Abstract
γδ T cells lie at the interface between innate and adaptive immunity, sharing features with both arms of the immune system. The vast majority of γδ T cells reside in epithelial layers of tissues such as skin, gut, lung, tongue and reproductive tract. Here they provide a first line of defense against environmental attack. While the existence of epithelial resident γδ T cells has been known for over 20 years, understanding the molecular events regulating development and function of these cells is still in progress. Here we review recent advances in the field, with a particular emphasis on the γδ T cell population resident in mouse epidermis. These studies have enhanced our knowledge and understanding of the life cycle of this enigmatic population of cells.
Epithelial resident γδ T cells
Epithelial resident γδ T cells were first described in the epidermal layer of the skin [1]. γδ T cells were subsequently found to reside in other epithelial tissues, such as the gut, lung, tongue and reproductive tract [1]. In fact, all epithelial tissues examined contain a resident population of T cells bearing the γ and δ chains of the T cell receptor (TCR) [2]. While most species contain epithelial resident γδ T cells, including humans and rodents, their composition varies between the species. In humans, γδ T cells account for 10% or more of the T cell populations in the epithelium, whereas in the mouse, anywhere from 50-100% of the T cells are γδ T cells [1,3]. Because epithelial tissues have large surface areas, epithelial γδ T cells thus constitute a major T cell population.
γδ T cells residing in epithelial tissues are distinct from conventional αβ T cells in development, phenotype and function. Firstly, unlike αβ T cells, many epithelial γδ T cell TCR rearrangements occur only in the fetal thymus and not postnatally [1], although some rearrangements do occur both before and after birth, such as rearrangement of the Vγ5 gene (nomenclature used is that of Garman [4]) expressed by intestinal γδ T cells [1]. Secondly, many of the molecules that define αβ T cell maturation and regulate function, such as CD4, CD8 and CD28, are not found on epithelial resident γδ T cells [5,6]. Finally, while αβ T cells express diverse T cell receptors and recognize a vast array of foreign antigens, epithelial resident γδ T cells have limited to no receptor diversity [7]. They are believed to recognize tissue-specific stress- or damage-induced self ligands and numerous studies have demonstrated crucial roles for these unconventional T cells in tissue homeostasis and immune surveillance [8].
The largely sessile epithelial resident γδ T cells are quite distinct from circulating peripheral γδ T cells. As discussed in this review, the development, selection and effector functions differ substantially. Epithelial γδ T cell TCRs are generally much less diverse than those of circulating γδ T cells [7]. Signals through these TCRs during intrathymic development appear, on the one hand, to favor developmentof epithelial γδ T cells, but, on the other hand lead to expansion of autoreactive peripheral γδ T cells [9-11]. Furthermore, costimulation requirements of epithelial resident and peripheral γδ T cells differ, most notably in their use of JAML and CD28, respectively [12].
It is thus clear that many of the well defined rules that apply to αβ T cells and peripheral γδ T cells, do not hold true for tissue resident γδ T cells and currently no paradigm exists for development and activation of γδ T cells resident in epithelial tissues. Concomitant with our increased understanding of the functional significance of these cells, the complex nature of their molecular regulation has received much attention in recent years. In this article, we review new advances in the field, with a particular emphasis on γδ T cells resident in the epidermal layer of the skin, known as dendritic epidermal T cells (DETC) [Box 1], We discuss how current understanding ties in with earlier observations on epithelial γδ T cell biology. It is anticipated that many of the rules that are being deciphered for DETC will also hold true for γδ T cells resident in other epithelial tissues. As much of the recent work has been done in the mouse, this review will focus primarily on these studies.
Box 1 Dendritic Epidermal T Cells.
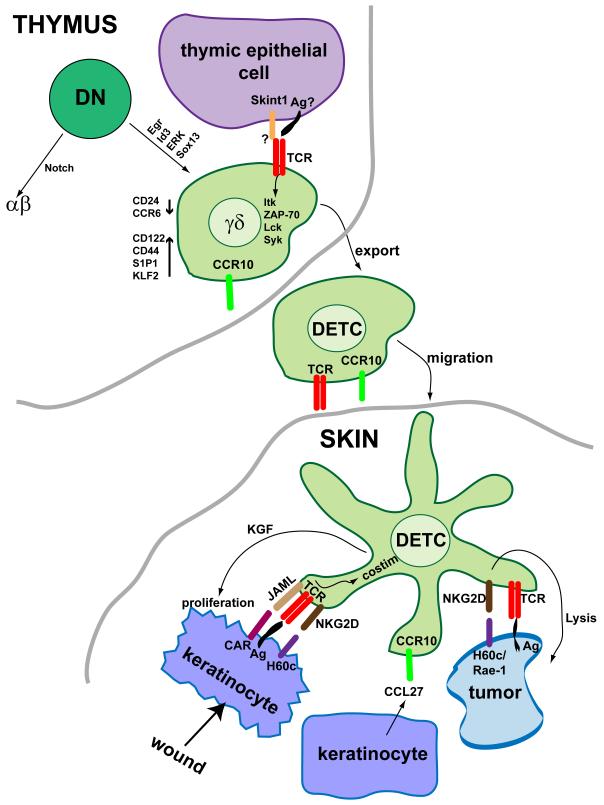
Dendritic epidermal T cells in mice (Figure 1), also known as DETC, express a canonical γδ T cell receptor composed of Vγ3 and Vδ1 chains [1]. These cells represent a prototypical epithelial resident γδ T cell population. These cells are the first to develop in the thymus, appearing around day 13 of embryonic development, and are the exclusive T cell population in the thymus at this time [1]. Additionally, Vγ3Vδ1 expressing cells are the only resident T cell population found in the epidermis [1]. Studies on the development and function of these cells can thus be performed isolated from the effects of other T cell populations. The development and effector function of later arising γδ populations are clearly effected by the neighboring αβ T cells through “trans-conditioning” [60,61]. As such, much of the work described in this review focuses on this subset of epithelial γδ T cells.
αβ versus γδ lineage choice
Commitment to the γδ lineage, as for αβ T cells, occurs in the thymus. At the double-negative (DN) stage of thymocyte development, gene rearrangement at the TCRβ, γ and δ gene loci is initiated. Both γδ and αβ T cells subsequently proofread their TCRs and this is accompanied by upregulated expression of CD5 and CD27 [13]. A key question in the field is what determines the choice to be a γδ or αβ T cell.
Signaling through the Notch transmembrane receptor is used to determine a wide variety of cell fates [14]. One of the earliest signals delivered to T cells in the thymus is through Notch. In the mouse, signals through Notch in immature thymocytes are thought to favor the development of the αβ T cell lineage at the expense of γδ T cells [15]. This has recently been brought into question by studies in humans where high Notch activity promotes the formation of γδ T cells [16]. Whether this reflects a true difference between human and mouse, or is merely due to the different systems used to assess the role of Notch, warrants further investigation.
Recent findings have supported a model where signal strength determines lineage fate [17,18]. Strong TCR signaling favors γδ T cell lineage development, whereas weak TCR signaling favors αβ lineage development [17,18]. This idea is further supported by single-cell analyses in the OP9-DL1 coculture system [19] where irreversible acquisition of a mature γδ lineage phenotype follows a strong TCR signal in otherwise dual potential T cells. Precisely how γδ T cells receive a stronger TCR signal in vivo is unknown. Greater amounts of cell surface expression of the γδ TCR compared with the pre-TCR [17] may well contribute. The distinct structure of the γδ TCR complex [20] may also contribute to its ability to transduce a stronger signal. Alternatively, it may be through ligand binding that a stronger signal is delivered to the γδ TCR.
At the molecular level, signaling through the γδ TCR activates the ERK MAPK pathway [17] and ERK is more highly phosphorylated in γδ than in αβ lineage cells [17,18]. The downstream effectors Egr and Id3 have thus been of recent focus in studies of γδ lineage choice. Alterations in the expression levels of either Egr1 or Id3 [9,17] in DN thymocytes has a significant effect on αβ and γδ lineage commitment. Overexpression of Egr1 augments γδ T cell differentiation at the expense of αβ T cells [9,17]. Similarly, Id3 deficiency results in defective γδ T cell maturation [17]. Id3 deficient mice, show severe reductions in the number of both splenic Vγ2+ T cells and epidermal Vγ3+ T cells [9]. Interestingly, total γδ thymocyte numbers are reduced in the fetal thymus of these mutant mice [17], but are augmented in the adult thymus [9,11], largely due to the expansion of autoreactive Vγ1+ γδ T cells in the adult thymus, that are thought to have been selected on high-affinity self ligands [9,11]. Thus, Id3 is not only required for γδ lineage choice, but also for the development of a normal repertoire of γδ T cells.
The HMG transcription factor Sox13 has also been shown to provide lineage cues to developing thymocytes. Sox13 expression is upregulated in immature γδ thymocytes and subsequently downregulated upon maturation [19]. The generation of αβ T cells is inhibited by Sox13 and, conversely, Sox13 is important for the development of at least some γδ T cells [21]. While Vγ3+ DETC [Box 1] precursors are apparently normal in numbers in the absence of Sox13, other Vγ expressing T cells are diminished [21]. Whether this represents a reduction in other epithelial subsets of γδ T cells or peripheral γδ T cell precursors is unclear. Furthermore, additional experimentation is required to understand whether these transcription factors contribute directly to γδ lineage choice or simply promote the survival or expansion of γδ T cell subsets.
Data so far support signal strength as an important determinant of αβ versus γδ T cell lineage fate. However, the precise mechanism by which the γδ TCR is able to transduce a strong signal has yet to be deciphered. While ligand binding appears to play a role for some γδ T cell subsets, whether this is true of all epithelial and peripheral subsets or represents a unique feature of particular γδ T cell populations requires further clarification. Once committed to the γδ lineage, distinct γδ subsets show marked differences in subsequent development
Programmed TCR gene rearrangement in epithelial γδ T cells
One of the most striking features of γδ T cell development is the highly organized manner in which the γδ T cell receptor is rearranged [Box 2]. What directs this ordered gene rearrangement has been the subject of numerous studies, with the current hypothesis being that in the fetal thymus chromosomal positioning determines rearrangements [22], whereas in the adult, rearrangement is regulated by locus accessibility [23]. During fetal thymic development, histone acetylation is high throughout the entire TCRγ locus [24]. The locus is thought to be open for rearrangement and it is the most J proximal genes (Vγ3 and Vγ4) that are rearranged first [22], similar to what has been seen for VH gene rearrangement [25,26]. In contrast, in the adult thymus, the upstream Vγ2 and Vγ5 genes are believed to be maintained in an open and accessible state while the acetylation levels and accessibility of Vγ3 and Vγ4 is suppressed [23,24]. This shifts the balance of rearrangements to the Vγ2 and Vγ5 genes in the adult thymus. It is currently thought that the transcription factors E2A and STAT5 are important players in this process. STAT5 has been implicated in accessibility control of Vγ5 [27], while analysis of E2A-deficient mice suggests involvement of E2A in regulating accessibility of Vγ2 [28]. The precise mechanism by which these and other transcription factors regulate the highly ordered rearrangement process requires further clarification.
Box 2 Ordered Vγ gene rearrangement.
It has been known for some time that different subsets of γδ T cells arise in the thymus at different stages of ontogeny [1]. The Vγ3 chain is the first to be rearranged in the fetal thymus at around day 13 of gestation. These Vγ3+ γδ T cells migrate to the epidermis where they expand locally to reach adult numbers. Vγ4+ cells are the next to be produced, also in the fetal thymus and they migrate to the reproductive tract, tongue and lung. Vγ3 and Vγ4 TCRs are essentially invariant, in large part due to the absence of terminal deoxynucleotidyl transferase (TdT) in the fetal thymus [1]. Both Vγ3+ and Vγ4+ cells also express an invariant Vδ1 chain. In the late fetal and the adult thymus, Vγ3+ and Vγ4+ T cells are no longer produced. Instead, Vγ5+ cells emerge and localize to the intestinal epithelium. In addition, Vγ2+, Vγ1.1+ and Vγ1.2+ cells are generated. These cells have diverse junctional sequences and localize primarily to the secondary lymphoid organs [1].
Selection in epithelial γδ T cell development
It has long been thought that mechanisms in addition to ordered rearrangement of TCR-γ and δ genes contribute to appearance of distinct γδ T cell subsets in the thymus and subsequently in their specific tissue location. Cellular selection processes are also believed to be required. Previous studies using specific knock-out mice found that multiple TCR signaling molecules, including Lck, Syk and ZAP-70 are important for DETC development [29-32]. However, the mechanisms by which these signaling molecules affect tissue-specific development of γδ T cells are poorly understood and whether this represents a signal generated by ligand-induced selection is unknown. While αβ T cells undergo positive and negative selection events before becoming mature T cells able to exit the thymus, a role for such selection processes in γδ T cell maturation has been more difficult to address. The restricted TCR usage of γδ T cell subsets was originally thought to indicate intrathymic selection had taken place. It has since been demonstrated that programmed rearrangement mechanisms restrict the TCR diversity of γδ T cell subsets. However, recent studies still point to intrathymic selection events restricting the TCR usage of γδ T cells resident in epithelial tissues, in particular those in the skin [10].
Skint1 is a recently identified transmembrane protein expressed by thymic stroma and keratinocytes. In a substrain of FVB mice, the Skint1 gene is non-functional due to a point mutation in codon 324 resulting in premature termination of the protein [33]. In these mice, Vγ3Vδ1 cells are present in normal numbers in the fetal thymus, but do not acquire a mature phenotype and do not take up residence in the skin [10]. Instead, the skin is populated by γδ T cells bearing alternate γ and δ chains. Transgenic expression of Skint1 is able to restore thymic maturation of Vγ3Vδ1 cells and epidermal residence of these mature DETC [34]. Evidence that Skint1 may be a selecting molecule for Vγ3Vδ1 cells comes from maturation of Skint1-deficient DETC precursors following antibody-mediated TCR ligation, which indicates that TCR-Skint1 interaction is required for selection of Vγ3Vδ1 T cells [10]. However, it remains to be determined if direct ligation of Skint1 is required or whether Skint1 acts indirectly, for example, by regulating expression of a Vγ3Vδ1 TCR ligand in the thymus.
Early work suggested that another γδ T cell subset also requires ligand engagement during development. The KN6 γδ TCR recognizes the nonclassical MHC class 1b molecule T22 and these T cells are found in both peripheral lymph node and the intestine [35]. Engagement of KN6 transgenic thymocytes by ligand during development favors the appearance of a mature CD24lo γδ population [17]. Attenuating KN6 γδ TCR signaling interferes with this γδ fate and promotes an αβ fate [17]. These data suggest ligand recognition is important for both lineage choice and maturation. This idea remains somewhat controversial however, as recent analysis, using T22 tetramers in non-transgenic animals, found no decrease in the numbers of T22-specific γδ T cells in the absence of thymic T22 signals [36].
The current prevailing view is that development of tissue-specific subsets of γδ T cells requires some form of intrathymic selection, with an outcome distinct from equivalent signals in αβ T cells and peripheral γδ T cells. Strong signals appear to favor development of epithelial γδ T cells [10], but induce negative selection in αβ T cells [37] and expansion of autoreactive peripheral γδ T cells [9,11]. Emerging evidence points to a role for positive selection in conferring tissue specificity to epithelial γδ T cells through the induction of distinct molecules directing the specific homing of individual subsets.
Tissue-specific homing
The majority of Vγ3+ T cells in the E16 fetal thymus of wild-type mice display a mature activated or memory phenotype, including upregulated expression of CD122 [38] and CD44 [39] and downregulation of CD24 [40]. CD122+ γδ thymocytes also express increased levels of the sphingosine 1-phosphate receptor 1 (S1P1) [38], a molecule previously shown to be important for exit of thymocytes from the thymus [41], and decreased CCR6 expression [38]. As such, upregulation of S1P1 and down regulation of CCR6 may signify readiness of DETC precursors for thymic export. The molecules directing these mature γδ thymocytes to their appropriate epithelial residence has been under recent investigation.
CD122+ Vγ3+ thymocytes also express CCR10, a molecule whose ligand, CCL27, is specifically expressed in the skin [38]. CCR10 was thus proposed to represent a homing receptor for thymic γδ T cells destined to migrate to the skin epithelium. This idea has been supported by recent work using CCR10-deficient animals [42]. In these animals, Vγ3+ cells develop normally in the thymus, but are defective in migrating into skin. CCR10-deficient γδ T cells that are found in the skin, display an abnormal morphology and accumulate in the dermis rather than epidermis. Although an earlier report did not find defective homing of γδ T cells to the skin in CCR10-deficient animals [43], morphology and localization of the cells that had accumulated in the skin were not examined. Interestingly, while CCR10-deficient animals do show reduced numbers of DETC in the epidermis, significant numbers of epidermal T cells are present in these animals suggesting the involvement of additional molecules in the skin-homing process. E and P selectin ligands and CCR4 have also been implicated in skin homing [43]. Vγ3+Vδ1+ cells in the thymus and skin express both E and P selectin ligands. Mice deficient in these ligands show a dramatic reduction in skin γδ T cell numbers, while thymic numbers are unaffected [43]. Thus, these molecules may well support the correct localization of DETC in the skin, however the possibility that they are required for survival or retention in the epidermis still needs to be addressed.
The role of CCR4 in skin homing is also unclear at this stage. The vast majority of immature Vγ3+ thymocytes are negative for CCR4 whereas virtually all mature epidermal resident DETC express CCR4 [43]. While Ccr4−/− animals do show reduced numbers of CD3+Vγ3+ T cells in the epidermis [43], a population of CD3+Vγ3− cells is apparent. Therefore, if CCR4 is essential for skin homing, how its deficiency allows migration of other CD3+Vγ3− T cells to the epidermis is unclear. The thymic populations in these CCR4-deficient animals were not examined. Thus, whether there is a true homing defect or alternatively a developmental abnormality in these animals awaits clarification.
The transcription factor Kruppel-like factor 2 (KLF2) has recently been shown to regulate Sphingosine-1-phosphate receptor 1 (S1P1) expression by thymic γδ T cells and play a role in localization of γδ T cells to the gut [44], supporting the idea that S1P1 is important for thymic egress of γδ T cells, but contrasting with a previous report indicating no effect of S1P1 on gut homing [45]. This earlier report was based on long-term treatment with the S1P1 analog FTY720 having no effect on gut γδ T cell numbers. However, this could be due to a S1P1-independent mechanism for retention of these cells in the intestinal epithelium. However, whether S1P1 is truly a marker of tissue homing, or simply thymocyte egress, remains to be determined experimentally.
Using the epidermal-specific DETC population, it was recently demonstrated that TCR-mediated signaling in the thymus plays an important role in the acquisition of a specific skin-homing property [46]. In the absence of TCR signaling due to deletion of Itk, Vγ3+ thymocytes fail to upregulate S1P1 and CCR10 and express lower levels of KLF2 than wild-type animals [46]. They do however upregulate CD122 and downregulate CD24, suggesting their maturation is unimpaired. Itk, on the other hand is dispensible for DETC maintenance. The small numbers of DETC that do home to the epidermis in Itk-deficient animals proliferate locally and take up residence [46], indicating that Itk is important for skin homing, but not for maintenance of DETC in the epidermis. Two additional studies support the idea that a TCR signal during the window of epidermal T cell development can direct a CCR10+CD122+ skin-homing phenotype [38,47]. In these studies, homing was independent of TCR composition or specificity. Thus, at least for skin-homing γδ T cells, TCR signals are critical for inducing expression of proper homing and cytokine receptors for epidermal localization and expansion.
Specificity in gut homing of γδ T cells has also been described. It has been known for a number of years, and recently confirmed, that γδ T cell homing to the small intestinal epithelium relies on the interaction between CCR9 on T cells and its ligand CCL25 expressed by intestinal epithelial cells [48-50]. Mice deficient in CCR9 have a significantly reduced γδ+ intraepithelial lymphocyte (IEL) compartment [48-50]. However, unlike for DETC homing to the skin, TCR-ligand interaction appears to inhibit acquisition of CCR9 and subsequent gut homing. In the thymus of β2m−/− animals, where T10 and T22 IEL TCR ligands are absent, most T10 and T22 specific IEL are CCR9hi, indicating a gut-homing phenotype [36]. In wild-type animals, where a TCR ligand is present, the CCR9hi T22-specific γδ thymocytes are CD122lo, suggestive of a pre-selection phenotype, whereas the CCR9lo cells are CD122hi [36]. As such, IEL precursors that have not encountered a thymic TCR ligand may have a greater potential to home to the gut.
Thus, the situation for IEL seems quite different from that for DETC. A lack of selection appears to favor gut homing for IEL precursors, whereas selection seems to favor skin-homing in DETC precursors. Whether this represents a difference in the adult versus fetal thymus or is cell intrinsic for the diverse versus canonical TCRs remains an open question. Additionally, precisely how signals through one γδ TCR can induce CCR10 and through another γδ TCR can inhibit CCR9 is completely unknown.
Costimulation
The unique nature of epithelial γδ T cells is perhaps best exemplified by their antigen recognition and activation. It has long been recognized that the rules for αβ T cell recognition of antigen and subsequent activation do not hold true for γδ T cells. Unlike αβ T cells, epithelial γδ T cells do not recognize antigen in the context of MHC class I or class II molecules [5,6]. In wild-type animals, most do not express the CD4 or CD8 coreceptors, except for a population of CD8αα+ γδ T cells in the intestine. In addition, they do not express the αβ costimulatory molecule CD28 [5,6], a molecule well defined for its essential role in effective αβ T cell activation [51]. Whether equivalent molecules exist for γδ T cells has been controversial and somewhat difficult to address as the rules for antigen recognition by epithelial resident γδ T cells are undefined. Recent evidence suggests that for activation of rapid and robust effector functions, whether it be proliferation and cytokine production or cell killing, epithelial γδ T cells, like αβ T cells, require accessory molecules to enhance their TCR-mediated signals.
NKG2D is an activating receptor expressed by NK cells, αβ T cell subsets and γδ T cells [52]. Signals through NKG2D by interaction with its ligands Rae-1 and H60c can stimulate DETC to kill tumor cells [53,54]. However, whether NKG2D can stimulate without the need for TCR signals or functions as a costimulatory molecule has been controversial. Early studies favored NKG2D as an activating receptor that did not require simultaneous TCR triggering [55]. A more recent study however, found that DETC are only able to lyse cultured keratinocyte cell lines following interaction between NKG2D and its skin-specific ligand, H60c, together with TCR-mediated signals [54]. NKG2D signals alone do not activate in this system. The reason for these differences is not entirely clear and the role for NKG2D and Rae-1/H60c interactions in DETC responses in vivo remains an open question.
The Junctional Adhesion Molecule Like protein (JAML) is a costimulatory receptor specific for epithelial γδ T cells [12]. The interaction of JAML with its ligand Coxsackie and Adenovirus Receptor (CAR) induces PI3K activation [56] leading to potent costimulation of epithelial γδ T cells as measured by MAP kinase activation, proliferation and growth factor and cytokine production [12]. This interaction is crucial for effective DETC-mediated wound closure in the skin of mice. The unique property of the JAML-CAR interaction is its specificity for the epithelial subsets of γδ T cells. Both epidermal and intestinal γδ T cells show strong costimulation through JAML, while peripheral γδ T cells are completely unresponsive [12]. Peripheral γδ T cells are similar to αβ T cells in this respect, responding well to costimulation through CD28. What is the role of costimulation in epithelial γδ T cells which already exist in a partially activated state? Whether it perhaps functions to give directionality to the response, provides additional specificity and context to the response [57], or plays some other role is completely unknown at this stage. Nevertheless, the demonstration of an epithelial γδ T cell-specific costimulatory molecule opens the door for a more thorough dissection of the molecular events involved in the activation of this important population of T cells.
One cannot overlook however the increasing number of accessory molecules identified as important for the function of peripheral γδ T cells, as many of these may well also play a role in epithelial γδ T cell function. There has been much recent focus on the IL-17 producing subset of γδ T cells that provide a rapid inflammatory response to infection. IL-17 producing γδ T cells express the Toll-like receptors TLR1 and TLR2, as well as dectin-1, but not TLR4 [58]. In addition, these cells produce IL-22 upon activation of the aryl hydrocarbon receptor (AhR). Activation of these cells to produce IL-17 does not appear to require TCR recognition [58] (although prior TCR-mediated activation of these cells cannot be ruled out), thus does not constitute a costimulatory response. In contrast, IFN-γ producing peripheral γδ T cells lack the pattern recognition receptors TLR2, dectin-1 and AhR [58]. Instead these cells express CD27 and costimulatory signals through CD27 synergize with TCR signals to induce proliferation and survival of these cells [59]. Whether similar mechanisms are in play for IL-17 and IFN-γ production by epithelial subsets of γδ T cells warrants investigation.
Concluding remarks
Epithelial γδ T cells are important regulators of tissue homeostasis and play vital roles in tumor surveillance and wound repair. Studies over the past few years have provided valuable insight into the molecular regulation of the development, activation and function of these unique T cells, in particular the DETC subset resident in the epidermis (Figure 1). Their intrathymic development, while sharing a number of phenotypic similarities with αβ T cell development, relies on unique signals, at least some of which are through the T cell receptor. While these signals appear to induce some form of intrathymic selection, whether they are in fact TCR ligand induced, comparable to αβ T cells, remains to be determined. What clearly is unique to tissue resident γδ T cells is their activation requirements, which differ from both conventional αβ T cells and peripheral circulating γδ T cells. Not only do epithelial γδ T cells exhibit non-MHC restricted activation, their costimulatory requirements are also unique utilizing JAML and NKG2D as opposed to molecules such as CD28. Recent studies strongly support a vital role for costimulation in epithelial γδ T cell activation, however as epithelial γδ T cells do exhibit a partially activated phenotype, whether the purpose of costimulation in epithelial γδ T cells is equivalent to that of αβ and/or peripheral γδ T cells is currently unknown. Identification of the cognate ligands for the TCRs expressed by epithelial resident γδ T cells will allow for a more thorough understanding of precisely how the molecules described in the recent literature, as well as others not yet identified, regulate this unique population of T cells.
Figure 1.
Molecular regulation of DETC development and function. DETC precursors develop in the fetal thymus from DN thymocytes by way of signals through Egr, Id3, ERK and Sox13. Subsequent maturation involves interaction with Skint1 expressed by thymic epithelial cells. It is unknown whether this interaction is directly through the TCR and whether TCR ligand is also required. Mature DETC precursors, upon exit from the thymus express low levels of CD24 and CCR6, but have increased CD122, CD44, S1P1 and KLF2 expression. These cells also require expression of CCR10 to migrate to the skin where they take up residence in the epidermal layer. Upon epidermal insult, DETC encounter with antigen expressed by damaged or malignant epithelial cells. DETC are activated through coordination of TCR and costimulatory signals, although the detailed nature of these signals remains under investigation. Subsequently, activated DETC are able to kill tumor cells or, alternatively, produce cytokines and growth factors, such as keratinocyte growth factor (KGF), to repair the damaged epithelial layer [53,62].
Acknowledgements
Supported by NIH grants AI036964 and AI64811 (W.L.H. and D.A.W.) and GM080301 (W.L.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison JP, Havran WL. The immunobiology of T cells with invariant γδ antigen receptors. Annu Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- 2.Havran WL, et al. Epithelial cells and their neighbors. III. Interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G627–630. doi: 10.1152/ajpgi.00224.2005. [DOI] [PubMed] [Google Scholar]
- 3.Toulon A, et al. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raulet DH. The structure, function, and molecular genetics of the γ/δ T cell receptor. Annu Rev Immunol. 1989;7:175–207. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- 5.Havran WL, et al. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 6.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien RL, et al. γδ T-cell receptors: functional correlations. Immunol Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 8.Jameson J, Havran WL. Skin γδ T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 9.Lauritsen JP, et al. Marked induction of the helix-loop-helix protein Id3 promotes the γδ T cell fate and renders their functional maturation Notch independent. Immunity. 2009;31:565–575. doi: 10.1016/j.immuni.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JM, et al. Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 11.Ueda-Hayakawa I, et al. Id3 restricts the developmental potential of γδ lineage during thymopoiesis. J Immunol. 2009;182:5306–5316. doi: 10.4049/jimmunol.0804249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witherden DA, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial γδ T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taghon T, et al. Developmental and molecular characterization of emerging β- and γδ-selected pre-T cells in the adult mouse thymus. Immunity. 2006;24:53–64. doi: 10.1016/j.immuni.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 15.Washburn T, et al. Notch activity influences the αβ versus γδ T cell lineage decision. Cell. 1997;88:833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 16.Van de Walle I, et al. An early decrease in Notch activation is required for human TCR-αβ lineage differentiation at the expense of TCR-γδ T cells. Blood. 2009;113:2988–2998. doi: 10.1182/blood-2008-06-164871. [DOI] [PubMed] [Google Scholar]
- 17.Haks MC, et al. Attenuation of γδTCR signaling efficiently diverts thymocytes to the αβ lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Hayes SM, et al. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Kreslavsky T, et al. T cell receptor-instructed αβ versus γδ lineage commitment revealed by single-cell analysis. J Exp Med. 2008;205:1173–1186. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes SM, Love PE. Distinct structure and signaling potential of the γδTCR complex. Immunity. 2002;16:827–838. doi: 10.1016/s1074-7613(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 21.Melichar HJ, et al. Regulation of γδ versus αβ T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 22.Xiong N, et al. The genomic arrangement of T cell receptor variable genes is a determinant of the developmental rearrangement pattern. Proc Natl Acad Sci U S A. 2004;101:260–265. doi: 10.1073/pnas.0303738101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong N, Raulet DH. Development and selection of γδ T cells. Immunol Rev. 2007;215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 24.Agata Y, et al. Histone acetylation determines the developmentally regulated accessibility for T cell receptor γ gene recombination. J Exp Med. 2001;193:873–880. doi: 10.1084/jem.193.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams GS, et al. Unequal VH gene rearrangement frequency within the large VH7183 gene family is not due to recombination signal sequence variation, and mapping of the genes shows a bias of rearrangement based on chromosomal location. J Immunol. 2001;167:257–263. doi: 10.4049/jimmunol.167.1.257. [DOI] [PubMed] [Google Scholar]
- 26.Yancopoulos GD, et al. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984;311:727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- 27.Tani-ichi S, et al. Accessibility control of TCR Vγ region by STAT5. Int Immunol. 2010;22:693–703. doi: 10.1093/intimm/dxq054. [DOI] [PubMed] [Google Scholar]
- 28.Bain G, et al. Positive and negative regulation of V(D)J recombination by the E2A proteins. J Exp Med. 1999;189:289–300. doi: 10.1084/jem.189.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colucci F, et al. A new look at Syk in αβ and γδ T cell development using chimeric mice with a low competitive hematopoietic environment. J Immunol. 2000;164:5140–5145. doi: 10.4049/jimmunol.164.10.5140. [DOI] [PubMed] [Google Scholar]
- 30.Endo Y, et al. Zeta-chain-associated protein-70 molecule is essential for the proliferation and the final maturation of dendritic epidermal T cells. Exp Dermatol. 2005;14:188–193. doi: 10.1111/j.0906-6705.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 31.Kawai K, et al. Impaired development of Vγ3 dendritic epidermal T cells in p56lck protein tyrosine kinase-deficient and CD45 protein tyrosine phosphatase-deficient mice. J Exp Med. 1995;181:345–349. doi: 10.1084/jem.181.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallick-Wood CA, et al. Disruption of epithelial γδ T cell repertoires by mutation of the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 1996;93:9704–9709. doi: 10.1073/pnas.93.18.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyden LM, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbee SD, et al. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci U S A. 2011;108:3330–3335. doi: 10.1073/pnas.1010890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer C, et al. Ligand recognition during thymic development and γδ T cell function specification. Semin Immunol. 2010;22:207–213. doi: 10.1016/j.smim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen KD, et al. Cutting edge: γδ intraepithelial lymphocytes of the small intestine are not biased toward thymic antigens. J Immunol. 2009;182:7348–7351. doi: 10.4049/jimmunol.0900465. [DOI] [PubMed] [Google Scholar]
- 37.Gascoigne NR, Palmer E. Signaling in thymic selection. Curr Opin Immunol. 2011 doi: 10.1016/j.coi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong N, et al. Positive selection of dendritic epidermal γδ T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Prinz I, et al. Visualization of the earliest steps of γδ T cell development in the adult thymus. Nat Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 40.Leclercq G, et al. Intrathymic differentiation of Vγ3 T cells. J Exp Med. 1993;178:309–315. doi: 10.1084/jem.178.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 42.Jin Y, et al. CCR10 is important for the development of skin-specific γδT cells by regulating their migration and location. J Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang X, et al. Embryonic trafficking of γδ T cells to skin is dependent on E/P selectin ligands and CCR4. Proc Natl Acad Sci U S A. 2010;107:7443–7448. doi: 10.1073/pnas.0912943107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odumade OA, et al. Kruppel-like factor 2 regulates trafficking and homeostasis of γδ T cells. J Immunol. 2010;184:6060–6066. doi: 10.4049/jimmunol.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunisawa J, et al. Sphingosine 1-phosphate dependence in the regulation of lymphocyte trafficking to the gut epithelium. J Exp Med. 2007;204:2335–2348. doi: 10.1084/jem.20062446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia M, et al. Differential roles of IL-2-inducible T cell kinase-mediated TCR signals in tissue-specific localization and maintenance of skin intraepithelial T cells. J Immunol. 2010;184:6807–6814. doi: 10.4049/jimmunol.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin Y, et al. Cutting edge: Intrinsic programming of thymic γδT cells for specific peripheral tissue localization. J Immunol. 2010;185:7156–7160. doi: 10.4049/jimmunol.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chennupati V, et al. Intra- and intercompartmental movement of γδ T cells: intestinal intraepithelial and peripheral γδ T cells represent exclusive nonoverlapping populations with distinct migration characteristics. J Immunol. 2010;185:5160–5168. doi: 10.4049/jimmunol.1001652. [DOI] [PubMed] [Google Scholar]
- 49.Uehara S, et al. Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3highCD69+ thymocytes and γδTCR+ thymocytes preferentially respond to CCL25. J Immunol. 2002;168:134–142. doi: 10.4049/jimmunol.168.1.134. [DOI] [PubMed] [Google Scholar]
- 50.Wurbel MA, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor γδ+ gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 51.Watts TH. Staying alive: T cell costimulation, CD28, and Bcl-xL. J Immunol. 2010;185:3785–3787. doi: 10.4049/jimmunol.1090085. [DOI] [PubMed] [Google Scholar]
- 52.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 53.Girardi M, et al. Regulation of cutaneous malignancy by γδ T cells. Science. 2001;294:605–609. [PubMed] [Google Scholar]
- 54.Whang MI, et al. Costimulation of dendritic epidermal γδ T cells by a new NKG2D ligand expressed specifically in the skin. J Immunol. 2009;182:4557–4564. doi: 10.4049/jimmunol.0802439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nitahara A, et al. NKG2D ligation without T cell receptor engagement triggers both cytotoxicity and cytokine production in dendritic epidermal T cells. J Invest Dermatol. 2006;126:1052–1058. doi: 10.1038/sj.jid.5700112. [DOI] [PubMed] [Google Scholar]
- 56.Verdino P, et al. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science. 2010;329:1210–1214. doi: 10.1126/science.1187996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Witherden DA, Havran WL. Costimulating epithelial γδ T cells. Cell Cycle. 2011;10:4–5. doi: 10.4161/cc.10.1.14290. [DOI] [PubMed] [Google Scholar]
- 58.Martin B, et al. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 59.Ribot JC, et al. Cutting edge: adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-γ- or IL-17-producing γδ T cells upon infection. J Immunol. 2010;185:6421–6425. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pennington DJ, et al. The inter-relatedness and interdependence of mouse T cell receptor γδ+ and αβ+ cells. Nat Immunol. 2003;4:991–998. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- 61.Pennington DJ, et al. Early events in the thymus affect the balance of effector and regulatory T cells. Nature. 2006;444:1073–1077. doi: 10.1038/nature06051. [DOI] [PubMed] [Google Scholar]
- 62.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]