Abstract
Purpose
We examined estradiol and testosterone effects on thermoregulation in women with and without Polycystic Ovary syndrome (PCOS). We hypothesized that core temperature (Tc) threshold for sweating during exercise is delayed in women with PCOS and that testosterone delays the Tc set point for sweating during exercise.
Methods
For 16 d, we suppressed estrogens, progesterone, and testosterone with a gonadotropin-releasing hormone antagonist (GnRHant) in seven women with and seven women without PCOS (control); we added 17β-estradiol (0.2 mg·d-1, two patches) on days 4–16 (E2) and testosterone (2.5 mg·d-1, orally) on days 13–16 (E2 + T). Under each hormone condition, subjects cycled in a temperature of 35°C at 60% of age-predicted HRmax for 40 min.
Results
Tc sweating threshold was lower in women in the PCOS group compared with those in the control during GnRHant (37.21°C ± 0.51°C vs 37.70°C ± 0.12°C, P < 0.05); neither E2 nor E2 + T influenced the thermoregulatory responses in PCOS. E2 decreased Tc sweating threshold in control (37.06°C ± 0.69°C, P < 0.05), but E2 + T attenuated this response (37.53°C ± 0.19°C). Peak sweating rate was greater in women in the PCOS group compared with those in the control group during GnRHant (1.06 ± 0.47 vs 0.47 ± 0.11 mg·cm-2·min-1) and E2 + T (0.85 ± 0.41 vs 0.44 ± 0.10 mg·cm-2·min-1, P < 0.05). Compared with the control group, total sweat losses were greater in the PCOS group during GnRHant (0.614 ± 0.189 vs 0.419 ± 0.098 L) and during E2 + T (0.696 ± 0.281 vs 0.434 ± 0.164 L, P < 0.05) but not during E2 (0.639 ± 0.231 and 0.505 ± 0.214 L for PCOS and control groups, respectively, P = 0.09).
Conclusions
Thermoregulation was adequate in women with PCOS; however, the women with PCOS achieved thermoregulation at the expense of producing higher sweat volumes.
Keywords: GnRH ANTAGONIST, TEMPERATURE REGULATION, POLYCYSTIC OVARY SYNDROME, TESTOSTERONE, ESTROGEN
Polycystic ovary syndrome (PCOS) is the most common reproductive endocrinopathy in young women (5), affecting 6%–10% of reproductive-age women (36). Although the fundamental defect of PCOS is an ongoing area of research, there is a consensus that common features include insulin resistance and obesity, abnormal gonadotropin dynamics and androgen excess, inadequate ovulation, static estrogen exposure similar to the late follicular phase in ovulatory women, and infrequent progesterone exposure. Exercise can be an important intervention for people with insulin resistance and obesity because exercise has direct effects on the muscle to improve glucose uptake and decrease body fat. Thus, exercise is a common recommendation to women with PCOS to reduce obesity, improve insulin resistance (4,22,34), and improve cardiopulmonary function. However, participation in exercise programs in women with PCOS is low. We propose that an important contributing factor to this low participation is a failure to prescribe exercise programs specific to women with PCOS that are safe, effective, and comfortable.
Aerobic exercise increases core temperature (Tc) due to heat produced by the working muscle. The Tc threshold for the onset of sweating and peripheral vasodilation is defined as the Tc above which the effector response is greater than that of baseline. A shift in the Tc threshold has been called a change in the ‘‘set point’’ for temperature regulation. A delay in sweating threshold during increases in Tc is a classic indicator of compromised thermoregulation because a greater increase in Tc is required to induce sweating at the skin, the evaporation of which is the primary cooling mechanism in humans. Conversely, an earlier Tc onset for sweating indicates improved thermoregulation, and it is seen in heat-acclimatized people. Although increases in sweating provide a thermoregulatory advantage, profuse sweating requires careful consideration of hydration status. Several variables affect thermoregulation during exercise, including duration and intensity of exercise, environmental conditions, acclimatization to exercise heat stress, work capacity, physical conditioning, hydration status, and personal factors such as medications, supplements, sleep, and illness. Although athletes have received a great deal of attention regarding thermal illness, individuals who participate in sports more casually are also at risk for heat illnesses, particularly individuals who are not acclimated to the heat, whose fitness levels are low, or those with body mass index (BMI) > 27 kg·m-2 (2,14).
Estrogens and progesterone have long been recognized to affect thermoregulation. These reproductive hormones affect temperature-sensitive neurons in the brain (21,30); thus, any estradiol or progesterone effects on the set point change in Tc may occur at the level of the CNS in much the same way as that of a fever (21,30). In general, estrogens improve the sweating response as indicated by a lower Tc threshold for the onset of sweating and peripheral (skin) vasodilation, whereas progesterone has the opposite effect of delaying sweating until higher Tc (31). The effect of testosterone or other androgens on thermoregulation in women is unclear. Under certain conditions, heat- or exercise-induced sweating is greater in women compared with men (3,27,28), although it is unclear whether the sex differences are related to hormones, body size, or both. Despite the influence of testosterone on temperature regulatory neurons in the brain, the effect of testosterone on thermoregulation has not been examined in women with PCOS, even in the face of their chronically elevated testosterone and estradiol exposure.
Temporary suppression of sex hormones with a gonadotropin-releasing hormone (GnRH) antagonist can be useful to examine the effects of estradiol, progesterone, or testosterone in both eumenorrheic women and women with PCOS. In eumenorrheic women, administration of a GnRH antagonist suppresses blood estrogens and progesterone concentrations to postmenopausal levels after 36–48 h of administration. We can use this same method to suppress hypothalamically stimulated estradiol and testosterone in women with PCOS. When endogenous production of these hormones is temporarily suppressed, we can study the individual and/or combined effects of estradiol and testosterone in young women by selectively adding these hormones back.
The purpose of this study was to examine the effects of estradiol and the combined effects of estradiol with testosterone on thermoregulation in women with PCOS by suppressing the hypothalamic–pituitary–ovarian axis, followed by sequential administration of estradiol and testosterone. We were interested in the combined effects of these hormones because tonically elevated estrogens and androgens usually accompany PCOS. Testosterone, the primary androgen elevated in PCOS, can counteract or attenuate the effects of estrogen (37), so when testosterone and estradiol are increased concurrently, testosterone may attenuate the effects of estradiol on thermoregulation. Because of their chronically high testosterone exposure, we hypothesized that the Tc threshold for sweating during exercise would be delayed in obese women with PCOS compared with obese healthy controls under low hormone conditions and during estrogen administration. We further hypothesized that testosterone administration combined with estradiol administration would delay the Tc set point for sweating during heat stress within women both with and without PCOS.
METHODS
Study Design
We recruited seven nonsmoking, obese women with PCOS and seven obese women without PCOS. All women were interviewed to obtain their medical history. Women without PCOS reported regular menstrual cycles and no gynecological abnormalities and provided written confirmation of a normal physical examination within 1 yr of being recruited to the study. Subjects in both groups were not taking any medications. PCOS subjects were recruited into the study on the basis of the presence of hyper-androgenism (either clinical, on the basis of a history of documented hirsutism, or biochemical, due to elevated serum testosterone concentrations) in addition to one of two cardinal characteristics of PCOS on the basis of the Rotterdam Criteria (35). To be defined as having PCOS, in addition to androgen excess, at least one of the following two criteria were present: be oligo/anovulatory, defined as intermenstrual period of ≥45 d, or a total of eight menses or fewer per year; or have polycystic ovaries. Polycystic ovaries were defined by the morphological appearance of 12 small follicles in the range of 3–9 mm mean diameter in the ovary on day 3 as determined by transvaginal ultra-sound. We excluded other disorders of the ovaries, adrenal and pituitary. All subjects gave written informed consent to participate in the study, which had previous approval from the Human Investigation Committee of the Yale University School of Medicine.
Experimental design
Each subject participated in three heat stress sessions: one during GnRH antagonist alone, one while taking GnRH antagonist with estradiol (E2), and one while taking GnRH antagonist with estradiol and testosterone (E2 + T). To suppress reproductive function for the duration of the study, the subjects received the GnRH antagonist, ganirelix acetate (GnRH antagonist, 250 μg·d-1; Antagon, Organon, Inc., West Orange, NJ) each day for 16 d. Beginning on the fifth day of GnRH antagonist administration, the women received estradiol for 11 d (17β-estradiol, two transdermal patches, 0.1 mg·d-1 each; Mylan Pharmaceuticals, Morgantown, WV). Beginning on the 12th day of GnRH antagonist administration, the women received methyl testosterone at an oral dosage of 2.5 mg·d-1 (Compounded Solutions, Monroe, CT) for the final 4 d of GnRH antagonist treatment. We chose methyl testosterone in this study because it is less rapidly metabolized due to its 17-methyl group and because it is not aromatized to estradiol.
Experimental protocols were performed after 48 h of GnRH antagonist administration, on the ninth day (GnRH antagonist with estradiol, E2), and on the 16th day of administration (GnRH antagonist with estradiol and testosterone, E2 + T). This design permitted within-subject comparisons concerning the effects of estradiol and testosterone on changes in temperature regulation and on hormonal variables. This hormone-suppression add-back design eliminated other potential confounders such as GnRH and the gonadotropins as well as other ovarian products, thus providing a direct assessment of the variables of interest.
In women without PCOS, the GnRH antagonist administration began on days 25–28 of their menstrual cycle. Women with PCOS who participated in this study were not menstruating so they were scheduled to begin the GnRH antagonist administration at their convenience. The subjects self-administered daily subcutaneous injections of the GnRH antagonist after training by qualified study personnel. This method of GnRH antagonist administration is easily discontinued in the event of uncomfortable adverse effects, such as headaches or vasomotor symptoms (i.e., “hot flashes”). The hypothalamic–pituitary–ovarian axis suppression is reversed on cessation of drug therapy.
GnRH antagonist (ganirelix acetate)
Ganirelix acetate is a synthetic decapeptide with high antagonistic activity against naturally occurring GnRH. Ganirelix acetate is derived from native GnRH with substitutions at positions 1, 2, 3, 6, 8, and 10. When ganirelix acetate is given in therapeutic doses, it acts by competitively blocking the GnRH receptors on the pituitary gonadotroph and subsequent transductions pathway. It induces a rapid, reversible suppression of gonadotropin secretion (23,24). In young, cycling women, continued administration of ganirelix acetate leads to suppression of estrogens and progesterone to postmenopausal levels. These decreases occur after 36–48 h of administration, and the suppression of the hypothalamic–pituitary–ovarian axis is reversed on cessation of drug therapy (23,24).
Heat Stress Tests
Exercise protocol
Volunteers arrived at the laboratory between 7:00 and 8:00 a.m. after having eaten only a prescribed low-fat breakfast (~300 kcal). The subjects refrained from alcohol and caffeine for 12 h before the experiment. Subjects prehydrated by drinking 7 mL·kg-1 body weight of tap water at home before arrival at the laboratory and 10 mL·kg-1 body weight the night before the study. On arriving at the laboratory, each subject gave a baseline urine sample and was weighed to the nearest 10 g on a beam balance. The subject then sat on the contour chair of a semirecumbent cycle ergometer in the test chamber (27°C and 30% relative humidity) and was instrumented for the measurement of esophageal and skin temperatures, sweat rate, and blood pressure. An indwelling catheter (21-gauge) was inserted into an arm vein for blood sampling, and a heparin block (20 U·mL-1) maintained catheter patency. Subjects were semirecumbent during placement of the catheter and were seated for at least 45 min before sampling to ensure a steady state in plasma volume and constituents. We recorded resting blood pressure (Colin Medical Instruments Corp., Komaki, Japan) and HR (ECG) at the end of the 45-min control period. At the end of the control period, a blood sample was drawn. Hydration state was assessed from the specific gravity of the baseline urine sample (mean = 1.002 ± 0.001). After the control measurements, the chamber temperature was increased to 35°C, and the subject sat quietly for 20 min before exercise to establish a new steady state.
Immediately after this heated resting period, the subjects exercised at 55%–60% of their age-predicted HRmax for 40 min. None of our subjects was used to exercising, so on the day of the first experiment, many of them began the protocol at a higher level of intensity but were not able to complete the entire session at that intensity. We therefore lowered the intensity as time went on to ensure they could complete the full 40 min. Thus, many subjects began the protocol at 60% of HRmax but were exercising at 50% HRmax by the end of the 40-min exercise period. All sessions were identical about work rate within each subject across the three hormone conditions. The subjects exercised with a fan positioned directly in front of the bike with a fan speed of 1.6 m·s-1 to promote continuous evaporative sweating (1). Blood pressure was measured every 10 min, and sweating rate and esophageal and skin temperatures were monitored continuously. Blood samples were drawn at 10, 20, and 40 min of exercise.
Measurements
Body Tc was measured from an esophageal thermocouple at the level of the left atrium. Skin temperatures were measured on the forehead, chest, upper arm, lateral abdomen, lower back, forearm, thigh, and calf. Core and skin temperatures were collected at a rate of five data points per second. Data were stored in a computer through an analog-to-digital converter system (ACRO 931; Daisylab, National Instruments, Austin, TX) as a mean of every 3 s and were downloaded into an online data collection system (Labview; National Instruments). Mean skin temperature was calculated from the following equation that takes into consideration surface area (13) and the thermo-sensitivity of each skin area (20):
| [1] |
where subscripts refer to mean skin (sk), chest (ch), forehead (fh), abdomen (ab), upper arm (ua), forearm (fa), lower back (lb), thigh (th), and calf (ca). An automatic dew point sensor enclosed in a ventilated, Plexiglas capsule was placed on the forearm and was secured with surgical glue to determine sweating rate (12). The subjects’ clothing was weighed before and after exercise. We determined total sweat loss using the change in body weight after correcting for the change in weight of the subjects’ clothing (there was no fluid intake or urine output between the beginning and the end of exercise). The preexercise and final blood samples were analyzed for 17β-estradiol (S[E2]), progesterone (S[P4]) testosterone (S[T]), aldosterone (S[ALD]), and atrial natriuretic peptide (P[ANP]) concentrations; endothelin-1 (1–23, P[ET-1]) concentration was determined before exercise.
Blood and Urine Analysis
An aliquot was transferred to a into a tube without the anticoagulant for the determination of S[ALD], S[E2], S[P4], and S[T]. All other aliquots were placed in chilled tubes containing EDTA and were analyzed for P[ANP] and P[ET-1]. The samples were centrifuged, frozen immediately, and stored at –80°C until analysis. Serum concentrations of 17β-estradiol, progesterone, bound and free testosterone, and plasma ET-1 were analyzed within one assay kit for each hormone. Intra-assay coefficient of variation for the midrange standard for S[E2] (182.7 ± 4.1 pg·mL-1 was 2.3% (Diagnostic Products, Los Angeles, CA), that for S[P4] (1.9 ± 0.8 ng·mL-1) was 8.9% (Diagnostic Products), that for bound S[T] (26.2 ± 1.8 ng·dL-1) was 2.0%, and that for free S[T] (9.3 ± 0.2 pg·dL-1) was 2.3% (Diagnostic Products). Intra- and interassay coefficients of variation for the midrange standard for S[ALD] (136 pg·mL-1) were 1.5% and 1.8% and those for P[ANP] (29.8 pg·mL-1) were 6.2% and 7.5% (Siemens Healthcare Diagnostics, Los Angeles, CA).
Statistics
For Tc, each subject's 3-s averages were collapsed into 30-s averages to determine individual Tc thresholds for the onset of sweating. Each subject's sweating rate was plotted as a function of Tc during exercise, and the Tc threshold for sweating (i.e., the Tc above which the effector response is greater than that of baseline) was determined visually by an experienced investigator who was blinded to the hormone condition and group to which the subject belonged. The investigator identified the threshold, using the “first clear inflection point” when graphing mean sweating rate versus esophageal temperature (Tes). Although this method is somewhat subjective, these inflection points are clear and are consistent with other objective methods (9). For other analyses, before statistical treatment, the independent variable was partitioned into 5-min bins. We used repeated-measures ANOVA models to test differences in Tc, sweating rate, and the Tc sweating threshold and slopes due to group (PCOS vs control) and hormone treatment (GnRH, E2, and E2 + T)over time (10). On the basis of on an α level of 0.05 and a sample size of seven, our β level (power) was ≥0.80 for detecting effect sizes at 0.28°C. Our low sample size did not permit analysis of interactions, but we looked for them in the data and interpreted our data accordingly. Data were analyzed using SPSS statistical software (SPSS version 16.0 for Mac; SPSS, Chicago, IL) and were expressed as mean ± SD in the text and tables but were shown as mean T SEM in graphs for clarity.
RESULTS
None of the women in either the control or the PCOS group reported adverse effects or symptoms due to the GnRH antagonist or hormone administration. Compared with GnRH antagonist administration alone, 4 d of transdermal estradiol administration increased S[E2] by approximately six- to eightfold in both groups (Table 1; P < 0.05). Again, compared with GnRH antagonist administration alone, during combined estradiol (12 d) with testosterone for 4 d, S [E2] remained increased by approximately eightfold, and both free and bound S[T] increased by approximately sixfold (Table 1; P < 0.05). Finally, P[ET-1] increased in both groups during E2 + T, but this increase was exaggerated in the women with PCOS (Table 1; P < 0.05).
TABLE 1.
Subjects’ characteristics and sex hormones during GnRH antagonist (ganirelix acetate) and estradiol (E2) and E2 + testosterone (E2 + T) administration.
| Control Women (n = 7) |
PCOS Women (n = 7) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | GnRH ant | E2 | E2 + T | Baseline | GnRH ant | E2 | E2 + T | |
| Age (yr) | 25.0 ± 4.6 | 24.3 ± 5.6 | ||||||
| Height (cm) | 160.7 ± 5.6 | 169.7 ± 7.1 | ||||||
| BMI (kg·m–2) | 33.8 ± 1.9 | 29.8 ± 5.0 | ||||||
| BSA (m2) | 1.97 ± 0.03 | 1.99 ± 0.18 | ||||||
| Weight (kg) | 87.3 ± 3.9 | 87. ± 14.8 | 87.6 ± 4.8 | 87.7 ± 4.6 | 84.3 ± 14.6 | 85.4 ± 13.5 | 85.9 ± 13.6 | 84.7 ± 13.2 |
| S[E2] (pg·mL–1) | 34.1 ± 18.7 | 26.3 ± 22.5 | 173.5 ± 118.3* | 163.0 ± 66.2* | 48.7 ± 31.9 | 29.9 ± 35.3 | 232.0 ± 188.5* | 178.5 ± 170.5* |
| S[P4] (ng·mL–1) | 1.1 ± 0.8 | 0.6 ± 0.1** | 0.6 ± 0.1** | 0.6 ± 0.2** | 1.0 ± 0.4 | 0.9 ± 0.2 | 1.1 ± 0.7 | 0.8 ± 0.2 |
| Total S[T] (ng·dL–1) | 0.3 ± 0.2 | 0.2 ± 0.4 | 0.2 ± 0.4 | 1.4 ± 0.6* | 0.4 ± 0.1 | 0.3 ± 0.9 | 0.3 ± 0.4 | 1.2 ± 0.7* |
| Free S[T] (ng·dL–1) | 0.03 ± 0.09 | 0.02 ± 0.03 | 0.6 ± 0.02 | 0.36 ± 0.18* | 0.12 ± 0.04 | 0.05 ± 0.04 | 0.06 ± 0.03 | 0.26 ± 0.16* |
| P[ET-1] (fmol·mL–1) | 2.01 ± 0.71 | 1.45 ± 2.6 | 3.68 ± 0.90* | 3.25 ± 1.78 | 3.58 ± 2.07 | 7.10 ± 2.48*,** | ||
BSA (m2) = ([height (cm) × weight (kg)]/3600)1/2.
Different from GnRH antagonist within the group, P < 0.05.
Different from baseline within the group, P < 0.05.
Different from control, P < 0.05.
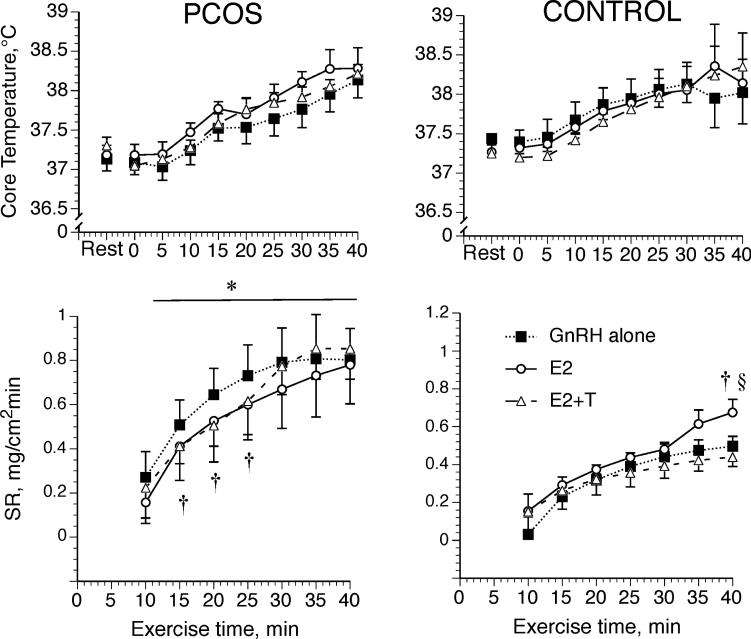
Our protocol led to similar average work outputs in the two groups (68 ± 9 and 66 ± 3 W for PCOS and controls, respectively (range = 55–80 W)) and across the three hormonal conditions (the latter was controlled). Neither group (PCOS and control) nor hormone administration affected Tc at rest or during exercise (Fig. 1). Sweating rate was elevated in the women with PCOS compared with the control women during all hormone treatments (Fig. 1; P < 0.05). Within women with PCOS, sweat rate was lower during E2 and E2 + T compared with GnRH antagonist during much of exercise, but peak sweating rates were unaffected by hormone treatments (Fig. 1; P < 0.05). Finally, within control subjects, sweating rate increased during E2 relative to GnRH antagonist and E2 + T but only at the end of exercise (Fig. 1; P < 0.05). Compared with the control group, total sweat losses were greater in the PCOS group during GnRH antagonist (0.614 ± 0.189 vs 0.419±0.098 L, P < 0.05) and during E2 + T (0.696±0.281 vs 0.434 ± 0.164 L, P < 0.05), but group differences only showed a trend during E2 (0.639 ± 0.231 and 0.505 ± 0.214 L for PCOS and control groups, respectively, P = 0.09) and may have failed to attain statistical significance because of our small sample size. Because the women with PCOS were slightly smaller than the control women, we also compared their total sweat losses as a function of body surface area (BSA): compared with the control group, women with PCOS lost more sweat per square meter of BSA only during E2 + T (365.1 ± 160.1 vs 219.8 ± 80.6 mL·m-2, P < 0.05), but differences failed to attain significance under the other two hormone conditions (GnRH = 314.0 ± 100.2 vs 213.5 ± 50.6 mL·m-2, P = 0.19, and E2 = 302.8 ± 132.6 and 255.8 ± 105.4 mL·m-2 for PCOS and control groups, respectively, P = 0.45).
FIGURE 1.
Core temperature at rest (27°C) and arm sweat rate (SR) during 40 min of semirecumbent cycle exercise in the heat (35°C). Women with (PCOS) and without (Control) PCOS were studied while taking GnRH antagonist alone, while taking GnRH antagonist with estradiol (E2), and while taking GnRH antagonist with estradiol + testosterone (E2 + T). Data are expressed as mean ± SEM. *Different from control, †different from GnRH within the group, §different from E2 + ± within the group, P < 0.05.
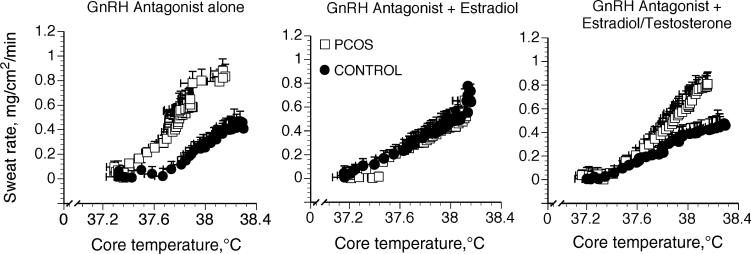
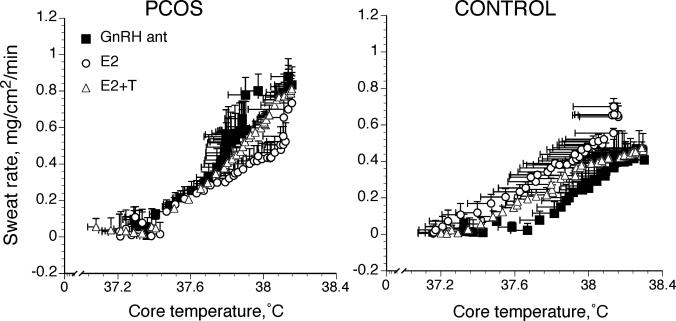
The Tc threshold for sweating onset was lower in PCOS women compared with control women during GnRH antagonist (Fig. 2 and Table 2; P < 0.05). In control women, E2 administration was associated with a lower Tc sweating threshold compared with GnRH antagonist (Fig. 3 and Table 2; P G 0.05), but the change in threshold was absent when T was administered along with E2. In contrast, sex hormone administration had no effect on the Tc threshold for sweating in women with PCOS (Fig. 3 and Table 2).
FIGURE 2.
Sweating rate as a function of core temperature under three different hormone conditions in women with (PCOS) and without (control) PCOS. Hormonal conditions were while taking GnRH antagonist alone, while taking GnRH antagonist with estradiol, and while taking GnRH antagonist with estradiol + testosterone. Data are expressed as mean ± SEM.
TABLE 2.
Cardiovascular and temperature responses to exercise under different hormonal conditions.
| GnRH Antagonist | E2 | E2 + T | |
|---|---|---|---|
| Tc threshold (°C) | |||
| PCOS | 37.28 ± 0.27* | 37.34 ± 0.39 | 37.31 ± 0.71 |
| Control | 37.70 ± 0.12 | 37.22 ± 0.19** | 37.53 ± 0.19 |
| Slope, ΔSR/ΔTes | |||
| PCOS | 1.05 ± 0.54 | 0.72 ± 0.31 | 1.69 ± 1.34 |
| Control | 0.88 ± 0.17 | 0.60 ± 0.22 | 0.97 ± 0.43 |
| r2 | |||
| PCOS | 0.84 ± 0.11 | 0.81 ± 0.15 | 0.88 ± 0.10 |
| Control | 0.81 ± 0.07 | 0.84 ± 0.11 | 0.71 ± 0.09 |
| Peak SR (mg·cm–2min–1) | |||
| PCOS | 1.09 ± 0.55* | 0.96 ± 0.42 | 0.85 ± 0.41* |
| Control | 0.47 ± 0.11 | 0.78 ± 0.15**,*** | 0.44 ± 0.10 |
| Peak HR (bpm) | |||
| PCOS | 148 ± 16 | 153 ± 13 | 155 ± 11 |
| Control | 150 ± 10 | 144 ± 12 | 145 ± 10 |
| Peak MAP | |||
| PCOS | 97 ± 8 | 101 ± 5 | 98 ± 3 |
| Control | 97 ± 18 | 96 ± 13 | 95 ± 16 |
| Tsk at 27°C before exercise (°C) | |||
| PCOS | 34.6 ± 0.3 | 33.7 ± 1.0 | 33.7 ± 0.9 |
| Control | 33.9 ± 1.3 | 33.7 ± 1.6 | 33.7 ± 1.0 |
| Tsk at 35°C before exercise (°C) | |||
| PCOS | 34.9 ± 0.8 | 34.9 ± 0.3 | 34.8 ± 0.4 |
| Control | 34.3 ± 1.3 | 34.9 ± 0.2 | 35.0 ± 0.5 |
Data are mean ± SD.
Core temperature (Tc) threshold for sweating onset and slopes correspond to those shown in Figures 2 and 3. Peak sweating rate (SR), HR, and mean arterial pressure (MAP) were at 40 min of exercise under all conditions except during GnRH antagonist administration in the PCOS group, when peak HR occurred at 35 min of exercise.
Tes, esophageal temperature; Tsk, mean skin temperature; r2, correlation of Tc versus SR slope; GnRH antagonist, gonadotropin-releasing antagonist (ganirelix acetate) administration; E2, 17β-estradiol administration; T, testosterone.
Different from control, P < 0.05.
Different from GnRH antagonist within the group, P < 0.05.
Different from E2 + T within the group, P < 0.05.
FIGURE 3.
Sweating rate as a function of core temperature in women with (PCOS) and without (control) PCOS under three different hormone conditions. Women with and without PCOS were studied while taking GnRH antagonist alone (GnRH ant), while taking GnRH antagonist with estradiol (E2), and while taking GnRH antagonist with estradiol + testosterone (E2 + T). Data are expressed as mean ± SEM.
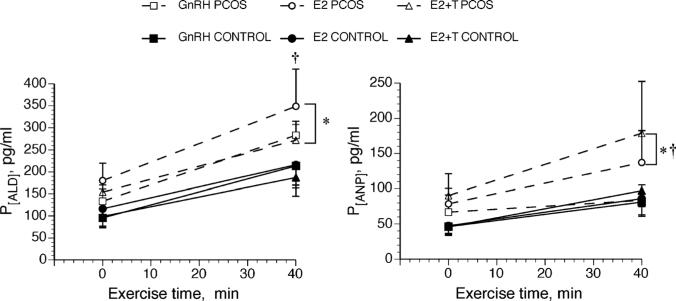
Baseline HR (74 ± 12 and 84 ± 4 bpm for PCOS and control groups, respectively, P = 0.15) and blood pressure were similar across treatments for both groups (mean arterial pressure = 85 ± 14 and 95 ± 9 mm Hg for PCOS and control groups, respectively, P = 0.24). Peak HR and blood pressure were similar at the end of exercise between the two groups (Table 2). Baseline P[ALD] and P[ANP] were similar between groups and across hormone conditions. However, both of these fluid-regulating hormones increased more dramatically in the women with PCOS in response to exercise and were therefore higher in this group at the end of exercise compared with controls (Fig. 4; P < 0.05).
FIGURE 4.
Changes in plasma concentration of serum aldosterone (S[ALD]) and plasma atrial natriuretic peptide (P[ANP]) during 40 min of semirecumbent cycle exercise in the heat (35°C). Women with and without PCOS were studied while taking GnRH antagonist alone, while taking GnRH antagonist with estradiol (E2), and while taking GnRH antagonist with estradiol + testosterone (E2–T). Data are expressed as mean ± SEM. *Different from control, †different from GnRH within PCOS group, P < 0.05.
DISCUSSION
Our most important finding is that obese women with PCOS regulated temperature adequately during an exercise/ temperature challenge and maintained similar core temperature to their counterparts without PCOS. However, the women with PCOS achieved thermal regulation at the expense of producing high volumes of sweat, even at mild exercise intensity. Indeed, the exercise intensity in these sessions was light and elicited a very low sweating rate in our obese subjects without PCOS. Thus, women with PCOS sweated earlier and more profusely relative to women without PCOS to maintain their core temperature. Both groups were obese (BMI ≥ 29.8 kg·m-2) in this study, suggesting that the thermoregulatory changes seen in our PCOS women were independent of obesity. Finally, although estradiol administration increased sweating in the control women, women with PCOS were insensitive to changes in estradiol exposure, with or without testosterone.
The women with PCOS were smaller than the control women, which may have indicated a greater fitness level. Thus, it is possible that the earlier sweating threshold reflected improved fitness in our group with PCOS. We consider this difference in fitness unlikely because both groups described similar levels of physical activity in their daily lives; none of the women exercised regularly in either group, and there is nothing to suggest greater fitness other than lower body weight. BSA was similar between the two groups, and adjusting their sweating rates for BSA did not affect the findings in a meaningful way.
In the control women, the earlier Tc threshold for sweating during estradiol administration extends earlier findings in young, healthy, lean women (31) to obese women. The temperature regulatory actions of estradiol and testosterone under these conditions likely originate in the preoptic/ anterior hypothalamus, the primary temperature regulation center. Estradiol inhibits cold-sensitive neurons and stimulates warm-sensitive neurons in the rat (30) and should, therefore, inhibit heat-retaining mechanisms and excite heat loss mechanisms, thus causing a decrease in the regulated body temperature. Similarly, testosterone excites warm-sensitive neurons in the rat hypothalamus, although estradiol and testosterone do not excite the same neurons (30). Extending these findings to human physiology, in young healthy women, estradiol alters thermoregulatory responses so that temperature-lowering effector mechanisms occur at a lower Tc during exercise or heat exposure (7,8,31,33). Our data are the first to study the effects of testosterone on temperature regulation in women and demonstrate that testosterone attenuates the Tc-lowering effect of estrogens during exercise in overweight to obese women. Finally, in support of a central, rather a peripheral, mechanism, mean skin temperature was similar across group and hormone condition. Nadel et al. (19) demonstrated that the threshold for the onset of sweating is affected by mean Tsk, such that this threshold will shift to a lower Tc when mean Tsk is elevated. These findings introduced the concept that, although sweating is primarily controlled by the central brain temperature, it is affected by mean skin temperature (29). In our present study, mean Tsk is similar between groups and across conditions, indicating that any group or hormone differences in these studies were due to central (hypothalamic) drive rather than changes in local skin temperature.
The effect of estrogens and progesterone on temperature regulation in women has been studied using a variety of conditions in which estrogens and progesterone levels are altered, most notably comparing different phases of the menstrual cycle (31,33) and during oral contraceptive administration (31). In general, these studies demonstrate an increase in the thermoregulatory set point when progesterone exposure is high but demonstrate a lower thermoregulatory set point when estrogens are elevated and progesterone exposure is low. Although estradiol and progesterone may have opposite effects on temperature regulation, the progesterone effects seem to predominate when the two are concomitantly increased (7,8,15,17,32). The greater progesterone exposure in the midluteal phase of the menstrual cycle is associated with an increase in resting Tc and a delay in the Tc threshold for thermoregulatory peripheral effector responses (15,17,31,32). Conversely, during the preovulatory phase of the menstrual cycle, the cycle phase characterized by rising estrogen levels, resting Tc and the thresholds for cutaneous vaso-dilation and sweating are shifted to a lower Tc during exercise (33). We did not see an effect of our hormone administration on the women with PCOS, but we speculate that, because these women are chronically exposed to both estrogen and testosterone, our hormone administration may not have been a large-enough stimulus. Progesterone is chronically suppressed in women with PCOS, which may contribute to their generally improved thermoregulation apparent in the present investigation.
Our findings of enhanced exercise S[ALD] in women with PCOS are similar to an earlier investigation demonstrating greater resting S[ALD] in lean women with PCOS (6). These earlier findings also suggested an association between S[ALD] and cardiovascular risk in women with PCOS (6). Our data extend these findings to obese women with PCOS and suggest a greater renin–angiotensin–aldosterone system response to exercise in women with PCOS compared with women without PCOS. The renin–angiotensin–aldosterone system is an important component of the pathogenesis of hypertension and endothelial dysfunction in overweight and obese populations and may play an important role in the development of cardiovascular dysfunction in the metabolic syndrome (25). In the present study, the women are still young and with normal blood pressure, but the heightened S[ALD] response may be a harbinger of hypertension in PCOS women as they age.
Limitations
Using our current design, we were unable to isolate the effects of testosterone on thermoregulation in these experiments. We designed the study in this way for two reasons: first, the extent to which testosterone and estradiol metabolites remain in the tissue after the subjects have stopped taking the hormones is unpredictable and variable, so isolating these hormones would have required four instead of two groups (for cross-sectional studies) or long washout periods between studies within our two groups. Although these studies would have been interesting, it is difficult to recruit obese women (with or without PCOS) into exercise studies, and we suspect our compliance would have suffered dramatically with a long washout period. Second, women with PCOS typically see chronically high estradiol exposure concomitant with testosterone exposure, so administering them together would best mimic their natural state. For a similar reason (the risk of low compliance), we were unable to perform a fourth (baseline or pre–GnRH antagonist) study.
Perspectives
Exercise is routinely prescribed for women with PCOS to induce weight loss and improve insulin resistance (18), but participation in exercise programs in women with PCOS remains low. To entice women with PCOS to exercise and improve their compliance, the program must be as safe, comfortable, and enjoyable as possible. We therefore need to prescribe exercise programs specific to women with PCOS. Moderate to high levels of exercise of long duration are recommended for weight loss (11,16). However, the present investigation demonstrated excess sweating in women with PCOS during mild exercise lasting only 40 min, indeed mild enough that it initiated very little sweating in their control counterparts. Clear recommendations have been established for the general population regarding exercise in the heat (26); we now require such guidelines for women with PCOS. Perfuse sweating can increase the risk of dehydration, so special attention to hydration should be paid during longer exercise periods or during exercise in more extreme environmental conditions. Moreover, excessive sweating can be uncomfortable or embarrassing, which may cause women with PCOS to stop exercising long before they have reached the intensity and duration recommended for weight loss or achieved their fitness goals.
Footnotes
Drs. Stachenfeld and Taylor receive salary support from National Institutes of Health.
The authors declare that they have no conflict of interest.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
The authors thank the technical support of Andrew Grabarek, BS, and Cheryl Leone, MS, The John B. Pierce Laboratory, and the cooperation of the volunteer subjects.
REFERENCES
- 1.Adams WC, Mack GW, Langhans GW, Nadel ER. Effects of varied air velocity on sweating and evaporative rates during exercise. J Appl Physiol. 1992;73:2668–74. doi: 10.1152/jappl.1992.73.6.2668. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong L, Casa D, Millard-Stafford M, Moran D, Pyne S, Roberts W. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–72. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- 3.Avellini BA, Shapiro Y, Pandolf KB, Pimental NA, Goldman RF. Physiological responses of men and women to prolonged dry heat exposure. Aviat Space Environ Med. 1980;51:1081–5. [PubMed] [Google Scholar]
- 4.Balen AH, Dresner M, Scott EM, Drife JO. Should obese women with polycystic ovary syndrome receive treatment for infertility? BMJ. 2006;332:434–5. doi: 10.1136/bmj.332.7539.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barontini M, Garcia-Rudaz MC, Veldhuis JD. Mechanisms of hypothalamic–pituitary–gonadal disruption in polycystic ovarian syndrome. Arch Med Res. 2001;32:544–52. doi: 10.1016/s0188-4409(01)00325-3. [DOI] [PubMed] [Google Scholar]
- 6.Cascella T, Palomba S, Tauchmanova L, et al. Serum aldosterone concentration and cardiovascular risk in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2006;91:4395–400. doi: 10.1210/jc.2006-0399. [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N, Johnson JM. Modification of active cutaneous vasodilation by oral contraceptive hormones. J Appl Physiol. 1997;83:2012–8. doi: 10.1152/jappl.1997.83.6.2012. [DOI] [PubMed] [Google Scholar]
- 8.Charkoudian N, Johnson JM. Reflex control of cutaneous vasoconstrictor system is reset by exogenous female reproductive hormones. J Appl Physiol. 1999;87:381–5. doi: 10.1152/jappl.1999.87.1.381. [DOI] [PubMed] [Google Scholar]
- 9.Cheuvront SN, Bearden SE, Kenefick RW, et al. A simple and valid method to determine thermoregulatory sweating threshold and sensitivity. J Appl Physiol. 2009;107:69–75. doi: 10.1152/japplphysiol.00250.2009. [DOI] [PubMed] [Google Scholar]
- 10.Colton T. Statistics in Medicine. Little Brown & Co; Boston (MA): 1974. pp. 142–6. [Google Scholar]
- 11.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 12.Graichen H, Rascati R, Gonzalez RR. Automated dew-point temperature sensor. J Appl Physiol. 1982;52:1658–60. doi: 10.1152/jappl.1982.52.6.1658. [DOI] [PubMed] [Google Scholar]
- 13.Hardy JD. Heat transfer. In: Newburgh LH, editor. Physiology of Heat Regulation and Science of Clothing. Saunders; Philadelphia (PA): 1949. pp. 78–108. [Google Scholar]
- 14.Hart LE, Sutton JR. Environmental considerations for exercise. Cardiol Clin. 1987;5:245–58. [PubMed] [Google Scholar]
- 15.Hirata K, Nagasaka T, Hirashita M, Takahata T, Nuriomura T. Effects of human menstrual cycle on thermoregulatory vasodilation during exercise. Eur J Appl Physiol. 1986;54:559–65. doi: 10.1007/BF00943341. [DOI] [PubMed] [Google Scholar]
- 16.Jakicic JM, Clark K, Colmena E, et al. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33(12):2145–56. doi: 10.1097/00005768-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Kolka MA, Stephenson LA. Effect of luteal phase elevation in core temperature on forearm blood flow during exercise. J Appl Physiol. 1997;82:1079–83. doi: 10.1152/jappl.1997.82.4.1079. [DOI] [PubMed] [Google Scholar]
- 18.Moran LJ, Brinkworth G, Noakes M, Norman RJ. Effects of lifestyle modification in polycystic ovarian syndrome. Reprod Biomed Online. 2006;12:569–78. doi: 10.1016/s1472-6483(10)61182-0. [DOI] [PubMed] [Google Scholar]
- 19.Nadel ER, Bullard RW, Stolwijk JA. Importance of skin temperature in the regulation of sweating. J Appl Physiol. 1971;31:80–7. doi: 10.1152/jappl.1971.31.1.80. [DOI] [PubMed] [Google Scholar]
- 20.Nadel ER, Mitchell JW, Stolwijk JAJ. Differential thermal sensitivity in the human skin. Pflugers Arch. 1973;340:71–6. doi: 10.1007/BF00592198. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama T, Suzuki M, Ishizuka N. Action of progesterone on thermosensitive neurons. Nature. 1975;258:80. doi: 10.1038/258080a0. [DOI] [PubMed] [Google Scholar]
- 22.Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13:251–7. doi: 10.1016/s1043-2760(02)00612-4. [DOI] [PubMed] [Google Scholar]
- 23.Oberye JJL, Mannaerts BMJL, Huisman JAM, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part I. Absolute bioavailability of 0.25 mg of ganirelix after a single subcutaneous injection in healthy female volunteers. Fertil Steril. 1999;72:1001–5. doi: 10.1016/s0015-0282(99)00413-6. [DOI] [PubMed] [Google Scholar]
- 24.Oberye JJL, Mannaerts BMJL, Huisman JAM, Timmer CJ. Pharmacokinetic and Pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril. 1999;72:1006–12. doi: 10.1016/s0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- 25.Rossi GP, Belfiore A, Bernini G, et al. Body mass index predicts plasma aldosterone concentrations in overweight–obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–71. doi: 10.1210/jc.2008-0251. [DOI] [PubMed] [Google Scholar]
- 26.Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39(2):377–90. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro Y, Moran D, Epstein Y. Acclimatization strategies—preparing for exercise in the heat. Int J Sports Med. 1998;19(2 suppl):S161–3. doi: 10.1055/s-2007-971986. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro Y, Pandolf KB, Avellini BA, Pimental NA, Goldman RF. Physiological responses of men and women to humid and dry heat. J Appl Physiol. 1980;49:1–8. doi: 10.1152/jappl.1980.49.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Shibasaki M, Davis SL, Cui J, et al. Neurally mediated vasoconstriction is capable of decreasing skin blood flow during orthostasis in the heat-stressed human. J Physiol. 2006;575:953–9. doi: 10.1113/jphysiol.2006.112649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva NL, Boulant JA. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am J Physiol. 1986;250:R625–32. doi: 10.1152/ajpregu.1986.250.4.R625. [DOI] [PubMed] [Google Scholar]
- 31.Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. J Appl Physiol. 2000;88:1643–9. doi: 10.1152/jappl.2000.88.5.1643. [DOI] [PubMed] [Google Scholar]
- 32.Stephenson LA, Kolka MA. Thermoregulation in women. In: Holloszy JO, editor. Exercise and Sports Science Reviews. Williams & Wilkins; Philadelphia (PA): 1993. pp. 231–62. [PubMed] [Google Scholar]
- 33.Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol. 1999;86:22–8. doi: 10.1152/jappl.1999.86.1.22. [DOI] [PubMed] [Google Scholar]
- 34.Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod. 2006;21:80–9. doi: 10.1093/humrep/dei311. [DOI] [PubMed] [Google Scholar]
- 35.Trivax B, Azziz R. Diagnosis of polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50:168–77. doi: 10.1097/GRF.0b013e31802f351b. [DOI] [PubMed] [Google Scholar]
- 36.Tsilchorozidou T, Overton C, Conway GS. The pathophysiology of polycystic ovary syndrome. Clin Endocrinol. 2004;60:1–17. doi: 10.1046/j.1365-2265.2003.01842.x. [DOI] [PubMed] [Google Scholar]
- 37.Tuomela T, Miettinen P, Viinikka L, Perheentupa J. Estrogen–androgen antagonism in the regulation of epidermal growth factor in mouse submandibular salivary gland and kidneys. Life Sci. 1990;47:1925–32. doi: 10.1016/0024-3205(90)90404-f. [DOI] [PubMed] [Google Scholar]