patients in both patient groups
| Control group (n = 24) | Intervention group (n = 24) | |
|---|---|---|
| Overall adherence | ||
| <80% | 5 (21%) | 0 |
| <90% | 6 (25%) | 2 (8%) |
| Daily adherence | ||
| <80% | 6 (25%) | 0 |
| <90% | 7 (28%) | 2 (8%) |
Duration of capecitabine treatment and persistence
Figure 4 illustrates the comparison of the time period patients were kept on capecitabine chemotherapy between the control and intervention group. At the end of the observation period of 126 days the probability of still being treated with capecitabine in the control group was found to be 48% and in the intervention group 83%. This difference was statistically significant (p = 0.019, log-rank test).
Fig. 4.
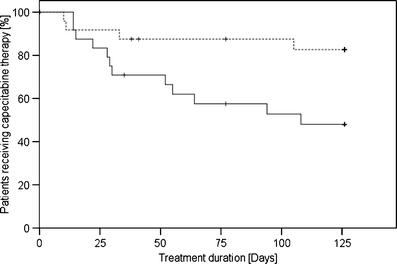
Kaplan–Meier plot of treatment duration in the control (solid line) and intervention group (dotted line)
The data of two control patients (B12 and C08) were censored because their treatment stop was due to their neo-adjuvant chemotherapy regimens and neither due to toxicity nor tumour progress. Ten of 12 treatment aborts in the control group were linked to serious adverse events (nine) and non-response to chemotherapy associated with disease progression (one). One patient stopped his therapy arbitrarily due to lack of motivation and in one case the reason for treatment disruption remained unknown.
In the intervention group, data of three patients were censored. In one case, therapy was stopped due to the death of the patient (BI05) and in two other cases it was stopped according to the originally scheduled treatment plan (CI05 and CI11). The four treatment aborts in this group were linked to adverse events (two) and disease progression (two).
The studied patient population did not demonstrate noteworthy problems with regard to arbitrary non-persistence. Only three patients of the control group stopped taking their capecitabine chemotherapy without prior consent of their oncologist. In all three cases the oncologist approved this treatment interruption a few days later and chemotherapy prescription with capecitabine was ultimately stopped.
Drug intake intervals
Table 5 shows the 2 × 2 table used to calculate the relative risk to exhibit an irregular intake interval, i.e. outside the defined borders (>14 or <10 h). The relative risk for the intervention group was 0.51 (confidence interval, 95% 0.46–0.56). The Chi-square test indicated that the difference was significant (p < 0.05, one degree of freedom). Additionally, the ‘number needed to treat’ was calculated to prevent one non-adherent intake interval. By calculation of the reciprocal of the absolute risk reduction the number needed to treat was found to be 5.7, i.e. six intake intervals need to be supported by the evaluated pharmaceutical care intervention in order to prevent one interval >14 or <10 h.
Table 5.
A 2 × 2 table to calculate relative risk for intake intervals outside defined borders
| Intervals | Intervals | Total | |
|---|---|---|---|
| <10 h/>14 h | ≥10 h/≤14 h | ||
| Intervention group | 480 | 2,187 | 2,667 |
| Control group | 676 | 1,221 | 1,897 |
| Total | 1,156 | 3,408 | 4,564 |
Discussion
Our study revealed the potential of adherence enhancement by providing intensified pharmaceutical care. However, a number of limitations have to be considered before interpreting the data. Apart from the relatively small number of patients, we chose a non-randomized study design due to the fact that pharmaceutical care must be regarded as a highly complex intervention [22]. A parallel design could have led to significant contamination bias because of the interaction that occurs between clinic patients. In addition, since blinding is not possible with this intervention, healthcare professionals might have adopted an approach to patient follow-up counseling that was dependent upon the intervention received. Therefore, we decided to recruit first the control group and subsequently the intervention group in each participating centre.
The basis for the evaluation of adherence in this study were the two parameters overall and daily adherence as proposed by Vrijens and Goetghebeur [24]. It is remarkable that median overall adherence of 96% in our control group was the same as that reported by Mayer et al. for 13 metastatic breast cancer patients using the same method [19]. Median daily adherence of 94% was found to be a bit lower compared with overall adherence in the control group. Even if taking into account that MEMS™ results can be biased by an altered behaviour of the patients being aware of the observation (‘Hawthorne effect’), one may conclude that adherence does not represent a major problem in this patient population. A closer look into the data, however, shows that there are individual patients with relatively low adherence. Two of our patients in the control group took only 50% or less of their prescribed doses which may most likely endanger therapeutic efficacy. Six (25.0%) patients in the control group did not take their medication as prescribed on at least every fifth day. In the study of Muss et al. in elderly patients, only 76% exhibited an overall adherence to capecitabine of 80% or higher [18]. These results indicate that there are subgroups of patients with low adherence that might benefit from adherence-enhancing measures. From a health professional’s perspective it must be desirable to identify such patients as early as possible in order to initiate adherence-enhancing activities. Using the data of electronic monitoring devices during routine patient consultations could be one way to ensure early detection of potential non-compliers and discuss adherence-enhancing measures with the patient [25].
The pharmaceutical care intervention led to an improvement of both overall and daily adherence although only the difference in daily adherence was significantly different. The intervention resulted in a decrease of variability with regard to all assessed adherence parameters. This outcome of the adherence-enhancing intervention is further documented by the fact that none of the patients of the intervention group showed an overall or daily adherence below 80%. As limiting factor for the interpretation of our data it has to be pointed out that the adherence limit of 80% is a purely arbitrary cut-off which is not based upon any objective dose response data. It would be desirable to define an adequate adherence as result of dose efficacy studies which would allow the assessment of results of adherence-enhancing interventions with regard to their clinical significance. The importance of demonstrating positive influence of clinically relevant outcomes has been discussed by Kripalani et al. who concluded that only very few adherence studies fulfil this demand [26].
When regarding the adherence parameters in this study, some patients demonstrated an overall adherence above 100%. However, as a result of the analysis of the individual MEMS™ adherence profiles it can be concluded that more doses than prescribed were only taken in very few cases. These patients reported that they took an additional dose during the evening because they simply couldn’t remember the earlier intake, which resulted in an overestimation of overall adherence coupled with a decrease of daily adherence. The studied population did not show the often discussed phenomenon of over-adherence, i.e. that patients are over-motivated and take more doses than prescribed by the physician [27].
The significant effect of the pharmaceutical care intervention on the duration of capecitabine chemotherapy is noteworthy. In nine patients of the control group the therapy was stopped due to the development of severe toxicity, compared to only two patients in the intervention group. This could be a consequence of the intensive patient education during the pharmaceutical care consultations. Patients who are well informed and know what to expect during the course of therapy are better prepared how to manage adverse effects and the development of a therapy-limiting dimension of toxic symptoms is less likely [28]. However, this observation may be due to chance imbalances in this small population and requires confirmation in a large randomized trial.
Last but not the least, the intervention also demonstrated a significant effect on the regularity of drug intake intervals. The ‘number needed to treat’ of six could allow the interpretation that a pharmaceutical care programme for patients receiving twice daily capecitabine would prevent one non-adherent intake interval every three days. The authors are aware that it is not clear yet which extent of variability of the dosage interval is acceptable without consequences for efficacy and toxicity. However, the result underlines the potential of pharmaceutical care programmes. It is certainly worth developing such programmes as a multidisciplinary approach to assure safe and effective oral anticancer therapy.
Conclusions
The results of this study demonstrate the potential of intensified pharmaceutical care provision to improve treatment outcome of oral chemotherapy. The pharmaceutical care intervention resulted in a significant improvement of patient adherence. Moreover, patients receiving intensified pharmaceutical care were kept longer on their capecitabine regimen and showed better regularity with regard to drug intake intervals. The development of an adherence monitoring and enhancing infrastructure is a necessary prerequisite to exploit the full potential of orally administrable chemotherapies. Screening systems to detect potential non-adherers would support the rational utilization of the required resources.
Acknowledgements
We thank all patients who were willing to participate in our study. We also thank all oncologists and nurses who supported our study.
Financial disclosure
Partial funding was provided by Roche, Basel. However, the researchers were entirely independent during all phases of this work.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Relevance of the manuscript
Efficacy of an orally administered anticancer therapy depends on a high level of patient adherence. There is still a lack of strategies to assure patient adherence in this particular group of patients. This study presents a systematic and detailed analysis on patient adherence to capecitabine chemotherapy. For the first time, the impact of an intensified pharmaceutical care provision on the adherence of patients receiving oral chemotherapy has been studied.
References
- 1.Parsad SD, Ratain MJ. Prescribing oral chemotherapy. BMJ. 2007;334:376. doi: 10.1136/bmj.39128.449317.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weingart SN, Flug J, Brouillard D, Morway L, Partridge A, Bartel S, et al. Oral chemotherapy safety practices at US cancer centres: questionnaire survey. BMJ. 2007;334:407. doi: 10.1136/bmj.39069.489757.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weingart SN, Brown E, Bach PB, Eng K, Johnson SA, Kuzel TM, et al. NCCN task force report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(Suppl 3):S1–14. [PubMed] [Google Scholar]
- 4.Bedell CH. A changing paradigm for cancer treatment: the advent of new oral chemotherapy agents. Clin J Oncol Nurs. 2003;7:5–9. doi: 10.1188/03.CJON.S6.5-9. [DOI] [PubMed] [Google Scholar]
- 5.Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27:23–44. doi: 10.1016/j.clinthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Breekveldt-Postma NS, Herings RM. Persistence with antihypertensives related to formulation: the case of nifedipine. Ann Pharmacother. 2005;39:237–242. doi: 10.1345/aph.1E163. [DOI] [PubMed] [Google Scholar]
- 7.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 8.Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoes AW, Leufkens HG. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail. 2003;9:404–411. doi: 10.1054/S1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 9.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waeber B, Burnier M. Differential persistence with initial antihypertensive therapies: a clue for understanding the needs of hypertensive patients. J Hypertens. 2006;24:1021–1022. doi: 10.1097/01.hjh.0000226189.28934.e1. [DOI] [PubMed] [Google Scholar]
- 11.Cramer JA. Enhancing patient compliance in the elderly. Role of packaging aids and monitoring. Drugs Aging. 1998;12:7–15. doi: 10.2165/00002512-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Cantrell CR, Eaddy MT, Shah MB, Regan TS, Sokol MC. Methods for evaluating patient adherence to antidepressant therapy: a real-world comparison of adherence and economic outcomes. Med Care. 2006;44:300–303. doi: 10.1097/01.mlr.0000204287.82701.9b. [DOI] [PubMed] [Google Scholar]
- 13.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 14.Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11:1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 15.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 16.Lebovits AH, Strain JJ, Schleifer SJ, Tanaka JS, Bhardwaj S, Messe MR. Patient noncompliance with self-administered chemotherapy. Cancer. 1990;65:17–22. doi: 10.1002/1097-0142(19900101)65:1<17::AID-CNCR2820650106>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.Lee CR, Nicholson PW, Souhami RL, Slevin ML, Hall MR, Deshmukh AA. Patient compliance with prolonged low-dose oral etoposide for small cell lung cancer. Br J Cancer. 1993;67:630–634. doi: 10.1038/bjc.1993.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer EL, Partridge AH, Harris LN, Gelman RS, Schumer ST, Burstein HJ, et al. Tolerability of and adherence to combination oral therapy with gefitinib and capecitabine in metastatic breast cancer. Breast Cancer Res Treat. 2009;117:615–623. doi: 10.1007/s10549-009-0366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beney J, Bero LA, Bond C (2000) Expanding the roles of outpatient pharmacists: effects on health services utilisation, costs, and patient outcomes. Cochrane Database Syst Rev CD000336 [DOI] [PubMed]
- 21.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 22.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 24.Vrijens B, Goetghebeur E. Comparing compliance patterns between randomized treatments. Control Clin Trials. 1997;18:187–203. doi: 10.1016/S0197-2456(96)00235-8. [DOI] [PubMed] [Google Scholar]
- 25.Rosen MI, Rigsby MO, Salahi JT, Ryan CE, Cramer JA. Electronic monitoring and counseling to improve medication adherence. Behav Res Ther. 2004;42:409–422. doi: 10.1016/S0005-7967(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 26.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 27.Boza RA, Milanes F, Slater V, Garrigo L, Rivera CE. Patient noncompliance and overcompliance. Behaviour patterns underlying patients failure follow doctors orders. Postgrad Med. 1987;81:163–170. doi: 10.1080/00325481.1987.11699750. [DOI] [PubMed] [Google Scholar]
- 28.Chau I, Legge S, Fumoleau P. The vital role of education and information in patients receiving capecitabine (Xeloda) Eur J Oncol Nurs. 2004;8(Suppl 1):S41–S53. doi: 10.1016/j.ejon.2004.06.008. [DOI] [PubMed] [Google Scholar]


