Abstract
Background
Dermatologists, paediatricians, and general practitioners are often consulted by worried parents for the evaluation of a cutaneous tumor.
Methods
Selective literature review.
Results
Only 1–2% of skin tumors excised in children turn out to be malignant when examined histologically. Warning signs of malignancy include rapid growth, firm consistency, diameter exceeding 3 cm, ulceration, a non-movable mass, and presence in the neonatal period. The more common malignant skin tumors in adults—basal cell carcinoma, cutaneous squamous cell carcinoma, and melanoma—are very rare in childhood. Congenital melanocytic nevi and sebaceous nevi bear a lower malignant potential than previously believed; nevertheless, their excision is often indicated. A Spitz nevus can mimic a melanoma both clinically and histologically. Some benign skin tumors of childhood tend to regress spontaneously within a few years but may cause complications at particular locations and when multiple. For infantile hemangiomas requiring systemic treatment because of imminent obstruction or ulceration, propranolol seems to have a far more favorable risk-benefit ratio than corticosteroids.
Conclusion
Physicians need specialized knowledge in order to decide whether a skin tumor in a child should be excised, non-surgically treated, or further evaluated, or whether it can be safely left untreated because of the likelihood of spontaneous remission.
The early detection and—mostly surgical—treatment of malignant skin tumors and their precursor lesions represent an important area in dermatology today. In adults, the most common skin cancers by far are melanoma, basal cell carcinoma, and squamous cell carcinoma (1), and their incidence is steadily rising. In children, the situation could not be more different: pediatric dermatologists are confronted with a multitude of mostly benign skin tumors that may require treatment, but excision is required more rarely.
This review article briefly explains the role of the most important adult malignant cutaneous neoplasias in childhood. We then discuss three types of nevi that are important for the development of malignant skin tumors and that constitute the most important differential diagnosis to pediatric melanoma. In the third part we focus on four skin tumor types that are typical for the first decade of life. This is based on a selective literature search on PubMed, Medline, and PubMed Central. The tumors under discussion were selected because of their great importance in the authors’ everyday clinical practice.
Malignant skin tumors in childhood
Only 1% to 2% of all skin tumors excised from infants and children are malignant.
They include
Fibrosarcoma
Rhabdomyosarcoma
Angiosarcoma
Neuroblastoma
Malignant peripheral nerve sheath tumor
Cutaneous T-cell lymphoma and other lymphomas
as well as the semi-malignant
Pediatric fibromatoses
Hemangioendothelioma
Tufted angioma
Dermatofibrosarcoma protuberans.
Warning signs include rapid growth, ulceration, fixation or deep localization on the fascia, rough texture, size larger than 3 cm, and manifestation in neonates (2).
Melanoma
The rising incidence of melanoma affects young people only after puberty. 1% to 4% of all melanomas develop in those younger than 20 years of age, and only 0.3% to 0.4% in prepubertal children. Except for the large congenital melanocytic nevi, most of the predisposing factors shown in Box 1 lead to melanoma only in adulthood, although they are already present in childhood, which means that preventive measures should be adhered to from an early age. In children as in adults, most melanomas develop de novo, but in children, atypical, amelanotic, and nodular melanomas are more common (4). The fact that diagnosis is difficult may account for the fact that melanomas in children often have a greater tumor thickness at the time of excision; another reason may be greater reluctance to make a diagnosis of suspected melanoma and perform a diagnostic excision. Melanoma simulators include the Spitz nevus and atypical melanocytic nevi.
Box 1. Risk factors for the development of melanoma (3).
Light skin, red or blond hair, light eyes
Freckles, actinic lentigines (sun spots)
Tendency to sunburn when exposed to UV light
Intermittent intensive exposure to UV light
Large number of common melanocytic nevi
Several atypical melanocytic nevi
Congenital melanocytic nevus, especially giant nevus
Melanoma in the family
Impaired DNA repair, especially xeroderma pigmentosum
Immunosuppression
Previous malignant disease
The therapeutic approach in childhood melanoma is the same as in adults, including the nowadays routinely performed sentinel lymph node biopsy in tumors of ≥1 mm thickness. Although positive sentinel nodes are more common in children, the prognosis for the preservation of life hardly differs from that in adults with tumors of the same thickness. 5-year survival in pediatric melanomas is generally reported to be 74% to 80% (5).
Malignant epithelial skin tumors
Genetic and environmental risk factors that are relevant early in life are known for the semi-malignant basal cell carcinoma (BCC) and for cutaneous squamous cell carcinoma (SCC) (Box 2). The most important risk factors for both types of cancer are light skin and exposure to ultraviolet light, including UV light from artificial sources. Intermittent exposure to strong sunlight accompanied by sunburn is the determining factor for BCC and the cumulative lifetime exposure for SCC (1). In the absence of further trigger factors, these come into play only after decades of latency. Malignant epithelial skin tumors in children are mostly observed on the basis of predisposing genodermatoses (7).
Box 2. Risk factors for the development of malignant epithelial skin tumors (6).
Light skin
UV light
Ionizing radiation
-
Predisposing genodermatoses, for example:
Basal cell nevus syndrome in basal cell carcinoma
Epidermodysplasia verruciformis in squamous cell carcinoma
Xeroderma pigmentosum in both tumors
Sebaceous nevus
Long term immunosuppression, for example after organ transplantation
High risk human papillomaviruses
Chemical carcinogens, such as inorganic arsenic
Chronic inflammation, ulceration, scars, and scleroses
Nevi and their relation to malignant skin tumors
Congenital melanocytic nevi
The malignant potential of congenital melanocytic nevi (CMN) has been known for more than a century. However, it is becoming increasingly clear that the dimension of the risk of malignant degeneration differs widely on a case by case basis and depends on the size of the nevus, among other factors. CMN are therefore classified by the largest diameter that they are likely to reach in adulthood (Table 1).
Table 1. Established classification for congenital melanocytic nevi (CMN) (8).
| Description | Size*1 | Incidence |
| Small | <1.5 cm | roughly 1: 100 |
| Medium | 1.5–19.9 cm | roughly 1: 1000 |
| Large | ≥ 20 cm | roughly 1: 20 000 |
| Giant | >40–50 cm*2 | roughly 1: 500 000 |
*1Relates to the largest diameter that is reached in adulthood. The largest diameter of CNM increases between birth and adulthood by a factor of 1.7 on the head, by a factor of 3.3 on the legs, and a factor of 2.8 on the trunk, arms, and feet (8).
*2No general consensus
The risk of malignant degeneration of small and medium sized CMN is low. In practice melanomas almost never develop before puberty and arise from the epidermis, which enables early detection. However, the indication for surgical removal also needs to consider psychosocial and cosmetic aspects. For this reason, but also because of greater ease of removal, excision of larger nevi—or even serial excision—is often indicated in childhood.
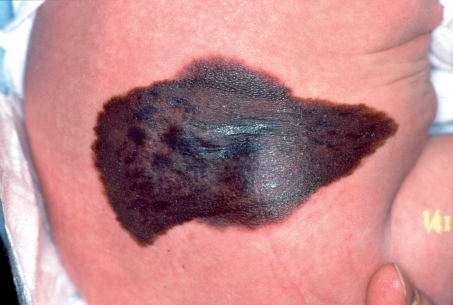
The problems with larger CMN (Figure 1) and giant nevi in particular are more complex (8):
Figure 1.
Large congenital melanocytic nevus in a neonate
Cutaneous melanomas arising from the nevus—and, more rarely, other malignant tumors—develop in the first decade of life in 70% of cases and often develop in the deeper strata of the nevus and consequently are noticed only later.
Melanomas can develop not only from the nevus but also on other locations—for example, the central nervous system.
Especially giant and large CMN may be associated with neurocutaneous melanosis, a leptomeningeal seeding of nevus cells (9). The most important risk factor in this context is a large number of so-called satellite nevi. Furthermore, malformations of the central nervous system may be present. In giant nevi that overlie the dorsal median body axis and/or have multiple satellites, cranial and spinal magnetic resonance tomography in the first 4–6 months of life is recommended (10). Symptomatic neurocutaneous melanosis typically manifests in the first 2–3 years of life, with signs of raised intracranial pressure or spinal compression. The prognosis is poor. Not all patients with a positive finding on MRI go on to develop neurological symptoms, however.
The risk that one of these complications may develop is estimated at 5% to 15% for giant CMN and is highest in the first 5 to 10 years of life.
The treatment approach to CNM is the subject of continuing discussion, particularly because only the risk of tumor development on the cutaneous nevus can be reduced. If no neurocutaneous melanosis is present, then most experts advise early and complete excision of large and giant CMN or at least to remove particularly striking or difficult to control areas at the end of the first year of life. Independently of the treatment, quarterly follow-up is recommended in such cases.
Superficially ablative procedures, such as dermabrasion, serve primarily the purpose of cosmetic improvement and make sense only in the first few months of life and in lesions that cannot be excised. Dermabrasion does not reach the deeper nevus cell layers; furthermore, it does not prevent hypertrichosis, which typically accompanies nevi. According to the current state of knowledge, laser treatment should be considered only in particular cases and special localizations (for example, the face).
Spitz nevus
Histological examination shows that 1% to 2% of all excised melanocytic lesions are Spitz (spindle cell) nevi. The clinical variation of Spitz nevi ranges from a dome shaped, skin colored, or reddish papule in the face of a toddler to a brown-black plaque on the proximal extremity of an adolescent. Typical of Spitz nevi, and of differential diagnostic importance, are their initially rapid growth and subsequent persistence.
The Spitz nevus is neither a melanoma precursor nor a melanoma, but it is clinically and histologically the most important melanoma simulator, as its original description as “juvenile melanoma” suggests. Even experienced dermatohistopathologists using immunohistological techniques have difficulty in determining the dignity of some skin tumors with Spitz nevus-like histology (“atypical Spitz tumor”). In such cases, comparative genomic hybridization or fluorescence in situ hybridization have proved helpful, but they are not yet routinely available (11).
In case of doubt, the therapeutic approach to be used should be that for confirmed melanoma. Depending on the thickness of the tumor, this entails staging measures and sentinel lymph node extirpation, which will be positive in 50% of spitzoid melanocytic tumors. A confirmed finding of melanocytes in the lymph nodes, however, does not necessarily mean metastasis (12).
Sebaceous nevus
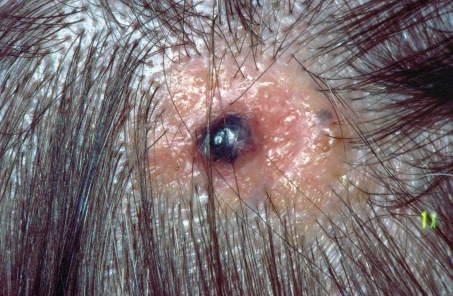
With a prevalence of 0.3%, sebaceous nevus is the most common organoid epidermal nevus. “Organoid” means that more than one tissue structure—in this case mainly sebaceous glands and sweat glands—is involved in the malformation. More than 90% of sebaceous nevi are localized on the head, mainly on the scalp. The fact that alopecia is associated with the nevus is particularly objectionable in this setting. In 10% to 30% of cases, skin tumors will develop on the nevus, albeit usually only in adulthood. These are mainly benign tumors of the hair follicles, sebaceous glands, apocrine and eccrine sweat glands, primarily the (pigmented) trichoblastoma (Figure 2) and the syringocystadenoma papilliferum (papillary syringadenoma). BCCs will develop on the nevus much more rarely than assumed in the past—retrospective studies have shown this happens in 0% to 3.5% of cases—and, exceptionally, also SCCs and adnexal carcinomas of the skin. In children, only 15 cases of BCC on a sebaceous nevus have been documented (13); consequently most authors do not see the need for an indication to remove a sebaceous nevus in childhood for reasons of prophylaxis. Early removal is advantageous, however, because of easier excisability (scalp) and for cosmetic reasons (face) (14). Excision is the method of choice.
Figure 2.
Pigmented trichoblastoma on a sebaceous nevus
A selection of common benign skin tumors
Hemangioma
Hemangiomas are the most common tumors in childhood, with a prevalence of 3% to 5% in infants. Girls are affected twice or three times as often as boys. Premature babies have an up to 10-fold increased risk of hemangioma. Most infantile hemangiomas are caused by a hyperactive vascular endothelial growth factor (15), which is triggered by peripartal hypoxia. In diffuse hemangiomatoses, endothelial cell emboli arising from placental hemangiomas (chorangiomas) are a causative factor (16). A difference to other vascular tumors that is of importance for the differential diagnosis is that “genuine” hemangiomas express the glucose transport-1 protein (GLUT-1), which can be confirmed immunohistologically.
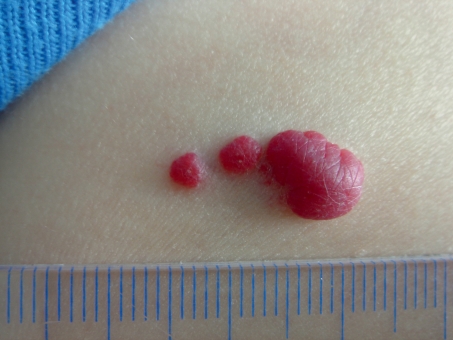
Pediatric hemangiomas have a characteristic growth dynamic, which makes it easy to distinguish them from vascular malformations: shortly after birth, an initially white or bright red macula (differential diagnosis: vascular nevus) will develop into a proliferating, plump nodule that is either of intense red color (intracutaneous) or of pale-livid color (subcutaneous), depending on its location (Figure 3). The proliferation phase usually lasts about 6 to 9 months and is followed by a halt in growth, before spontaneous regression sets in at an age of 12 to 14 months in 80% to 90% of hemangiomas, which can take several years. Most hemangiomas do not require treatment. If there is a risk of obstruction (especially in periocular, paratracheal, or intratracheal hemangiomas), of ulceration (especially intertriginously), or if the hemangiomas are very large (risk of heart failure, rarely also hypothyroidism), then an urgent and absolute indication for treatment exists; a relative indication exists for localization on the face without obstruction because of “cosmetic” impairment and possible later psychosocial stigmatization (Table 2).
Figure 3.
Hemangioma
Table 2. Treatment indication and options for the therapy of infantile hemangiomas (18).
| Localisation or type of hemangioma | Method of choice | Alternatives |
| 1. Absolute treatment indication | ||
| Risk of obstruction (for example, eye, trachea) | Propranolol*1 | Systemic corticosteroids, excision of circumscibed hemangiomas |
| Nd:YAG-laser*2 | ||
| Ulceration | Propranolol*1 | |
| Diffuse neonatal hemangiomatosis | Propranolol*1 | Systemic corticosteroid |
| 2. Relative treatment indication | ||
| Localisation on the face | Contact cryotherapy*3 | Nd:YAG-laser*2 |
| 3. No treatment indication | ||
| Unproblematic hemangiomas (for example, on the trunk or proximal extremities) | ||
*1currently not licensed (see text)
*2 in shallow hemangiomas (maximum depth 1.1 mm) pulsed color laser is also possible
*3maximum depth 3–4 mm, max diameter 1 cm
Contact cryotherapy is a simple and repeatable method for the early treatment of proliferating hemangiomas of up to 1 cm diameter and 3 to 4 mm thickness. Using equipment where the metal applicator is electrically cooled down to merely –32°C makes occurrence of undesirable side effects such as hypopigmentation, blistering, and ulceration with subsequent scarring far less likely than using metal probes cooled with liquid nitrogen.
The surprise discovery of the strong effect of the non-selective betablocker propranolol on proliferating hemangiomas (17) has resulted in this treatment becoming increasingly standard in the absolute indications listed above, replacing treatment with high-dose corticosteroids, which had routinely been used in the past. However, no randomized controlled studies have been done for this treatment, which is therefore not yet licensed. For this reason alone, the treatment should be initiated on an inpatient basis. Because of the obviously favorable risk-benefit profile of propranolol, the guideline of the Association of Scientific Medical Societies in Germany (AWMF) (18) has recently been revised. Close attention should be paid to possible adverse effects such as hypoglycemia and, more rarely, hypotension or bradyarrhythmias.
Juvenile xanthogranuloma
Juvenile xanthogranuloma (JXG) is the most common form of the non-Langerhans cell histiocytoses. JXGs usually present as benign, mostly solitary, firm, initially reddish, then yellow-orange colored papules and nodules of 0.5 to 2 cm diameter, which are mostly located on the head, neck, and upper body. They are mostly observed in neonates (5% to 17%), infants, and toddlers, only exceptionally in older children or adults. In very rare cases they can manifest extracutaneously. In periocular or multiple JXGs there is a risk of involvement of the frontal sections of the eye, and an ophthalmological examination is indicated in such cases. The list of further possible affected organs is long: central nervous system, muscles, bones, lungs, liver, spleen, kidneys, adrenal glands, pericardium, testes, and larynx can all be affected (19). The association with Langerhans cell histiocytosis (20) or neurofibromatosis type I and juvenile myelomonocytic leukemia (21) is common above average. Most JXG regress spontaneously in the first 4 to 6 years of life and thus do not require treatment.
Mastocytoma
Mastocytomas occur either as solitary lesions or in the context of mastocytoses, a heterogeneous group of disorders. They are characterized by an increase in mast cells in the skin and in some forms—mostly affecting adults—also in the bone marrow, lymphatic organs, and other organ systems. In childhood the following forms have been observed (22):
Solitary mastocytomas (20% of all forms of mastocytosis in children);
Maculopapular mastocytosis with more than 5 mastocytomas (urticaria pigmentosa, 70% of cases); and
Very rarely, the diffuse cutaneous mastocytosis (5%).
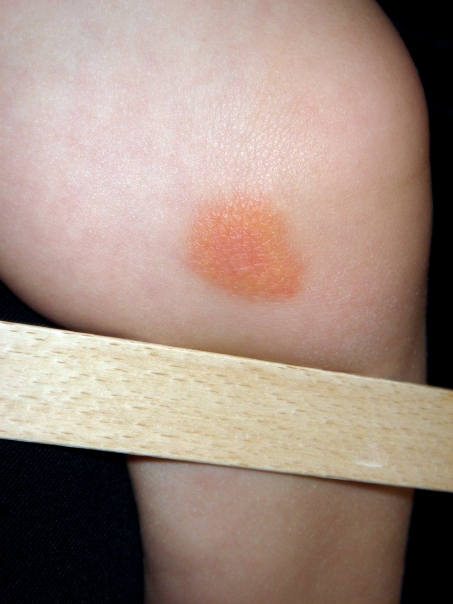
The different forms are caused by activating mutations of the proto-oncogene c-KIT on chromosome 4q12, which encodes for a mast cell growth factor receptor (stem cell factor, SCF) (23). Solitary mastocytomas are present at birth in about 25% of cases, others manifest within the first 2 years of life. They are recognizable as pink or brownish-orange papules, nodules, or plaques, and their diameter ranges from a few mm to several cm (Figure 4). Their surface is mostly smooth, occasionally it may resemble orange skin.
Figure 4.
Solitary mastocytoma
The diagnosis is made by using the Darier friction test: after rubbing, notable swelling and reddening will develop in and around the mastocytoma within 3 to 5 minutes; occasionally, the skin will blister. This may happen spontaneously and lead to flushing symptoms and a drop in blood pressure, especially in multiple mastocytomas. A skin biopsy is usually unnecessary unless the diagnosis is unclear. In order to assess the proliferation of mast cells—which may even be systemic in rare cases—measuring the serum concentration of tryptase (a mast cell product) is useful. If clinical symptoms are scarce, no extracutaneous symptoms are present, and serum tryptase levels are normal, further diagnostic evaluation in children is not necessary, whereas in adults it is recommended.
In more than 70%, mastocytomas regress spontaneously by the time a child reaches puberty. Excision of solitary tumors may be considered in case of recurring episodes or blistering and superinfection.
Avoiding mast cell degranulating factors is important:
Non-immunological stimuli: friction, pressure, sunlight exposure, sudden exposure to hot or warm water
Immunological stimuli—for example, insect bites, vaccinations, raised temperature
Histamine releasing substances: for children it is mainly codeine (in cough mixtures), non-steroidal anti-inflammatory drugs such as ibuprofen, and, more rarely anesthetic drugs and iodine containing contrast media that are relevant.
Pilomatricoma
Although pilomatricoma is among the most common skin tumors excised in children (10%), the diagnosis is often made only on histology (14). Pilomatricomas are slow growing, mostly asymptomatic, benign skin tumors that arise from the matrix cells of the hair follicle and are localized mostly on the head, neck, or arms (24). The clinical presentation is a firm to rock-hard, mostly subcutaneous nodule that adheres to the skin but can be easily moved on its base. Extent, lobular surface, and an often blueish hue are more easily detected by stretching the skin over the tumor (“tent sign”). The clinical diagnosis can be sonographically supported by detecting echogenic internal echoes, which correspond to calcification areas (hence the antiquated name, epithelioma calcificans Malherbe). Since no spontaneous remission occurs, the therapy of choice is complete excision. The recurrence rate is low at 0% to 6%, because of the easy delineation of the tumor margins. In 2% to 3.5% of cases, there are multiple tumors, and associated disorders may be present, in particular Curschmann-Steinert myotonic dystrophy, Gardner syndrome, Rubinstein-Taybi syndrome, and chromosomal aberrations (25).
Key Messages.
Malignant skin tumors in childhood are very rare. Most pediatric skin tumors do not require further diagnostic evaluation or treatment, only clinical observation.
Large and giant congenital melanocytic nevi have a higher risk of malignant degeneration or complications, which may take effect in the first few years of life. Sebaceous nevi will give rise to certain, mostly benign, skin tumors in 10% to 30%, mostly after the 20th year of life.
Spitz nevus is the most important melanoma simulator in children.
By contrast to most infantile hemangiomas, those that threaten complications require rapid, if necessary systemic, treatment.
Mastocytomas and juvenile xanthogranulomas regress spontaneously in most cases. Usually, neither biopsy nor excision is required.
Syndromal associations are possible in multiple juvenile xanthogranulomas and pilomatricomas.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
We thank Professor Dr Eva-B. Bröcker, director of the Dermatology Department of the University Hospital of Würzburg, for her critique of the manuscript.
Footnotes
Conflict of interest statement
Professor Höger is participating in a multicenter study of the efficacy of propranolol in hemangiomas, which receives funding from Pierre Fabre, Toulouse/France. Professor Hamm declares that no conflict of interest exists.
References
- 1.Diepgen TL. Szeimies RM, Hauschild A, Garbe C, Kaufmann R, Landthaler M, editors. Epidemiologie von Hauttumoren: Epitheliale Hauttumoren. Tumoren der Haut. Stuttgart. Thieme. 2010:87–92. [Google Scholar]
- 2.Knight PJ, Reiner CB. Superficial lumps in children: what, when, and why? Pediatrics. 1983;72:147–153. [PubMed] [Google Scholar]
- 3.Bauer J, Garbe C. Melanozytäre Nävi als Präkursoren und Risikomarker für das maligne Melanom. In: Szeimies RM, Hauschild A, Garbe C, Kaufmann R, Landthaler M, editors. Thieme. Stuttgart: Tumoren der Haut; 2010. p. 293.p. 299. [Google Scholar]
- 4.Mills O, Messina JL. Pediatric melanoma: a review. Cancer Control. 2009;16:225–233. doi: 10.1177/107327480901600304. [DOI] [PubMed] [Google Scholar]
- 5.Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735–4741. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 6.Griffin JR, Cohen PR, Tschen JA, et al. Basal cell carcinoma in childhood: case report and literature review. J Am Acad Dermatol. 2007;57(Suppl):97–102. doi: 10.1016/j.jaad.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Holman JD, Dyer JA. Genodermatoses with malignant potential. Curr Opin Pediatr. 2007;19:446–454. doi: 10.1097/MOP.0b013e3282495939. [DOI] [PubMed] [Google Scholar]
- 8.Kovalyshyn I, Braun R, Marghoob A. Congenital melanocytic naevi. Australas J Dermatol. 2009;50:231–240. doi: 10.1111/j.1440-0960.2009.00553_1.x. [DOI] [PubMed] [Google Scholar]
- 9.Pavlidou E, Hagel C, Papavasilliou A, Giouroukos S, Panteliadis C. Neurocutaneous melanosis: report of three cases and up-to-date review. J Child Neurol. 2008;23:1382–1391. doi: 10.1177/0883073808319069. [DOI] [PubMed] [Google Scholar]
- 10.Kinsler VA, Chong WK, Aylett SE, Atherton DJ. Complications of congenital melanocytic naevi in children: analysis of 16 years’ experience and clinical practice. Br J Dermatol. 2008;159:907–914. doi: 10.1111/j.1365-2133.2008.08775.x. [DOI] [PubMed] [Google Scholar]
- 11.Gerami P, Jewell SS, Morrison LE, et al. Fluorescence in situ hybridization (FISH) as an ancillary diagnostic tool in the diagnosis of melanoma. Am J Surg Pathol. 2009;33:1146–1156. doi: 10.1097/PAS.0b013e3181a1ef36. [DOI] [PubMed] [Google Scholar]
- 12.Busam KJ, Pulitzer M. Sentinel lymph node biopsy for patients with diagnostically controversial Spitzoid melanocytic tumors? Adv Anat Pathol. 2008;15:253–262. doi: 10.1097/PAP.0b013e31818323ac. [DOI] [PubMed] [Google Scholar]
- 13.Altaykan A, Ersoy-Evans S, Erkin G, Ozkaya O. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616–619. doi: 10.1111/j.1525-1470.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 14.Price HN, Zaenglein AL. Diagnosis and management of benign lumps and bumps in childhood. Curr Opin Pediatr. 2007;19:420–424. doi: 10.1097/MOP.0b013e328224b8ee. [DOI] [PubMed] [Google Scholar]
- 15.Boye E, Olsen BR. Signaling mechanisms in infantile hemangioma. Curr Opin Hematol. 2009;16:202–208. doi: 10.1097/MOH.0b013e32832a07ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeger PH, Maerker JM, Kienast AK, Syed SB, Harper JI. Neonatal haemangiomatosis associated with placental chorioangiomas: report of three cases and review of the literature. Clin Exp Dermatol. 2009;34:e78–e80. doi: 10.1111/j.1365-2230.2009.03221.x. [DOI] [PubMed] [Google Scholar]
- 17.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 18.Deutsche Gesellschaft für Kinderchirurgie, Deutsche Gesellschaft für Kinder- und Jugendmedizin, Deutsche Dermatologische Gesellschaft und Arbeitsgemeinschaft Pädiatrische Dermatologie. Leitlinie Hämangiome im Säuglings- und Kleinkindesalter. www.uni-duesseldorf.de/AWMF/ll/006-100.htm.
- 19.Dehner LP. Juvenile xanthogranulomas in the first two decades of life: a clinicopathologic study of 174 cases with cutaneous and extracutaneous manifestations. Am J Surg Pathol. 2003;27:579–593. doi: 10.1097/00000478-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hoeger PH, Diaz C, Malone M, Malone M, Pritchard J, Harper JI. Juvenile xanthogranuloma as a sequel to Langerhans cell histiocytosis: a report of three cases. Clin Exp Dermatol. 2001;26:391–394. doi: 10.1046/j.1365-2230.2001.00842.x. [DOI] [PubMed] [Google Scholar]
- 21.Raygada M, Arthur DC, Wayne AS, Rennert OM, Toretsky JA, Stratakis CA. Juvenile xanthogranuloma in a child with previously unsuspected neurofibromatosis type 1 and juvenile myelomonocytic leukemia. Pediatr Blood Cancer. 2010;54:173–175. doi: 10.1002/pbc.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briley LD, Phillips CM. Cutaneous mastocytosis: a review focusing on the pediatric population. Clin Pediatr (Phila) 2008;47:757–761. doi: 10.1177/0009922808318344. [DOI] [PubMed] [Google Scholar]
- 23.Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804–815. doi: 10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
- 24.Pirouzmanesh A, Reinisch JF, Gonzalez-Gomez I, Smith EM, Meara JG. Pilomatrixoma: a review of 346 cases. Plast Reconstr Surg. 2003;112:1784–1789. doi: 10.1097/01.PRS.0000091160.54278.64. [DOI] [PubMed] [Google Scholar]
- 25.Wachter-Giner T, Bieber I, Warmuth-Metz M, Bröcker EB, Hamm H. Multiple pilomatricomas and gliomatosis cerebri - a new association? Pediatr Dermatol. 2009;26:75–78. doi: 10.1111/j.1525-1470.2008.00827.x. [DOI] [PubMed] [Google Scholar]